Abstract
Purpose
We previously showed that metabolic tumor volume (MTV) on PET-CT predicts for disease recurrence and death in head and neck cancer (HNC). We hypothesized that increases in MTV over time would correlate with tumor growth and biology, and predict outcome. We sought to examine tumor growth over time in serial pre-treatment PET-CT scans.
Methods and Materials
From 2006–2009, 51 patients had two PET-CT scans prior to HNC treatment. MTV was defined as the tumor volume ≥50% of maximum SUV (SUVmax). MTV was calculated for the primary tumor, nodal disease, and composite (primary tumor + nodes). MTV and SUV velocity were defined as the change in MTV or SUVmax over time respectively. Cox regression analyses were used to examine correlations between SUV, MTV velocity, and outcome (disease progression and overall survival [OS]).
Results
Median follow-up time was 17.5 months. Median time between PET-CT scans was 3 weeks. Unexpectedly, 51% of cases demonstrated a decrease in SUVmax (average −0.1cc/week) and MTV (average −0.3cc/week) over time. Despite the variability in MTV, primary tumor MTV velocity predicted disease progression (hazard ratio [HR] 2.94; p=0.01), and OS (HR 1.85; p=0.03).
Conclusions
Primary tumor MTV velocity appears to be a better prognostic indicator of disease progression and survival compared to nodal MTV velocity. However, substantial variability was found in PET-CT biomarkers between serial scans. Caution should be used when integrating PET-CT biomarkers into clinical protocols in HNC.
Keywords: metabolic tumor volume, functional imaging, head and neck cancers, PETCT
Introduction
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging has been increasingly used for staging, radiotherapy target definition, and for determining treatment response in head-and-neck cancers (HNC) (1–3). When combined with computed tomography (CT) imaging, PET-CT has an improved sensitivity and specificity compared to standard CT or magnetic resonance imaging (MRI) (4–6).
Differential FDG uptake between tumor cells and normal tissue measured via maximum standardized uptake value (SUVmax) is an independent prognostic factor in several HNC subsites (7–9). We recently reported on a more functional pre-radiation PET-CT measurement tool, metabolic tumor volume (MTV), which quantifies the metabolic tumor burden. Pre-treatment MTV predicted for disease progression and survival in HNC whereas SUVmax did not (10). We also showed that post-radiation MTV is a prognostic factor in HNC (11). These findings suggest that metabolic tumor burden may be a stronger independent prognostic factor compared to SUV (12–15).
Presumably, HNC increases in size and metabolic activity over time. The rate of growth should correlate with tumor biology, with faster growing tumors intuitively being more aggressive and fatal. The anatomic and functional imaging characteristics of PET-CT make it an excellent candidate to capture tumor growth rate – which has not been evaluated in HNC. This study’s purpose was to examine tumor growth over time without any intervening treatment, measured with SUVmax and MTV in serial pre-radiation PET-CT scans.
Methods and Materials
Patients
After Institutional Review Board approval, we reviewed the medical records of HNC patients treated with definitive radiation at Stanford University (June 2006 through November 2009). Patients with two pre-radiotherapy PET-CT scans without treatment between the two scans were included. Patients were excluded for the following reasons: distant metastatic disease at diagnosis; prior definitive surgery, chemotherapy or radiotherapy; or salivary gland, paranasal sinus, thyroid or skin primary tumors. Fifty-one patients met the above criteria. Patient characteristics are provided in Table 1.
Table 1.
Patient characteristics
| Characteristic | n (%) |
|---|---|
| Gender | |
| Female | 6 (12) |
| Male | 45 (88) |
| Age (median (range)) | 58y (37–89) |
| Site | |
| Larynx | 8 (16) |
| Hypopharynx | 6 (12) |
| Nasopharynx | 8 (16) |
| Oropharynx | 29 (57) |
| T stage | |
| T1 | 15 (29) |
| T2 | 17 (33) |
| T3 | 10 (20) |
| T4 | 9 (18) |
| N stage | |
| N0 | 6 (12) |
| N1 | 5 (10) |
| N2 | 37 (73) |
| N3 | 3 (6) |
| Grade | |
| Low | 2 (4) |
| Intermediate | 19 (37) |
| High | 23 (45) |
| Unknown | 7 (14) |
| Karnofsky performance status | |
| 70 | 3 (6) |
| 80 | 9 (18) |
| 90 | 34 (67) |
| Unknown | 5 (10) |
| p16 (oropharynx only, n=29) | |
| p16 negative | 5 (17) |
| p16 positive | 23 (79) |
| Unknown | 1 (3) |
| Chemotherapy | |
| Yes | 43 (84) |
| No | 8 (16) |
PET-CT scans
The first PET-CT scan was typically done for diagnostic purposes, and the second for radiotherapy planning. With both scans, patients fasted at least 6 hours prior to imaging. Blood sugar was confirmed to be < 180mg/dL. The prescribed 18F FDG dose was 15 mCi. After 45–60 minutes, patients underwent imaging. Whole-body diagnostic PET/CT scans were obtained in 2D mode using a GE Discovery LS scanner (GE Healthcare). The radiation therapy planning PET/CT scans were acquired using a GE Discovery ST scanner (GE Healthcare). CT data were collected in helical acquisition mode. PET data were collected with 3–5 minutes of acquisition time per bed position. PET images were reconstructed with a standard iterative algorithm (ordered-subset expectation maximization, 2 iterative steps, 28 subsets) using the CT images for attenuation correction, and subsequently converted into units of SUV using the patient weight and injected dose.
PET-CT endpoints
Image analysis was accomplished with MIM Software Suite, along with MIM fusion, and MIM contouring packages (MIMvista Corporation, Cleveland, OH). The MTVs of interest were generated retrospectively with the aid of the diagnostic nuclear medicine reports. Maximum SUV (SUVmax) was determined for the primary tumor and the involved lymph nodes individually and as a composite (SUVmax for combined tumor and nodal volumes). MTV was defined as the volume of hypermetabolic tissue within the region of the gross tumor with an SUV greater than 50% of the SUVmax. To test inter-user reproducibility we randomly selected a subset of 30 PET-CT scans from our cohort and had two co-investigators (K.C. and J.M.) independently determine the MTV for the primary tumor and nodal volumes.
SUV and MTV velocity were defined as the absolute change in SUVmax and MTV divided by the time in weeks between PET scans, respectively. We also calculated a corrected maximum SUV (SUVmax-corrected), which accounted for differences in background SUV levels between PET scans. Since the aortic arch was not included in treatment planning scans, we used the average SUV of the cerebellum (SUVcerebellum patient) with each scan for each patient to estimate the background SUV, and then normalized each individual SUVmax to a common background SUV with the following transformation:
where SUVcerebellum population represented the average cerebellar SUV of our entire patient cohort (SUVcerebellum population = 8.0).
Similar to corrected SUV, we also calculated a corrected MTV to reduce MTV dependence on SUVmax. The uncorrected MTV was defined as the tumor volume with an SUV above the threshold of 50% of the SUV max. The corrected MTV relied on the SUV threshold from the first PET-CT (50% * SUVmax 1) to define MTV for both the first and the second PET scans. It accounted for differences in background SUV levels by normalizing the SUV threshold for the second scan MTV to the cerebellar SUV ratio. The corrected MTVs were defined as the volume of tumor above the following SUV thresholds:
where SUVmax 1 is the maximum SUV of the first PET-CT scan; and SUVcerebellum 1 and SUVcerebellum 2 represent the average SUV of the cerebellum on the first and second PET-CT, respectively.
Statistics
Disease progression, cancer-specific survival, and overall survival were calculated from the date of diagnosis. Disease progression was defined as local-regional failure, or distant metastases. Associations between PET-CT endpoints (SUV and MTV velocity) and outcome (disease progression, cancer-specific survival, and overall survival) were assessed with Cox proportional hazard models (16).
Intra-user reproducibility was determined separately for primary tumor and nodal MTV, and was measured with a Pearson correlation coefficient (R2). Correlations between MTV and other patient characteristics were assessed with t-tests (gender, pathology grade, p16 status), ANOVA (tumor site, Karnofsky performance status - KPS, T-stage, N-stage), and linear regression (age). Statistical analysis was performed with SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
PET-CT velocity
The median time between PET-CT scans was 3.0 weeks (range 6 days to 10.7 weeks). Of the first PET-CT scans, the median primary tumor SUVmax was 11.2 (range 2.8 – 53), and the median nodal SUVmax was 10.4 (range 0 – 49). The median primary tumor MTV was 4.5 cc (range 0.5 – 35 cc), and the median nodal MTV was 5.9 cc (range 0.7 – 29 cc). Figure 1 demonstrates the change in SUV and MTV over time. We observed significant variability between each patient’s two PET-CT scans. One would expect SUV and MTV to increase over time as tumors grow larger and more metabolically active, however more than half the cohort (n=26; 51%) had SUVs or MTVs that decreased over time. The mean composite SUV velocity for the entire cohort was negative (−0.1/week; standard deviation [SD] = 2.0), and the mean composite MTV velocity was negative (−0.3 cc/week; SD = 1.7).
Figure 1. Change in uncorrected SUV and MTV between serial PET-CT scans.
Plots represent the absolute change in SUV (A, C and E) and MTV (B, D and F) over time in the primary tumor (A and B), lymph nodes (C and D), and a composite volume (primary tumor + lymph nodes; E and F). Patients with disease progression are represented by filled squares, and those without progression by open circles. Abbreviations: SUV = standardized uptake value; MTV = metabolic tumor volume.
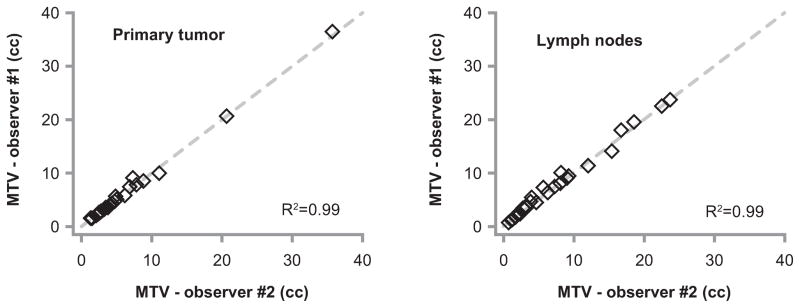
We then explored the potential causes of the variation observed between sequential PET-CT scans. Figure 2 demonstrates inter-user reproducibility by comparing the MTV for the primary tumor and lymph nodes from two independent and blinded observers in 30 PET-CT scans. The mean difference in primary tumor MTV between observers was 4% (range 0–24%) and the difference was within +/− 1% in 18 tumors. With nodal MTV, the mean difference between observers was 8% (range 0–35%) and the difference was within +/− 1% in 9 nodal MTVs. Overall, MTV calculation appears to be highly reproducible, and poor inter-user reproducibility unlikely explains the variability between serial PET-CT scans.
Figure 2. MTV inter-user reproducibility.
Plots represent the results of two blinded observers who independently determined MTV of the primary tumor (left), and lymph nodes (right) on a subset of 30 randomly selected PET-CT scans. Clear diamonds represent individual patients, and the dashed gray line represents a perfect correlation. R2 represents the Pearson correlation coefficient. Abbreviations: MTV = metabolic tumor volume.
Next we sought to evaluate error introduced from the PET scanner and imaging technique. All patients had their first and second PET-CT scans done on different scanners. In an attempt to partially account for this source of error, we corrected SUVmax to account for differences in background SUV, and re-calculated SUV velocity. Similar to the uncorrected SUV velocity, the corrected SUV velocity was negative in several patients (n=20; 39%) (Figure 3), however the average corrected SUV velocity for the entire cohort was positive (+0.4/week) compared to the uncorrected SUV velocity which was negative (−0.1/week). This suggests that the imaging techniques may have contributed to the overall variability between sequential PET scans.
Figure 3. Change in corrected SUV and MTV between serial PET-CT scans.
Plots represent the absolute change in corrected SUV (A, C and E) and corrected MTV (B, D and F) over time in the primary tumor (A and B), lymph nodes (C and D), and a composite volume (primary tumor + lymph nodes; E and F). Patients with disease progression are represented by filled squares, and those without progression by open circles. Abbreviations: SUV = standardized uptake value; MTV = metabolic tumor volume.
The third source of error we examined relates to the instability of SUVmax, which is inherently sensitive to image processing techniques and random statistical noise. Our uncorrected MTV definition depends explicitly on SUVmax, whereas corrected MTV depends less on a single SUVmax. Unfortunately, as with uncorrected MTV velocity, the corrected MTV velocity was negative in a large number of patients (n=23; 45%) (Figure 3). With the entire population, the average MTV velocity did not significantly change with correction (−0.2 cc/week for corrected MTV velocity, vs. −0.3 cc/week for uncorrected MTV velocity). These findings suggest that the random fluctuations in SUVmax were less likely to explain the observed variability in serial PET scans.
Correlation between PET-CT velocity and outcome
The median follow-up time for the population was 17.5 months (range 3.9–40 months). Four patients (8%) suffered disease progression (three with distant metastatic disease, and one with locoregional disease progression). The patients with disease progression had a slightly shorter interval between PET/CT scans compared to patients without progression (2.6 vs. 3.1 months), however the difference was not significant (p=0.39 by Wilcoxon rank sum test). Of note, in patients who ultimately progressed, there was no clinical suspicion of disease progression between the first and second PET/CT. Eight patients died in total, three of intercurrent illness, and five of head-and-neck cancer. Despite the limited number of events and the PET velocity variation, we did see a correlation between uncorrected MTV velocity and patient outcome (Table 2). A 1 cc/week increase in primary tumor MTV velocity was associated with a 194% increase in the risk of disease progression (p=0.009), a 143% increase in cancer-specific mortality (p=0.004), and an 85% increase in the risk of death (p=0.032). Thirteen patients (25%) had primary tumor MTVs that increased by at least 1 cc/week. Neither SUV velocity, nor nodal MTV velocity were associated with disease progression or death.
Table 2.
Correlation between PET velocity and outcome
| Outcome | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Progression alone | ||
| SUV velocity | ||
| Primary tumor | 0.75 (0.45–1.25) | 0.27 |
| Lymph nodes | 0.69 (0.42–1.16) | 0.16 |
| Composite | 0.74 (0.45–1.22) | 0.24 |
| MTV velocity | ||
| Primary tumor | 2.94 (1.31–6.62) | 0.009 |
| Lymph nodes | 1.38 (0.61–3.12) | 0.44 |
| Composite | 2.14 (1.12–4.09) | 0.021 |
| Cancer-specific survival | ||
| SUV velocity | ||
| Primary tumor | 0.77 (0.46–1.28) | 0.31 |
| Lymph nodes | 0.78 (0.48–1.28) | 0.33 |
| Composite | 0.79 (0.49–1.27) | 0.32 |
| MTV velocity | ||
| Primary tumor | 2.43 (1.32–4.48) | 0.004 |
| Lymph nodes | 0.91 (0.42–1.94) | 0.80 |
| Composite | 1.66 (0.96–2.84) | 0.068 |
| Overall survival | ||
| SUV velocity | ||
| Primary tumor | 0.81 (0.56–1.16) | 0.25 |
| Lymph nodes | 0.88 (0.57–1.36) | 0.57 |
| Composite | 0.83 (0.58–1.18) | 0.30 |
| MTV velocity | ||
| Primary tumor | 1.85 (1.05–3.24) | 0.032 |
| Lymph nodes | 0.79 (0.41–1.52) | 0.48 |
| Composite | 1.27 (0.8–2.02) | 0.31 |
Correlation between PET-CT velocity and patient characteristics
There was no significant correlation between composite MTV velocity and any of the following patient or tumor characteristics: age (p=0.56); gender (p=0.48); KPS (p=0.79); primary tumor site (p=0.97). There was no correlation between primary tumor MTV velocity and T-stage (p=0.36), or between nodal MTV velocity and N-stage (p=0.25). In oropharyngeal cancer patients, there was no correlation between p16 status and composite MTV velocity (p=0.95).
Discussion
Neither SUVmax nor MTV measured on serial PET-CT scans adequately captured tumor progression over time. Our results did not support the intuitive concept that HNC tumors increase in size and metabolic activity over time. As we move towards risk-adapted therapy, there will be interest to integrate PET-CT as a risk-stratifying biomarker, and incorporate serial FDG PET-CT scans to assess treatment response. Here, we highlight potential limitations in serial PET-CT reproducibility. The temporal variability we observed from a single institution will only be magnified with PET-CT scans obtained from different institutions. Further investigations involving PET-CT requires strict standardization to elucidate its role as a marker of HNC response.
The literature is sparse on serial PET-CT scan reproducibility; however decreases in SUV over time have been described in other sites. In non-small cell lung cancer, upwards of 25% of tumors can have decreased SUVmax between PET-CT scans (17), and factors such as tumor necrosis, proximity to lung consolidation, and respiratory motion could account for the SUV reduction.
Several technical or procedural factors have been shown to affect the results of FDG imaging (18). Technical factors include resolution variability, integrity and stability of different PET scanners, FDG dose injected, timing between injection and scanning, attenuation correction algorithm employed, image analysis software, and region of interest (ROI) determination. At least two guidelines for standardized PET acquisition and analysis have been proposed in attempt to minimize the impact of technical factors (18, 19). Both guidelines stress the importance of image reconstruction and attenuation consistency and the need to account for artifacts in imaging. Because PET camera specifications can be variable, Shankar et al also recommends that patients should be scanned using the same scanner or the same scanner model, ideally at the same center. This could not be achieved in our study as most patients had staging PET-CT scans upon presentation to our clinic. However, PET-CT scanners at our institution are routinely calibrated and typically differ by an average of 5 – 7%, similar to studies comparing inter-scan variability (20, 21).
Our attempts to correct SUV and MTV velocity to account for differences in baseline SUV and the instability in SUVmax failed. While the corrected SUV velocity increased (+0.4/week) compared to the uncorrected SUV velocity (−0.1/week), a significant number of patients demonstrated a negative corrected SUV velocity. Similarly, our attempt to correct MTV fared no better. Our methods to account for technical factors, which likely affected baseline SUV and SUVmax in serial scans, proved insufficient.
Another potential source of error in comparing serial PET-CT scans is the subjectivity of ROI delineation. Shankar et al recommends using a thresholding or edge finding algorithm to eliminate such subjectivity (18). Likewise, with MTV investigators only have to identify the tumor and involved nodes, and the remainder is computer generated. In fact, we found excellent inter-observer MTV reproducibility, which suggests human bias unlikely accounts for the observed variability.
Several patient-related factors could influence FDG uptake and contribute to our observed results. Impaired glucose metabolism or diabetes can affect FDG uptake and decrease PET sensitivity in detecting smaller lesions (22–24). Patients with a large volume of body fat may alter FDG uptake in normal tissues and the tumor (25, 26). Other factors that can influence FDG bio-distribution include steroids, sedative use to induce muscle relaxation during scanning, and the patient’s hydration status (18).
Finally, several tumor-related factors could affect SUV or MTV. Tumor necrosis, cystic nodes, or inflammatory changes in adjacent normal tissue all could influence FDG uptake (27, 28). These changes could be triggered by tumor biopsy, or dental extractions. Tumor necrosis or cystic nodes could lead to decreased FDG uptake, and thus decreased SUV or MTV. Peri-tumoral inflammation on the other hand could cause increased peri-tumoral SUV, which would lead to MTV overestimation.
Despite the variability in our results, we still observed a significant correlation between primary tumor MTV velocity and disease progression (HR: 2.94, p <0.01), cancer-specific survival (HR=2.43; p=0.004), and overall survival (HR: 1.85, p 0.03). The observation that primary tumor MTV velocity, and not nodal MTV velocity, drives the correlation deserves further attention. While an explanation of this observation remains unclear, it lends support to the hypothesis that primary tumor burden, not nodal tumor burden, predicts overall disease progression. Perhaps the prognostic utility of nodal involvement is better captured with the classic nodal staging system that incorporates size, number, and location of involved nodes. MTV fails to capture number and location of involved lymph nodes. To study this further, we are currently conducting a separate independent study analyzing the prognostic utility of primary tumor MTV versus nodal MTV.
In summary, our data shows significant variability in SUV and MTV from serial PET-CT scans in the same patient at two different time points without any therapeutic intervention. This variability could be mistakenly interpreted as tumor regression or tumor progression in cases where PET-CT is used to assess therapeutic response. This study highlights the challenges of incorporating PET/CT into clinical protocols, and into clinical practice. Prospective studies with standardized protocols on well-calibrated scanners are needed to define the role of serial FDG PET-CT in head-and-neck cancer.
Highlights.
PET/CT shows promise as a risk-stratifying biomarker in head-and-neck cancer; however the optimal method to implement this imaging modality remains unclear. This study evaluates the prognostic impact of metabolic tumor volume (MTV) velocity, recorded on serial pre-radiotherapy PET/CT scans, which could act as a surrogate for tumor growth rate. While we found that primary tumor MTV velocity predicted outcome, we unexpectedly found significant variability in PET/CT biomarkers between serial PET/CT scans. This study highlights the challenges of interpreting serial PET/CT images in clinical practice, and emphasizes the limitations of integrating PET/CT into clinical protocols.
Acknowledgments
This work was supported in part by 1R01 CA118582-05 (QTL, EEG) and P01 CA67166-15 (QTL, EEG, TEK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ciernik IF, Dizendorf E, Baumert BG, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys. 2003;57:853–863. doi: 10.1016/s0360-3016(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 2.Fleming AJ, Jr, Smith SP, Jr, Paul CM, et al. Impact of [18F]-2-fluorodeoxyglucose-positron emission tomography/computed tomography on previously untreated head and neck cancer patients. Laryngoscope. 2007;117:1173–1179. doi: 10.1097/MLG.0b013e31805d017b. [DOI] [PubMed] [Google Scholar]
- 3.Paulino AC, Koshy M, Howell R, et al. Comparison of CT- and FDG-PET-defined gross tumor volume in intensity-modulated radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61:1385–1392. doi: 10.1016/j.ijrobp.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Daisne JF, Duprez T, Weynand B, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. 2004;233:93–100. doi: 10.1148/radiol.2331030660. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues RS, Bozza FA, Christian PE, et al. Comparison of whole-body PET/CT, dedicated high-resolution head and neck PET/CT, and contrast-enhanced CT in preoperative staging of clinically M0 squamous cell carcinoma of the head and neck. J Nucl Med. 2009;50:1205–1213. doi: 10.2967/jnumed.109.062075. [DOI] [PubMed] [Google Scholar]
- 6.Roh JL, Pae KH, Choi SH, et al. 2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography as guidance for primary treatment in patients with advanced-stage resectable squamous cell carcinoma of the larynx and hypopharynx. Eur J Surg Oncol. 2007;33:790–795. doi: 10.1016/j.ejso.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Allal AS, Dulguerov P, Allaoua M, et al. Standardized uptake value of 2-[(18)F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. J Clin Oncol. 2002;20:1398–1404. doi: 10.1200/JCO.2002.20.5.1398. [DOI] [PubMed] [Google Scholar]
- 8.Lee SW, Nam SY, Im KC, et al. Prediction of prognosis using standardized uptake value of 2-[(18)F] fluoro-2-deoxy-d-glucose positron emission tomography for nasopharyngeal carcinomas. Radiother Oncol. 2008;87:211–216. doi: 10.1016/j.radonc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Minn H, Lapela M, Klemi PJ, et al. Prediction of survival with fluorine-18-fluoro-deoxyglucose and PET in head and neck cancer. J Nucl Med. 1997;38:1907–1911. [PubMed] [Google Scholar]
- 10.La TH, Filion EJ, Turnbull BB, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335–1341. doi: 10.1016/j.ijrobp.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy JD, La TH, Chu K, et al. Post-Radiation Metabolic Tumor Volume Predicts Outcome in Head-and-Neck Cancer. Int J Radiat Oncol Biol Phys. 2011;80:514–521. doi: 10.1016/j.ijrobp.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyun SH, Choi JY, Shim YM, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115–122. doi: 10.1245/s10434-009-0719-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee P, Weerasuriya DK, Lavori PW, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007;69:328–333. doi: 10.1016/j.ijrobp.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Seol YM, Kwon BR, Song MK, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol. 2010;49:201–208. doi: 10.3109/02841860903440270. [DOI] [PubMed] [Google Scholar]
- 15.Xie P, Yue JB, Zhao HX, et al. Prognostic value of 18F-FDG PET-CT metabolic index for nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2010;136:883–889. doi: 10.1007/s00432-009-0729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society Series B. 1972;34:187–220. [Google Scholar]
- 17.Everitt S, Herschtal A, Callahan J, et al. High rates of tumor growth and disease progression detected on serial pretreatment fluorodeoxyglucose-positron emission tomography/computed tomography scans in radical radiotherapy candidates with nonsmall cell lung cancer. Cancer. 2010;116:5030–5037. doi: 10.1002/cncr.25392. [DOI] [PubMed] [Google Scholar]
- 18.Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 19.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 20.Lockhart CM, MacDonald LR, Alessio AM, et al. Quantifying and reducing the effect of calibration error on variability of PET/CT standardized uptake value measurements. J Nucl Med. 2011;52:218–224. doi: 10.2967/jnumed.110.083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804–1808. doi: 10.2967/jnumed.108.054239. [DOI] [PubMed] [Google Scholar]
- 22.Hara T, Higashi T, Nakamoto Y, et al. Significance of chronic marked hyperglycemia on FDG-PET: is it really problematic for clinical oncologic imaging? Ann Nucl Med. 2009;23:657–669. doi: 10.1007/s12149-009-0288-7. [DOI] [PubMed] [Google Scholar]
- 23.Rabkin Z, Israel O, Keidar Z. Do hyperglycemia and diabetes affect the incidence of false-negative 18F-FDG PET/CT studies in patients evaluated for infection or inflammation and cancer? A Comparative analysis. J Nucl Med. 2010;51:1015–1020. doi: 10.2967/jnumed.109.074294. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang HM, Cortes-Blanco A, Pourdehnad M, et al. Do high glucose levels have differential effect on FDG uptake in inflammatory and malignant disorders? Nucl Med Commun. 2001;22:1123–1128. doi: 10.1097/00006231-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara Y, Zasadny KR, Neuhoff AW, et al. Reevaluation of the standardized uptake value for FDG: variations with body weight and methods for correction. Radiology. 1999;213:521–525. doi: 10.1148/radiology.213.2.r99nv37521. [DOI] [PubMed] [Google Scholar]
- 26.Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 (Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chun EJ, Lee HJ, Kang WJ, et al. Differentiation between malignancy and inflammation in pulmonary ground-glass nodules: The feasibility of integrated (18)F-FDG PET/CT. Lung Cancer. 2009;65:180–186. doi: 10.1016/j.lungcan.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Simons KS, Pickkers P, Bleeker-Rovers CP, et al. F-18-fluorodeoxyglucose positron emission tomography combined with CT in critically ill patients with suspected infection. Intensive Care Med. 2010;36:504–511. doi: 10.1007/s00134-009-1697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]