Abstract
The significance of interethnic variation in CD4 counts between Asian and Caucasian populations is not known. Patients on combination antiretroviral therapy from Treat Asia and Australian HIV Observational Databases (TAHOD, predominantly Asian, n = 3356; and AHOD, predominantly Caucasian, n = 2312, respectively) were followed for 23 144 person-years for AIDS/death and all-cause mortality endpoints. We calculated incidence-rates and used adjusted Cox regression to test for the interaction between cohort (TAHOD/AHOD) and time-updated CD4 count category (lagged by 3 months) for each of the endpoints. There were 382 AIDS/death events in TAHOD (rate: 4.06, 95%CI: 3.68–4.50) and 305 in AHOD (rate: 2.39, 95%CI: 2.13–2.67), per 100 person-years. At any given CD4 count category, the incidence-rates of endpoints were found to be similar between TAHOD and AHOD (in the adjusted models, P > .05 for the interaction term between cohort type and latest CD4 counts). At any given CD4 count, risk of AIDS or death was not found to vary by ethnicity, suggesting that the CD4 count thresholds for predicting outcomes defined in Caucasian populations may be equally valid in Asian populations.
Keywords: Asians, Caucasian race, CD4 counts, cohort analysis, ethnicity
Introduction
South-East and East Asian countries contribute over 10% of global HIV burden, with over 4 million people living with HIV infection.1 Traditionally, most clinical guidelines in HIV have been developed on the basis of data collected from Caucasian populations in Western countries and are extrapolated to Asian HIV-infected populations.2–4 However, the clinical significance of interethnic variation in immunological profile between the 2 populations is not known.3
The CD4 T-cell lymphocyte count (CD4 count), one of the most important clinical markers of HIV disease progression,2 is known to have significant interethnic variation. For example, several reference-range finding studies on healthy HIV-uninfected individuals from the Asian region indicated that Asians tend to have lower total lymphocyte and CD4 counts as compared to Caucasians.5–8 A recent study comparing HIV-infected untreated patients from the Treat Asia HIV Observational Database (TAHOD) (predominantly Asian) to those from a French cohort showed that at any given CD4 percentage, Asians had lower CD4 counts, as compared to their Caucasian counterparts.9 Furthermore, another study which included patients from the TAHOD and the Australian HIV Observational Database (AHOD) cohorts, suggested that Asians have slightly lower CD4 count response after starting combination antiretroviral therapy (cART), as compared to Caucasians, even after accounting for baseline CD4 counts.10 These findings give rise to the question of whether a given low CD4 count implies a different magnitude of the risk of opportunistic infections or death in Asian and Caucasian HIV-infected individuals.3 If prognostic significance of a given level of CD4 count is found to be different between the 2 populations, it would imply that the CD4 thresholds defined on Caucasian populations may not be applicable to Asian populations.
In the present study, we model the relationship between CD4 counts and AIDS or death and examine for possible heterogeneity in this relationship between the Asian and Caucasian populations. We use data from the TAHOD and AHOD, both being large prospective cohorts from the Asia-Pacific region, founded on similar methodology and with well-validated clinical endpoints.11,12
Methods
Study Population
The Treat Asia and Australian HIV Observational databases (TAHOD and AHOD, respectively) are clinical cohort studies of patients with HIV infection in Asia and Australia, respectively, as a part of International Epidemiologic Databases to Evaluate AIDS initiative. Both the cohorts have similar methodologies, which have been published elsewhere.11,12 Briefly, prospective data collection for TAHOD commenced in 2003, with retrospective data provided where available. In TAHOD, data are collected from 17 clinical sites in Asian region. Written consent was not a requirement of sites in TAHOD unless required by the site’s local ethics committee because data are collected in an anonymous form. Prospective data collection for AHOD commenced in 1999, with retrospective data provided where available. Data for AHOD are collected from 27 clinical sites throughout Australia, including hospitals, sexual health clinics, and general medical practices. Written, informed consent is obtained from all patients recruited to AHOD at the time of enrolment. Ethical approval for both TAHOD and AHOD was obtained from the University of New South Wales, Sydney, Australia, and all other relevant institutional review boards. All TAHOD and AHOD study procedures were developed in accordance with the revised 1975 Helsinki Declaration Data for both cohorts are transferred electronically to the National Centre in HIV Epidemiology and Clinical Research (NCHECR) every March and September and include the same set of core variables. All data are subject to standardized quality control procedures.
For this analysis, we included patients recruited to the TAHOD, and the AHOD, whoever started cART (defined as 3 or more antiretroviral drugs in combination). Ethnicity is consistently reported in TAHOD and more than 95% are known to be Asian (29% Chinese, 25% Thai, and 14% Indian) and <1%are known to be White/Caucasian. In AHOD, ethnicity is inconsistently available (known in about 48% of patients, n = 1103). Where available, greater than 90% are known to be Caucasians in AHOD. HIV seroconversion surveys in Australia consistently report White/Caucasian ethnicity at greater than 80%.13,14 We therefore assume that AHOD largely represents a Caucasian population. We defined study entry date as later of cohort entry date or cART start date. Patients were also required to have at least one follow-up visit after the study entry date.
Study Endpoints
The primary endpoint of this study was a combined endpoint of progression to newly diagnosed AIDS-defining illness or death from any cause. The definition of AIDS diagnosis was adapted using a modified version of the revised 1993 CDC case definition for AIDS,15 excluding CD4 counts less than 200/mm3. The secondary endpoint was all-cause mortality. We considered patients with no contact for at least 12 months prior to March 31, 2009 (the censorship date), who were not known to meet the study endpoints, to be lost to follow-up (LTFU). Follow-up data were censored at the first of: lost to follow-up or the censorship date.
Statistical Analysis
We compared the baseline data between the TAHOD and AHOD cohorts using Mann-Whitney Rank sum test or Student t-test for continuous variables and chi-square test for categorical variables. For the follow-up analysis, we used CD4 count/mm3 and HIV RNA load copies/mL as time-updated variables, lagged by 3 months (ie, not updated until 3 months even if a more recent value was measured) since a clinical event may influence the latest (most recent) values. We then calculated incidence of AIDS or death and all-cause mortality in each cohort, stratified by latest CD4 counts (lagged by 3 months and categorized as <50, 50–100, 100–200, 200–350, 350–500, and >500 cells/mm3). We used Cox regression models with time-updated variables to model the relationship between latest CD4 count lagged by 3 months (categorized as above) and each of the endpoints. To examine for heterogeneity in this relationship between the 2 cohorts, we included an interaction term, as a product of cohort (TAHOD/AHOD) and latest CD4 counts lagged by 3 months. Models were a priori adjusted for fixed covariates, including cohort (TAHOD/AHOD), gender, hepatitis B and C coinfection (defined as HBV surface antigen and HCV antibody positive, respectively), HIV exposure category (homosexual contact± Intravenous drug user [IDU], IDU ± heterosexual, heterosexual, and other), and prior AIDS; and time-updated covariates including HIV RNA category lagged by 3 months (categorized as <500, 500–10 000, and >10 000 copies/mL), age, on or off cART, calendar year (categorized as earlier than 2000, 2000–02, 2002–04, 2004–06, 2006–08, and later than 2008) and new AIDS (for mortality endpoint only). For all time-updated variables, missing data were imputed by carrying forward (but not backward) the last observation, till the last follow-up date. The proportional hazard assumption for cohort variable and each of the endpoints, assessed by Schoenfeld residuals test, was not found to be violated.
We conducted the following sensitivity analysis: (1) Since the rate of LTFU in those with CD4 counts <50 cells/mm3 was found to be different between TAHOD and AHOD, we used the method suggested by Egger et al,16 to assess the possible impact of differential pattern in LTFU. First, we calculated the mortality rate in those who remained in follow-up with latest CD4 counts <50 cells/mm3 in TAHOD. We assumed the mortality rate of 40% (95% CI 33%–48%) in LTFU patients from TAHOD with latest CD4 counts <50 cells/mm3.17 We then calculated the overall mortality in this group weighted by the proportion LTFU and not LTFU, which is presented in Figure 2b; (2) Since AHOD tend to have laboratory measurements more frequently, we adjusted the frequency of CD4 count and HIV RNA measurements in AHOD to mirror TAHOD, by excluding the every second measurement in AHOD; (3) censoring tuberculosis-related AIDS diagnosis or deaths with tuberculosis as an underlying cause; (iv) using latest (time-updated) CD4 counts and HIV RNA levels, without lagging (ie, using the most recent measurements); and (v) including only those from AHOD with known Caucasian ethnicity.
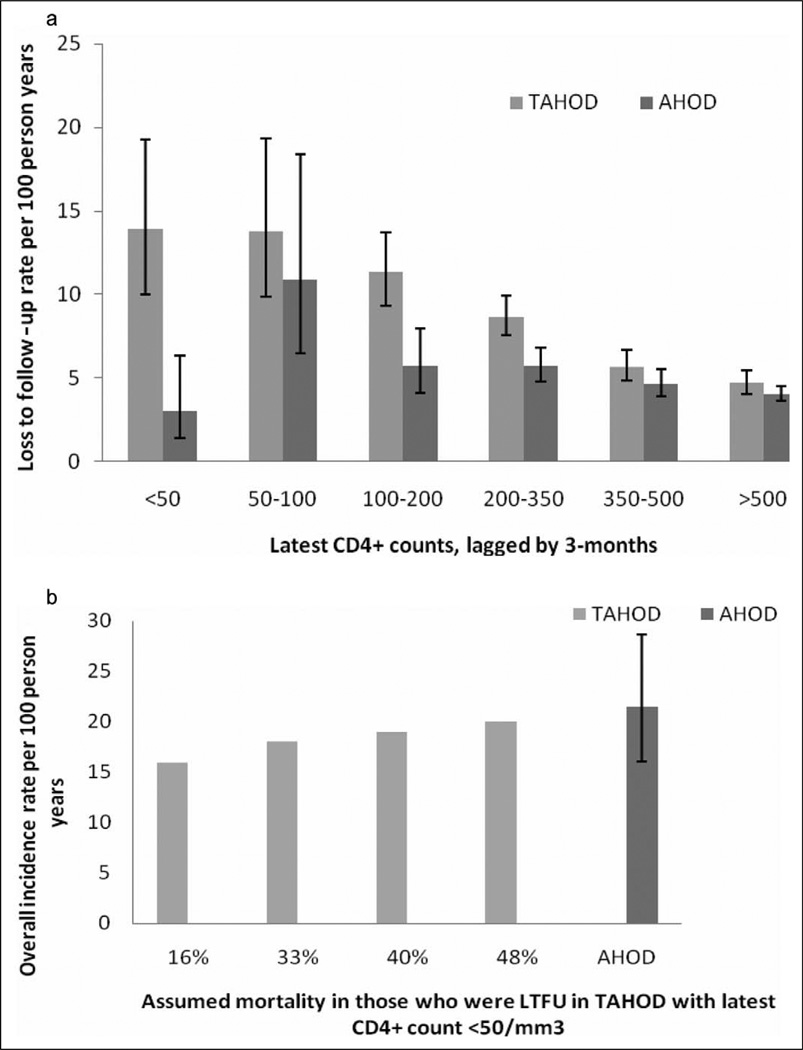
Figure 2.
a, Rate of loss to follow-up, per 100 person years, by last known CD4 count per mm3 (lagged by 3 months), in TREAT Asia HIV Observational Database (TAHOD) and Australian HIV Observational Database (AHOD). Error bars represent 95% confidence intervals. b, Overall estimated all-cause mortality rate in TAHOD (with latest CD4 count <50 cells/mm3), after accounting for assumed mortality rates in those who were lost to follow-up (LTFU), and the comparison with observed rate in AHOD. The higher the assumed mortality in LTFU patients, the similar are the mortality rates between TAHOD and AHOD. Error bars represent 95% confidence intervals.
Data were analyzed using Stata version 10 (Stata Corporation, College Station, Texas).
Results
Patient Characteristics at Study Entry
A total of 5668 patients (3356 from TAHOD and 2312 from AHOD) were included in the analysis, contributing 23 144 person years of follow-up (PYFU). The patient characteristics at study entry in TAHOD and AHOD are shown in Table 1. Participants from TAHOD were more likely than those from AHOD, respectively, to be: younger (mean age in years: 38 vs. 43), female (28.6% vs. 5.8%), report as heterosexually acquired infection (69.3% vs. 9.2%), have hepatitis B coinfection (7.1% vs. 5.1%), prior AIDS (46.8% vs. 20.3%), detectable HIV RNA load (52% vs. 40.6%), lower median CD4 count (259 cells/mm3 vs. 432 cells/mm3), less time spent on cART, and to be on nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimen (75.6%vs 48.7%). Also, a higher proportion of TAHOD participants had missing hepatitis B and C coinfection status (Table 1).
Table 1.
Patient Characteristics at Study Entry
| Characteristics | TAHOD, n = 3356 n (%) |
AHOD, n = 2312 n (%) |
Pa |
|---|---|---|---|
| Age (years) | |||
| Mean | 38 | 43 | <.001 |
| <30 | 565 (16.8) | 154(6.6) | <.001 |
| 30–40 | 1564 (46.6) | 852 (36.8) | |
| 40–50 | 838 (25.0) | 800 (34.6) | |
| 50–60 | 274 (8.1) | 376 (16.3) | |
| >60 | 115 (3.4) | 130 (5.6) | |
| Sex | |||
| Male | 2393(71.3) | 2171(93.9) | <.001 |
| Female | 960 (28.6) | 134 (5.8) | |
| Transgender | 3 (0.09) | 7 (0.3) | |
| HIV exposure category | |||
| Homosexual contact ± IDU | 652 (19.4) | 1891 (81.8) | <.001 |
| IDU ± heterosexual | 123 (3.7) | 56 (2.4) | |
| Heterosexual | 2326 (69.3) | 212 (9.2) | |
| Other | 248 (7.4) | 127 (5.5) | |
| Missing | 7 (0.2) | 26 (1.1) | |
| Hepatitis B coinfection | |||
| Negative | 2024 (60.3) | 1823 (78.8) | <.001 |
| Positive | 239 (7.1) | 119 (5.1) | |
| Missing/never tested | 1093 (32.5) | 370 (16.0) | |
| Hepatitis C coinfection | |||
| Negative | 1817 (54.1) | 1782 (77.0) | <.001 |
| Positive | 191 (5.7) | 273 (11.8) | |
| Missing/never tested | 1348 (40.2) | 257 (11.1) | |
| HIV RNA <500 copies/mL | 1615 (48.1) | 1373(59.4) | <.001 |
| Prior AIDS at study entry | 1825 (46.8) | 511 (20.3) | <.001 |
| Median CD4 count per mm3 (IQR) | 259 (144–399) | 432 (262–640) | <.001b |
| ART Class at baseline | |||
| NRTI | 3192 (95.1) | 2030 (87.8) | <.005 |
| NNRTI | 2549 (75.6) | 1126 (48.7) | |
| PI | 760 (22.6) | 971 (42.0) | |
| Other | 9 (0.3) | 20 (0.9) | |
| Median duration of cART in months (IQR) | 13 (2.3–30.3) | 32 (10.3–45.6) | <.001b |
Comparison by t-test for continuous variables and the χ2 test for noncontinuous variables.
Comparison by Wilcoxon rank-sum test.
Abbreviations: TAHOD, TREAT Asia HIV Observational Database; AHOD, Australian HIV Observational Database; IDU, Intravenous drug user; IQR, inter-quartile range; CD4 count refers to CD4 T-cell count; ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
CD4 Count and Risk of AIDS or Death in TAHOD and AHOD
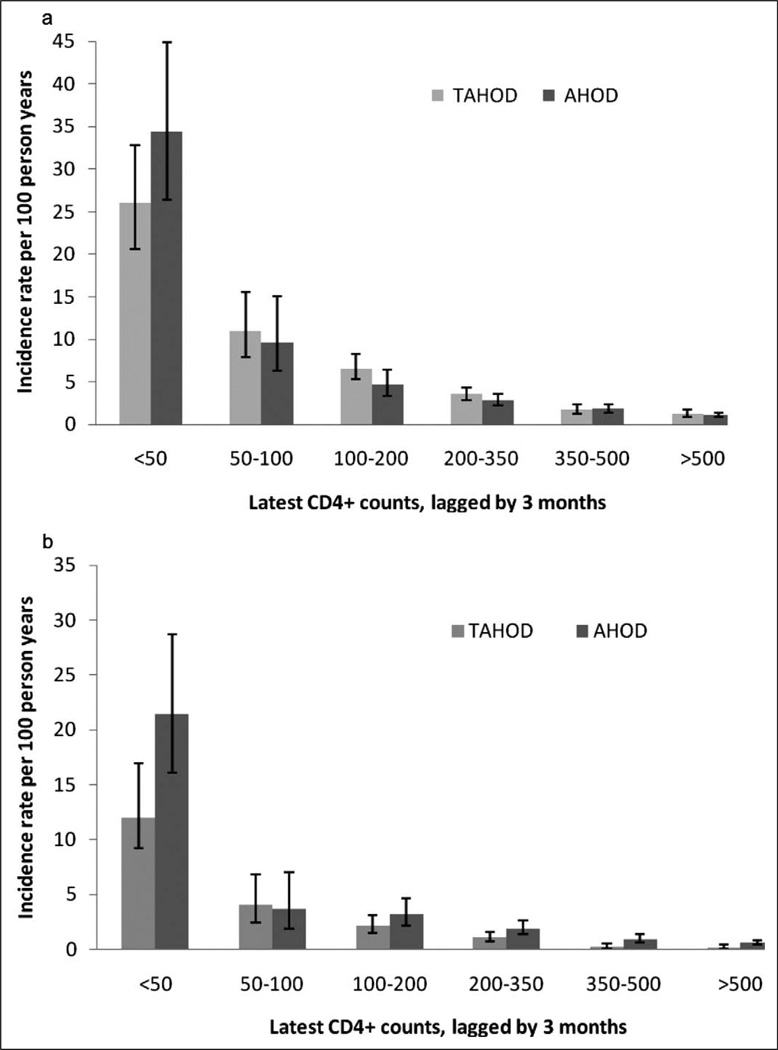
There were a total of 382 AIDS or death events in TAHOD (incidence rate per 100 PYFU: 4.06, 95% CI: 3.68–4.50) and 305 events in AHOD (incidence rate per 100 PYFU: 2.39, 95% CI: 2.13–2.67). Incidence rates of AIDS or death, stratified by latest CD4 count (lagged by 3 months), in TAHOD and AHOD are plotted in Figure 1a. At any given CD4 count category, the rates of AIDS or death were found to be similar between TAHOD and AHOD, except in lowest category (<50 cells/mm3), where TAHOD tended to have lower rate (Figure 1a).
Figure 1.
Incidence rates per 100 person years of (a) AIDS or death and (b) all-cause mortality by latest CD4 count per mm3 (lagged by 3 months) in TREAT Asia HIV Observational Database (TAHOD) and Australian HIV Observational Database (AHOD). Error bars represent 95% confidence intervals.
In the multivariable model (Table 2), the risk of AIDS or death increased with decreasing CD4 counts. There was no statistically significant interaction by cohort (TAHOD/AHOD) and time-updated CD4 count category (lagged by 3 months) in predicting AIDS or death (P = .256 for the interaction term) (Table 2). Among the other covariates included in the model, age, HIV RNA, hepatitis B and C coinfection, and prior AIDS were associated with higher risk and being on cART was associated with lower risk of AIDS or death (data not shown).
Table 2.
Adjusted Analyses for AIDS or Death
| Parameters | Adjusted Analysisa | |||
|---|---|---|---|---|
| HR | 95% CI | P | ||
| Latest CD4 count per mm3 (lagged by 3-months) | >500 | Reference | ||
| 350–500 | 1.43 | 1.00–2.05 | .05 | |
| 200–350 | 2.02 | 1.42–2.86 | <.001 | |
| 100–200 | 3.07 | 2.03–4.66 | <.001 | |
| 50–100 | 5.35 | 3.19–8.97 | <.001 | |
| <50 | 15.44 | 10.34–23.08 | <.001 | |
| Cohort | AHOD | Reference | ||
| TAHOD | 1.53 | 0.92–2.55 | 0.100 | |
| Interaction termb | 0.256b | |||
Abbreviations: HR, Hazard ratio; TAHOD, TREAT Asia HIV Observational Database; AHOD, Australian HIV Observational Database; CD4 count refers to CD4 T-cell count.
Models were a priori adjusted for fixed covariates, including cohort (TAHOD/AHOD), gender, hepatitis B and C coinfection, HIV exposure category, and prior AIDS; and time-updated covariates including HIV RNA category lagged by 3 months (categorized as <500, 500–10 000, and >10 000 copies/mL), age, on or off cART, calendar year (categorized as earlier than 2000, 2000–2002, 2002–2004, 2004–2006, 2006–2008, and later than 2008) and new AIDS (for mortality endpoint only).
P value is for the interaction term (product of cohort [TAHOD/AHOD] and categorized CD4 count lagged by 3 months), obtained in the adjusted model predicting respective endpoint. Excluding the interaction term from the multivariable model changes the HR for cohort (TAHOD vs. AHOD) to 1.43 (95% CI: 1.02–2.00) for AIDS or death and 0.84 (95% CI: 0.53–1.33) for all-cause mortality.
CD4 Count and Risk of All-Cause Mortality in TAHOD and AHOD
There were a total of 135 deaths in TAHOD (incidence rate per 100 PYFU: 1.36, 95% CI: 1.15–1.61) and 206 deaths in AHOD (incidence rate per 100 PYFU: 1.56, 95% CI: 1.36–1.78). Rates of death in TAHOD and AHOD, stratified by latest CD4 count (lagged by 3 months), are plotted in Figure 1b. Similar to the AIDS or death endpoint, the mortality rates were found to be similar between TAHOD and AHOD at any given CD4 count category, except in lowest category (<50 cells/mm3), where TAHOD tended to have lower rate (incidence-rate: 12, 95% CI: 9.2–17) as compared to AHOD (incidence-rate: 21.5, 95% CI: 16–28.7), per 100 PYFU (Figure 1b).
In the multivariable model (Table 3), the risk of death increased with decreasing CD4 counts. Overall risk of death in the adjusted model (excluding the interaction term) was similar between TAHOD and AHOD (HR for TAHOD vs AHOD: 0.84, 95% CI: 0.53–1.33). Similar to the AIDS or death endpoint, there was no statistically significant interaction by cohort (TAHOD/AHOD) and time-updated CD4 count category (lagged by 3 months) in predicting death (P = .254 for the interaction term; Table 2). The other predictors associated with increased risk of death included age, HIV RNA, hepatitis B and C coinfection, and prior AIDS (data not shown).
Table 3.
Adjusted Analyses for All-Cause Mortality
| Parameters | Adjusted Analysisa | |||
|---|---|---|---|---|
| HR | 95% CI | P | ||
| Latest CD4 count per mm3 (lagged by 3-months) | >500 | Reference | ||
| 350–500 | 1.36 | 0.83–2.19 | .212 | |
| 200–350 | 2.44 | 1.58– 3.77 | <.001 | |
| 100–200 | 3.81 | 2.30–6.31 | <.001 | |
| 50–100 | 4.11 | 1.94–8.69 | <.001 | |
| <50 | 21.56 | 13.12–35.45 | <.001 | |
| Cohort | AHOD | Reference | ||
| TAHOD | 0.48 | 0.18–1.32 | .157 | |
| Interaction termb | .254b | |||
Abbreviations: HR, Hazard ratio; TAHOD, TREAT Asia HIV Observational Database; AHOD, Australian HIV Observational Database; CD4 count refers to CD4 T-cell count.
Models were a priori adjusted for fixed covariates, including cohort (TAHOD/AHOD), gender, hepatitis B and C coinfection, HIV exposure category, and prior AIDS; and time-updated covariates including HIV RNA category lagged by 3 months (categorized as <500, 500–10,000, and >10,000 copies/mL), age, on or off cART, calendar year (categorized as earlier than 2000, 2000–02, 2002–04, 2004–06, 2006–08, and later than 2008) and new AIDS (for mortality endpoint only).
P value is for the interaction term (product of cohort (TAHOD/AHOD) and categorized CD4 count lagged by 3-months), obtained in the adjusted model predicting respective endpoint. Excluding the interaction term from the multivariable model changes the HR for cohort (TAHOD vs. AHOD) to 1.43 (95% CI: 1.02–2.00) for AIDS or death and 0.84 (95% CI: 0.53–1.33) for all-cause mortality.
Loss to Follow-Up in TAHOD and AHOD and Its Impact on Mortality Estimates
The risk of both the endpoints, AIDS or death and all-cause mortality, tended to be lower in TAHOD in the lowest CD4 category (<50 cells/mm3). Since this was unexpected, we examined for possible differential patterns in loss to follow-up.
A total of 727 patients from TAHOD and 618 patients from AHOD were lost to follow-up, at the rate of 7.33 (95% CI: 6.81–7.88) and 4.67 (95% CI: 4.31–5.05), per 100 PYFU, respectively. To investigate the possibility that the patients with severe disease were more likely to be lost to follow-up in TAHOD, we compared the rates of loss to follow-up, stratified by latest CD4 count (lagged by 3 months), between TAHOD and AHOD, as illustrated in Figure 2a. The rates of loss to follow-up tended to be markedly higher in TAHOD as compared to AHOD in the lowest CD4 count category (Figure 2a). The loss to follow-up rate for TAHOD and AHOD patients with last knownCD4 count (lagged by 3 months) <50 cells/mm3 was 13.90 (95% CI: 10.03–19.28) and 3.03 (95% CI: 1.44–6.36), per 100 PYFU, respectively.
The mortality in those who remained in the follow-up in TAHOD, with latest CD4 count <50 cells/mm3, was found to be 16 per 100 PYFU (or 16%). After adjusting for assumed mortality in the LTFU group, the overall mortality rate in TAHOD with latest CD4 counts <50 cells/mm3 was estimated to range from 18% to 20%, which is similar to 21.5% reported for AHOD in the same CD4 count category (Figure 2b).
Other Sensitivity Analyses
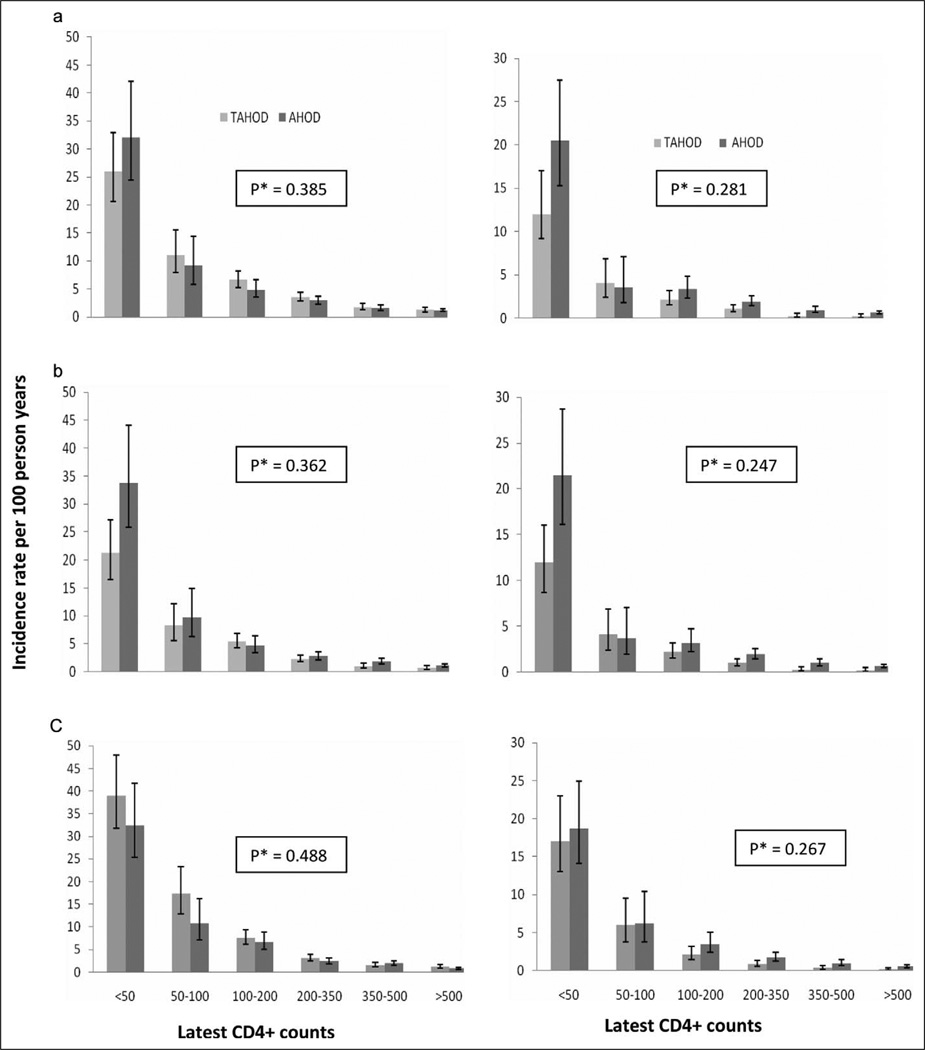
Results from all of the other sensitivity analyses were broadly similar (Figure 3). Censoring tuberculosis-related AIDS diagnosis or deaths with tuberculosis as an underlying cause (n = 180 in TAHOD and 4 in AHOD) slightly increased the difference in absolute risk of AIDS or death at lowest CD4 count category (lagged by 3 months) between TAHOD and AHOD (Figure 3); since tuberculosis was more common in TAHOD in lowest CD4 category. Also, the hazard of AIDS or death in TAHOD, relative to AHOD, became closer to 1 (HR: 1.26, 95%CI: 0.88–1.80), suggesting that the overall higher rate of AIDS events in TAHOD were largely explained by tuberculosis. More importantly, the interaction term in the multivariate model remained nonsignificant in all of the sensitivity analyses (Figure 3) and also if only those from AHOD with known Caucasian ethnicity were included (P = .25 for AIDS or death).
Figure 3.
Incidence rate of AIDS or death (left vertical panel) and all-cause mortality (right vertical panel), by latest CD4 count (cells/mm3), in TREAT Asia HIV Observational Database (TAHOD) and Australian HIV Observational Database (AHOD). *P value is for the interaction term (product of cohort [TAHOD/AHOD] and categorized CD4 count lagged by 3 months), obtained in the multivariate models predicting respective endpoint, adjusted for same covariates as listed in Tables 2 and 3. The data are when (a) every second time-dependent CD4 count excluded in AHOD; (b) censoring tuberculosis-related AIDS diagnosis or deaths with tuberculosis as an underlying cause; and (c) using latest (time-updated) CD4 counts and HIV RNA levels, without lagging (ie, using the recent most measurements). The latest CD4 counts were lagged by 3 months in (a) and (b). Error bars represent 95% confidence intervals.
Discussion
In this study, we examined the clinical significance of previously reported interethnic variations in the CD4 count between Asian and Caucasian populations. We found that the prognostic significance of the CD4 count, in terms of AIDS or death and all-cause mortality, was not found to vary between Asian and Caucasian HIV-infected individuals. In other words, a given low CD4 count level engenders a similar magnitude of risk of AIDS or death and all-cause mortality in the 2 populations.
The TAHOD patients tended to have lower rates of AIDS or death and all-cause mortality at very low CD4 counts (<50 cells/mm3), possibly suggesting that Asian populations may have some survival advantage at low CD4 counts. However, we believe these findings are a result of differential rate of loss to follow-up between cohorts, as patients with TAHOD having lower CD4 counts were more likely to be lost to follow-up than patients with AHOD. Higher rate of loss to follow-up in those with severer disease has previously been reported from other cohorts in low-income countries.17–19 Our sensitivity analyses showed if we adjusted for this potential excess of events in those who were LTFU, then the rates in TAHOD tend to be more similar to that in AHOD (Figure 2b). Also, in the multivariate model, the cohort type was not found to be an effect modifier of CD4 counts, suggesting that other factors in the model may have explained this difference. Lastly, even if there is a true difference in risk of endpoints between TAHOD and AHOD, it is unlikely to be clinically significant, as cART initiation or modification would be indicated at these low CD4 counts, irrespective of the magnitude of the risk.
A study on an Ethiopian cohort, an ethnicity known to have lower CD4 counts compared with Caucasians, suggested that the lower preseroconversion CD4 counts (ie, lower CD4 counts in healthy state) do not imply shorter survival times in Ethiopians, presumably because of slower decline in CD4 counts.20 Further, the previously reported differences in CD4 counts between HIV-infected Asians and Caucasians were only significant at higher CD4 percentages, where clinical events are less likely to occur.9 In addition, mortality rates in Asian (and African populations who are known to have lower CD4 counts in HIV-negative individuals) are reported to approach those in Caucasian populations within few months after cART initiation.19,21,22 Our findings support these observations, which collectively suggest that the ethnicity may not be an important factor and the CD4 thresholds defined for Caucasian HIV-infected populations should be broadly appropriate for Asian HIV-infected populations. Although 2 studies from Hong Kong suggested that lower CD4 thresholds for cART initiation may be appropriate among Asian populations, these studies were limited by small sample size and a smaller number of clinical endpoints (<30 in both studies).23,24 Our data do not support the need for different thresholds for the 2 populations.
This comparison between Asian and Caucasian populations was performed on treated HIV-infected individuals and may have limited generalizability to untreated HIV-infected individuals. Further, our cohort (especially TAHOD) may represent a selected group who has survived the initial few months on cART, as mortality is known to be higher in patients from low-income countries in first 3 to 4 months of treatment, as compared to those from high-income countries.19,22 However, this increased mortality in earlier months of cART is due mainly to comorbidities, cART toxicities, lack of access to health care, and the immune reconstitution inflammatory syndrome (IRIS).19,22,25–28 Consequently, our analysis is protected from confounding by these factors and is likely to represent the true risk of endpoints at various CD4 strata. Further, we also performed sensitivity analysis by censoring tuberculosis-related events, as tuberculosis is known to be more prevalent in Asian countries29 and is a common form of IRIS.25,28 The results from sensitivity analyses were largely similar.
There were a few other limitations to our analysis. Firstly, the 2 populations are highly heterogeneous, including different treatment patterns, and our multivariable models may not have accounted for all factors. Ideally, such a comparison should be performed between Caucasians and Asians who are raised in Western countries. Also, ascertaining of ethnicity is incomplete in AHOD and it is likely that there may be some patients of Asian ethnicity in the AHOD, possibly resulting in misclassification. However, where ethnicity is recorded in AHOD, more than 90% are Caucasians and also, sensitivity analysis with only those AHOD patients with known Caucasian ethnicity yielded similar results. Further, we did not account for different subtypes of HIV-1 that may be prevalent in the 2 cohorts.30 However, there is little evidence of subtype differences in disease progression or response to cART.31,32 Second, we did not divide Asian ethnicity into its various subtypes (eg, Indians, Thai, Chinese, etc), to avoid loss of power. However, this is unlikely to influence our results as CD4 counts are known to be similar among various Asian populations.5,6,33 Third, hepatitis coinfection status was missing from a significant proportion of TAHOD participants, which is especially important as hepatitis C coinfection is known to be responsible for significant mortality in HIV/HCV coinfected population.34,35
The study has several strengths. Firstly, this is a prospective study including a large group of patients on a variety of antiretroviral agents followed for a prolonged period of time. Also, we analyzed the broad endpoints of AIDS or death and all-cause mortality, which include non-AIDS deaths; as recent data suggest that latest CD4 counts are associated with risk of non-AIDS events.36–38 Lastly, use of data from 2 observational cohort studies founded on the same methodology is a major strength of our study, reducing the likelihood of confounding due to methodological differences.
In summary, the risk of AIDS or death and all-cause mortality at any given CD4 count strata was not found to vary between Asian and Caucasian populations. These findings suggest, albeit indirectly, that the CD4 thresholds defined on Caucasian populations are broadly appropriate in Asian populations. Further, these findings provide an appropriate evidence base on Asian populations for the CD4 count thresholds recommended for initiating cART and prophylaxis for opportunistic infections.
Acknowledgments
The writing committee would like to acknowledge several thousands of patients, the TAHOD Steering Committee [The TREAT Asia HIV Observational Database: CV Mean, V Saphonn* and K Vohith, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia; FJ Zhang*, HX Zhao and N Han, Beijing Ditan Hospital, Beijing, China; PCK Li* and MP Lee, Queen Elizabeth Hospital, Hong Kong, China; N Kumarasamy*, S Saghayam and C Ezhilarasi, YRG Centre for AIDS Research and Education, Chennai, India; S Pujari*‡, K Joshi, and A Makane, Institute of Infectious Diseases, Pune, India; TP Merati*, DN Wirawan and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia; E Yunihastuti* and O Ramadian, Working Group on AIDS Faculty of Medicine, University of Indonesia/ Ciptomangunkusumo Hospital, Jakarta, Indonesia; S Oka*, J Tanuma and M Honda, National Center for Global Health and Medicine, Tokyo, Japan; JY Choi*, SH Han, and JM Kim, Division of Infectious Diseases, Dept. of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea; C KC Lee*, B HL Sim and R David, Hospital Sungai Buloh, Kuala Lumpur, Malaysia; A Kamarulzaman* and A Kajindran, University of Malaya Medical Centre, Kuala Lumpur, Malaysia; G Tau, Port Moresby General Hospital, Port Moresby, Papua New Guinea**; R Ditangco*, E Uy and R Bantique, Research Institute for Tropical Medicine, Manila, Philippines; YMA Chen*, WW Wong and LH Kuo, Taipei Veterans General Hospital and AIDS Prevention and Research Centre, National Yang-Ming University, Taipei, Taiwan; PL Lim*, A Chua and E Foo, Tan Tock Seng Hospital, Singapore; P Phanuphak*, Kiat Ruxrungtham and M Khongphattanayothin, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; S Sungkanuparph*, S Kiertiburanakul and B Piyavong, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; T Sirisanthana*†, R Chaiwarith and W Kotarathititum, Research Institute for Health Sciences, Chiang Mai, Thailand; AH Sohn*, L Messerschmidt* and B Petersen, TREAT Asia, amfAR—The Foundation for AIDS Research, Bangkok, Thailand; J Chuah*, Gold Coast Sexual Health Clinic, Miami, Queensland, Australia; DA Cooper, MG Law*, J Zhou* and A Jiamsakul, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, Australia. *TAHOD Steering Committee member; **Inactive site; † Steering Committee Chair; ‡ co-Chair. TAHOD reviewers: PCK Li, MP Lee, S Vanar, S Faridah, A Kamarulzaman, JY Choi, B Vannary, R Ditangco, K Tsukada, SH ?A3B2 twb=0.39w?> Han, FJ Zhang, YMA Chen, N Kumarasay, A Dravid, OT Ng, C Duncombe, S Sungkanuparph, T Sirisanthana. Independent reviewers: F Drummond, M Boyd] and the AHOD Steering Committee [The Australian HIV Observational Database: D Ellis, General Medical Practice, Coffs Harbour, NSW; J Chuah*, M Ngieng, B Dickson, Gold Coast Sexual Health Clinic, Miami, QLD;M Bloch, T Franic, S Agrawal, N Cunningham Holdsworth House General Practice, Darlinghurst, NSW; R Moore, S Edwards, P Locke, Northside Clinic, North Fitzroy, VIC; D Nolan, C Forsdyke, J Skett, Department of Clinical Immunology, Royal Perth Hospital, Perth, WA; NJ Roth*†, J Nicolson, Prahran Market Clinic, South Yarra, VIC; D Allen, P Maudlin Holden Street Clinic, Gosford, NSW; D Smith, C Mincham, C Gray Lismore Sexual Health & AIDS Services, Lismore, NSW; D Baker*, R Vale, East Sydney Doctors, Darlinghurst, NSW; D Russell, S Downing, Cairns Sexual Health Service, Cairns, QLD; D Templeton, C O’Connor, Royal Prince Alfred Hospital Sexual Health, Camperdown, NSW; D Sowden, K McGill, Clinic 87, Sunshine Coast & Cooloola HIV Sexual Health Service, Nambour, QLD; D Orth; D Youds, Gladstone Road Medical Centre, Highgate Hill, QLD; E Jackson, Blue Mountains Sexual Health and HIV Clinic, Katoomba, NSW; T Read, J Silvers, Melbourne Sexual Health Centre, Melbourne, VIC; A Kulatunga, P Knibbs, Communicable Disease Centre, Royal Darwin Hospital, Darwin, NT; J Hoy, K Watson*, M Bryant, S Price, The Alfred Hospital, Melbourne, VIC; M Gotowski, S Taylor, L Stuart-Hill, Tamworth Sexual Health Service, Tamworth, NSW; D Cooper, A Carr, K Hesse, R Norris St Vincent’s Hospital, Darlinghurst, NSW; R Finlayson, I Prone, Taylor Square Private Clinic, Darlinghurst, NSW; MT Liang, Nepean Sexual Health and HIV Clinic, Penrith, NSW; M Kelly, A Gibson, H Magon, AIDS Medical Unit, Brisbane, QLD; K Brown, N Skobalj, Illawarra Sexual Health Clinic, Warrawong, NSW; L Wray, H Lu, Sydney Sexual Health Centre, Sydney, NSW; W Donohue, The Care and Prevention Programme, Adelaide University, Adelaide, SA; I Woolley, M Giles, T Korman, Monash Medical Centre, Clayton, VIC; Dubbo Sexual Health Centre, Dubbo, NSW; P Canavan*, National Association of People Living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; B Mulhall*, School of Public Health, University of Sydney, Sydney, NSW; M Law*, K Petoumenos*, S Marashi Pour*, Courtney Bendall*, National Centre in HIV Epidemiology and Clinical Research, University of NSW, Sydney; NSW.*Steering Committee member 2010, † Current Steering Committee chair. Cause of Death (CoDE) reviewers AHOD reviewers: D Sowden, D Templeton, A Carr, J Hoy, L Wray, J Chuah, K Morwood, T Read, N Roth, I Woolley, M Kelly, J Broom.]
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: The TREAT Asia HIV Observational Database and the Australian HIV Observational Database are part of the Asia Pacific HIV Observational Database and are initiatives of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the following institutes of the U.S. National Institutes of Health (NIH): National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), the Office of the Director (OD), and the National Cancer Institute (NCI), as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) (grant no. U01AI069907). Additional support is provided by the Dutch Ministry of Foreign Affairs through a partnership with Stichting Aids Fonds. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales.
Footnotes
This study has been presented at the 14th International Workshop of HIV Observational Databases (IWHOD), Barcelona, Spain, 2010 (abstract no.:92) and at 22nd Annual Conference for the Australasian Society for HIV Medicine, Sydney, 2010 (abstract no. 356).
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
References
- 1.World Health Organisation. HIV /AIDS in the South-East Asia Region. [Accessed June 15, 2010];2009 http://www.searo.who.int/LinkFiles/Publications_HIV_AIDS_Report2009.pdf.
- 2.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300(5):555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 3.Kadhiravan T, Sharma SK. Mortality of HIV-infected patients in low-income countries. Lancet. 2006;368(9554):2207–2207. doi: 10.1016/S0140-6736(06)69886-3. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation. Rapid advice: Antiretroviral therapy for HIV infectionin adults and adolescents. [Accessed June 15, 2010];2009 http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf.
- 5.Chng WJ, Tan GB, Kuperan P. Establishment of adult peripheral blood lymphocyte subset reference range for an Asian population by single-platform flow cytometry: influence of age, sex, and race and comparison with other published studies. Clin Diagn Lab Immunol. 2004;11(1):168–173. doi: 10.1128/CDLI.11.1.168-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das BR, Bhanushali AA, Khadapkar R, Jeswani KD, Bhavsar M, Dasgupta A. Reference ranges for lymphocyte subsets in adults from western India: influence of sex, age and method of enumeration. Indian J Med Sci. 2008;62(10):397–406. [PubMed] [Google Scholar]
- 7.Jiang W, Kang L, Lu HZ, et al. Normal values for CD4 and CD8 lymphocyte subsets in healthy Chinese adults from Shanghai. Clin Diagn Lab Immunol. 2004;11(4):811–813. doi: 10.1128/CDLI.11.4.811-813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster HK, Pattanapanyasat K, Phanupak P, et al. Lymphocyte immunophenotype reference ranges in healthy Thai adults: implications for management of HIV/AIDS in Thailand. Southeast Asian J Trop Med Public Health. 1996;27(3):418–429. [PubMed] [Google Scholar]
- 9.Achhra AC, Zhou J, Dabis F, et al. Difference in absolute CD4+count according to CD4 percentage between Asian and Caucasian HIV-Infected patients. J AIDS Clinic Res. 2010;1:101. doi: 10.4172/2155-6113.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egger S, Petoumenos K, Kamarulzaman A, et al. Long-term patterns in CD4 response are determined by an interaction between baseline CD4 cell count, viral load, and time: The Asia Pacific HIV Observational Database (APHOD) J Acquir Immune Defic Syndr. 2009;50(5):513–520. doi: 10.1097/qai.0b013e31819906d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Australian HIV Observational Database. Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV Med. 2002;3(1):28–36. doi: 10.1046/j.1464-2662.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Kumarasamy N, Ditangco R, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38(2):174–179. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prestage G, Bradley J, Down I, et al. HIV Seroconversion Study. [Accessed August 9, 2010];Newly diagnosed men in Australia. 2009 http://www.nchecr.unsw.edu.au/nchecrweb.nsf/resources/QldSurv/$file/SeroconReportOct09.pdf. [Google Scholar]
- 14.Volk JE, Prestage G, Jin F, et al. Risk factors for HIV seroconversion in homosexual men in Australia. Sexual Health. 2006;3(1):45–51. doi: 10.1071/sh05020. [DOI] [PubMed] [Google Scholar]
- 15.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 16.Egger M, Spycher BD, Sidle J, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-saharan Africa. PLoS Med. 2011;8(1) doi: 10.1371/journal.pmed.1000390. e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anglaret XMDP, Toure SMDM, Gourvellec GM, et al. Impact of Vital Status Investigation Procedures on Estimates of Survival in Cohorts of HIV-Infected Patients From Sub-Saharan Africa. [Report] JAIDS. 2004;35(3):320–323. doi: 10.1097/00126334-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 19.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 20.Mekonnen Y, Geskus RB, Hendriks JC, et al. Low CD4T cell counts before HIV-1 seroconversion do not affect disease progression in Ethiopian factory workers. J Infect Dis. 2005;192(5):739–748. doi: 10.1086/432545. [DOI] [PubMed] [Google Scholar]
- 21.Brinkhof MWG, Egger M, Schechter M, Dabis F. Mortality of HIV-infected patients in low-income countries–Authors’response. Lancet. 2006;368(9554):2207–2208. doi: 10.1016/S0140-6736(06)69886-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Dou Z, Ma Y, et al. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151(4):241–251. doi: 10.7326/0003-4819-151-4-200908180-00006. W-252. [DOI] [PubMed] [Google Scholar]
- 23.Ho C, Lee S, Wong K, Cheng L, Lam M. Setting a minimum threshold CD4 count for initiation of highly active antiretroviral therapy in HIV-infected patients. HIV Med. 2007;8(3):181–185. doi: 10.1111/j.1468-1293.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 24.Wong KH, Chan KC, Cheng KL, Chan WK, Kam KM, Lee SS. Establishing CD4 thresholds for highly active antiretroviral therapy initiation in a cohort of HIV-infected adult Chinese in Hong Kong. AIDS Patient Care STDS. 2007;21(2):106–115. doi: 10.1089/apc.2006.0037. [DOI] [PubMed] [Google Scholar]
- 25.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruxrungtham K, Brown T, Phanuphak P. HIV/AIDS in Asia. Lancet. 2004;364(9428):69–82. doi: 10.1016/S0140-6736(04)16593-8. [DOI] [PubMed] [Google Scholar]
- 27.Seyler C, Toure S, Messou E, Bonard D, Gabillard D, Anglaret X. Risk factors for active tuberculosis after antiretroviral treatment initiation in Abidjan. Am J Respir Crit Care Med. 2005;172(1):123–127. doi: 10.1164/rccm.200410-1342OC. [DOI] [PubMed] [Google Scholar]
- 28.Manosuthi W, Chaovavanich A, Tansuphaswadikul S, et al. Incidence and risk factors of major opportunistic infections after initiation of antiretroviral therapy among advanced HIV-infected patients in a resource-limited setting. J Infect. 2007;55(5):464–469. doi: 10.1016/j.jinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. TB in South-East Asia. [Accessed April 19, 2010];2009 http://www.searo.who.int/EN/Section10/Section2097/Section2100_10639.htm.
- 30.Osmanov S, Pattou C, Walker N, Schwardlander B, Esparza J. Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000. J Acquir Immune Defic Syndr. 2002;29(2):184–190. doi: 10.1097/00042560-200202010-00013. [DOI] [PubMed] [Google Scholar]
- 31.Alaeus A, Lidman K, Bjorkman A, Giesecke J, Albert J. Similar rate of disease progression among individuals infected with HIV-1 genetic subtypes A-D. AIDS. 1999;13(8):901–907. doi: 10.1097/00002030-199905280-00005. [DOI] [PubMed] [Google Scholar]
- 32.Geretti Anna M, Harrison L, Green H, et al. Effect of HIV-1 subtype on virologic and immunologic response to starting highly active antiretroviral therapy. Clin Infect Dis. 2009;48(9):1296–1305. doi: 10.1086/598502. [DOI] [PubMed] [Google Scholar]
- 33.Ramalingam S, Kannangai R, Zachariah A, Mathai D, Abraham C. CD4 counts of normal and HIV-infected south Indian adults: do we need a new staging system? Natl Med J India. 2001;14(6):5. [PubMed] [Google Scholar]
- 34.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end–stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32(3):492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 35.Miller MF, Haley C, Koziel MJ, Rowley CF. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis. 2005;41(5):713–720. doi: 10.1086/432618. [DOI] [PubMed] [Google Scholar]
- 36.Inferior Clinical Outcome of the CD4+ Cell Count-Guided Antiretroviral Treatment Interruption Strategy in the SMART Study: Role of CD4+ Cell Counts and HIV RNA Levels during Follow-up. J Infect Dis. 2008;197(8):1145–1155. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 37.Achhra AC, Amin J, Law MG, et al. Immunodeficiency and the risk of serious clinical endpoints in a well studied cohort of treated HIV-infected patients. AIDS. 2010;24(12):1877–1886. doi: 10.1097/QAD.0b013e32833b1b26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22(7):841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]