Abstract
Angiogenin, a 14-kDa multi-functional pro-angiogenic growth factor, is up-regulated in several types of cancers. Anti-angiogenin monoclonal antibodies used as antagonists inhibited the establishment, progression, and metastasis of human cancer cells in athymic mice (Olson et al. 1994). Silencing angiogenin and inhibition of angiogenin’s nuclear translocation blocked cell survival and induced cell death in B-lymphoma and endothelial cells latently infected with Kaposi sarcoma associated herpesvirus (KSHV) (Sadagopan et al. 2009) suggesting that actively proliferating cancer cells could be inducing angiogenin for inhibiting apoptotic pathways. However, the mechanism of cell survival and apoptosis regulation by angiogenin and their functional significance in cancer is not known. We demonstrate that angiogenin interacts with p53 and colocalizes in the nucleus. Silencing endogenous angiogenin induced p53 promoter activation and p53 target gene (p53, p21 and Bax) expression, down-regulated anti-apoptotic Bcl-2 gene expression and increased p53 mediated cell death. In contrast, angiogenin expression blocked pro-apoptotic Bax and p21 expression, induced Bcl-2 and blocked cell death. Angiogenin also co-immunoprecipitated with p53 regulator protein Mdm2. Angiogenin expression resulted in the inhibition of p53 phosphorylation, increased p53-Mdm2 interaction, and consequently increased ubiquitination of p53. Taken together these studies demonstrate that angiogenin promotes the inhibition of p53 function to mediate anti-apoptosis and cell survival. Our results reveal for the first time a novel p53 interacting function of angiogenin in anti-apoptosis and survival of cancer cells and suggest that targeting angiogenin could be an effective therapy for several cancers.
Keywords: Angiogenin, p53, Mdm2, KSHV, cancer, anti-apoptosis, p53 ubiquitination
INTRODUCTION
The process of apoptosis is regulated by several cellular proteins and the tumor suppressor protein p53 is one of the major players in the regulation of apoptosis. p53 function is consistently altered in different types of cancer cells (Vogelstein et al. 2000). p53 is inactivated by mutation in approximately half of the human cancers and most of the remaining malignancies deactivate the p53 pathway with a variety of methods such as an increase in p53 inhibitors, sequestration of p53, inactivation of p53, reduction of p53 activators and down-regulation of p53 target genes (Foulkes 2007). All of these results in anti-apoptosis, cell survival and cell proliferation. Under normal conditions, p53 remains inactive due to its rapid degradation by the ubiquitin ligase Mdm2 (Kubbutat et al. 1997). However, upon cellular stress, p53 is phosphorylated resulting in the shutdown of Mdm2 mediated degradation and accumulation of transcriptionally active p53 which activates several targets including cell cycle inhibitors and pro-apoptotic proteins resulting in apoptosis or proliferation arrest.
Angiogenin, a multifunctional 14-kDa angiogenic protein, was first isolated based on its angiogenic activity from the conditioned media of HT-29 human colon adenocarcinoma cells (Fett et al. 1985). Our studies and others have shown that angiogenin is up-regulated in cancer cells (Li et al. 1994; Chopra et al. 1998; Montero et al. 1998; Sadagopan et al. 2009). Angiogenin is detected in normal human plasma at a concentration of 250–360 ng/ml (Shimoyama et al. 1996). However, its expression is often up-regulated in various cancers including pancreatic (Shimoyama et al. 1996), breast (Montero et al. 1998), cervical (Chopra et al. 1998), ovarian (Barton et al. 1997), colon, colorectal, gastric (Li et al. 1994), urothelial, and endometrial cancers (Chopra et al. 1997), and is associated with cancer progression and poor prognosis. In the nucleolus, angiogenin mediates rRNA transcription by binding to CT repeats abundant in the promoter region of the rRNA gene (Xu et al. 2003). Angiogenin also exerts its ribonucleolytic activity by catalyzing the generation of 18S and 28S rRNA. Nuclear translocation of angiogenin in endothelial cells has been shown to be necessary for the angiogenic potentials of not only angiogenin but also for VEGF and bFGF (Kishimoto et al. 2005). Inhibition of nuclear translocation of angiogenin by the aminoglycoside antibiotic neomycin(Liu et al. 2001) or mutagenesis of angiogenin’s nuclear localization sequence (Moroianu and Riordan 1994) both abolished the angiogenic activity of angiogenin. Activation of PLCγ is required for nuclear translocation for angiogenin (Hu 1998) and neomycin inhibits this nuclear translocation by inhibiting PLCγ activation. In contrast, paromomycin, an analogue aminoglycoside, does not inhibit nuclear translocation of angiogenin (Sadagopan et al. 2011). Significant amounts of angiogenin (250 to 400 pg/ml) were detected in the supernatants of KSHV positive B-cell lymphoma BCBL-1, BC-3, BJAB-KSHV, and JSC-1 cells (Sadagopan et al. 2011). Angiogenin increased 45S rRNA gene transcription, anti-apoptosis and proliferation in KSHV infected endothelial cells (Sadagopan et al. 2009). Inhibition of nuclear translocation of angiogenin and angiogenin silencing blocked KSHV infected endothelial and lymphoma cell survival and induced cell death (Sadagopan et al. 2011).
Although angiogenin was up-regulated in almost all cancers and possess anti-apoptotic effects, whether angiogenin targets p53 for its anti-apoptotic effects leading into cancer progression has not been examined before. Hence, we reasoned that deciphering the mechanism involved in apoptosis regulation by angiogenin would provide valuable insights in identifying targets to counteract the measures taken by cancerous or transformed cells for their survival. Our studies demonstrate that angiogenin physically interacts with p53 and angiogenin silencing induces p53-luc activation, p53 target gene expression and increases cell death. In contrast, angiogenin expression blocks p53 target gene expression and p53 mediated cell death. Angiogenin expression results in the phosphorylation of p53, increased p53-Mdm2 interaction and ubiquitination of p53. These results suggest that proliferating cells could be inducing the multi-functional angiogenin to block cell death mediated by p53 and thus giving cancerous cells a survival advantage.
RESULTS
Angiogenin interacts with p53 in primary normal endothelial cells
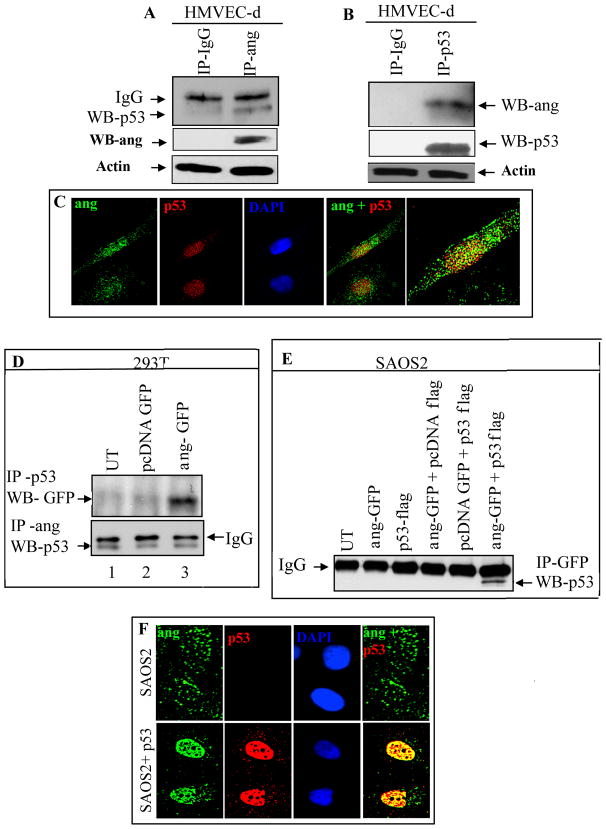
Angiogenin is critical for the proliferation, tube formation and anti-apoptosis of endothelial cells (Sadagopan et al. 2009). Since angiogenin is already established as a potent growth factor and angiogenin inhibitors were reported to inhibit cancer cell survival and tumor growth (Sadagopan et al. 2011; Tsuji et al. 2005), we hypothesized that up-regulation of angiogenin and interaction of angiogenin with p53 might be one of the mechanisms employed by cancer cells to block p53 mediated apoptosis. When we first determined the interaction between endogenous angiogenin with endogenous p53 in primary human dermal microvascular endothelial cells (HMVEC-d), p53 was immunoprecipitated (IP) with an anti-angiogenin antibody (Fig. 1A) and angiogenin was immunoprecipitated with a p53 antibody (Fig. 1B). No reaction was seen with mouse IgG used as a control (Fig. 1A and 1B). In immunofluoresence analysis, angiogenin colocalized with p53 predominantly in the nucleus of HMVEC-d cells (Fig. 1C) which supported the IP results. These results indicated that angiogenin interacts with p53 in normal endothelial cells probably to maintain a check and balance on apoptosis and cell survival.
Figure 1. Detection of angiogenin interaction with p53 in primary endothelial cells and in transformed 293T cells.
(A) HMVEC-d cells were immunoprecipitated (IP) with either IgG or anti-angiogenin (ang) antibody and Western blotted (WB) for p53. Western blots for angiogenin show the immunoprecipitated protein and actin was used as loading control. (B) HMVEC-d cells were immunoprecipitated with either IgG or anti-p53 antibody and Western blotted for angiogenin. Western blots for p53 show the immunoprecipitated protein and actin was used as loading control. (C) Immunofluorescence analysis demonstrating interaction between endogenous p53 and endogenous angiogenin in HMVEC-d cells. (D) 293T cells were transfected with angiogenin-GFP (ang-GFP) for 48h, immunoprecipitated with anti-p53 antibody and Western blotted for GFP (top panel), immunoprecipitated with anti-angiogenin antibody and Western blotted for p53 (bottom panel). (E) SAOS2 cells were transfected with p53-flag and angiogenin-GFP and the lysates were immunoprecipitated with anti-GFP antibody and Western blotted with p53 antibody. (F) Immunofluorescence analysis demonstrating interaction between p53 and endogenous angiogenin in SAOS2 cells transfected without p53 (first panels) or with p53-flag plasmid (second panels).
Angiogenin interacts with p53 in transformed cells
Determination of angiogenin expression by quantitative real-time PCR and angiogenin-ELISA demonstrated that transformed human embryonic kidney 293T (HEK 293T) cells and p53 null human osteosarcoma cells (SAOS2 cells) express high angiogenin levels compared to HMVEC-d cells (supplementary figures S1A and S1B). This is not surprising since the in vitro cultured primary HMVEC-d cells grow as a monolayer whose growth is contact inhibited while the transformed or cancerous cells, characterized by sustained cell growth, require continuous protein synthesis and a constant supply of ribosomes (Hanahan and Weinberg 2000). During tumorigenesis, the transcription of ribosomal proteins is known to be up-regulated through the Akt-PI3-K-mTOR-S6K pathway. Ribosome biogenesis is a multistep process involving assembly of ribosomal proteins and rRNA in an equal molar ratio. Since angiogenin is known to regulate ribosome biogenesis by mediating rRNA transcription (Tsuji et al. 2005), the increased angiogenin in transformed and cancer cells could be attributed to the increased protein synthesis and proliferation observed in these cells compared to primary HMVEC-d cells.
To further examine the interaction between angiogenin and p53, 293T cells were transfected with either pcDNA-GFP or angiogenin-GFP (ang-GFP) and co-immunoprecipitation (co-IP) experiments were performed. In co-IP reactions with anti-p53 antibodies, GFP was detected in Western blot reactions only in angiogenin-GFP transfected cells (Fig. 1D). Co-IP of p53 with angiogenin was also observed in untransfected, pcDNA-GFP and angiogenin-GFP transfected cells (Fig. 1D) which suggested an interaction of endogenous angiogenin with p53 in 293T cells. These results clearly demonstrated that the physical association of p53 and angiogenin occurs in angiogenin expressing cells.
To validate these findings, we transfected p53-flag and angiogenin-GFP in p53 null SAOS2 cells, immunoprecipitated with anti-GFP antibody and Western blotted for p53. We observed that p53 co-IPed with angiogenin only in cells transfected with both p53-flag and angiogenin-GFP and not in other control plasmid transfected cells (Fig. 1E). These results further demonstrated that angiogenin physically interacts with p53 in transformed cells.
To further analyze the association between p53 and angiogenin, we performed immunofluorescence colocalization experiments using SAOS2 cells exogenously transfected with p53-flag plasmids. Endogenous angiogenin was localized both in the cytoplasm and nucleus of p53 null SAOS2 cells (Fig. 1F, first panels). In contrast, in p53-flag transfected SAOS2 cells, we observed a strong nuclear colocalization between p53 and endogenous angiogenin (Fig. 1F, second panels). These results demonstrated that the over-expression of p53 in SAOS2 cells is sufficient to localize the endogenous angiogenin predominately in the nucleus. Compared to HMVEC-d cells, the colocalization of angiogenin with p53 in SAOS2 cells transfected with p53-flag was significantly higher which could probably be due to elevated angiogenin as well as p53 expression in these cells.
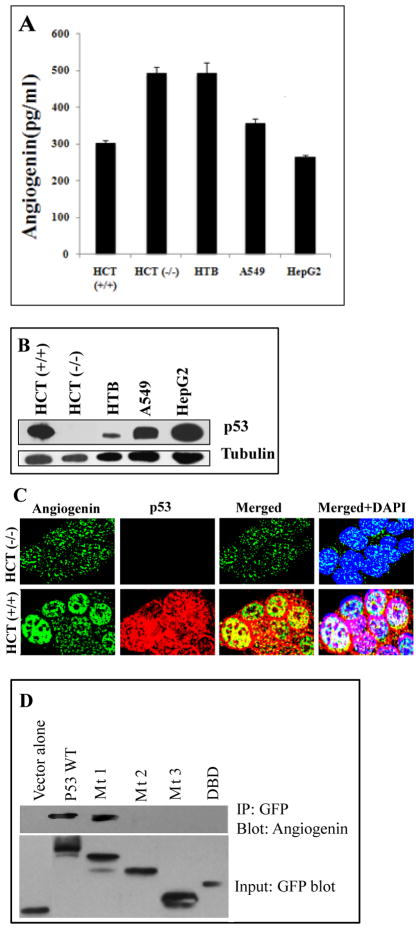
Angiogenin is detected in human colon carcinoma, neuroblastoma, hepatocellular carcinoma and lung adenocarcinoma cell lines
Angiogenin analysis in p53+/+ and p53−/− human colon carcinoma HCT116 cell lines, p53 positive HTB (human neuroblastoma), A549 (human lung adenocarcinoma), and HepG2 (human hepatocellular carcinoma) cell lines revealed an increase in the level of secreted angiogenin in p53−/− HCT116 cells compared to p53+/+ cells (Fig. 2A). Similarly, HepG2 and A549 cells expressing high levels of p53 showed decreased angiogenin in the supernatants compared to a low level of p53 expression and high angiogenin levels in the supernatants of HTB and p53−/− HCT116 cells (Fig. 2A and B). Increased nuclear retention of angiogenin and interaction with p53 could be one of the possible reasons for the decreased angiogenin in the supernatants of cancer cell lines p53+/+ HCT116, HepG2 and A549 that express increased levels of p53 (Fig 2A and B).
Figure 2. Angiogenin colocalizes with p53 in human colon carcinoma cell line HCT and angiogenin is detected in cancer cell lines.
(A) Supernatants from cultured p53+/+ HCT116, p53−/−HCT 116, HTB, A549 and HepG2 cells were tested by angiogenin ELISA to determine the concentrations of angiogenin. Values shown represent mean ± SD of three independent experiments. (B) Cell lysates of p53+/+ HCT116, p53−/−HCT 116, HTB, A549 and HepG2 cells were Western blotted with p53 to analyze the level of p53 expression in different cancer cells. Bottom panel: loading control with anti-tubulin antibody. (C) p53+/+ and p53−/− HCT116 cells cultured in 8 well chamber slides were fixed and stained with anti-p53 and anti-angiogenin antibodies. Yellow or white colors indicate the colocalization of p53 and angiogenin in the nucleus of p53+/+ HCT116 cells. (D) Interaction of endogenous angiogenin with p53 deletion constructs. HEK293T cells were transfected with EGFP-tagged empty vector or p53 wild-type, p53 mt1, p53 mt2, p53 mt3 or DBD plasmid constructs. Cells were lysed after 48h of transfection, immunoprecipitated with anti-GFP antibodies followed by immunoblotting with anti-angiogenin antibody (top). Western blotting for input was carried out with anti-GFP antibody using the same lysates (bottom).
Angiogenin colocalizes with p53 in a human colon carcinoma cell line
To determine whether co-localization of angiogenin and p53 is also observed in cancer cell lines, we examined p53+/+ and p53−/− HCT116 cell lines for p53 and angiogenin colocalization. A punctuate fluorescence pattern of endogenous angiogenin was detected in both the nucleus and cytoplasm of p53−/− HCT116 cells (Fig. 2C). In contrast, a strong nuclear localization of angiogenin was observed in p53+/+ HCT116 cells which predominately colocalized with p53 (Fig. 2C).
The AD2 domain of p53 is required for its interaction with angiogenin
To determine which domain of p53 is responsible for its interaction with angiogenin, we used 293T cell lysates transfected with vector alone, pEGFP-p53-WT (wild type) and deletion mutants of p53 with EGFP tags such as pEGFP-p53-mt1 (first activation domain AD1 deleted), pEGFP-p53-mt2 (both activation domains AD1 and AD2 deleted), pEGFP-p53-mt3 (both AD domains and the DNA binding domain [DBD] deleted), and pEGFP-p53-DBD (containing only the DBD domain) (Hu et al.,2010). Co-IP analysis demonstrated that the AD1 deletion mutant p53-mt1 interacts with angiogenin similar to p53-WT (Fig. 2D). In contrast, the interaction of p53 with angiogenin was abrogated in AD1 and AD2 deletion mutant p53-mt2 transfected cells. The deletion mutants p53-mt3 and p53-DBD which lack the AD2 domain, also did not show any detectable level of interaction with angiogenin (Fig. 2D). These results suggested that the AD2 domain of p53 is required for p53 interaction with angiogenin.
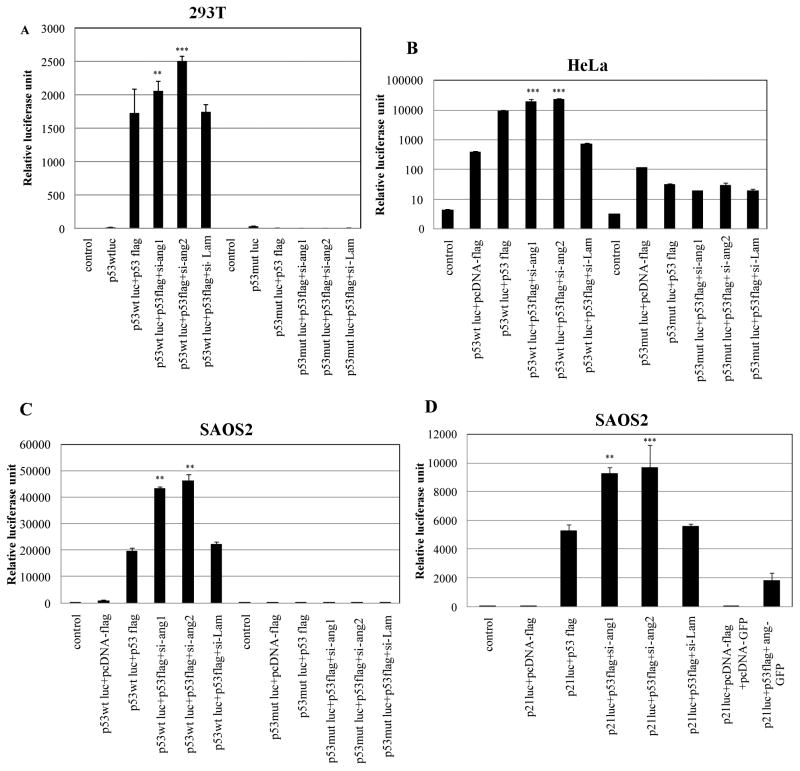
Silencing endogenous angiogenin induces p53 consensus DNA binding sequence activation
To determine the functional consequences of angiogenin interaction with p53, we reasoned that if the angiogenin-p53 interaction is involved in p53 mediated anti-apoptosis, then silencing angiogenin should increase p53 target gene expression resulting in cell death. A prerequisite for p53 target gene expression is p53 promoter activation. To determine the effect of angiogenin silencing on p53 promoter activity, we transfected 293T cells with a luciferase gene under the p53 consensus DNA binding sequence (p53wt-luc) or p53 DNA binding sequence mutated (p53mut-luc) along with flag tagged full length p53 (p53-flag) in combination with two different lentivirus angiogenin shRNAs (si-ang1 and 2) and analyzed for luciferase reporter activity. Transduction with lentivirus encoding nuclear lamin shRNA (si-lamin), a functionally unrelated molecule, was used as control. We have previously validated the efficacy of lentivirus based si-angiogenin constructs in which > 80% reduction in angiogenin expressionand secretion were observed (Sadagopan et al. 2009). The induction of wt p53-luc activity with p53-flag increased significantly by silencing angiogenin, while it had no impact on mut-p53-luc activity (Fig. 3A). The control si-lamin did not induce p53-luc activity (Fig. 3A). Similar effects of angiogenin silencing over p53-luc induction by p53 were observed in HeLa cells (Fig. 3B) that are known to have high levels of endogenous angiogenin (Tsuji et al. 2005) as well as in p53 null SAOS2 cells transfected with p53 (Fig. 3C). Compared to the vector control, angiogenin knockdown cells showed about 1.5, 1.9 and 2.3-fold increase in p53-luc activity in 293T, HeLa and SAOS2 cells, respectively (Fig. 3A,B and C).. These results suggested that angiogenin could be inhibiting p53 functions and angiogenin silencing could potentially be relieving this inhibition.
Figure 3. Effect of endogenous angiogenin silencing on p53 consensus DNA binding sequence promoter (p53-luc) and p53 target p21 gene promoter.
(A, B and C) 293T, HeLa and SAOS2 cells, respectively, were transfected with p53 wt-luc or p53 mut-luc promoters along with p53 flag in the presence of two different lentivirus angiogenin shRNAs (si-ang1 and si-ang2) or si-lamin. Luciferase reporter activity was measured by ELISA as per manufacturer’s protocol and normalized to renilla luciferase. Untransfected cells were used as control. (D) SAOS2 cells were transfected with p21-luc promoter along with p53 flag constructs in the presence of si-ang, si-ang2, si-lamin or ang-GFP and luciferase reporter activity was measured and normalized to renilla luciferase activity. Empty vectors pcDNA-flag and pcDNA-GFP was transfected as control. Values shown represent mean ± SD of three independent experiments. (**) p<0.001, (***) p<0.0001 compared with the control.
Silencing endogenous angiogenin induces p53 target p21 gene promoter
The master regulator of apoptosis is p53 and it carries out its function by transcriptionally regulating proteins like p21, GADD and the Bcl-2 family of proteins. To determine whether p53 promoter activation by angiogenin silencing also results in the activation of p53 dependent promoters, SAOS2 cells were transfected with p21 –luc promoter along with p53-flag, both with and without angiogenin or si-angiogenin. There was a significant increase in p21-luc activity when si-angiogenin was transfected along with p53-flag (Fig. 3D). In contrast, transfection of ang-GFP inhibited p21-luc activity considerably (Fig. 3D). This result further supported our finding that angiogenin inhibits p53 function and silencing angiogenin rescued p53 thus increasing the transcriptional upregulation of p53 dependent promoters.
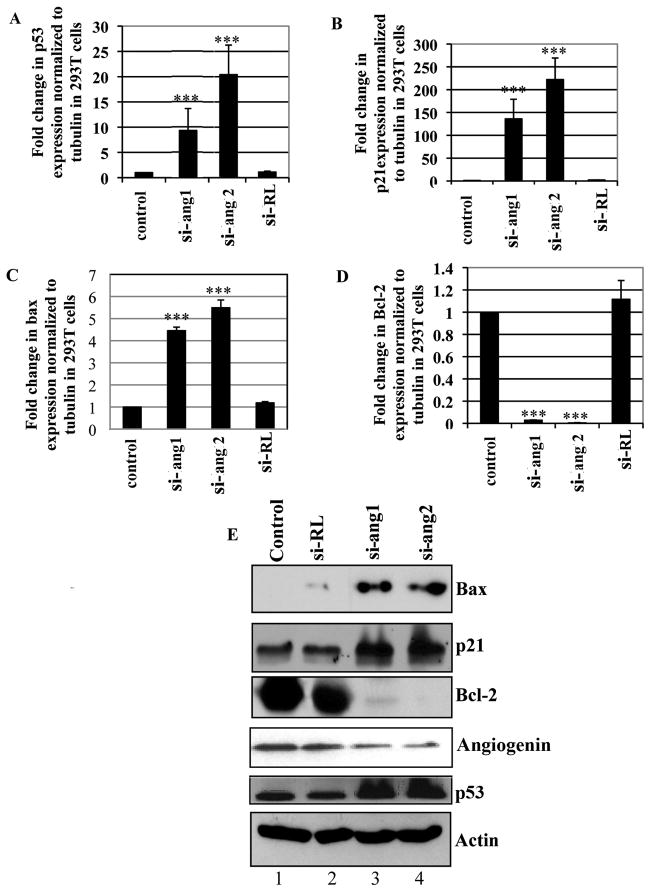
Silencing endogenous angiogenin induces the pro-apoptotic p53 target genes Bax and p21 and down-regulates anti-apoptotic Bcl-2 gene expression
Activation of p53 regulates extrinsic and intrinsic pathways of apoptosis. The extrinsic apoptotic pathway involves binding of death ligands to death receptor and a resulting cell death cascade (Ashkenazi and Dixit 1998; Adams and Cory 2007). In contrast, in the intrinsic apoptotic pathway, mitochondrial membrane potential is altered by a pro-apoptotic member of the Bcl-2 family. Bax, a member of the Bcl-2 family, forms heterodimers with Bcl-2 and Bcl-XL, both anti-apoptotic proteins, and these interactions serve to maintain a balance favoring the anti-apoptotic factors (Adams and Cory 2007). Since p53-luc activity increased by silencing angiogenin, we next tested the impact of silencing angiogenin on p53 target genes p21, Bax and Bcl-2 expression. 293T cells were transfected with si-ang 1, si-ang 2 or renilla lentivirus shRNA (si-RL), RNA was extracted and subjected to p53 target gene expression analysis. Quantitative real-time PCR analysis revealed an increase in p53 (Fig. 4A), p21 (Fig. 4B) and Bax (Fig. 4C) gene expression in si-angiogenin transfected cells but not in control or si-RL transfected cells (Fig. 4A, B and C). In contrast, the expression of Bcl-2, a pro-survival molecule was down-regulated when angiogenin was silenced (Fig. 4D). Western blot analysis supported this observation where we observed the up-regulation of p53 target genes Bax and p21, and inhibition of Bcl-2 by angiogenin silencing in 293T cells (Fig. 4E). These results further suggested that si-angiogenin treatment might be releasing the inhibition on p53 thus leading to increased expression of the p53 target genes Bax and p21 and decreased expression of Bcl-2.
Figure 4. Effect of endogenous angiogenin silencing on the expression of p53 target genes and pro-survival Bcl-2 gene.
(A–D) cDNA prepared from 293T cells transfected with si-ang or si-RL (renilla luciferase) were used to measure p53 (A) and p53 target genes p21 (B), Bax (C) and Bcl-2 (D) expression by quantitative real-time PCR. Untransfected cells were used as control. For all the above, each reaction was done in triplicate and each bar represents the mean ± SD from three independent experiments. (**) p<0.001, (***) p<0.0001 compared with the control. (E) Western blot analysis showing p53 target gene p21, Bax and Bcl-2 expression in 293T cells transfected with si-RL or si-ang. Actin was used as loading control.
Angiogenin expression blocks pro-apoptotic p53 target Bax and p21 expression and induces anti-apoptotic Bcl-2 gene expression
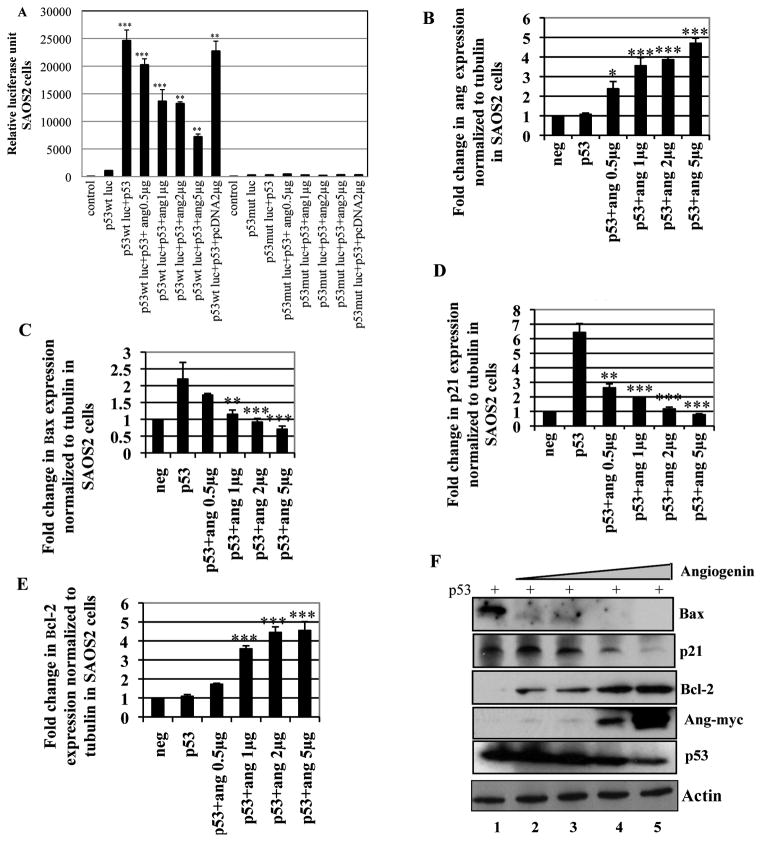
To further validate the above findings and to determine whether angiogenin addition could block p53 function, we used the p53 null SAOS2 cell line. Western blot analysis confirmed the absence of p53 expression in SAOS2 cells (Supplementary Fig. 2A). SAOS2 cells transfected with p53 wt-luc or p53 mut-luc promoters along with p53 flag in the presence of increasing concentrations of angiogenin plasmid were analyzed for luciferase activity. Wild type p53-luc activity was minimal in p53 null SAOS2 cells which increased significantly to a much higher level upon p53 expression (Fig. 5A). This increased p53 dependent p53-promoter activity was inhibited significantly by transfection of angiogenin expression plasmids in a dose dependent manner (Fig. 5A). Mutant p53-luc activity was not affected either by p53 expression or with increasing angiogenin expression (Fig. 5A).
Figure 5. Effect of increased angiogenin expression over p53-luc activity, p53 target gene expression and Bcl-2 expression in SAOS2 cells.
(A) SAOS2 cells were transfected with p53 wt-luc or p53 mut-luc promoters along with p53 flag in the presence of increasing concentrations of angiogenin (0.5–5μg) and the luciferase activity was measured by ELISA. Values are represented as mean ± SD of three independent experiments. (**) p<0.001, (***) p<0.0001 compared with the control. (B–E) cDNA prepared from SAOS2 cells transfected with p53-flag with or without increasing concentrations of angiogenin (0.5–5μg) was used to measure angiogenin expression (B) and p53 target gene expression Bax (C), p21 (D) and Bcl-2 (E) by quantitative real-time PCR. Untransfected cells were used as negative control (neg). For all the above, each reaction was done in triplicate and each bar represents the mean ± SD from three independent experiments. (*) p<0.01, (**) p<0.001, (***) p<0.0001. (F) Western blot analysis showing p53 target gene (Bax, p21, and Bcl-2) expression in SAOS2 cells transfected with p53-flag along with increasing concentrations of angiogenin (0.5–5μg Ang-myc plasmid). Western blots with myc and p53 antibody show the expression of angiogenin (Ang-myc) and p53, respectively. Actin was used as loading control.
To ascertain the role played by angiogenin in regulating p53 target gene expression, SAOS2 cells were transfected with p53flag along with increasing concentrations of angiogenin plasmids. The expression of angiogenin (ang-myc) and p53 target genes in the transfected cells were monitored by real-time PCR and Western blotting. Angiogenin expression (Fig. 5B) inhibited p53-depedent Bax (Fig. 5C) and p21 (Fig. 5D) gene expression in a dose-dependent manner while up-regulating Bcl-2 expression (Fig. 5E). Western blot analysis supported this observation (Fig. 5F). There was a clear dose dependent inhibition of pro-apoptotic protein Bax in angiogenin expressing cells. In contrast, activation of the anti-apoptotic protein Bcl-2 was increased which indicated that the cells expressing angiogenin trigger anti-apoptosis in a p53 dependent manner. Similarly, a dose dependent inhibition of cell cycle regulator p21 protein was observed in angiogenin expressing cells and increased expression of p21 has been shown to lead into apoptosis and cell death (Waldman et al. 1995). Taken together, these results demonstrated that angiogenin inhibits p53 activity, decreases the expression of pro-apoptotic proteins and increases the expression of anti-apoptotic proteins.
Angiogenin inhibits DNA-binding activity of p53 and angiogenin silencing increases p53 binding to its consensus sequence
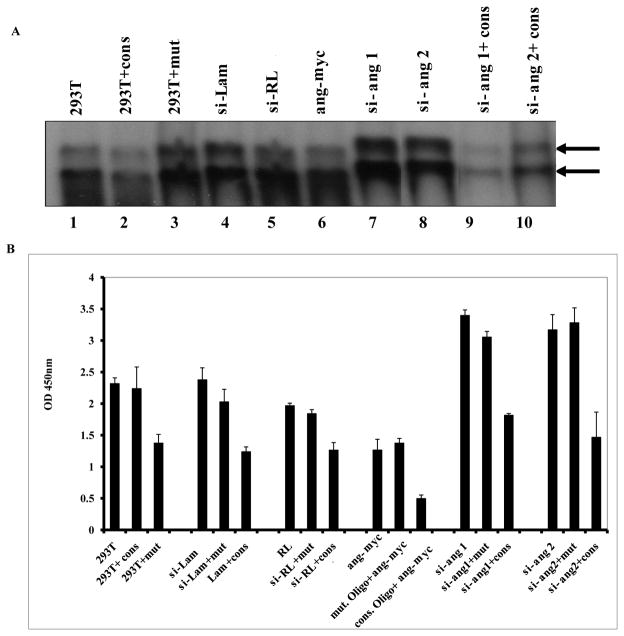
Since our results suggested that the interaction of angiogenin with p53 probably sequesters p53 and reduces its ability to bind to consensus DNA sequences, we used an electromobility shift assay (EMSA) to examine whether angiogenin expression affects the DNA binding activity of p53, and conversely whether silencing angiogenin increases p53’s DNA binding activity. EMSA was done using nuclear extracts from 293T cells transduced with si-Lamin, si-RL, si-ang1, si-ang2, and 293T cells transfected with myc tagged angiogenin. The nuclear extract from 293T cells showed binding activity to [γ-32P] ATP labled-WT–p53 consensus (cons) oligonucleotide probe (WT-p53) by EMSA (Fig. 6A, lane 1). This binding was reduced in the extracts premixed for 30 min with cold WT-p53 probe (Fig. 6A, lane 2) compared to the mut-p53 probe (Fig. 6A, lane 3) which demonstrated the specificity of probe. Nuclear extracts from si-lamin and si-RL transduced 293T cells showed effective binding (Fig 6A, lanes 4 and 5). Binding of p53 to its consensus sequence was reduced in cells transfected with angiogenin (Fig. 6A, lane 6). In contrast, angiogenin silencing effectively induced higher levels of p53 binding to the WT-p53 probe (Fig 6A, lanes 7 and 8). This was reduced when the nuclear extracts from si-ang1 (Fig. 6A, lane 9) or si-ang2 (Fig. 6A, lane 10) transduced cells were premixed with cold WT-p53 probe. When the effect of angiogenin on p53 DNA binding was examined by quantifying the reactions with a p53 transcription factor ELISA (Fig. 6B), results similar to EMSA were observed. Taken together these results demonstrated that angiogenin blocks the DNA binding activity of p53.
Figure 6. Effect of angiogenin silencing and angiogenin expression on p53 binding to its consensus sites.
(A) EMSA experiment showing binding of nuclear extracts prepared from 293T cells (lanes 1–3), 293T cells transduced with si-Lamin (lane 4), si-RL (lane 5), si-ang1 (lanes 7 and 9), si-ang2 (lanes 8 and 10), or 293T cells transfected with angiogenin (ang-myc) (lane 6). Specificity of the DNA-protein interaction was assessed by competition EMSA as mentioned in the methods. Arrows indicate the specific protein:DNA complexes. (B) Nuclear extracts prepared from 293T cells described in A were tested for their p53 binding by ELISA as per the procedure described in materials and methods. For competition, soluble oligonucleotides containing various transcription factor-specific sites (wild-type) or their mutant form were added to the nuclear extracts. Positive controls provided for each transcription factor were used simultaneously to check the functionality of the assay. Data represent the mean ± SD of two experiments. Binding in si-ang cells was calculated with respect to the DNA binding activities in angiogenin expressing cells.
Angiogenin expression blocks p53 mediated cell death
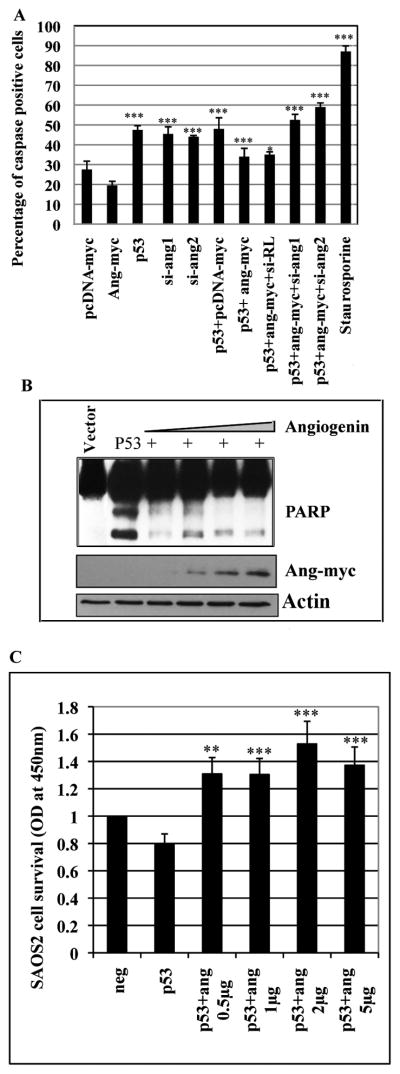
The p53 target protein Bax controls apoptosis by driving the release of cytochrome C from mitochondria and cytochrome C release activates caspase-3 (Liu et al. 1996). Cleaved caspase-3 is considered as a primary indicator of apoptotic cells (Kroemer et al. 1997). To determine whether angiogenin mediated p53 target gene inhibition leads to caspase-3 activation, SAOS2 cells were transfected with p53 and increasing concentrations of angiogenin plasmid, stained for cleaved caspase-3 and analyzed by flow cytometry. There was a basal level of caspase cleavage in untransfected and pcDNA transfected SAOS2 cells, which was inhibited upon angiogenin (ang-myc) expression (Fig. 7A and Supplementary figure 3 A, B and C). There was a two-fold increase in cleaved caspase levels with p53 expression which was reduced when p53 was expressed along with angiogenin (Fig. 7A and Supplementary figure 3 D, and E). Interestingly, caspase staining during angiogenin silencing was significantly higher when compared to cells transfected with p53 alone indicating that si-angiogenin could have silenced the endogenous angiogenin as well as angiogenin that was overexpressed with about 50–70% cell death (Fig. 7A and Supplementary figure 3 G and H). Control si-RL had little effect on increasing cleaved caspase staining observed with p53+angiogenin-myc transfected cells (Fig. 7A and Supplementary figure 3F). These results suggested that angiogenin could protect the cells from p53 mediated apoptosis.
Figure 7. Effect of angiogenin on p53 expressing SAOS2 cell survival.
(A) SAOS2 cells were transfected with the indicated plasmids and the cells were used for measuring cleaved caspase-3 by flow cytometry (supplementary figure S3) and the percentage of cells positive for cleaved caspase is represented as a bar graph. Staurosporin, a well known inducer of apoptosis, was used as a positive control. Each reaction was done in triplicate and each bar represents the mean ± SD from three independent experiments. (*) p<0.01, (***) p<0.0001. (B) SAOS2 cells were transfected with p53 and increasing concentrations of angiogenin (0.5–5μg Ang-myc plasmid) and Western blotted for PARP. Western blots with myc show the expression of angiogenin (Ang-myc). Actin was used as a loading control. (C) SAOS2 cells were transfected with p53 along with increasing concentrations of myc-angiogenin (0.5–5μg), and assayed for cell survival by MTT assay. Untransfected cells were used as negative control (neg). Data are expressed as mean ± SD of three independent experiments done in triplicate. (**) p<0.001, (***) p<0.0001.
During cell death, caspase-3 plays a central role in the execution of the apoptotic program by cleaving the poly (ADP-ribose) polymerase (PARP) that is involved in a number of cellular processes, particularly DNA repair and programmed cell death (Yu et al. 2002). Since we observed a reduction in caspase-3 positive SAOS2 cells transfected with p53 in the presence of angiogenin we studied the impact of this on PARP cleavage. P53 expression resulted in increased PARP cleavage while angiogenin (ang-myc) dose dependently inhibited PARP cleavage in these cells (Fig. 7B). By MTT cell survival assay, we observed significant increases in SAOS2 cell survival with increasing angiogenin concentrations (Fig. 7C). Compared to the control, there was about 1.6-fold increase in cell survival when angiogenin was transfected along with p53. These results demonstrated that angiogenin expression rescued the SAOS2 cells from p53 mediated cell death in a dose dependent manner which further supported our hypothesis that angiogenin inhibits the apoptotic functions mediated by p53.
Angiogenin expression inhibits p53 phosphorylation and induces ubiquitination of p53
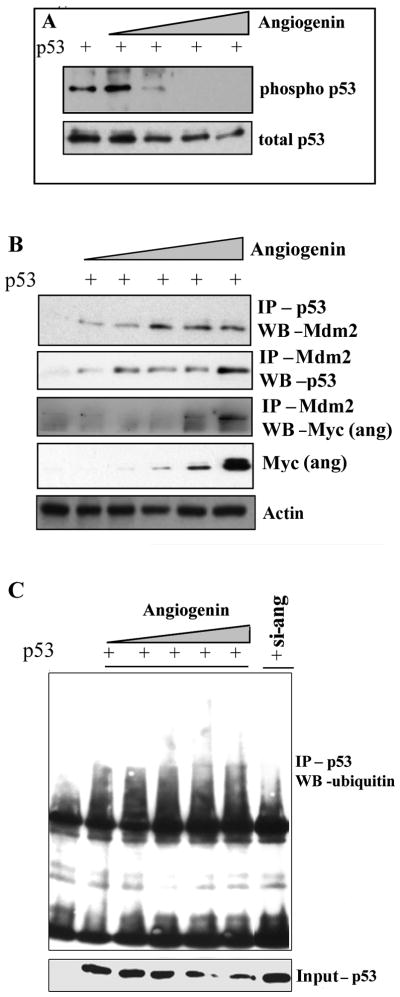
The default pathway adopted by tumor promoting agents in blocking the tumor suppressor protein p53 is by mutation, deactivation of the p53 pathway or by sequestering p53, rendering it inactive, reducing its activators or down-regulating p53 target genes resulting in cell cycle arrest or apoptosis (Riley et al. 2008). Under normal conditions, p53 remains inactive due to its rapid degradation by the ubiquitin ligase Mdm2 (Haupt et al. 1997). However, upon cellular stress, p53 is phosphorylated resulting in the halting of Mdm2 mediated degradation and accumulation of transcriptionally active p53, which activates several targets including cell cycle inhibitors and pro-apoptotic proteins leading to apoptosis or proliferation arrest. Since it has been shown that phosphorylation of p53 at serine 15 inhibits the ability of Mdm2 to bind to p53, we hypothesized that angiogenin could possibly be inhibiting the phosphorylation of p53 at serine 15, as it is a prerequisite for targeting p53 function. p53 is phosphorylated at multiple sites in vivo and by several protein kinases in vitro (Meek 1994; Milczarek et al. 1997). We did not observe any p53 expression in SAOS2 cells (Fig. S2). To understand the mechanism involved in angiogenin mediated inhibition of p53 function and to analyze the phosphorylation status of p53, SAOS2 cells were transfected with p53 and increasing concentrations of angiogenin (ang-myc) and Western blotted for phospho-p53 at serine 15. There was a dose dependent inhibition in p53 phosphorylation in these cells (Fig. 8A).
Figure 8. Effect of angiogenin treatment on p53 phosphorylation at serine 15 and ubiquitination of p53 in SAOS2 cells.
(A) SAOS2 cells transfected with p53 and increasing concentrations of angiogenin (0.5–5μg Ang-myc plasmid) were Western blotted for phospho p53 (top panel), stripped and reprobed for total p53 (bottom panel). (B) SAOS2 cells were transfected with p53-flag and increasing concentrations of myc-angiogenin and immunoprecipitated with p53 antibody and Western blotted for Mdm2 (top panel), immunoprecipitated with Mdm2 antibody and blotted for p53 (second panel), and immunoprecipitated with Mdm2 and Western blotted with myc (angiogenin; third panel). The lysates were Western blotted for myc (angiogenin) for input control (bottom panel). Actin was used as loading control. (C) SAOS2 cells transfected with p53 and increasing concentrations of angiogenin, or the cells transfected with p53 in the presence of si-angiogenin, were immunoprecipitated for p53 and Western blotted for ubiquitin. Bottom panel shows input p53 blot.
Phosphorylation of p53 at serine 15 impairs the ability of Mdm2 to bind p53 in response to DNA damage and p53 is stabilized to control apoptotic and cell cycle regulation functions (Shieh et al. 1997; Shieh et al. 2000). Since p53 phosphorylation at serine 15 was blocked by angiogenin, next we investigated whether inhibition in p53 phosphorylation results in increased Mdm2 association. SAOS2 cells with p53 and increasing concentrations of myc-angiogenin plasmids were immunoprecipitated with p53 and Western blotted for Mdm2. As expected, with increasing angiogenin concentrations there was a corresponding increase in Mdm2 – p53 association, when the lysates were immunoprecipitated with p53 and Western blotted for Mdm2 (Fig. 8B, top panel) or while immunoprecipitating with Mdm2 and Western blotting for p53 (Fig. 8B, middle panel).
The interaction between Mdm2 and p53 is well documented (Kubbutat et al. 1997) and our current study showed an interaction between angiogenin and p53. To determine whether angiogenin interacts with Mdm2 and thus forms a ternary complex, SAOS2 cells transfected with p53 and with increasing concentrations of myc-angiogenin plasmid were immunoprecipitated with Mdm2 antibody and Western blotted for myc (angiogenin). SAOS2 cell lysates were Western blotted using anti-myc antibody for input control (Fig 8B, bottom panel). At high angiogenin concentrations, we observed the interaction of Mdm2 with angiogenin (Fig. 8B, third panel). These results suggested that angiogenin could be forming complex with Mdm2 and p53.
Since we observed increased interaction between p53 and Mdm2 with increasing levels of angiogenin, next we analyzed whether this results in p53 ubiquitination. SAOS2 cells transfected with p53 and increasing concentrations of myc-angiogenin plasmid were immunoprecipitated with p53 and Western blotted for ubiquitin. There was a basal level of ubiquitination of p53 in p53 transfected cells probably due to endogenous angiogenin (Fig. 8C). We observed an increase in ubiquitination with increasing angiogenin expression (Fig. 8C). Conversely, a decrease in ubiquitination level was observed when p53 was expressed along with si-ang (Fig. 8C). Since there could be other molecules involved, we cannot conclusively state that Mdm2 is solely involved in angiogenin mediated p53 degradation. This needs further characterization which is beyond the scope of the current study. Nevertheless, taken together, these results suggested that angiogenin inhibition of phosphorylation of p53 could result in increased association with Mdm2 leading to p53 ubiquitination and hence a block in p53 functions.
DISCUSSION
Our comprehensive results presented here suggest that the distinct survival advantage of angiogenin secreting transformed or cancer cells tested could be mediated by angiogenin’s ability to inactivate p53. We found that the angiogenin induced inactivation of p53 involves the interaction of angiogenin with p53, inhibition of p53 serine 15 phosphorylation and the resultant binding of Mdm2, which is well known for its involvement in the ubiquitination of p53 (Haupt et al. 1997; Kubbutat et al. 1997). Ubiquitination of p53 in the presence of angiogenin clearly suggests that angiogenin’s ability to prevent cell death and increase cell survival could potentially be mediated by its capacity to inactivate p53 functions.
Several significant observations shown here clearly indicate that angiogenin is one of the regulators of the p53 mediated anti-apoptosis signaling cascade. Our initial observations showed that the interaction of p53 with angiogenin occurred only in cells that expressed angiogenin. The physical interactions between angiogenin and p53 suggested that angiogenin probably exerts its role in anti-apoptosis by sequestering p53 in a complex. Angiogenin is known to be localized in the cytoplasm, nucleus and nucleolus and the angiogenin-p53 interaction occurs predominantly in the nucleus. Although retention of p53 in the cytoplasm was thought to be a passive way to block the nuclear functions of p53, accumulating evidence suggests that cytoplasmic localization of p53 plays critical roles in p53 mediated apoptosis and autophagy (Marchenko et al. 2007). Hence, angiogenin could possibly be retaining p53 in the nucleus and blocking the cytoplasmic functions of p53 thus promoting anti-apoptosis. Whether the angiogenin-p53 interaction occurs in the cytoplasm and the resulting complex moves into the nucleus or whether the interaction occurs only in the nucleus needs to be analyzed further.
Angiogenin efficiently up-regulated the p53 target genes Bcl-2 (anti-apoptotic protein), that protects against apoptosis, and down-regulated Bax and p21 (pro-apoptotic proteins), while silencing angiogenin reversed this phenomenon. Though Bcl-2 is also a p53 target gene, its expression is repressed by silencing angiogenin and enhanced by angiogenin expression. This could be due to p53’s ability to repress rather than enhances the transcription of Bcl-2 since the promoter element of Bcl-2 contains a p53-negative response element (Hemann and Lowe 2006). Increased expression of Bcl-2, but not Bax and p21, shows that the angiogenin expressing cells attain an anti-apoptotic state. The p53 target genes Bcl-2 and Bax are regarded as two important regulators of the mitochondrial apoptosis pathway, and these two proteins homodimerize and heterodimerize with each other. It is very well known that the Bcl-2/Bax ratio in mitochondria decides the apoptotic or non apoptotic fate of the cell. High Bcl-2/Bax ratios lead to an elevated pro-apoptotic activity (Oltvai et al. 1993). With increased angiogenin expression, we observed decreased Bax expression and a corresponding increase in Bcl2 levels suggesting that angiogenin expression leads to a decreased Bcl2/Bax ratio and the cells become non-apoptotic. The pro-apoptotic protein Bax translocates into the mitochondria and oligomerize promoting cytochrome C release that activates a caspase cascade leading to apoptosis (Antonsson et al. 2001). The decreased Bax expression and a corresponding decrease in cleaved caspase-3 levels with increasing angiogenin expression suggest that angiogenin is actively involved in the regulation of p53 mediated anti-apoptosis and cancer cell survival.
Phosphorylation of serine 15 on the N-terminus of p53 contributes to the disruption of the p53-Mdm2 complex resulting in the stabilization of the p53 protein (Shieh et al. 1997). We identified that the inhibition of p53 serine phosphorylation, which occurs in angiogenin stimulated cells, induced the binding of p53 with Mdm2. By inhibiting serine 15 phosphorylation angiogenin might induce p53-Mdm2 association resulting in p53 ubiquitination. Our studies suggest that the angiogenin mediated decrease of p53 phosphorylation contributed to its effects on the induction of anti-apoptotic pathways. Although the inhibition of p53 phosphorylation could be potentially responsible for the deregulation of apoptosis, we cannot exclude the likelihood that dephosphorylation of p53 by angiogenin is also required to maintain apoptosis. It has been proposed that the dephosphorylation of p53 regulates the apoptosis related proteins Bcl-2 and Bax and changes the apoptotic function of p53 (Li et al. 2006). Since the angiogenin-GFP and myc constructs used in our study have a signal peptide, angiogenin may also become secreted. Studies by us and others have shown that secreted angiogenin induces the AKT and ERK1/2 signal pathways (Hu, et al., 1998; Sadagopan et al., 2011). Hence, it is also possible that angiogenin could be regulating p53 phosphorylation and apoptosis via AKT and ERK1/2 pathways as these two molecules have been reported to be involved in the transcriptional activity of p53 and the regulation of apoptotic functions of p53 (Gottlieb et al. 2002; Perfettini et al. 2005). Our results also do not rule out the possibility that angiogenin’s observed effects on p53 could also be mediated by the paracrine and autocrine activity of secreted angiogenin. Nevertheless, collectively our studies suggest that angiogenin could be inhibiting apoptosis by more than one pathway and the p53 deregulation observed could be one of the mechanisms involved in anti-apoptosis mediated by angiogenin.
These new insights that angiogenin induces anti-apoptosis through the ubiquitination of p53 may have a wide range of clinical implications in several human cancers. Further studies are required to fully characterize angiogenin interaction with p53 and to understand the significance of this interaction in different in vivo tumor models. Designing strategies for blocking the interaction between angiogenin and p53 might be of significant therapeutic value in cancer treatment.
MATERIALS and METHODS
Cells
Primary human dermal microvascular endothelial (HMVEC-d) cells (CC-2543; Clonetics Walkersville, MD) were grown in endothelial basal medium (EBM-2) with growth factors. A549 (human lung adenocarcinoma), HepG2 (human hepatocellular carcinoma), HTB (human neuroblastoma) and HeLa (human cervical carcinoma) cell lines were from ATCC. All these cell lines were culturedin DMEM (Gibco BRL, Grand Island, NY) supplementedwith 10% heat-inactivated FBS (HyClone, Logan, UT), 2 mM L-glutamine, and antibiotics. p53+/+ and p53−/−HCT116 (human colon carcinoma) cells were a kind gift from Dr. B. Vogelstein (Johns Hopkins University). p53+/+ and p53−/− HCT116 and SAOS2 cells (ATCC) were cultured in McCoy 5A media with 10% FBS.
Plasmid constructs
p53wt-luc, p53mut-luc, and p53-flag were purchased from Addgene, Cambridge, MA. Si-angiogenin1 and si-angiogenin2 were from Open Biosystems, Huntsville, AL. The myc-DDK-angiogenin construct was obtained from Origene (RC208874), and angiogenin-GFP was created by sub-cloning the full length angiogenin gene into the pcDNA TOPO-GFP expression vector (Invitrogen, Carlsbad, CA). Both the myc and GFP constructs of angiogenin consist of the whole open reading frame including signal peptide region of angiogenin. EGFP tagged wild type p53 (pEGFP-p53-WT) and deletion constructs of p53 (pEGFP-p53-mt1, pEGFP-p53-mt2, pEGFP-p53-mt3, and pEGFP-p53-DBD) were generously provided by Dr. AP Rapoport (University of Maryland).
All other materials and methods are included with the supplementary information.
Supplementary Material
Acknowledgments
This study was supported in part by Public Health Service grants, AI 091767 and the RFUMS—H.M. Bligh Cancer Research Fundto B.C. We thank Dr. AP Rapoport (University of Maryland) for providing p53 constructs. We thank Dr. B. Vogelstein (Johns Hopkins University) for the p53+/+ and p53−/− HCT116 cell lines. We thank Keith Philibert for critically reading the manuscript and Bob Dickinson for FACS analysis at the RFUMS core facility.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Author Contributions: SS, MVV and BC designed the experiments. SS, MVV, SC, NP, VB and NSW performed the experiments. MVV, SS, and BC wrote the paper.
References
- Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19(5):488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276(15):11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Barton DP, Cai A, Wendt K, Young M, Gamero A, et al. Angiogenic protein expression in advanced epithelial ovarian cancer. Clin Cancer Res. 1997;3(9):1579–1586. [PubMed] [Google Scholar]
- Chopra V, Dinh TV, Hannigan EV. Serum levels of interleukins, growth factors and angiogenin in patients with endometrial cancer. J Cancer Res Clin Oncol. 1997;123(3):167–172. doi: 10.1007/BF01214669. [DOI] [PubMed] [Google Scholar]
- Chopra V, Dinh TV, Hannigan EV. Circulating serum levels of cytokines and angiogenic factors in patients with cervical cancer. Cancer Invest. 1998;16(3):152–159. doi: 10.3109/07357909809050029. [DOI] [PubMed] [Google Scholar]
- Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, et al. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Foulkes WD. p53--master and commander. N Engl J Med. 2007;357(25):2539–2541. doi: 10.1056/NEJMp0707422. [DOI] [PubMed] [Google Scholar]
- Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene. 2002;21(8):1299–1303. doi: 10.1038/sj.onc.1205181. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387(6630):296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Lowe SW. The p53 – Bcl-2 connection. Cell Death and Differentiation. 2006;13:1256–1259. doi: 10.1038/sj.cdd.4401962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Gartenhaus RB, Eichberg D, Liu Z, Fang HB, et al. PBK/TOPK interacts with the DBD domain of tumor suppressor p53 and modulates expression of transcriptional targets including p21. Oncogene. 2010;29(40):5464–5474. doi: 10.1038/onc.2010.275. [DOI] [PubMed] [Google Scholar]
- Hu GF. Neomycin inhibits angiogenin-induced angiogenesis. Proc Natl Acad Sci U S A. 1998;95(17):9791–9795. doi: 10.1073/pnas.95.17.9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005;24(3):445–456. doi: 10.1038/sj.onc.1208223. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18(1):44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387(6630):299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- Li D, Bell J, Brown A, Berry CL. The observation of angiogenin and basic fibroblast growth factor gene expression in human colonic adenocarcinomas, gastric adenocarcinomas, and hepatocellular carcinomas. J Pathol. 1994;172(2):171–175. doi: 10.1002/path.1711720203. [DOI] [PubMed] [Google Scholar]
- Li DW, Liu JP, Schmid PC, Schlosser R, Feng H, et al. Protein serine/threonine phosphatase-1 dephosphorylates p53 at Ser-15 and Ser-37 to modulate its transcriptional and apoptotic activities. Oncogene. 2006;25(21):3006–3022. doi: 10.1038/sj.onc.1209334. [DOI] [PubMed] [Google Scholar]
- Liu S, Yu D, Xu ZP, Riordan JF, Hu GF. Angiogenin activates Erk1/2 in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2001;287(1):305–310. doi: 10.1006/bbrc.2001.5568. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Marchenko ND, Wolff S, Erster S, Becker K, Moll UM. Monoubiquitylation promotes mitochondrial p53 translocation. Embo J. 2007;26(4):923–934. doi: 10.1038/sj.emboj.7601560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek DW. Post-translational modification of p53. Semin Cancer Biol. 1994;5(3):203–210. [PubMed] [Google Scholar]
- Milczarek GJ, Martinez J, Bowden GT. p53 Phosphorylation: biochemical and functional consequences. Life Sci. 1997;60(1):1–11. doi: 10.1016/s0024-3205(96)00479-1. [DOI] [PubMed] [Google Scholar]
- Montero S, Guzman C, Cortes-Funes H, Colomer R. Angiogenin expression and prognosis in primary breast carcinoma. Clin Cancer Res. 1998;4(9):2161–2168. [PubMed] [Google Scholar]
- Moroianu J, Riordan JF. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc Natl Acad Sci U S A. 1994;91(5):1677–1681. doi: 10.1073/pnas.91.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KA, French TC, Vallee BL, Fett JW. A monoclonal antibody to human angiogenin suppresses tumor growth in athymic mice. Cancer Res. 1994;54(17):4576–4579. [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Perfettini JL, Castedo M, Nardacci R, Ciccosanti F, Boya P, et al. Essential role of p53 phosphorylation by p38 MAPK in apoptosis induction by the HIV-1 envelope. J Exp Med. 2005;201(2):279–289. doi: 10.1084/jem.20041502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9(5):402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Sadagopan S, Valiya Veettil M, Paudel N, Bottero V, Chandran B. Kaposi’s sarcoma-associated herpesvirus-induced angiogenin plays roles in latency via the phospholipase C gamma pathway: blocking angiogenin inhibits latent gene expression and induces the lytic cycle. J Virol. 2011;85(6):2666–2685. doi: 10.1128/JVI.01532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopan S, Sharma-Walia N, Veettil MV, Bottero V, Levine R, et al. Kaposi’s sarcoma-associated herpesvirus upregulates angiogenin during infection of human dermal microvascular endothelial cells, which induces 45S rRNA synthesis, antiapoptosis, cell proliferation, migration, and angiogenesis. J Virol. 2009;83(7):3342–3364. doi: 10.1128/JVI.02052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, George Paul A, Patel K, Chandran K, Ahmad W, et al. NFAT and CREB regulate Kaposi’s sarcoma-associated herpesvirus-induced cyclooxygenase 2 (COX-2) J Virol. 2011;84(24):12733–12753. doi: 10.1128/JVI.01065-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, et al. ERK1/2 and MEK1/2 induced by Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J Virol. 2005;79(16):10308–10329. doi: 10.1128/JVI.79.16.10308-10329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91(3):325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- Shimoyama S, Gansauge F, Gansauge S, Negri G, Oohara T, et al. Increased angiogenin expression in pancreatic cancer is related to cancer aggressiveness. Cancer Res. 1996;56(12):2703–2706. [PubMed] [Google Scholar]
- Tsuji T, Sun Y, Kishimoto K, Olson KA, Liu S, et al. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Res. 2005;65(4):1352–1360. doi: 10.1158/0008-5472.CAN-04-2058. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55(22):5187–5190. [PubMed] [Google Scholar]
- Xu ZP, Tsuji T, Riordan JF, Hu GF. Identification and characterization of an angiogenin-binding DNA sequence that stimulates luciferase reporter gene expression. Biochemistry. 2003;42(1):121–128. doi: 10.1021/bi020465x. [DOI] [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297(5579):259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.