Abstract
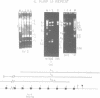
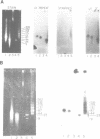
Lambda phage clones containing multiple copies of the 1.1 kb tandemly repeated unit of the sea urchin (S. purpuratus) U1 RNA genes were isolated from a gene library. The 1.1 kb repeat unit encodes a single copy of the predominant U1 RNA expressed in oocytes and embryos prior to the blastula stage. The tandem repeat unit is about 80 kb in size and is probably present one time per haploid genome as judged by pulsed-field electrophoresis of sperm DNA digested with restriction enzymes which do not cut in the repeat unit. Two of the phage contained DNA flanking the repeat unit as well as several repeat units. The tandem repeat unit ends just 3' to the U1 coding region. There is only limited homology in the 5' flanking region with U1 snRNA genes from the sea urchin L. variegatus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ach R. A., Weiner A. M. The highly conserved U small nuclear RNA 3'-end formation signal is quite tolerant to mutation. Mol Cell Biol. 1987 Jun;7(6):2070–2079. doi: 10.1128/mcb.7.6.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ares M., Jr, Mangin M., Weiner A. M. Orientation-dependent transcriptional activator upstream of a human U2 snRNA gene. Mol Cell Biol. 1985 Jul;5(7):1560–1570. doi: 10.1128/mcb.5.7.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Brown D. D. How a simple animal gene works. Harvey Lect. 1980;76:27–44. [PubMed] [Google Scholar]
- Brown D. T., Morris G. F., Chodchoy N., Sprecher C., Marzluff W. F. Structure of the sea urchin U1 RNA repeat. Nucleic Acids Res. 1985 Jan 25;13(2):537–556. doi: 10.1093/nar/13.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card C. O., Morris G. F., Brown D. T., Marzluff W. F. Sea urchin small nuclear RNA genes are organized in distinct tandemly repeating units. Nucleic Acids Res. 1982 Dec 11;10(23):7677–7688. doi: 10.1093/nar/10.23.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzi M., Rohrer U., Birnstiel M. L. Analysis of a sea urchin gene cluster coding for the small nuclear U7 RNA, a rare RNA species implicated in the 3' editing of histone precursor mRNAs. Proc Natl Acad Sci U S A. 1986 May;83(10):3243–3247. doi: 10.1073/pnas.83.10.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley J. M., 3rd, Roebuck K. A., Stumph W. E. Three linked chicken U1 RNA genes have limited flanking DNA sequence homologies that reveal potential regulatory signals. Nucleic Acids Res. 1984 Oct 11;12(19):7411–7421. doi: 10.1093/nar/12.19.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K., Laas W., Patterson D. Fractionation of large mammalian DNA restriction fragments using vertical pulsed-field gradient gel electrophoresis. Somat Cell Mol Genet. 1986 Mar;12(2):185–195. doi: 10.1007/BF01560665. [DOI] [PubMed] [Google Scholar]
- Hardin S. H., Carpenter C. D., Hardin P. E., Bruskin A. M., Klein W. H. Structure of the Spec1 gene encoding a major calcium-binding protein in the embryonic ectoderm of the sea urchin, Strongylocentrotus purpuratus. J Mol Biol. 1985 Nov 20;186(2):243–255. doi: 10.1016/0022-2836(85)90101-9. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Differential accumulation of U1 and U4 small nuclear RNAs during Xenopus development. Genes Dev. 1987 Mar;1(1):39–46. doi: 10.1101/gad.1.1.39. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E., Forbes D. J. The two embryonic U1 small nuclear RNAs of Xenopus laevis are encoded by a major family of tandemly repeated genes. Mol Cell Biol. 1984 Dec;4(12):2580–2586. doi: 10.1128/mcb.4.12.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. True genes for human U1 small nuclear RNA. Copy number, polymorphism, and methylation. J Biol Chem. 1984 Feb 10;259(3):2013–2021. [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Dathan N. A., Parry H. D., Carbon P., Krol A. Changing the RNA polymerase specificity of U snRNA gene promoters. Cell. 1988 Nov 4;55(3):435–442. doi: 10.1016/0092-8674(88)90029-3. [DOI] [PubMed] [Google Scholar]
- Maxson R., Cohn R., Kedes L., Mohun T. Expression and organization of histone genes. Annu Rev Genet. 1983;17:239–277. doi: 10.1146/annurev.ge.17.120183.001323. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Skuzeski J. T., Lund E., Steinberg T. H., Burgess R. R., Dahlberg J. E. Functional elements of the human U1 RNA promoter. Identification of five separate regions required for efficient transcription and template competition. J Biol Chem. 1987 Feb 5;262(4):1795–1803. [PubMed] [Google Scholar]
- Nash M. A., Marzluff W. F. Structure of an unusual sea urchin U1 RNA gene cluster. Gene. 1988 Apr 15;64(1):53–63. doi: 10.1016/0378-1119(88)90480-5. [DOI] [PubMed] [Google Scholar]
- Nash M. A., Sakallah S., Santiago C., Yu J. C., Marzluff W. F. A developmental switch in sea urchin U1 RNA. Dev Biol. 1989 Aug;134(2):289–296. doi: 10.1016/0012-1606(89)90101-2. [DOI] [PubMed] [Google Scholar]
- Nijhawan P., Marzluff W. F. Metabolism of low molecular weight ribonucleic acids in early sea urchin embryos. Biochemistry. 1979 Apr 3;18(7):1353–1360. doi: 10.1021/bi00574a035. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C., Marzluff W. F. Expression of the U1 RNA gene repeat during early sea urchin development: evidence for a switch in U1 RNA genes during development. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2572–2576. doi: 10.1073/pnas.86.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Science. 1987 Feb 13;235(4790):766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- Skuzeski J. M., Lund E., Murphy J. T., Steinberg T. H., Burgess R. R., Dahlberg J. E. Synthesis of human U1 RNA. II. Identification of two regions of the promoter essential for transcription initiation at position +1. J Biol Chem. 1984 Jul 10;259(13):8345–8352. [PubMed] [Google Scholar]
- Strub K., Birnstiel M. L. Genetic complementation in the Xenopus oocyte: co-expression of sea urchin histone and U7 RNAs restores 3' processing of H3 pre-mRNA in the oocyte. EMBO J. 1986 Jul;5(7):1675–1682. doi: 10.1002/j.1460-2075.1986.tb04411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. C., Nash M. A., Santiago C., Marzluff W. F. Structure and expression of a second sea urchin U1 RNA gene repeat. Nucleic Acids Res. 1986 Dec 22;14(24):9977–9988. doi: 10.1093/nar/14.24.9977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller R., Carri M. T., Mattaj I. W., De Robertis E. M. Xenopus laevis U1 snRNA genes: characterisation of transcriptionally active genes reveals major and minor repeated gene families. EMBO J. 1984 May;3(5):1075–1081. doi: 10.1002/j.1460-2075.1984.tb01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]