Abstract
Objective
To compare the CT colonography (CTC) and double-contrast barium enema (DCBE) for colonic evaluation in patients with renal insufficiency.
Materials and Methods
Two sequential groups of consecutive patients with renal insufficiency who had a similar risk for colorectal cancer, were examined by DCBE (n = 182; mean ± SD in age, 51 ± 6.4 years) and CTC (n = 176; 50 ± 6.7 years), respectively. CTC was performed after colon cleansing with 250-mL magnesium citrate (n = 87) or 4-L polyethylene glycol (n = 89) and fecal tagging. DCBE was performed after preparation with 250-mL magnesium citrate. Patients with colonic polyps/masses of ≥ 6 mm were subsequently recommended to undergo a colonoscopy. Diagnostic yield and positive predictive value (PPV) for colonic polyps/masses, examination quality, and examination-related serum electrolyte change were retrospectively compared between the two groups.
Results
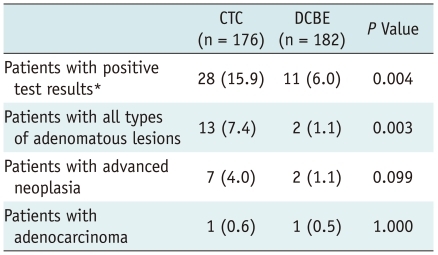
Both the CTC and DCBE were positive for colonic polyps/masses in 28 (16%) of 176 and 11 (6%) of 182 patients, respectively (p = 0.004). Among patients with positive findings, 17 CTC and six DCBE patients subsequently underwent a colonoscopy and yielded a PPV of 88% (15 of 17 patients) and 50% (3 of 6 patients), respectively (p = 0.089). Thirteen patients with adenomatous lesions were detected in the CTC group (adenocarcinoma [n = 1], advanced adenoma [n = 6], and non-advanced adenoma [n = 6]), as compared with two patients (each with adenocarcinoma and advanced adenoma) in the DCBE group (p = 0.003). Six (3%) of 176 CTC and 16 (9%) of 182 DCBE examinations deemed to be inadequate (p = 0.046). Electrolyte changes were similar in the two groups.
Conclusion
In patients with renal insufficiency, CTC has a higher diagnostic yield and a marginally higher PPV for detecting colorectal neoplasia, despite a similar diagnostic yield for adenocarcinoma, and a lower rate of inadequate examinations as compared with DCBE.
Keywords: CT colonography, Double-contrast barium enema, Diagnostic yield, Colonic neoplasia, Renal insufficiency
INTRODUCTION
Colonic evaluation for screening of colorectal neoplasms and diverticular diseases is generally a routine procedure before renal transplantation for end-stage renal disease (ESRD). ESRD has been associated with increased risks of malignancy and immunosuppressive therapy after renal transplantation may increase the risk of malignancy or accelerate the malignant progression of neoplasia, although their effects specifically on colorectal cancer still need to be further clarified (1-10). In addition, patients with diverticula/diverticulitis may be at higher risk of colonic perforation after renal transplantation, since immune suppression may mask the signs and symptoms of post-transplant diverticulitis, hence delaying diagnosis and increasing the risk of perforation (11-13).
Many patients with ESRD undergo a period of peritoneal dialysis before renal transplantation. Thus, radiological colonic examinations may be particularly useful for those who have developed peritoneal adhesion after dialysis, which could make colonoscopy difficult. Among the radiological colonic examinations, CT colonography (CTC) is particularly expected to be suitable for examining ESRD patients compared to double-contrast barium enema (DCBE). Unlike DCBE, CTC can be performed following a "wet" bowel preparation with polyethylene glycol (PEG) (14), the safest cathartic agent for ESRD patients (15, 16). CTC may also be able to potentially remove the risk of fluid/electrolyte disturbance in ESRD patients, adopting catharsis-free techniques. To our knowledge, no studies have compared CTC to DCBE in patients with renal insufficiency. In addition, although CTC is generally regarded to be more accurate diagnostically than DCBE, to date, there have been few direct comparisons of CTC and DCBE, and with the studies performed being before CTC techniques were optimized (17, 18). Therefore, the purpose of this study was to compare the CTC and DCBE for colonic evaluation in patients with renal insufficiency.
MATERIALS AND METHODS
The study was in accordance with the ethical standards of Declaration of Helsinki. The institutional review board of Asan Medical Center approved this retrospective study and waived patient informed consent.
Study Patients
At our institution, colorectal screening for neoplasms and diverticular diseases is a routine part of the diagnostic work-up prior to renal transplantation in patients older than 40 years. We used the DCBE until January 2008, and we then switched over to the CTC. Our study cohort consisted of 182 consecutive ESRD patients (109 men and 73 women; mean ± SD age, 50.9 ± 6.4 years) who underwent DCBE for pretransplant evaluation between January 2006 and December 2007, and 176 consecutive ESRD patients (101 men and 75 women; mean ± SD age, 49.6 ± 6.7 years) who underwent CTC between January 2008 and December 2009.
Review of Medical Records
We reviewed the history of colorectal cancer, colorectal adenoma, polyposis, inflammatory bowel disease, and hereditary nonpolyposis colorectal cancer syndrome of all patients and their family (including parents, siblings, and/or children) history of colorectal cancer. We additionally assessed patients' colorectal symptoms, including any changes in bowel habits and blood in stool. We also reviewed the CTC, DCBE, and colonoscopy results of all patients and their serum concentrations of sodium, potassium, calcium, and creatinine prior to and after the CTC and DCBE. The occurrence of any adverse events related to a CTC and DCBE was confirmed.
CTC Examination
Bowel preparation for a CTC consisted of two different protocols. All patients ingested a clear liquid diet the day before a CTC and underwent a cathartic preparation the night before the examination. The first 87 patients ingested 250 mL magnesium citrate (Magcorol; Taejoon Pharmaceuticals, Gyeonggido, Korea) and 10 mg bisacodyl for colon cleansing. Fecal tagging in these patients consisted of 200 mL of a 4.6% wt/vol barium suspension (Easy CT 4.6; Taejoon Pharmaceuticals, Gyeonggido, Korea) (19), taken after each meal the day before CTC (i.e. three doses for a total to 600 mL). Due to the occasional occurrence of a large amount of untagged stool with the magnesium citrate preparation and the reported higher safety of PEG in patients with renal insufficiency (15), the following 89 patients underwent bowel preparation by ingesting 4 L PEG (Colyte; Taejoon Pharmaceuticals, Gyeonggido, Korea). Fluid tagging in these patients consisted of 50 mL meglumine diatrizoate (Gastrografin; Bayer Schering Pharma AG, Berlin, Germany) ingested orally after a colon cleansing.
Colonic distention before CT scanning was performed using an automated carbon dioxide insufflator (PROTOCO2L; Bracco, Milan, Italy); spasmolytics were not used. Each patient underwent both supine and prone scans, performed using a 16-MDCT scanner (Somatom Sensation 16; Siemens Medical Solutions, Erlangen, Germany) with the following specifications: collimation, 0.75 mm × 16; pitch, 1; reconstructed slice thickness, 1 mm; reconstruction interval, 0.7 mm; field of view to fit; 120 kVp; and 50 mAs. Intravenous contrast was not administered.
The original clinical interpretations of the CTC results were used for this study and the results were interpreted by one of two board-certified experienced radiologists (S.H.P. and S.S.L., each of whom had interpreted more than 1000 CTC cases at the beginning of the study period). CTC results were interpreted primarily using three-dimensional (3D) endoluminal navigation with two-dimensional (2D) problem solving at a dedicated CTC workstation (Xelis Colon; Infinitt, Seoul, Korea) (20). Technical assessments, including adequacy of bowel preparation, fecal and fluid tagging, colonic distention, and CT artifacts, were made on a per-patient basis according to the CTC Reporting and Data System (C-RADS) (21). Inadequate examinations (C0), in which the radiologist was unable to exclude the presence of polyps ≥ 1 cm in maximal diameter, were recorded, along with the cause of each problem. Colonic masses and polyps ≥ 6 mm in maximal diameter and colonic diverticula or diverticulitis were reported. The segmental location and morphology (sessile, pedunculated, or flat) of the colonic mass/polyp were recorded. Extracolonic evaluation was performed as usual and also included confirmation of the absence/presence of any abnormal findings in the iliac fossae in which the transplanted kidney was intended to be placed. Any examination-related adverse reactions and complications were recorded.
Data on radiation dose were collected in each patient from the dose table provided by the scanner and were converted to an effective dose according to the International Commission on Radiological Protection publication 103 recommendations (22).
DCBE Examination
All patients ingested a clear liquid diet the day before a DCBE. A colon cleansing was performed by the oral ingestion of 250 mL magnesium citrate and 10 mg bisacodyl the night before the examination. PEG was not used as it leaves excess fluid in the colon and impairs mucosal barium coating (16). A DCBE was performed by first- or second-year radiology residents according to standard techniques (16) using digital fluoroscopic equipment (Shimavision 3200 HG; Shimadzu, Kyoto, Japan) and a transrectally administered 400 to 600 mL of 80% wt/vol barium suspension (Solotop Powder; Taejoon Pharmaceuticals, Gyeonggido, Korea). The original clinical interpretations of DCBE results were used for this study. DCBE results were interpreted in consensus by a junior instructor in gastrointestinal radiology and the resident who had performed the examination. The findings were reported in the same way as those of CTC. Radiation dose information was not available for the DCBE examinations.
Colonoscopy
The need for a colonoscopy following CTC or DCBE was ultimately determined by the referring clinicians, who considered the sizes of colonic lesions and the ability of the patient to undergo renal transplantation. A colonoscopy was performed by experienced board-certified gastroenterologists using a video colonoscope (CF 260; Olympus Optical Co., Tokyo, Japan). The colonoscopists had been informed of the CTC or DCBE results prior to performing the colonoscopy. Segmental location, size, and morphology of the detected lesions were recorded, and the lesions were removed or biopsied for pathologic examination. Lesion matching between CTC or DCBE and colonoscopy required both segmental and size agreement (i.e. within the same or adjacent segment and within ± 50% of the colonoscopic size) (23, 24). The results of colonoscopy served as the reference standard for the colonic masses/polyps.
Data and Statistical Analysis
In order to confirm the comparability between the CTC and DCBE groups (i.e. similar risk for colorectal cancer/neoplasia), we compared the demographic and clinical characteristics (i.e., age, sex distribution, and risk factors for colorectal cancers). As our retrospective clinical patients did not undergo subsequent colonoscopy if their CTC or DCBE had been negative, we could not calculate sensitivity and specificity. We instead focused primarily on analyzing the diagnostic yield of the CTC and DCBE, which is a well established method to compare diagnostic tests in a retrospective setting (25). The diagnostic yield is also a higher level index of clinical impact of a diagnostic test than sensitivity/specificity in the hierarchy of health technology assessment (26). We calculated the positive test rate, positive predictive value (PPV), and diagnostic yield of CTC and DCBE for colonic masses/polyps on a per-patient basis and compared the results of each parameter between the two groups. For the per-patient PPV, at least one relevant match between the CTC or DCBE and colonoscopy was required to be considered a true-positive examination. Positive test rate and PPV were calculated for two lesion size thresholds: ≥ 6 mm and ≥ 10 mm in maximal diameter. Diagnostic yields were obtained for three histological categories: all types of adenomatous lesions, advanced neoplasia, and adenocarcinoma. Advanced neoplasia represented both advanced adenomas (defined as adenomas ≥ 10 mm or by the presence of a substantial villous component or high-grade dysplasia) and adenocarcinomas. For the per-patient analysis of diagnostic yields, patients were categorized according to their most significant colonic lesion histology, regardless of the presence and the number of colonic lesions of less important histology (adenocarcinoma, advanced adenoma, non-advanced adenoma, and non-adenomatous lesions in decreasing order of importance).
The rates of diverticular diseases and clinically suspicious extracolonic findings for CTC and DCBE (i.e. extracolonic findings which were interpreted to require further diagnostic evaluation or treatment) were compared, as was the rate of inadequate quality examinations. We also compared changes in serum concentrations for electrolytes and creatinine if they had been measured prior to (within seven days) and after (either the same or the next day) the cathartic bowel preparation, among the DCBE and CTC patients prepared with magnesium citrate and CTC patients prepared with PEG.
Categorical variables of the CTC and DCBE groups were compared using Fisher's exact test, and continuous variables using Student t test. Comparisons among the three groups (DCBE patients and the two groups of CTC patients) were made using analysis of variance. Statistical analyses were performed with SPSS 15.0 for Windows (SPSS, Chicago, IL). p values less than 0.05 were considered to be statistically significant.
RESULTS
Patient Characteristics
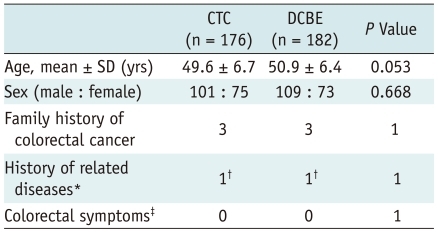
The demographic characteristics and colorectal cancer risks of patients in the CTC and DCBE groups are summarized in Table 1. There were no significant between group differences for gender and the known risk factors for colorectal cancer. Although the age difference was not statistically significanct (p = 0.053), the average age of DCBE patients was approximately 1 year older than the average age of CTC patients.
Table 1.
Characteristics of Study Patients
Note.- Data are number of patients except for age.
*History of colorectal cancer, colorectal adenoma, polyposis, inflammatory bowel disease, or hereditary nonpolyposis colorectal cancer syndrome, †History of polypectomy of tubular adenoma, ‡Changes in bowel habits and blood in stool.
CTC = CT colonography, DCBE = double-contrast barium enema
Colonic and Extracolonic Findings
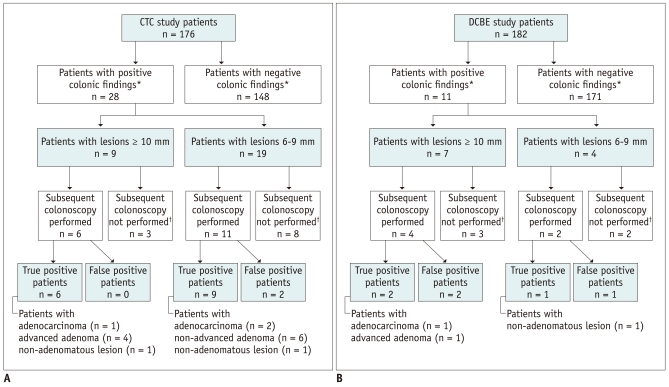
The outcomes regarding colonic masses/polyps of patients who underwent a CTC and DCBE are summarized in Figure 1. The CTC had a significantly higher positive test rate than did the DCBE for lesions ≥ 6 mm (15.9% [28/176] vs. 6% [11/182]; p = 0.004), but not for lesions ≥ 10 mm (5.1% [9/176] vs. 3.8% [7/182]; p = 0.616).
Fig. 1.
Enrollment and outcomes of patients undergoing CTC and DCBE.
A. Patients undergoing a CTC. *Positive for colonic masses/polyps ≥ 6 mm in size, †Seven patients were lost to follow-up without subsequent colonoscopy as they finally declined or were denied renal transplantation, and four patients did not undergo colonoscopy, most likely due to small lesion size. B. Patients undergoing DCBE. *Positive for colonic masses/polyps ≥ 6 mm in size, †Patients were lost to follow-up without subsequent colonoscopy as they finally declined or were denied to undergo renal transplantation. CTC = CT colonography, DCBE = double-contrast barium enema
Colonoscopic follow-up results were available in 17 of the 28 patients (60.7%) with positive CTC findings (i.e. lesions ≥ 6 mm), and a median time interval between the two examinations of 16 days (range, 1 to 165 days), and in 6 of the 11 patients (54.5%) with positive DCBE findings with a median time interval of 21 days (range, 11 to 69 days) (p = 0.734). Of the other 16 patients whose colonoscopic follow-up results were not available, 12 were lost to follow-up without subsequent colonoscopy as they finally declined or were denied a renal transplantation, and 4 did not undergo a colonoscopy most likely due to a small lesion size (Fig. 1). Colonoscopy results showed that PPVs for the CTCs and DCBEs were 88.2% (15/17) and 50% (3/6), respectively for detecting ≥ 6 mm lesions (p = 0.089); and were 100% (6/6 patients) and 50% (2/4 patients) for detecting lesions ≥ 10 mm (p = 0.133), indicating a trend that the CTC shows a higher PPV, albeit not statistically significant. The 17 patients who underwent a colonoscopy following positive CTC results turned out to be patients with adenocarcinoma (n = 1) (Fig. 2), advanced adenoma (n = 6), non-advanced adenoma (n = 6), non-adenomatous lesion (n = 2), and false-positive findings (n = 2). Both of the latter two false-positive patients underwent a colon preparation with magnesium citrate. The six patients who underwent colonoscopy following positive DCBE results turned out to be patients with adenocarcinoma (n = 1) (Fig. 3), advanced adenoma (n = 1), nonadenomatous lesion (n = 1), and false-positive findings (n = 3). The diagnostic yields relative to histological categories for both the CTC and DCBE are summarized in Table 2. CTC had a significantly higher diagnostic yield than DCBE for all adenomatous lesions (p = 0.003), and showed a trend for higher yield in advanced neoplasias, although not statistically significant (p = 0.099).
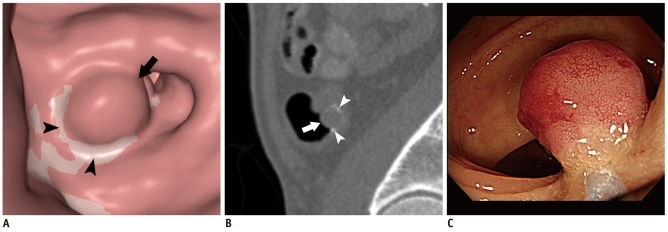
Fig. 2.
15-mm adenocarcinoma in sigmoid colon of 55-year-old man detected by CTC. CTC also detected another 9-mm cancerous polyp (not shown).
A. 3D endoluminal image of CTC showing rounded polyp in sigmoid colon (arrow). White curvilinear area (arrowheads) represents tagged fluid around polyp. B. Sagittal 2D CTC image showing polyp (arrow) and tagged fluid (arrowheads) adjacent to lesion. C. Colonoscopy performed next day showing corresponding 15-mm polyp. CTC = CT colonography
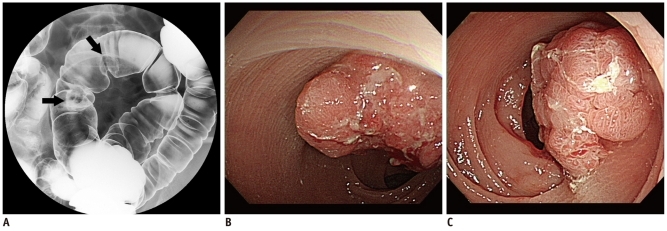
Fig. 3.
Two 25-mm and 20-mm adenocarcinomas in sigmoid colon of 65-year-old man detected by DCBE.
A. DCBE image of sigmoid colon showing two polypoid masses (arrows). B, C. Colonoscopy performed three weeks after DCBE showing 25-mm polypoid mass with ulceration in sigmoid colon (B) and another 20-mm polypoid mass located more proximally (C). DCBE = double-contrast barium enema
Table 2.
Diagnostic Yields of CTC and DCBE
Note.- Data are number of patients, with percentage in parentheses. *Positive tests were defined as presence of colonic polyps/masses ≥ 6 mm in diameter. Colonoscopic follow-up was performed in 17 of 28 patients (60.7%) with positive CTC findings and six of 11 (54.5%) with positive DCBE findings (p = 0.734). CTC = CT colonography, DCBE = double-contrast barium enema
Forty-four of 176 (25%) CTC patients (all without diverticulitis) and 41 of 182 (22.5%) DCBE patients (40 without and one with diverticulitis) had diverticular diseases, with no significant between group difference (p = 0.620).
Suspicious extracolonic abnormalities were noted in 14 of 176 (8.0%) CTC patients; of these, 11 were confirmed as true positive and one turned out to be a pseudolesion in the kidney at follow-up evaluations (4 benign pulmonary nodules, 1 pneumothorax, 1 gallbladder stone, 1 pancreatic cystic neoplasm, 1 chronic pancreatitis, 1 splenic hemangioma, 1 ovarian teratoma, and 1 benign lymphadenopathy). The remaining two patients, who had a suspected lymphadenopathy and a peritoneal nodule, respectively, have not been further characterized. Notable extracolonic findings were observed in only 2 of 182 DCBE patients (1.1%) (p < 0.001 compared with CTC), which were an extrinsic colon compression, suggesting that pelvic masses were present in both patients, but confirmed as false positive. Detection of the exracolonic findings did not affect renal transplantation in any patient but caused treatment in the patient with pneumothorax and interval clinical follow-ups in one each with a gallbladder stone, pancreatic cystic neoplasm, and chronic pancreatitis.
Examination Quality
Examination quality was inadequate in 6 of the 176 CTC patients (3.4%) and 16 of the 182 DCBE patients (8.8%) (p = 0.046). In these patients, the presence of large advanced cancer masses such as "apple-core" lesions and large ulcerofungating masses could be excluded, but smaller lesions could not be excluded. Causes of inadequate examinations in the CTC group include large amounts of untagged stool in 3 patients, all of which were prepared with magnesium citrate; collapse of the sigmoid colon in 2; and a respiratory motion artifact in 1. In the DCBE group, all inadequate examinations were due to poor bowel preparation and poor mucosal coating.
Laboratory Data and Adverse Effects
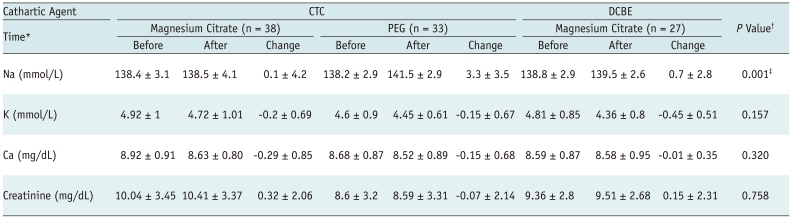
Serum concentrations of electrolytes and creatinine were not routinely measured before and after colonic examinations, since the safety of our bowel preparation methods was generally known. At the discretion of the attending physicians, electrolytes and creatinine were measured both before (within seven days) and after (either the same or the next day) colonic examinations in 27 DCBE patients, 38 CTC patients prepared with magnesium citrate, and 33 CTC patients prepared with PEG (Table 3). Changes in serum Na, K, Ca, and creatinine were all minimal and clinically insignificant in all three groups and did not differ significantly among groups (p ≥ 0.157), except for serum Na where CTC patients prepared with PEG showed greater change than the two other groups (p = 0.001). No patient had a serum electrolyte and/or fluid imbalance that required urgent treatment.
Table 3.
Serum electrolyte and creatinine concentrations before and after colon examinations
Note.- Data are expressed as mean ± SD. *Acquisition time of laboratory data with respect to time of colonic examination. "change" refers to concentration after minus concentration before colonic examination, †p values are for comparing changes in serum electrolytes and creatinine among three groups using analysis of variance, ‡Post hoc analysis using Bonferroni test showed significant difference in serum Na change between two CTC groups (p = 0.001) and between CTC group prepared with PEG and DCBE group (p = 0.016). CTC = CT colonography, DCBE = double-contrast barium enema, PEG = polyethylene glycol
All patients tolerated the examination well and there were no cases of colonic perforation or other significant complications related to CTC or DCBE.
Radiation Dose
The mean ± SD effective CTC dose for the combined supine and prone scans was 4.8 ± 0.4 mSv for male patients and 6.6 ± 0.4 mSv for female patients.
DISCUSSION
We observed that CTC had a higher diagnostic yield than DCBE for detecting colorectal neoplasia in ESRD patients, which was consistent with the generally regarded superior diagnostic accuracy of CTC compared to DCBE (27), and that the methods had a similar ability to detect diverticular diseases. These results may suggest that CTC is diagnostically superior to DCBE for pretransplant colorectal screening of ESRD patients. However, the greater resources are needed for the CTC compared to DCBE and the superiority of the CTC in our study was mostly attributed to the better detection of small non-cancerous lesions by CTCs, which are less of an immediate clinical concern. The difference was absent for adenocarcinoma and was only marginal for advanced neoplasia. Therefore, our results may not necessarily indicate that the CTC is a more cost-effective screening method for ESRD patients. In fact, the DCBE was shown to have reasonably high accuracy for detecting colorectal cancers approaching the lower-range performance of colonoscopy even though it is inferior in overall polyp detection (28-30). There was also some uncertainty in our study related to the lack of colonoscopy confirmation in some of the CTC- and DCBE-positive patients. Hence, the diagnostic yields of the two tests could be somewhat inaccurate. Since DCBEs had seemingly a lower PPV (50%) than CTCs (88.2% and 100% depending on lesion size), the difference in diagnostic yield between the two examinations might have been slightly greater if all patients positive on these examinations had undergone colonoscopy.
The overall superior diagnostic yield of CTCs for detecting colorectal neoplasia may be due to several factors. First, although direct comparative studies of CTCs and DCBEs are scarce (17, 18), CTCs may have a higher intrinsic diagnostic accuracy than DCBEs, as demonstrated by prior indirect comparisons (27). Second, CTCs were performed by experienced board-certified radiologists, whereas DCBEs were performed by relatively less experienced personnel (first- and second-year residents and a junior instructor). If DCBEs had been performed, they interpreted by more experienced practitioners and may have resulted in a higher diagnostic yield and PPV. However, our practice pattern reflects the current trend of declining quality of DCBEs at many institutions, due to examinations being performed by individuals with insufficient experience (31, 32). As referring clinicians increasingly dismiss DCBEs, many institutions do not have a large enough case volume for resident trainees to master the needed skills and for supervisors to maintain their expertise (33, 34). Third, the inferior results for DCBEs may be due in part to the less complete bowel cleansing in the DCBE group due to the use of moderate amounts of magnesium citrate, (i.e. 250 mL). This is reflected by the higher rates of technically inadequate (8.8%) and false-positive (50%) examinations for the DCBE group compared to the CTC group. Likewise, in the CTC group, all false-positive patients (n = 2) and patients with poor bowel preparation (n = 3) had been prepared with magnesium citrate. Magnesium citrate has a less potent cathartic effect than sodium phosphate or PEG, and a double dose of magnesium citrate (592 mL) was required to achieve the same level of colon cleansing as 45 mL of sodium phosphate in one study (35). Nevertheless, we only used a moderate amount of magnesium citrate in our patients, since it should be used with caution in patients with renal impairment, even if it is safer than sodium phosphate, which should not be used in patients with decreased renal function (15, 36-39). Our results showed that this moderate amount of magnesium citrate did not cause any greater changes in electrolytes than PEG or any substantial electrolyte imbalance.
The ability to perform a CTC with PEG is an advantage of this method for patients with renal impairment, as PEG is a potent and yet known to be the safest cathartic agent that causes the least fluid/electrolyte disturbance. PEG cannot be used for DCBE due to the large amount of retained fluid, which prevents mucosal coating, unless the procedure incorporates a delay of at least 12-18 hours and an additional administration of stimulant cathartics (16, 40). Interestingly enough, however, the only notable serum electrolyte change in our study, albeit clinically insignificant, occurred in patients prepared with PEG. Considering the well-established safety profile of PEG, the unexpected greater degree of elevation in serum Na in patients prepared with PEG might have been related to the use of Gastrografin for fluid tagging in the same group of patients, although it is a conjecture. Hypovolemia and/or electrolyte imbalance caused by Gastrografin-induced fluid loss from the intestine is a well-known adverse effect of Gastrografin and, therefore, its administration should be done with caution in patients with renal impairment who are at high risk for fluid/electrolyte imbalance (41). Our results suggest that the oral administration of 50 mL Gastrografin for fluid tagging in CTC is likely tolerable without any clinical consequences in ESRD patients. Nevertheless, the potential adverse effects of Gastrografin in patients with renal impairment may need to be further clarified. Additionally, as the ingestion of 4 L PEG is quite burdensome for patients, efforts are being made to decrease the volume of the cathartics and it may be worthwhile to investigate if CTC could be performed successfully with a smaller volume of PEG in patients with renal insufficiency.
A CTC may have several other potential advantages over DCBE; it allows for the seamless continuation to colonoscopy in cases where a colonoscopic polypectomy or biopsy is required. In contrast, colonoscopy has to be delayed after a DCBE, until the instilled barium is evacuated. Extracolonic evaluation of CTC has also been reported to detect a considerable number of clinically important extracolonic abnormalities, including malignancies, which would not be detectable with a DCBE, even though our CTC patients happened not to have any extracolonic malignancies or abnormalities that would have precluded renal transplantation. Moreover, radiation exposure caused by a CTC in our study was a bit lower than or at the lower margin of the radiation exposure from the radiation exposure from a DCBE reported in the literature (5 to 9 mSv) (42).
Our study had several limitations. First, as we did not randomize the patients for undergoing either a CTC or a DCBE, there may have been the potential for a selection bias, leading to a difference in prevalence for colorectal neoplasia in the CTC and DCBE groups. However, the potential bias may have been negligible as the groups were similar in several main known risk factors for colorectal cancer. In fact, considering that the DCBE group consisted of slightly older patients than the CTC group, the bias, if any, may have existed for a slightly higher prevalence of colorectal neoplasia in the DCBE group. Second, as mentioned previously, our study with the retrospective clinical population could not directly address the comparative sensitivity and specificity of DCBE and CTC. However, comparing the diagnostic yields is also a well established method to compare the clinical impact of diagnostic tests (25, 26). Third, as our study population only included ESRD patients, it is unclear if our results can be extrapolated to a general comparison of a CTC and a DCBE. A DCBE may perform better in patients without renal impairment, following more vigorous bowel cleansing and using a larger amount of magnesium citrate.
In conclusion, the CTC showed a higher diagnostic yield and a marginally higher PPV for detecting colorectal neoplasia, despite a similar diagnostic yield for adenocarcinoma, compared with a DCBE in patients with renal insufficiency. In addition, the two methods were similar in detecting diverticular diseases, and CTC had a lower rate of inadequate examinations than DCBE.
References
- 1.Lee S, Wasserberg N, Petrone P, Rosca J, Selby R, Ortega A, et al. The prevalence of colorectal neoplasia in patients with end-stage renal disease: a case-control study. Int J Colorectal Dis. 2008;23:47–51. doi: 10.1007/s00384-007-0379-7. [DOI] [PubMed] [Google Scholar]
- 2.Iseki K, Osawa A, Fukiyama K. Evidence for increased cancer deaths in chronic dialysis patients. Am J Kidney Dis. 1993;22:308–313. doi: 10.1016/s0272-6386(12)70323-2. [DOI] [PubMed] [Google Scholar]
- 3.Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 4.Marple JT, MacDougall M. Development of malignancy in the end-stage renal disease patient. Semin Nephrol. 1993;13:306–314. [PubMed] [Google Scholar]
- 5.Parikshak M, Pawlak SE, Eggenberger JC, Lee CS, Szilagy EJ, Margolin DA. The role of endoscopic colon surveillance in the transplant population. Dis Colon Rectum. 2002;45:1655–1660. doi: 10.1007/s10350-004-7254-1. [DOI] [PubMed] [Google Scholar]
- 6.Penn I. The effect of immunosuppression on pre-existing cancers. Transplantation. 1993;55:742–747. doi: 10.1097/00007890-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Saidi RF, Dudrick PS, Goldman MH. Colorectal cancer after renal transplantation. Transplant Proc. 2003;35:1410–1412. doi: 10.1016/s0041-1345(03)00478-0. [DOI] [PubMed] [Google Scholar]
- 8.Vamvakas S, Bahner U, Heidland A. Cancer in end-stage renal disease: potential factors involved -editorial- Am J Nephrol. 1998;18:89–95. doi: 10.1159/000013314. [DOI] [PubMed] [Google Scholar]
- 9.Penn I. Tumors after renal and cardiac transplantation. Hematol Oncol Clin North Am. 1993;7:431–445. [PubMed] [Google Scholar]
- 10.EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.6.3. Cancer risk after renal transplantation. Solid organ cancers: prevention and treatment. Nephrol Dial Transplant. 2002;17(Suppl 4):32, 34–36. [PubMed] [Google Scholar]
- 11.Coccolini F, Catena F, Di Saverio S, Ansaloni L, Faenza A, Pinna AD. Colonic perforation after renal transplantation: risk factor analysis. Transplant Proc. 2009;41:1189–1190. doi: 10.1016/j.transproceed.2009.02.064. [DOI] [PubMed] [Google Scholar]
- 12.Dalla Valle R, Capocasale E, Mazzoni MP, Busi N, Benozzi L, Sivelli R, et al. Acute diverticulitis with colon perforation in renal transplantation. Transplant Proc. 2005;37:2507–2510. doi: 10.1016/j.transproceed.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 13.Ponticelli C, Passerini P. Gastrointestinal complications in renal transplant recipients. Transpl Int. 2005;18:643–650. doi: 10.1111/j.1432-2277.2005.00134.x. [DOI] [PubMed] [Google Scholar]
- 14.Yun JY, Ro HJ, Park JB, Choi JB, Chung JE, Kim YJ, et al. Diagnostic performance of CT colonography for the detection of colorectal polyps. Korean J Radiol. 2007;8:484–491. doi: 10.3348/kjr.2007.8.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SH, Yee J, Kim SH, Kim YH. Fundamental elements for successful performance of CT colonography (virtual colonoscopy) Korean J Radiol. 2007;8:264–275. doi: 10.3348/kjr.2007.8.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubesin SE, Levine MS, Laufer I, Herlinger H. Double-contrast barium enema examination technique. Radiology. 2000;215:642–650. doi: 10.1148/radiology.215.3.r00jn36642. [DOI] [PubMed] [Google Scholar]
- 17.Rockey DC, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305–311. doi: 10.1016/S0140-6736(05)17784-8. [DOI] [PubMed] [Google Scholar]
- 18.Johnson CD, MacCarty RL, Welch TJ, Wilson LA, Harmsen WS, Ilstrup DM, et al. Comparison of the relative sensitivity of CT colonography and double-contrast barium enema for screen detection of colorectal polyps. Clin Gastroenterol Hepatol. 2004;2:314–321. doi: 10.1016/s1542-3565(04)00061-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim MJ, Park SH, Lee SS, Byeon JS, Choi EK, Kim JH, et al. Efficacy of barium-based fecal tagging for CT colonography: a comparison between the use of high and low density barium suspensions in a Korean population-a preliminary study. Korean J Radiol. 2009;10:25–33. doi: 10.3348/kjr.2009.10.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SS, Park SH, Kim JK, Kim N, Lee J, Park BJ, et al. Panoramic endoluminal display with minimal image distortion using circumferential radial ray-casting for primary three-dimensional interpretation of CT colonography. Eur Radiol. 2009;19:1951–1959. doi: 10.1007/s00330-009-1362-1. [DOI] [PubMed] [Google Scholar]
- 21.Zalis ME, Barish MA, Choi JR, Dachman AH, Fenlon HM, Ferrucci JT, et al. CT colonography reporting and data system: a consensus proposal. Radiology. 2005;236:3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 22.The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Pickhardt PJ, Taylor AJ, Leung WK, Winter TC, Hinshaw JL, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357:1403–1412. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 26.Hollingworth W, Jarvik JG. Technology assessment in radiology: putting the evidence in evidence-based radiology. Radiology. 2007;244:31–38. doi: 10.1148/radiol.2441051790. [DOI] [PubMed] [Google Scholar]
- 27.Sosna J, Sella T, Sy O, Lavin PT, Eliahou R, Fraifeld S, et al. Critical analysis of the performance of double-contrast barium enema for detecting colorectal polyps > or = 6 mm in the era of CT colonography. AJR Am J Roentgenol. 2008;190:374–338. doi: 10.2214/AJR.07.2099. [DOI] [PubMed] [Google Scholar]
- 28.Kung JW, Levine MS, Glick SN, Lakhani P, Rubesin SE, Laufer I. Colorectal cancer: screening double-contrast barium enema examination in average-risk adults older than 50 years. Radiology. 2006;240:725–735. doi: 10.1148/radiol.2403051236. [DOI] [PubMed] [Google Scholar]
- 29.Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology. 1997;112:17–23. doi: 10.1016/s0016-5085(97)70213-0. [DOI] [PubMed] [Google Scholar]
- 30.Tawn DJ, Squire CJ, Mohammed MA, Adam EJ. National audit of the sensitivity of double-contrast barium enema for colorectal carcinoma, using control charts For the Royal College of Radiologists Clinical Radiology Audit Sub-Committee. Clin Radiol. 2005;60:558–564. doi: 10.1016/j.crad.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Ferrucci JT. Double-contrast barium enema: use in practice and implications for CT colonography. AJR Am J Roentgenol. 2006;187:170–173. doi: 10.2214/AJR.05.0900. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson G. Colon imaging in radiology departments in 2008: goodbye to the routine double contrast barium enema. Can Assoc Radiol J. 2008;59:174–182. [PubMed] [Google Scholar]
- 33.Fletcher RH. The end of barium enemas? N Engl J Med. 2000;342:1823–1824. doi: 10.1056/NEJM200006153422409. [DOI] [PubMed] [Google Scholar]
- 34.Klabunde CN, Jones E, Brown ML, Davis WW. Colorectal cancer screening with double-contrast barium enema: a national survey of diagnostic radiologists. AJR Am J Roentgenol. 2002;179:1419–1427. doi: 10.2214/ajr.179.6.1791419. [DOI] [PubMed] [Google Scholar]
- 35.Borden ZS, Pickhardt PJ, Kim DH, Lubner MG, Agriantonis DJ, Hinshaw JL. Bowel preparation for CT colonography: blinded comparison of magnesium citrate and sodium phosphate for catharsis. Radiology. 2010;254:138–144. doi: 10.1148/radiol.09090398. [DOI] [PubMed] [Google Scholar]
- 36.Barkun A, Chiba N, Enns R, Marcon M, Natsheh S, Pham C, et al. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety--a Canadian Association of Gastroenterology position paper. Can J Gastroenterol. 2006;20:699–710. doi: 10.1155/2006/915368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fine A, Patterson J. Severe hyperphosphatemia following phosphate administration for bowel preparation in patients with renal failure: two cases and a review of the literature. Am J Kidney Dis. 1997;29:103–105. doi: 10.1016/s0272-6386(97)90015-9. [DOI] [PubMed] [Google Scholar]
- 38.Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy-a meta-analysis. Colorectal Dis. 2006;8:247–258. doi: 10.1111/j.1463-1318.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- 39.Wiberg JJ, Turner GG, Nuttall FQ. Effect of phosphate or magnesium cathartics on serum calcium: observations in normocalcemic patients. Arch Intern Med. 1978;138:1114–1116. [PubMed] [Google Scholar]
- 40.Smith C. Colorectal cancer. Radiologic diagnosis. Radiol Clin North Am. 1997;35:439–445. [PubMed] [Google Scholar]
- 41.Drugs.com Website. [Accessed October 5, 2011]. Available at: http://www.drugs.com/pro/gastrografin.html.
- 42.Kemerink GJ, Borstlap AC, Frantzen MJ, Schultz FW, Zoetelief J, van Engelshoven JM. Patient and occupational dosimetry in double contrast barium enema examinations. Br J Radiol. 2001;74:420–428. doi: 10.1259/bjr.74.881.740420. [DOI] [PubMed] [Google Scholar]