Abstract
We have used alpha-oligomers as antisense oligonucleotides complementary to three different sequences of the rabbit beta-globin mRNA: a region adjacent to the cap site, a region spanning the AUG initiation codon or a sequence in the coding region. These alpha-oligonucleotides were synthesized either with a free 5' OH group or linked to an acridine derivative. The effect of these oligonucleotides on mRNA translation was investigated in cell-free extracts and in Xenopus oocytes. In rabbit reticulocyte lysate and in wheat germ extracts oligomers targeted to the cap site and the initiation codon reduced beta-globin synthesis in a dose-dependent manner, whereas the target mRNA remained intact. The anti-cap alpha-oligomer was even more efficient that its beta-counterpart in rabbit reticulocyte lysate. In contrast, only the alpha-oligomer, linked to the acridine derivative, complementary to the cap region displayed significant antisense properties in Xenopus oocytes. Therefore initiation of translation can be arrested by oligonucleotide/RNA hybrids which are not substrates for RNase-H.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asseline U., Delarue M., Lancelot G., Toulmé F., Thuong N. T., Montenay-Garestier T., Hélène C. Nucleic acid-binding molecules with high affinity and base sequence specificity: intercalating agents covalently linked to oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3297–3301. doi: 10.1073/pnas.81.11.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J. R., Rayner B., Imbach J. L., Paoletti C., Malvy C. Comparative activity of alpha- and beta-anomeric oligonucleotides on rabbit beta globin synthesis: inhibitory effect of cap targeted alpha-oligonucleotides. Biochem Biophys Res Commun. 1989 Oct 16;164(1):311–318. doi: 10.1016/0006-291x(89)91719-1. [DOI] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Spitz S. A., Glave S. A., Reddy M. P., Ts'o P. O., Miller P. S. Hybridization arrest of globin synthesis in rabbit reticulocyte lysates and cells by oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1985 Oct 22;24(22):6139–6145. doi: 10.1021/bi00343a016. [DOI] [PubMed] [Google Scholar]
- Büsen W., Hausen P. Distinct ribonuclease H activities in calf thymus. Eur J Biochem. 1975 Mar 3;52(1):179–190. doi: 10.1111/j.1432-1033.1975.tb03985.x. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Chevrier M., Nguyen T. T., Hélène C. Rate of degradation of [alpha]- and [beta]-oligodeoxynucleotides in Xenopus oocytes. Implications for anti-messenger strategies. Nucleic Acids Res. 1987 Dec 23;15(24):10507–10521. doi: 10.1093/nar/15.24.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Thuong N. T., Toulmé J. J., Hélène C. Enzymatic amplification of translation inhibition of rabbit beta-globin mRNA mediated by anti-messenger oligodeoxynucleotides covalently linked to intercalating agents. Nucleic Acids Res. 1987 Jun 25;15(12):4717–4736. doi: 10.1093/nar/15.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Toulmé J. J., Hélène C. Anti-messenger oligodeoxynucleotides: specific inhibition of rabbit beta-globin synthesis in wheat germ extracts and Xenopus oocytes. Biochimie. 1986 Sep;68(9):1063–1069. doi: 10.1016/s0300-9084(86)80180-8. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Stein C. A., Loreau N., Thuong N. T., Neckers L. M., Subasinghe C., Hélène C., Cohen J. S., Toulmé J. J. Comparative inhibition of rabbit globin mRNA translation by modified antisense oligodeoxynucleotides. Nucleic Acids Res. 1989 Jun 12;17(11):4255–4273. doi: 10.1093/nar/17.11.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Yu Z., Shinozuka K., Zon G., Wilson W. D., Strekowska A. Comparative inhibition of ras p21 protein synthesis with phosphorus-modified antisense oligonucleotides. Anticancer Drug Des. 1989 Oct;4(3):221–232. [PubMed] [Google Scholar]
- Dash P., Lotan I., Knapp M., Kandel E. R., Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnor C., Bertrand J. R., Thenet S., Lemaître M., Morvan F., Rayner B., Malvy C., Lebleu B., Imbach J. L., Paoletti C. alpha-DNA. VI: Comparative study of alpha- and beta-anomeric oligodeoxyribonucleotides in hybridization to mRNA and in cell free translation inhibition. Nucleic Acids Res. 1987 Dec 23;15(24):10419–10436. doi: 10.1093/nar/15.24.10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnor C., Rayner B., Leonetti J. P., Imbach J. L., Lebleu B. Alpha-DNA.IX: Parallel annealing of alpha-anomeric oligodeoxyribonucleotides to natural mRNA is required for interference in RNase H mediated hydrolysis and reverse transcription. Nucleic Acids Res. 1989 Jul 11;17(13):5107–5114. doi: 10.1093/nar/17.13.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
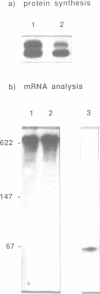
- Goodchild J., Carroll E., 3rd, Greenberg J. R. Inhibition of rabbit beta-globin synthesis by complementary oligonucleotides: identification of mRNA sites sensitive to inhibition. Arch Biochem Biophys. 1988 Jun;263(2):401–409. doi: 10.1016/0003-9861(88)90652-2. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Frank R., Dobberstein B. Translation arrest by oligodeoxynucleotides complementary to mRNA coding sequences yields polypeptides of predetermined length. Nucleic Acids Res. 1986 Feb 11;14(3):1427–1448. doi: 10.1093/nar/14.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Kean J. M., Murakami A., Blake K. R., Cushman C. D., Miller P. S. Photochemical cross-linking of psoralen-derivatized oligonucleoside methylphosphonates to rabbit globin messenger RNA. Biochemistry. 1988 Dec 27;27(26):9113–9121. doi: 10.1021/bi00426a008. [DOI] [PubMed] [Google Scholar]
- Lawson T. G., Ray B. K., Dodds J. T., Grifo J. A., Abramson R. D., Merrick W. C., Betsch D. F., Weith H. L., Thach R. E. Influence of 5' proximal secondary structure on the translational efficiency of eukaryotic mRNAs and on their interaction with initiation factors. J Biol Chem. 1986 Oct 25;261(30):13979–13989. [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Comparative hybrid arrest by tandem antisense oligodeoxyribonucleotides or oligodeoxyribonucleoside methylphosphonates in a cell-free system. Nucleic Acids Res. 1988 Apr 25;16(8):3341–3358. doi: 10.1093/nar/16.8.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus-Sekura C. J., Woerner A. M., Shinozuka K., Zon G., Quinnan G. V., Jr Comparative inhibition of chloramphenicol acetyltransferase gene expression by antisense oligonucleotide analogues having alkyl phosphotriester, methylphosphonate and phosphorothioate linkages. Nucleic Acids Res. 1987 Jul 24;15(14):5749–5763. doi: 10.1093/nar/15.14.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Hunt T. The use of single-stranded DNA and RNase H to promote quantitative 'hybrid arrest of translation' of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986 Aug 26;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Thenet S., Bertrand J. R., Paoletti J., Malvy C., Paoletti C. alpha-DNA II. Synthesis of unnatural alpha-anomeric oligodeoxyribonucleotides containing the four usual bases and study of their substrate activities for nucleases. Nucleic Acids Res. 1987 Apr 24;15(8):3421–3437. doi: 10.1093/nar/15.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Magarian C., Borun T. W. Resolution of hemoglobin subunits by electrophoresis in acid urea polyacrylamide gels containing Triton X-100. Anal Biochem. 1978 Apr;85(2):506–518. doi: 10.1016/0003-2697(78)90248-8. [DOI] [PubMed] [Google Scholar]
- Shakin S. H., Liebhaber S. A. Destabilization of messenger RNA/complementary DNA duplexes by the elongating 80 S ribosome. J Biol Chem. 1986 Dec 5;261(34):16018–16025. [PubMed] [Google Scholar]
- Shuttleworth J., Colman A. Antisense oligonucleotide-directed cleavage of mRNA in Xenopus oocytes and eggs. EMBO J. 1988 Feb;7(2):427–434. doi: 10.1002/j.1460-2075.1988.tb02830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. C., Dworkin M. B., Dworkin-Rastl E. Destruction of a translationally controlled mRNA in Xenopus oocytes delays progesterone-induced maturation. Genes Dev. 1988 Oct;2(10):1296–1306. doi: 10.1101/gad.2.10.1296. [DOI] [PubMed] [Google Scholar]
- Sun J. S., Asseline U., Rouzaud D., Montenay-Garestier T., Nguyen T. T., Hélène C. Oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent. Double helices with parallel strands are formed with complementary oligo-[beta]-deoxynucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6149–6158. doi: 10.1093/nar/15.15.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuong N. T., Asseline U., Roig V., Takasugi M., Hélène C. Oligo(alpha-deoxynucleotide)s covalently linked to intercalating agents: differential binding to ribo- and deoxyribopolynucleotides and stability towards nuclease digestion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5129–5133. doi: 10.1073/pnas.84.15.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidd D. M., Hawley P., Warenius H. M., Gibson I. Evaluation of N-ras oncogene anti-sense, sense and nonsense sequence methylphosphonate oligonucleotide analogues. Anticancer Drug Des. 1988 Aug;3(2):117–127. [PubMed] [Google Scholar]
- Toulmé J. J., Krisch H. M., Loreau N., Thuong N. T., Hélène C. Specific inhibition of mRNA translation by complementary oligonucleotides covalently linked to intercalating agents. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1227–1231. doi: 10.1073/pnas.83.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar G., Ahmed N. K. Modulation of drug resistance in a daunorubicin resistant subline with oligonucleoside methylphosphonates. Cancer Commun. 1989;1(4):225–232. [PubMed] [Google Scholar]
- Verspieren P., Cornelissen A. W., Thuong N. T., Hélène C., Toulmé J. J. An acridine-linked oligodeoxynucleotide targeted to the common 5' end of trypanosome mRNAs kills cultured parasites. Gene. 1987;61(3):307–315. doi: 10.1016/0378-1119(87)90194-6. [DOI] [PubMed] [Google Scholar]
- Verspieren P., Loreau N., Thuong N. T., Shire D., Toulmé J. J. Effect of RNA secondary structure and modified bases on the inhibition of trypanosomatid protein synthesis in cell free extracts by antisense oligodeoxynucleotides. Nucleic Acids Res. 1990 Aug 25;18(16):4711–4717. doi: 10.1093/nar/18.16.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A. R., Mol J. N., Stuitje A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques. 1988 Nov-Dec;6(10):958–976. [PubMed] [Google Scholar]