Abstract
A β-hydroxy hydroperoxide was obtained through base catalyzed disproportionation of a hydroperoxy endoperoxide available by singlet oxygenation of cyclohepta-1,4-diene. Vitamin E and C induce fragmentation of this β-hydroxy hydroperoxide generating aldehydes, especially in the presence of redox active metal ions such as those present in vivo, e.g., under conditions of “iron overload”. This chemistry may contribute to the oxidative cleavage of polyunsaturated fatty acyls that produces similar aldehydes, which damage proteins and DNA through covalent adduction resulting in “oxidative injury”.
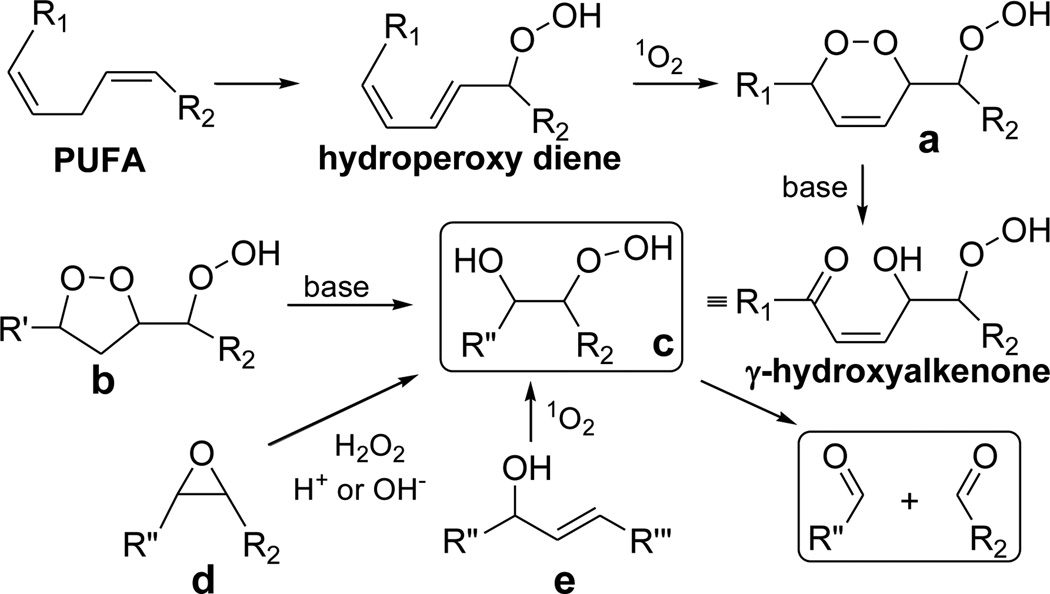
Oxidative cleavage of unsaturated fatty acyls produces aldehydes in vivo. Many of these aldehydes are toxic, causing “oxidative injury” owing to damage of proteins or DNA through covalent adduction. We postulated that β-hydroxy hydroperoxides (Scheme 1, c) are one type of intermediate that may be susceptible to fragmentation under mild physiological conditions. Possible routes to β-hydroxy hydroperoxides include Kornblum-DeLaMare rearrangement1 of β-hydroperoxy alkylperoxides (Scheme 1, a and b). Singlet oxygenation of conjugated hydroperoxy dienes generates the requisite endoperoxides (Scheme 1, a).2,3 Hydroperoxy dienes, that are major4 products of both enzymatic and free radical-promoted oxidation of polyunsaturated fatty acyls (PUFAs), accumulate in atherosclerotic lesions.5 Singlet oxygen can be generated in vivo through photosensitization6 by retinal7 in the eye as well as non photochemically.8–10 β-Hydroperoxy alkylperoxides (Scheme 1, b) are also produced by free radical cyclization of unsaturated peroxy radicals11–13 formed during the autoxidation of PUFAs.14,15 β-Hydroxy hydroperoxides (Scheme 1, c) are also available through addition of H2O2 to epoxides (Scheme 1, d)16–18 or through singlet oxygenation of allylic alcohols (Scheme 1, e).19,20
SCHEME 1.
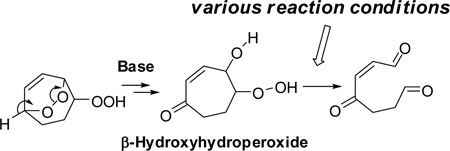
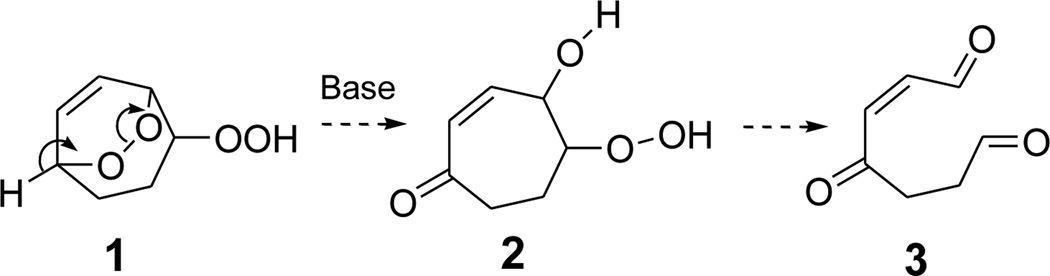
We chose the hydroperoxy endoperoxide 1 (Scheme 2) as a simple model system that would deliver a single product 3 by fragmentation of an intermediate β-hydroxy hydroperoxide 2. Notably, the electrophilic conjugated enedione functionality present in 3 is also found in certain, especially toxic, lipid oxidative fragmentation products, e.g., 4-oxo-2-nonenal, that damage proteins21 and DNA22 through covalent adduction.
SCHEME 2.
The hydroperoxy endoperoxide 1 is readily available through singlet oxygenation of cyclohepta-1,4-diene.3 Reaction conditions were defined that optimized the conversion of 1 into 2, an analogue of the PUFA-derived γ-hydroxyalkenone of Scheme 1. This model β-hydroxy hydroperoxide was exploited to define reaction conditions that promote fragmentation.
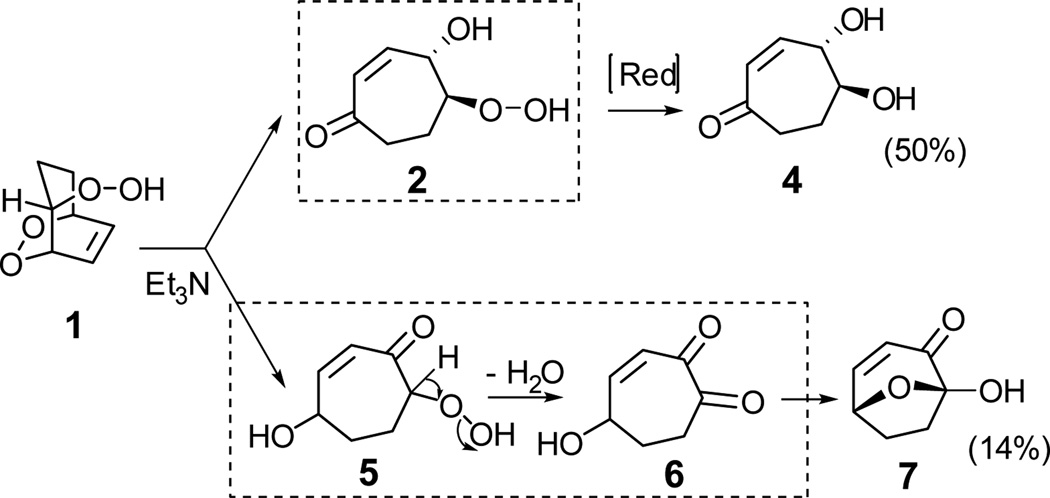
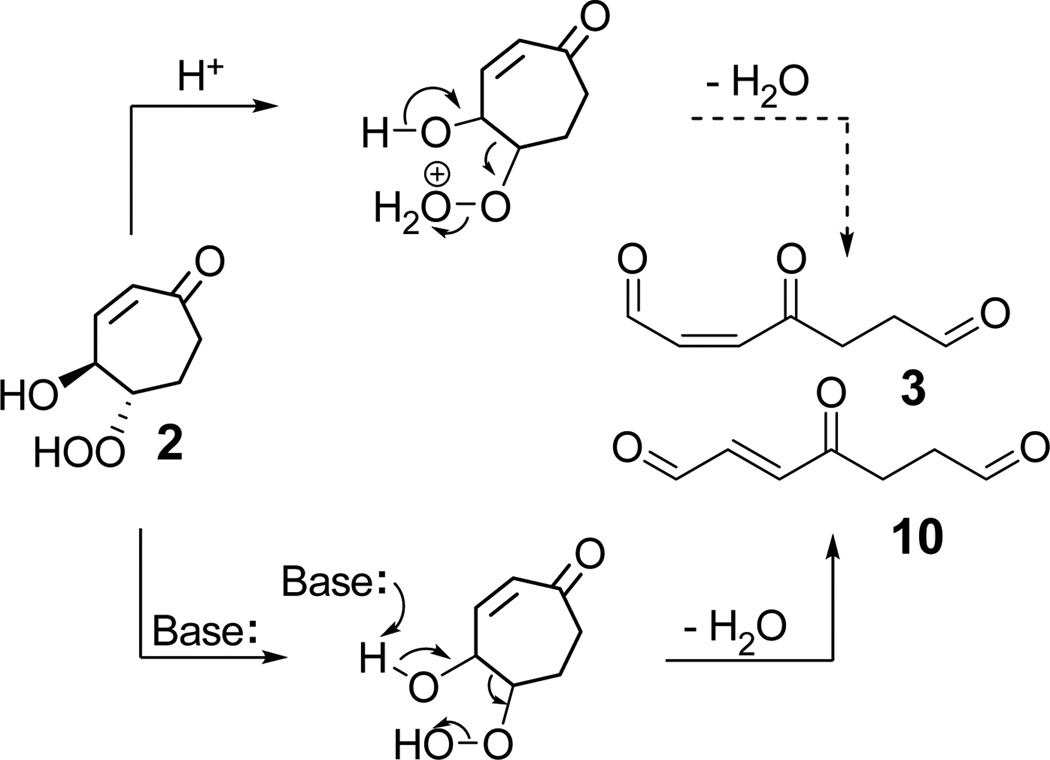
Disproportionation of the endoperoxide 1 was expected to produce β and δ hydroxyhydroperoxides 2 and 5 (Scheme 3). However, attempted use of triethylamine to catalyze23 the isomerization of endoperoxide 1 delivered a ketodiol 4 and a cyclic hydroxydione hemiacetal 7, in isolated yields of 50% and 14% respectively, and none of the desired β-hydroxy hydroperoxide 2. The production of 7 undoubtedly involves dehydration of the α-hydroperoxy ketone array in 5. Reduction of 2 by triethylamine presumably accounts for the generation of 4. It is known that amines can reduce hydroperoxides to alcohols.24 For example, tert-butyl hydroperoxide was reduced by tri-n-propylamine to give alcohol in good yield (~ 80%), and products generated through fragmentation of tri-propylamine which included di-n-propylamine (32%) and carbonyl compounds (mainly 2-methyl-2-pentenal, 17%).
SCHEME 3.
Other bases were also tested as catalysts for disproportionation. Proton sponge (N,N,N',N'-tetramethyl-naphthalene-1,8-diamine), DABCO (1,4-diaza-bicyclo-[2.2.2]-octane), or pyridine were added to solutions of 1 in CDCl3 and the reactions were monitored by NMR. Endoperoxide 1 is stable in the presence of pyridine. The main product generated through catalysis by proton sponge or DABCO is 7 (Scheme 3).
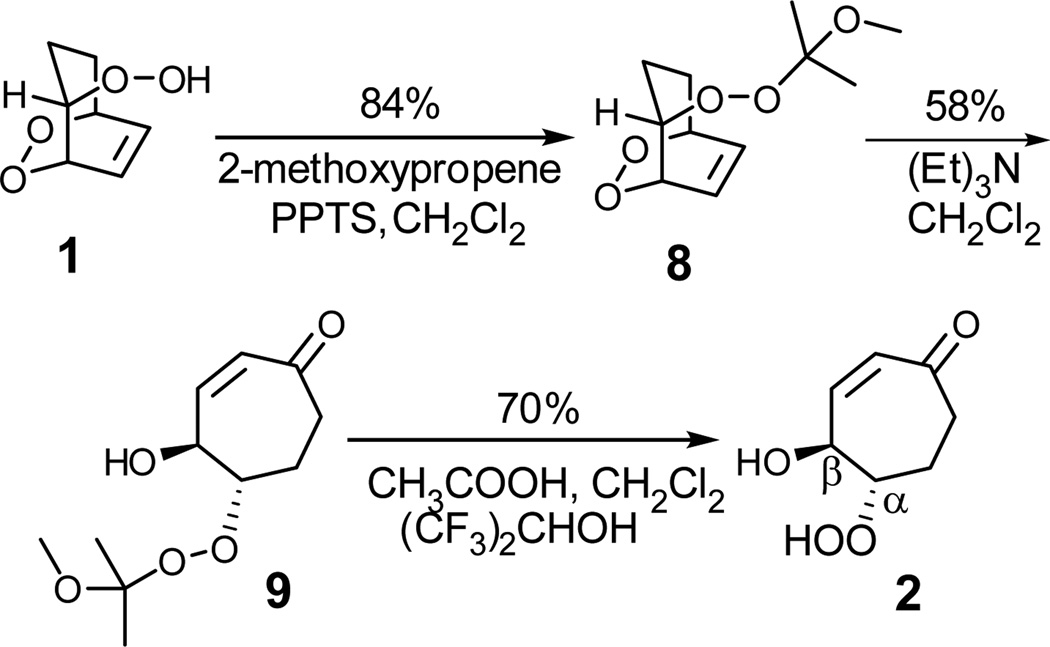
In an alternative route to 2 (Scheme 4), hydroperoxy endoperoxide 1 was first protected by treatment with 2-methoxypropene to give 8.25 In contrast with 1, triethylamine catalyzed isomerization of the methoxyisopropyl derivative 8 of hydroperoxide 1 delivered 9 in 58% yield together with 7 in 13% yield. Deprotection of 9 under mild acidic conditions delivered the requisite β-hydroxy hydroperoxide 2 in 70% yield. Acetic acid was used to catalyze the deprotection of 9 in a mixed solvent system of hexafluoro-2-propanol and methylene chloride. The isolation of 2 was facilitated by the ease with which acetic acid can be removed by rotary evaporation into a dry ice-cooled trap under high vacuum. This simple route provided ready access to the β-hydroxy hydroperoxide 2 for the proposed fragmentation reaction studies. The contrasting behavior of the hydroperoxide 1 and 2-methoxypropyl derivative 8 are especially striking. It provides a useful protection strategy. The discovery that such derivatives are not reduced by amines, in contrast with the hydroperoxide precursor, is valuable information for those engaged in synthetic manipulation of hydroperoxides.
SCHEME 4.
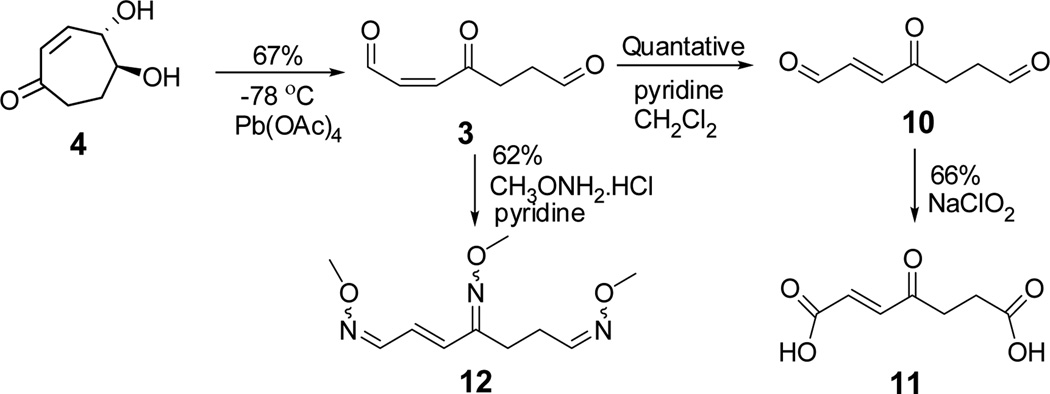
Cis-aldehyde 3, the expected product, from the fragmentation of β-hydroxy hydroperoxide 2 was synthesized through the oxidative cleavage of diol 4 by lead tetraacetate (Scheme 5).26 Trans-aldehyde 10 was readily obtained by treatment of 3 with pyridine.27 Trans-aldehyde 10 can be selectively oxidized to 4-oxo-2-heptenedioic acid 11, a known compound.28 The cis-aldehyde 3 reacts with methoxyamine hydrochloride to form an oxime derivative 12,29 which is stable, and whose structure was further confirmed by mass spectroscopy. Thus, the structures of cis and trans-aldehydes 3 and 10 were unambiguously confirmed.
SCHEME 5.
Previous studies showed that β-hydroxy hydroperoxides are subject to acid16,17 or base18,30 catalyzed fragmentation to carbonyl compounds. We examined the ability of amine bases or carboxylic acids (Table 1) to catalyze the fragmentation of 2 (Scheme 6). In the presence of pyridine, the β-hydroxy hydroperoxide 2 readily fragmented in CH2Cl2 solution to afford trans-aldehyde 10 (38%) and the reduction product 4 (16%). Treatment of 2 with triethylamine gave only diol 4 (57%) and no fragmentation products. The result supports the involvement of 2 in the mechanism postulated above for the conversion of 1 into 4 upon treatment with triethylamine, and further confirms the fact that 2 can be easily reduced. β-Hydroxy hydroperoxide 2 was partly decomposed when it was incubated in trifluoroacetic acid overnight, however the yield of aldehyde is very low.
TABLE 1.
Acid and base promoted decomposition of 2.
| catalyst (in CH2Cl2) |
reaction time | unreacted | product yields | ||
|---|---|---|---|---|---|
| 2 | 3 | 10 | 4 | ||
| pyridine | 12 h | / | / | 38% | 16% |
| Et3N | 5 h | / | / | / | 57% |
| CF3COOH | 12 h | 30% | 1% | / | / |
SCHEME 6.
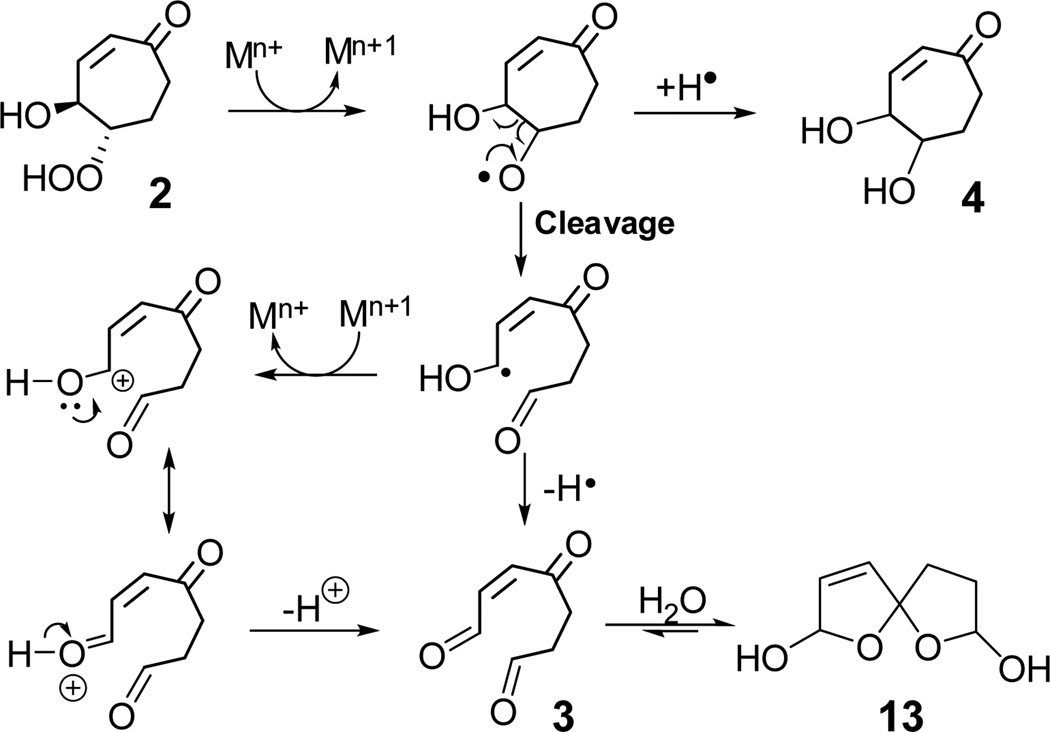
Although the β-hydroxy hydroperoxide 2 is stable in CHCl3 or CH2Cl2 solutions, it decomposed in D2O upon incubating at 37 °C for 24 h to form aldehyde 3 (as a hydrate in water) and diol 4 (Table 2). It is tempting to speculate that the effect of a strongly polar protic solvent indicates a polar mechanism for the decomposition in water. However, hydroperoxides are known to undergo transition metal ion-mediated decomposition through single electron transfer leading to the formation of an alkoxy radical.31,32 Possibly, there are traces of metal ions in water that are sufficient to promote the fragmentation (vide infra). The formation of diol 4 from the β-hydroxy hydroperoxide 2 may also involve homolytic cleavage of the hydroperoxide, promoted by trace metal ions, that generates an alkoxy radical which abstracts hydrogen or an alkoxide that abstracts a proton. Alternatively, β-scission of the alkoxy radical can result in the formation of cis-aldehyde 3 (Scheme 7). In aqueous solution, water adds to an aldehyde carbonyl in 3 to form a hydrate 13.
TABLE 2.
Metal ion-promoted decomposition of 2.a
| catalyst (in D2O) |
reaction time | unreacted | product yields | ||
|---|---|---|---|---|---|
| 2 | 3b | 4 | |||
| / | 24 h | / | 43% | 11% | |
| PBS buffer (200 mM) | 0.5 h | / | 15% | 30% | |
| Fe3+ (3.6 mM) | 0.5 h | / | 70% | 5% | |
| Cu2+ (3.6 mM) | 0.5 h | / | 80% | 9% | |
36 mM at 37 °C.
as the hydrate 13.
SCHEME 7.
Decomposition of 2 was much faster in phosphate buffered saline or in the presence of added metal ions (Cu2+ or Fe3+. 10 mol%) than in “pure” D2O. Incubation of 2 with Cu2+ or Fe3+ (0.1 equiv.) in aqueous solution at 37 °C for 0.5 h delivered aldehyde hydrate (~70% yield) and diol 4 (5%). Although pure cis-aldehdye 3 was not readily extracted from the aqueous solution even under acidic conditions, the aldehyde hydrate reacts with methoxyamine hydrochloride to form oxime derivatives which were readily isolated and characterized.
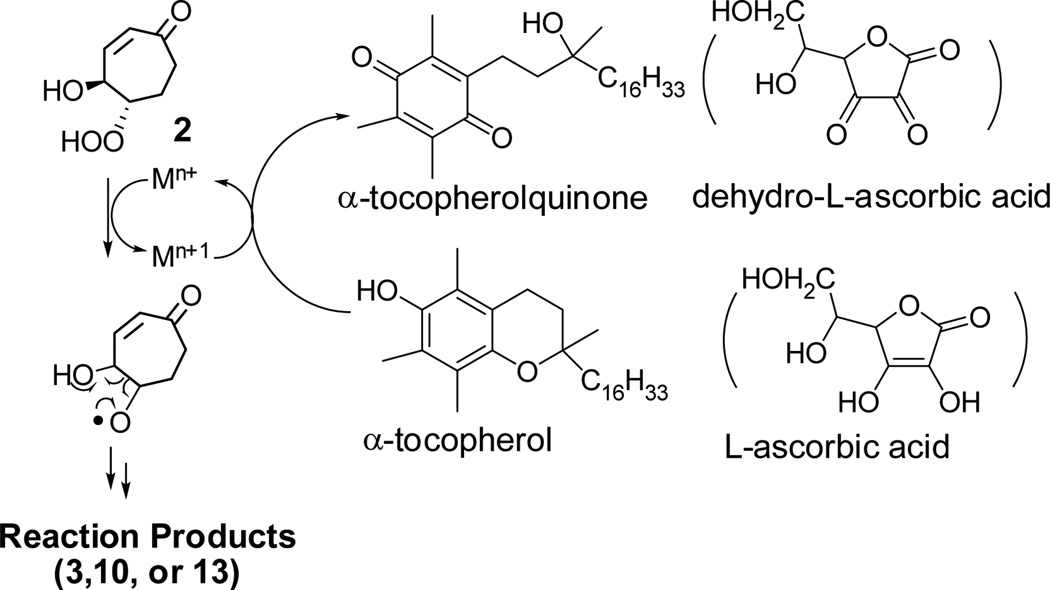
Vitamins E (α-tocopherol) and C (L-ascorbic acid) are generally considered to protect biological membranes from lipid peroxidation because of their ability to scavenge reactive oxygen species including peroxy radicals derived from unsaturated lipids.33–35 Vitamins E and C efficiently transfer a hydrogen atom to a peroxyl radical, and hence, break the chain reaction of lipid peroxidation. However, their potential to serve as pro-oxidants, rather than anti-oxidants in the presence of metal ions has also been recognized.35,36 Ascorbic acid is known to accelerate the decomposition of lipid hydroperoxides by reducing Fe3+ to Fe2+, that reduces the hydroperoxy group to generate alkoxy radicals and hydroxide.37 We tested the effects of vitamins E and C on the decomposition of β-hydroxy hydroperoxide 2 (Table 3). Both vitamins E and C promoted the fragmentation of 2 in the presence of metal ions (Fe2+, Cu2+). When reacted at room temperature for 4 h, β-hydroxy hydroperoxide 2 with only vitamin E or catalytic amounts (0.1 equiv.) of metal ions (Fe2+, Cu2+) gave very low yields (< 5%) of fragmentation product (3 or 10), and most of 2 was unreacted under these conditions. But when both vitamin E and metal ions were added, the reaction was accelerated. Decomposition of 2 was complete in 4 h. Aldehydes 3 or 10 were produced in about 40% yield. A possible mechanism for this effect is presented in Scheme 8. Reduced metal ions induce the homolysis of the β-hydroxy hydroperoxide 2 to form an alkoxy radical38, which undergoes β-scission to generate aldehydes. Vitamin E (α-tocopherol) is known to donate one electron to regenerate Fe2+ from Fe3+ and Cu1+ from Cu2+, and thus can promote the homolysis. The oxidation product of vitamin E is α-tocopheroquinone (α-TQ), which was also detected by NMR in the reaction product mixture. It is interesting that diol 4, the product of hydroperoxide reduction, was not detected in this reaction of 2. Apparently β-scission of the putative alkoxy radical intermediate is much faster than the abstraction of a hydrogen atom to form diol 4, even in an environment where a potential hydrogen atom donor (vitamin E) is abundant. This is a reasonable consequence of stabilization of the radical produced upon fragmentation by conjugation with both a C=C bond and an α oxygen. It also may reflect suppression of hydrogen atom transfer of the phenolic hydrogen owing to hydrogen bonding with the relatively strong hydrogen bond acceptor solvent CH3CN.39 Vitamin C also accelerated the fragmentation of 2 in water even without added redox active metal ions.40 In a D2O solution containing both vitamin C and Cu2+ or Fe3+, the β-hydroxy hydroperoxide 2 was completely decomposed in 5 min. The main product was the aldehyde hydrate (~60% yield). These results clearly demonstrate the ability of vitamins E and C to promote the formation of toxic aldehydes from β-hydroxy hydroperoxides.
TABLE 3.
Vitamin E and C-promoted fragmentation of 2.
| reagents a (slovent) |
reaction time temperature |
unreacted | products yields | ||
|---|---|---|---|---|---|
| 2 | 3b | 10 | 4 | ||
| Vit E (CH3CN) | 4 h, 25 °C | 96% | 4% | / | / |
| Vit E, Fe2+ (CH3CN) | 4 h, 25 °C | / | 26% | 14% | / |
| Vit E, Cu2+ (CH3CN) | 4 h, 25 °C | / | 36% | / | / |
| Fe2+ (CH3CN) | 4 h, 25 °C | 99% | 1% | / | / |
| Cu2+ (CH3CN) | 4 h, 25 °C | 98% | 2% | / | / |
| Vit C (D2O) | 3 h, 37 °C | / | 35% | / | 13% |
| Vit C, Fe3+ (D2O) | 5 min, 25 °C | / | 65% | / | 15% |
| Vit C, Cu2+ (D2O) | 5 min, 25 °C | / | 66% | / | 13% |
the concentration of vitamin E (or C): 2 : metal ions = 1.5 : 1 :0.1.
as an aldehyde hydrate 13.
SCHEME 8.
In conclusion, a β-hydroxy hydroperoxide, synthesized through base catalyzed disproportionation of a hydroperoxy endoperoxide, undergoes acid as well as base promoted fragmentations. Under neutral aqueous biomimetic conditions, fragmentation can be induced by redox active metal ions. Vitamins C and E, strongly promote fragmentation of β-hydroxy hydroperoxides by reducing catalytic metal ions. This chemistry may explain why vitamins E and C exhibit disappointingly low efficacy in some disease therapies that attempt to prevent oxidative injury mediated by toxic aldehydes generated through oxidative fragmentation of PUFAs. Thus, although these antioxidants may decrease the free radical-induced production of intermediates such as hydroperoxy endoperoxides and β-hydroxy hydroperoxides, they can promote – especially in the presence of redox active metal ions – the fragmentation reactions that convert such intermediates into toxic aldehydes which damage proteins and DNA. Further studies to evaluate the biological significance of the fragmentation of β-hydroxy hydroperoxides, demonstrated in the present investigation, should focus on detecting lipid-derived β-hydroxy hydroperoxides (Scheme 1, c) and their putative β-hydroperoxy alkylperoxide precursors (Scheme 1, a and b) in vivo.
Experimental Section
Triethylamine-catalyzed isomerization of exo-6,7-dioxabicyclo[3.2.2]non-8-en-2-yl-hydroperoxide (1)
Endoperoxide 1 (100 mg, 0.64 mmol) was dissolved in dry CH2Cl2 (10 mL) and the solution was cooled with an ice bath. To this solution was added Et3N (178 uL, 1.28 mmol) dropwise over 20 min. The mixture was stirred for 1 h at 0 °C and then 12 h at room temperature. Then the solution was neutralized with 1.0 M aqueous HCl. The organic layer was removed and the water layer was extracted with CH2Cl2 (2 × 10 mL). The combined organic layer was washed with water and dried with MgSO4. The crude product was chromatographed on silica gel by elution with ethyl acetate-hexanes (8:2) to afford 4,5-dihydroxy-cyclohept-2-enone (4) (46.1 mg, 0.32 mmol, yield = 50%, Rf = 0.20) and 1-hydroxy-8-oxa-bicyclo[3.2.1]oct-3-en-2-one (7) (12.5 mg, 0.09 mmol, yield = 14%, Rf = 0.51).
4,5-Dihydroxy-cyclohept-2-enone (4)
1H NMR (400 MHz, CDCl3) δ 6.48 (dd, J = 12.8, 3.2 Hz, 1H), 5.92 (ddd, J = 12.8, 2.6, 1.0 Hz, 1H), 4.44 (dt, J = 8.8, 2.6 Hz, 1H), 3.84 (ddd, J = 8.8, 6.4, 5.0 Hz, 1H), 3.12 (s, 1H), 2.85 (s, 1H), 2.75-2.61 (1H), 2.62-2.51 (1H), 2.20-2.10 (1H), 2.05-1.95 (1H); 13C NMR (100 MHz, CDCl3) δ 202.1, 146.3, 130.3, 75.0, 73.8, 38.8, 28.5. HRMS (FAB): m/z calcd for C7H11O3 (MH+), 143.0708, found 143.0715.
1-Hydroxy-8-oxa-bicyclo[3.2.1]oct-3-en-2-one (7)
1H NMR (400 MHz, CD3OD) δ 7.34 (dd, J = 9.6, 4.4 Hz, 1H), 5.99 (d, J = 9.6 Hz, 1H), 4.91 (dd, J = 6.8, 4.4 Hz, 1H), 2.40-2.20 (1H), 2.05-1.95 (2H), 1.80-1.70 (1H); 13C NMR (100 MHz, CD3OD) δ 196.5, 154.8, 125.1, 104.6, 74.8, 29.6, 26.9.
2-(1-Methoxy-1-methyl-ethylperoxy)-6,7-dioxabicyclo-[3.2.2]-non-8-ene (8)
Endoperoxide 1 (150 mg, 0.95 mmol) and pyridinium p-toluene sulfonate (15 mg) were dissolved in dry CH2Cl2 (15 mL) under argon. 2-Methoxypropene (200 µL, 2.09 mmol) was added dropwise. The resulting solution was stirred 10 min at room temperature. After removal the solvent by the rotary evaporation, the crude product was chromatographed on silica gel by elution with ethyl acetate-hexanes (3:7) to afford 2-(1-methoxy-1-methyl-ethylperoxy)-6,7-dioxabicyclo[3.2.2]non-8-ene (8) (184 mg, 0.80 mmol, yield = 84%, Rf = 0.28). 1H NMR (400 MHz, CDCl3) δ 6.42-6.30 (2H), 5.05-4.95 (1H), 4.70-4.60 (1H), 4.24 (ddd, J = 11.4, 5.0, 2.0 Hz, 1H), 3.22 (s, 3H), 1.90-1.70 (3H), 1.30 (d, J = 2.4 Hz, 6H), 1.30-1.10 (1H); 13C NMR (100 MHz, CDCl3) δ 129.0, 126.4, 105.0, 84.6, 76.6, 76.5, 49.4, 26.0, 23.0, 22.6, 22.0.
4-Hydroxy-5-(1-methoxy-1-methyl-ethylperoxy)-cyclohept-2-enone (9)
An approximately 0.2 M solution of protected endoperoxide 8 (216 mg, 0.94 mmol) in dry CH2Cl2 was cooled with an ice bath. To this solution was added Et3N (262 uL, 1.88 mmol, 2 equiv) dropwise over 20 min. The mixture was stirred at 0 °C for 3 h. Then the solution was neutralized with 1 M aqueous HCl. The organic layer was removed and the water layer was extracted with CH2Cl2 (2 × 20 mL). The combined organic layer was dried with MgSO4. The crude product was chromatographed on silica gel by elution with ethyl acetate-hexanes (4.5:5.5) to recover starting material 8 (93 mg, 0.40 mmol) and afford 4-hydroxy-5-(1-methoxy-1-methyl-ethylperoxy)-cyclohept-2-enone (9) (72 mg, 0.31 mmol, yield = 58%, Rf = 0.24) and 7 (10 mg, 0.07 mmol, yield = 13%). 1H NMR (400 MHz, CDCl3) δ 6.51 (ddd, J = 12.4, 3.2, 0.8 Hz, 1H), 5.91 (ddd, J = 12.4, 2.8, 1.2 Hz, 1H), 4.82-4.70 (1H), 4.23-4.15 (1H), 3.62 (s, 1H), 3.30 (s, 3H), 2.70-2.50 (2H), 2.25-2.15 (1H), 2.15-2.00 (1H), 1.40 (d, J = 2.4 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 202.2, 146.5, 129.9, 105.8, 86.0, 71.6, 50.1, 38.6, 24.5, 23.1, 22.8.
5-Hydroperoxy-4-hydroxy-cyclohept-2-enone (2)
The protected hydroperoxide 9 (180 mg, 0.78 mmol) was dissolved in a mixture of CH2Cl2 (3 mL) and hexfluro-2-propanol (1mL). Acetic acid (25 µL) was added to the reaction mixture as catalyst. The solution was stirred for 4 h under an argon atmosphere. After removal of the solvents by rotary evaporation, the crude product was separated on a silica gel column eluting with ethyl acetate-hexanes (1:1) to afford 5-hydroperoxy-4-hydroxy-cyclohept -2-enone (2) (52 mg, 0.33 mmol, yield = 70%, Rf = 0.14). 1H NMR (400 MHz, CDCl3) δ 8.20 (s, 1H), 6.54 (dd, J = 12.4, 3.2 Hz, 1H), 5.97 (ddd, J = 12.4, 2.8, 1.6 Hz, 1H), 4.86 (dm, J = 8.0 Hz, 1H), 4.30-4.20 (1H), 2.91 (s, 1H), 2.80-2.60 (2H), 2.30-2.00 (2H); 13C NMR (100 MHz, CDCl3) δ 202.0, 146.3, 130.1, 87.0, 71.8, 38.6, 24.0. This compound was further characterized by converting to the diol 4. HRMS (FAB for diol 4): m/z calcd for C7H11O3 (MH+), 143.0708, found 143.0715.
Oxidative cleavage of 4,5-dihydroxy-cyclohepta-2-enone (4)
Diol 4.4 (9.5 mg, 0.067 mmol) was dissolved in dry CH2Cl2 (1 mL) and was cooled with a Dry ice-acetone bath (−78 °C). Pb(OAc)4 (22 mg, 0.050 mmol) was also dissolved in dry CH2Cl2 (1 mL) and the solution was cooled to −78 °C. Then the Pb(OAc)4 solution was added dropwise through a cannula to the solution of diol 4. The reaction mixture was stirred for 30 min at −78 °C under an argon atmosphere. Then the solvent was removed and the residue was purified by flash chromatography on a silica gel column (ethyl acetate/hexanes, 8:2) to give cis-4-oxo-hept-2-enedial (3) (4.8 mg, 0.034 mmol, yield = 67%, Rf = 0.40). The cis-aldehyde 3 was dissolved in CH2Cl2 (1 mL) with 50 µL of pyridine. The mixture was stirred overnight at room temperature. The solvent was removed to deliver the pure trans-4-oxo-hept-2-enedial (10).
Cis-4-oxo-hept-2-enedial (3)
1H NMR (400 MHz, CDCl3) δ 10.19 (d, J = 7.2 Hz, 1H), 9.84 (s, 1H), 6.99 (d, J = 11.6 Hz, 1H), 6.22 (dd, J = 11.6, 7.2 Hz, 1H), 2.80-3.00 (4H); 13C NMR (100 MHz, CDCl3) δ 199.8, 198.4, 192.7, 139.7, 138.5, 37.7, 35.7. This compound was further characterized by conversion into the methoxime 12.
Trans-4-oxo-hept-2-enedial (10)
1H NMR (400 MHz, CDCl3) δ 9.84 (s, 1H), 9.80 (d, J = 7.2 Hz, 1H), 6.95-6.80 (2H), 3.10-3.00 (2H), 3.00-2.90 (2H); 13C NMR (100 MHz, CDCl3) δ 199.8, 197.8, 193.3, 144.4, 138.0, 37.5, 33.4. This compound was further characterized by conversion into the known acid 11.
Synthesis of 4-oxo-hept-2-enedioic acid (11)
To a magnetically stirred solution of aldehyde 10 (10.0 mg, 0.07 mmol) in t-BuOH-H2O (5:1, v/v, 1.5 mL) was added a solution containing NaH2PO4.H2O (29 mg, 0.21 mmol), 2-methyl-2-butene (400 uL, 0.8 mmol, 2 M solution in THF) and NaClO2 (40 mg, 0.42 mmol) in t-BuOH-H2O (5:1, v/v, 1.5 mL). The resulting mixture was stirred for 2 h at room temperature under argon. Then the pH of the solution was adjusted to 3.0 by adding 1 M aqueous HCl. The mixture was extracted with CH2Cl2/CH3OH (3:1) (3 × 15 mL). The combined organic layer was dried with MgSO4. After removal of the solvents by rotary evaporation, 4-oxo-hept-2-enedioic acid (11, 8.0 mg, 0.046 mmol, yield = 66%, Rf = 0.25) was purified by through a silica gel column eluting with CH2Cl2/CH3OH (3:1).
4-Oxo-hept-2-enedioic acid (11)
1H NMR (400 MHz, CD3OD) δ 7.02 (d, J = 16.0 Hz, 1H), 6.70 (d, J = 16.0 Hz, 1H), 2.98 (t, J = 6.4 Hz, 2H), 2.60 (t, J = 6.4 Hz, 2H); 13C NMR (100MHz, CD3OD) δ 199.1, 175.0, 167.6, 139.0, 131.7, 35.4, 27.3. These spectra agree with those reported previously.41
Preparation of oxime derivatives of cis-4-oxo-hept-2-enedial (3)
A solution of aldehyde 3 (4.2 mg, 0.03 mmol) and methoxyamine hydrochloride (45 mg, 0.54 mmol) in anhydrous pyridine (500 µL) was stirred at room temperature for 15 h. Pyridine was then removed with a steam of argon. Then water (1 mL) was added to the solid residue. The aqueous layer was extracted with ethyl acetate (3 × 5 mL), and the combined organic extracts were dried with MgSO4. The crude product was chromatographed on silica gel with 20% ethyl acetate in hexanes to afford 4-methoxyimino-hept-2-enedial bis-(O-methyl-oxime) (12) (4.2 mg, 0.018 mmol, yield = 62%, Rf = 0.38), which is mixture of 8 isomers.
4-Methoxyimino-hept-2-enedial bis-(O-methyl-oxime) (12)
HRMS (FAB): m/z calcd for C10H18N3O3 (MH+), 228.1348, found 228.1346. m/z calcd for C10H17N3O3 (M+)-OCH, 198.1243, found 198.1227. See appendix for the 1H NMR spectrum of 12.
Acid or base-catalyzed decomposition of 5-hydroperoxy-4-hydroxy-cyclohept-2-enone (2)
β-Hydroxyhydroperoxide 2 (4 mg, 0.025 mmol) was dissolved in CH2Cl2 (1 mL). Then pyridine (100 µL), triethylamine (50 µL), CF3COOH (50 µL) or HCl (20 µL, 37% in water) was added to catalyze the decomposition. The reaction mixtures were vortexed and then incubated at 37 °C under argon for various times. Then the solvents were removed with a steam of nitrogen. The residues were dissolved into 0.7 mL of CDCl3 (with 0.01 M phenyltrimethylsilane as internal standard) and transferred into NMR tubes. From the ratio of the integral areas of NMR signals belonging to decomposition products 3, 4, 10, and 13 to that for the internal standard (phenyltrimethylsilane), the yields of 3, 4, 10 and 4 were determined.
Decomposition of 5-hydroperoxy-4-hydroxy-cyclohept-2-enone (2) in deuterium oxide solutions
Cis-4-oxo-hept-2-enedial (3, 3 mg, 0.021 mmol) was dissolved in D2O (0.7 mL) and transferred into an NMR tube. The tube was mixed thoroughly by shaking and the 1H NMR spectrum was immediately recorded. The aldehyde peaks had totally disappeared and the peaks belonging to aldehyde hydrates (δ 5.40 – 5.80) appeared. It is difficult to extract the aldehyde from aqueous solution even under acidic conditions. Methoxyamine hydrochloride (45 mg, 0.54 mmol) was added to the aqueous solution and the resulting mixture was stirred overnight to deliver the methoxime derivative. HRMS (FAB): m/z calcd for C10H18N3O3 (MH+), 228.1348, found 228.1348.
β-Hydroxyhydroperoxide 2 (4 mg, 0.025 mmol) was dissolved into D2O (0.7 mL), phosphate buffered saline (pH 7.4, 200 mM in D2O), Cu2+ (3.6 mM CuSO4 in D2O) and Fe3+ (3.6 mM FeCl3 in D2O) solutions. Tert-butyl alcohol (5 µL) was added as internal standard. The solutions were transferred into NMR tubes and incubated at 37 °C under an argon atmosphere for various times. From the ratio of the integral areas of NMR signals belonging to decomposition products to that for the internal standard (tert-butyl alcohol), the yields of decomposition products were determined. Because the Fe3+ is paramagnetic, before obtaining the NMR spectrum, Fe3+ was removed by passing through chelex 100 resin (2 g) in a pipet.
Vitamin E and C promoted fragmentation of 5-hydroperoxy-4-hydroxy –cyclohept-2-enone (2)
β-Hydroxyhydroperoxide 2 (4 mg, 0.025 mmol) and vitamin E (16.4 mg, 0.038 mmol) were dissolved in CH3CN (0.8 mL). FeSO4·7H2O (0.7 mg, 0.0025 mmol) or CuSO4 (0.4 mg, 0.0025 mmol) in 20 µL of water was added to catalyze the reaction. The resulting solution was stirred for 4 h at room temperature under an argon atmosphere. Traces of water and metal salts were removed by passing the reaction mixture through a pipet containing anhydrous Na2SO4 (2 g). Then solvent was removed and the residue was dissolved into 0.7 mL CDCl3 (with 0.01 M phenyltrimethylsilane as internal standard) in an NMR tube. From the ratio of the integral areas of NMR signals belonging to decomposition products to that for the internal standard, the products yields were determined. For comparison, β-hydroxyhydroperoxide 2 with vitamin E or with Fe2+ or Cu2+ were reacted under the same conditions, and the products yields were also determined from the ratios of the integral areas of 1H NMR signals.
β-Hydroxyhydroperoxide 2 (4 mg, 0.025 mmol) with vitamin C (0.038 mmol), or with vitamin C (0.038 mmol) and with Cu2+ or Fe3+ (0.0025 mmol) was dissolved into D2O (0.7 mL). Tertbutyl alcohol (5 µL) was added as internal standard. Then the mixture was vortexed, transferred into NMR tubes, and incubated at 37 °C under an argon atmosphere for various times. From the ratio of the integral areas of NMR signals attributable to decomposition products to that for the internal standard, the yields of the products were determined.
Supplementary Material
Acknowledgement
We are grateful for support of this work by National Institute of Health Grants GM021249 and EY016813.
Footnotes
Supporting Information Available: General experimental methods, and NMR spectra of compounds 2–4 and 7–13. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kornblum N, Delamare HE. J. Am. Chem. Soc. 1951;73:880–881. [Google Scholar]
- 2.Neff WEFEN, Selke E, Weisleder D. Lipids. 1983;18:868–876. [Google Scholar]
- 3.Gu X, Zhang W, Salomon RG. J. Am. Chem. Soc. 2007;129:6088–6089. doi: 10.1021/ja0689785. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida Y, Niki E. Biofactors. 2006;27:195–202. doi: 10.1002/biof.5520270117. [DOI] [PubMed] [Google Scholar]
- 5.Folcik VA, Nivar-Aristy RA, Krajewski LP, Cathcart MK. J Clin Invest. 1995;96:504–510. doi: 10.1172/JCI118062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bensasson RV, Land EJ, Truscott TG. Excited states and free radicals in biology and medicine. New York: Oxford University Press; 1993. [Google Scholar]
- 7.Rozanowska M, Wessels J, Boulton M, Burke JM, Rodgers MA, Truscott TG, Sarna T. Free Radic. Biol. Med. 1998;24:1107–1112. doi: 10.1016/s0891-5849(97)00395-x. [DOI] [PubMed] [Google Scholar]
- 8.Steinbeck MJ, Khan AU, Karnovsky MJ. J Biol Chem. 1992;267:13425–13433. [PubMed] [Google Scholar]
- 9.Steinbeck MJ, Khan AU, Karnovsky MJ. J Biol Chem. 1993;268:15649–15654. [PubMed] [Google Scholar]
- 10.Tarr M, Valenzeno DP. Photochem Photobiol Sci. 2003;2:355–361. doi: 10.1039/b211778a. [DOI] [PubMed] [Google Scholar]
- 11.Funk MO, Isaac R, Porter NA. J. Am. Chem. Soc. 1975;(5):1281. doi: 10.1021/ja00838a074. [DOI] [PubMed] [Google Scholar]
- 12.Mihelich ED. J. Am. Chem. Soc. 1980;102:7141–7143. [Google Scholar]
- 13.Carless HAJ, Batten RJ. Chem. Soc. Perkin Trans. I. 1987:1999–2007. [Google Scholar]
- 14.Yin H, Morrow JD, Porter NA. J. Biol. Chem. 2004;279(5):3766–3776. doi: 10.1074/jbc.M307137200. [DOI] [PubMed] [Google Scholar]
- 15.Havrilla CM, Hachey DL, Porter NA. J. Am. Chem. Soc. 2000;122:8042–8055. [Google Scholar]
- 16.Payne G, Smith CW. J. Org. Chem. 1957;22:1682. [Google Scholar]
- 17.Subramanyam V, Brizuela C, Soloway AH. J. Chem. Soc. Chem. Commun. 1976:508. [Google Scholar]
- 18.Ogata Y, Sawaki Y, Shimizu H. J. Org. Chem. 1978;43:1760. [Google Scholar]
- 19.Adam W, Nestler B. J. Am. Chem. Soc. 1993;115:5041–5049. [Google Scholar]
- 20.Griesbeck AG, Adam W, Bartoschek A, El-Idreesy TT. Photochem. Photobiol. Sci. 2003;2:877–881. doi: 10.1039/b302255b. [DOI] [PubMed] [Google Scholar]
- 21.Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Drug Metab. Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Blair IA. Trends Cardiovasc. Med. 2001;11:148–155. doi: 10.1016/s1050-1738(01)00094-9. [DOI] [PubMed] [Google Scholar]
- 23.Pearson AJ, Lai YS, Lu W, Pinkerton AA. J. Org. Chem. 1989;54:3882–3893. [Google Scholar]
- 24.Harold E, Mare DL. J. Org. Chem. 1960;25:2114–2126. [Google Scholar]
- 25.Baba N, Yoneda K, Tahara S, Iwasa J, Kaneko T, Matsuo M. J. Chem. Soc. Chem. Commun. 1990:1281–1282. [Google Scholar]
- 26.Deng Y, Salomon RG. J. Org. Chem. 1998;63:7789. [Google Scholar]
- 27.Chill L, Miroz A, Kashman Y. J. Nat. Prod. 2000;63:523. doi: 10.1021/np990342m. [DOI] [PubMed] [Google Scholar]
- 28.Bal BS, Childers WE, Pinnick HW. Tetrahedron. 1981;37:2091. [Google Scholar]
- 29.Sha W, Salomon RG. J. Org. Chem. 2000;65:5315. doi: 10.1021/jo000537v. [DOI] [PubMed] [Google Scholar]
- 30.Richardson W, Heesen TC. J. Org. Chem. 1972;37:3416. [Google Scholar]
- 31.Lee SH, Blair IA. Chem. Res. Toxicol. 2000;13:698. doi: 10.1021/tx000101a. [DOI] [PubMed] [Google Scholar]
- 32.Pryor WA, Porter NA. Free Radical Biol. Med. 1990;8:541. doi: 10.1016/0891-5849(90)90153-a. [DOI] [PubMed] [Google Scholar]
- 33.Burton GW, Ingold KU. Acc. Chem. Res. 1986;19:194. [Google Scholar]
- 34.Burton GW, Ingold KU. J. Am. Chem. Soc. 1984;103:6472. [Google Scholar]
- 35.Proteggente AR, Rehman A, Halliwell B, Rice-Evans CA. Biochem. Biophys. Res. Commun. 2000;277:535. doi: 10.1006/bbrc.2000.3711. [DOI] [PubMed] [Google Scholar]
- 36.Stocker R. Trends Biochem. Sci. 1999;24:219. doi: 10.1016/s0968-0004(99)01404-8. [DOI] [PubMed] [Google Scholar]
- 37.Minotti G, Aust SD. Lipids. 1992;27:219. doi: 10.1007/BF02536182. [DOI] [PubMed] [Google Scholar]
- 38.Minotti G. Chem Res Toxicol. 1993;6:134–146. doi: 10.1021/tx00032a001. [DOI] [PubMed] [Google Scholar]
- 39.Litwinienko G, Ingold KU. Acc. Chem. Res. 2007;40:222–230. doi: 10.1021/ar0682029. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Oe T, Blair IA. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 41.Boughton BA, Griffin MD, O'Donnell PA, Dobson RC, Perugini MA, Gerrard JA, Hutton CA. Bioorg. Med. Chem. 2008;16:9975–9983. doi: 10.1016/j.bmc.2008.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.