Abstract
Cognitive performance is affected by motivation. Few studies, however, have investigated the neural mechanisms of the influence of motivation through potential monetary punishment on working memory. We employed functional MRI during a delayed recognition task that manipulated top‐down control demands with added monetary incentives to some trials in the form of potential losses of bonus money. Behavioral performance on the task was influenced by loss‐threatening incentives in the form of faster and more accurate performance. As shown previously, we found enhancement of activity for relevant stimuli occurs throughout all task periods (e.g., stimulus encoding, maintenance, and response) in both prefrontal and visual association cortex. Further, these activation patterns were enhanced for trials with possible monetary loss relative to nonincentive trials. During the incentive cue, the amygdala and striatum showed significantly greater activation when money was at a possible loss on the trial. We also evaluated patterns of functional connectivity between regions responsive to monetary consequences and prefrontal areas responsive to the task. This analysis revealed greater delay period connectivity between and the left insula and prefrontal cortex with possible monetary loss relative to nonincentive trials. Overall, these results reveal that incentive motivation can modulate performance on working memory tasks through top‐down signals via amplification of activity within prefrontal and visual association regions selective to processing the perceptual inputs of the stimuli to be remembered. Hum Brain Mapp , 2013. © 2011 Wiley Periodicals, Inc.
Keywords: event‐related fMRI, working memory, prefrontal cortex, top‐down processing, motivation
INTRODUCTION
Cognitive efficiency and performance level are affected by motivation. Working memory function is critical for goal‐directed behavior and the neural systems subserving working memory are influenced by motivation. Previous studies of the linkage between working memory and motivation have come from nonhuman primate electrophysiology [see Watanabe,2008 for review]. For example, it was demonstrated that taste rewards amplify working memory maintenance activity within prefrontal cortex (PFC) [Amemori and Sawaguchi2006; Hikosaka and Watanabe,2000; Kennerley and Wallis,2009a, b; Kobayashi et al.,2002; Leon and Shadlen,1999; Tsujimoto and Sawaguchi,2004]. In humans it has been demonstrated that incentivized conditions also amplify PFC activity associated with working memory with functional Magnetic Resonance Imaging (fMRI) [Beck et al.,2010; Krawczyk et al.,2007; Pochon et al,2002; Savine et al.,2010; Taylor et al; 2003]. More specifically, motivation has been demonstrated to influence several different working memory component processes including selective attention [Krawczyk et al.,2007; Small et al.,2005], encoding [Taylor et al.,2004], and active maintenance [Gilbert and Fiez,2004].
In this study, we investigated the extent to which motivation through monetary loss‐aversion influences neural systems subserving working memory. A wide array of studies of human judgment and decision making indicate that avoiding loss carries greater psychological impact than attaining an equivalent gain [Dreher,2007; Tversky and Kahneman,1981]. Additionally, processing losses is linked to differential brain activity relative to attaining gains [Knutson et al.,2000; Pessinglione et al., 2006; Wheeler and Fellows;2008]. However, few studies have systematically investigated the influence of possible monetary losses on cognitive performance specifically addressing the question of how monetary losses influence working memory. Thus, we tested human working memory performance under high and low motivational conditions during fMRI scanning. We employed a monetary loss manipulation in order to affect motivation to perform trials correctly using a delayed recognition paradigm in which modulation of attention can be measured based on instructional cues [Gazzaley et al.,2005a, b; Krawczyk et al.,2007]. Moreover, this paradigm allows us to investigate the role of motivation not only within PFC, but in posterior visual association areas that are likely influenced by PFC top‐down signals [Fuster et al.,1985; Miller and D'Esposito,2005]. We predicted that motivation in the form of monetary loss potential would influence working memory processes across all task periods (e.g., stimulus encoding, maintenance, and response). Such a result would be consistent with findings indicating increased performance [Heitz et al.,2007] and sustained activation of regions involved in cognitive control under incentive conditions [Locke et al.,2008]. Second we hypothesized that the PFC and visual association areas involved in working memory‐related processes would show amplified responses in loss trials relative to safe trials. Further, we predicted that brain regions sensitive to loss will show greater activation in the task period in which monetary incentive cues are delivered and greater task‐related functional connectivity with the PFC.
MATERIALS AND METHODS
Participants
Sixteen volunteers (five females) recruited from advertisements posted at the University of California, Berkeley, participated in this experiment. Age ranged from 19 to 32 (mean age, 22‐year‐old, standard deviation, 3.20). All participants provided written, informed consent prior to participating according to the guidelines of the Committee for the Protection of Human participants at the University of California, Berkeley. All participants were right‐handed, had normal or corrected vision, were free of neurological disorders, and were not taking any medications having a psychoactive, cardiovascular, or homeostatic effect.
Cognitive Task and Procedure
A working memory delayed recognition task was employed with picture stimuli. Performance incentives were manipulated through the use of a monetary endowment given to the participants prior to beginning the experiment. Participants were told that they would begin the experiment with a total of $40.00 in bonus money and that the total amount they would receive would be determined by their performance in the experiment. Participants were also informed that the bonus money would be converted into a total of 380 points. This enabled us to set arbitrary values for the trials and discourage participants being distracted from task performance by attempting to calculate their total bonus money throughout the experiment. A similar procedure was successfully used previously with performance rewards [Krawczyk et al.,2007]. Trials were designated to be either loss trials, in which the participant would lose 10 points if they responded incorrectly, or failed to enter a response within 1s, or safe trials, which had no monetary consequences for performance. Loss trials were cued with a number 10, indicating the point value at stake, presented before the encoding stimuli, while safe trials were preceded by a zero presented before the encoding period. Bonus pay was calculated and participants received the appropriate amount after the experiment was completed. The trial structure was identical in all conditions. Each trial began with the presentation of a point value (10 points loss trials and 0 points for safe trials). This cue was presented in green lettering for 2 s and was followed by a fixation cross presented for 4 s. A 4 s encoding period followed in which two pictures of faces and two pictures of scenes were presented for 400 ms per image with a 600 ms interstimulus interval consisting of a blank screen. All pictures were 225 pixels wide by 300 pixels tall and subtended ∼ 5 to 6° of visual angle. Each face image was cropped with blurred edges to include only the facial features and each face had a neutral expression. Three task conditions were included: remember faces/ignore scenes, remember scenes/ignore faces, and passive viewing (see Fig. 1). Remember faces/ignore scenes blocks required participants to remember the pictures of faces at encoding and to ignore pictures of scenes, while the remember scenes/ignore faces trials required them to do the opposite and remember scenes and ignore faces. In passive viewing trials participants viewed each picture with no mnemonic goals. A jittered delay period lasting 8, 10, or 12 s followed. A probe period began with the presentation of a test picture for 2 s (a face in remember faces, a scene in remember scenes) or an arrow in passive view. In the remember faces/ignore scenes and remember scenes/ignore faces conditions participants were instructed to judge if they had seen the picture in the encoding phase of that trial and to make a button press with the right thumb on a keypad if they had and a button press with the left thumb if they had not. In the passive viewing condition a right arrow prompted a right thumb press and a left arrow prompted a left thumb press. Participants were required to respond within 1 s of probe stimulus onset in order to get a correct answer in all trials and to retain all points for the trial in loss trials. After a 4 s fixation cross presentation, a 2 s feedback screen was presented indicating whether the participant's response had been correct, incorrect, or had exceeded the 1 s response window. If correct, a feedback statement was presented indicating that zero points had been lost in green type. If incorrect, or past the response time window, a loss total for the trial was presented in red type for 2 s. A fixation cross appeared for 4 s followed by a jittered intertrial interval of 4, 6, or 8 s.
Figure 1.
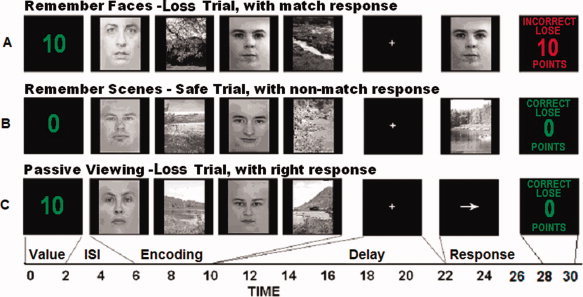
Behavioral task conditions. A: An example of a loss trial in which the instructions were to remember faces and ignore scenes and a match item appeared. Feedback shows the outcome slide for a loss due to an incorrect response. B: Example of a remember scenes/ignore faces safe trial with a nonmatch response. Feedback shows correct outcome screen. C: Passive viewing trial with a right button press response and correct feedback.
Three blocks of 16 trials were presented for the remember faces/ignore scenes and remember scenes/ignore faces conditions and 2 blocks of 16 trials were presented for the passive viewing condition. Participants were informed regarding the instructional condition of each block prior to the first trial. For four participants two remember scenes/ignore faces sets were completed and for three participants two remember faces/ignore scenes sets were completed due to time limitations. Data were acquired in 8 runs of 16 trials lasting 9 min 45 s each yielding a total of 128 trials.
We employed additional counterbalancing measures in order to minimize the differences between the conditions. Loss and safe trial types were pseudorandomly distributed throughout each block with the constraint that an equal number of loss and safe trials were included in each condition. The presentation of images in the encoding period was counterbalanced to control for order effects. Gender of the face images was experimentally controlled such that the two encoding pictures in any given trial were always the same gender. The probe stimulus contained an equal number of correct and incorrect trial types for the remember faces/ignore scenes and remember scenes/ignore faces conditions, as well as an equal number of left and right arrows in the passive viewing condition. Blocks were presented in a pseudorandomized order.
Participants performed a functional localizer task prior to the working memory task that allowed us to functionally define regions of interest (ROI) for use in our group analyses. The functional localizer consisted of seven 16 s blocks of grayscale faces, grayscale scenes, or a fixation cross. To insure that participants were attentive during the localizer task they were instructed to make simultaneous right and left button presses with the thumbs if they saw an image repeat. This localizer task has previously been shown to reliably activate scene and face‐selective regions of inferior temporal cortex [Gazzaley et al., 2005; Krawczyk et al.,2007].
MRI Data Acquisition
Images were acquired using a 4T Varian INOVA scanner using a gradient echoplanar sequence sensitive to BOLD contrast. A standard radiofrequency (RF) coil was used. Experimental stimuli were presented with E‐Prime software (PST, Pittsburgh, PA). This software also enabled recording of responses for accuracy and response time via a fiber optic response keypad consisting of four buttons. Participants used the right and left outside buttons to make their responses. Visual stimuli were presented using an Epson LCD projector (Long Beach, CA) onto a projection screen mounted above the participant's torso and viewed through a mirror mounted inside the RF coil. Each volume consisted of 40 coronal slices (3 mm thick, 0.5 mm slice gap) providing whole‐brain coverage. Images were acquired with specific parameters to optimize signal in ventral brain regions that are typically susceptible to drop‐out related to magnetic field inhomogeneities. This involved setting the phase encode orientation in the superior‐inferior direction. A one‐shot T2*weighted EPI sequence (TR = 2,000 ms, TE = 28 ms, FOV = 22.4 cm2, matrix size = 64 × 64) was used to acquire functional images. Head motion was limited using foam head padding.
Functional MRI Data Analysis
Detailed descriptions of the procedure used for analyzing activation within trials have been published previously [Zarahn et al.,1997] and are summarized below. Activation of each phase of the trials was assessed using multiple regression [Postle et al.,2000; Zarahn et al.,1997]. Preprocessing stages included correction for slice timing differences using a sync‐interpolation method, and interpolation of the data to 1‐s temporal resolution by combining each shot of half k‐space with the bilinear interpolation of the two flanking shots. Subsequent analyses were conducted using SMP2 run in Matlab 6.5 (http://www.mathworks.com). EPI images were realigned to the first volume of acquisition and then smoothed with a 8 mm 3D Gaussian kernel.
Separate regressors were used to model three phases of the task: encoding (6–10 s into the trial), delay (14–15 s into the trial) and response (1 s covariate capturing the first second of the response window). Note that the delay period regressor was placed 4 s after the delay period had begun in order to prevent contamination of the delay period activity from residual signal associated with the encoding period. Only correct trials were included in the analysis. Incorrect trials and those in which the participant exceeded the response window were modeled separately and excluded from group analyses. Each regressor was convolved with a canonical hemodynamic response function (HRF) provided in SPM2 and entered into the modified general linear model of SPM2. A high‐pass filter (cutoff 128 s) was applied to the data to remove frequency effects. Parameter estimates (e.g., β values) were extracted from this GLM analysis for the regressors modeling the encoding period of the task and averaged within functionally defined regions of interest (ROIs), or anatomically defined ROIs. Planned comparisons were conducted on these data using paired‐samples t‐tests (P < 0.05) to test our a priori hypotheses regarding regional activation. Data from all participants were co‐registerd to the MNI template brain and normalized for a group analysis of the encoding period data.
Functional ROI Analyses
Functional ROIs were chosen to test for differences in top‐down modulation and motivation in PFC and visual areas. In visual association cortex we isolated scene‐selective ROIs based on the localizer task GLM using a scene minus face contrast to obtain regions responsive to scene perception. Activation was restricted to the parahippocampal gyrus by masking the most active cluster with a spherical mask (5 mm radius) centered upon the voxel with the highest t‐value. This procedure yielded either 8 or 9 voxels for each ROI chosen. ROI masks for scene selective areas were obtained for all participants bilaterally. Face‐selective ROIs were obtained by conducting a face minus scene contrast on the localizer task GLM data to identify individual face‐selective ROIs in native space. For these analyses all active voxels for the contrast within the right and left fusiform gyri were included in the ROIs. For participants whose face localizer activation extended outside of the fusiform gyrus, the ROIs were trimmed using a cube shaped mask 10 mm in diameter and centered at MNI coordinates reported by Spiridon et al., [2006] corresponding to the right and left fusiform face areas (right: x = 31.6, y = −57.2, z = −10.4; left: x = −50.6, y = −70.8, z = −13.0). Mean parameter estimates from all face‐selective and scene‐selective and PPA ROIs were extracted for the encoding, delay, and response task periods.
Functionally defined ROIs were defined in the PFC for each participant by running a GLM contrast on the data from the experimental runs to obtain regions active for each task period collapsed across task conditions and incentive levels. The PFC ROIs were anatomically restricted in all participants to include only those voxels within the lateral PFC including middle and inferior frontal gyrus (anterior to primary motor cortex and superior to the orbital gyrus) falling into the following MNI coordinate ranges (x = −70 to 70, y = 5 to 70, z = −10 to 70). During the encoding period, PFC ROIs were bilateral in all participants. During the delay period, 15/16 participants had left PFC ROIs and 12/16 had right PFC ROIs. During the response period, 14/16 participants had left PFC ROIs and 15/16 had right PFC ROIs.
Structural ROI Analyses
Structural ROIs were defined in order to investigate brain regions engaged by incentive processing at the value cue and feedback period. These regions were defined bilaterally based on previous studies of monetary delay tasks [Knutson et al.,2002, 2003). They consisted of the amygdala (x, y, z = −26, 9, −27.6, and 26, 9, −27.6), insula (−24.5, 17, 4.1, and 27.3, 16, 4), caudate (−9.6, 1.5, 6.9, and 8.9, −0.41, 8.5), and putamen (−18.4, −5 2.4 and 18.5, −2.3, 4 ). These regions were localized with a spherical 8mm ROI applied to normalized MNI template images centered upon the coordinates listed above using Marsbar software (sourceforge.net/projects/marsbar).
Beta‐Series Analyses
Functional connectivity analyses were carried out using a beta‐series correlation approach. This analysis has been described in more detail elsewhere [Rissman et al.,2004] and has been used previously in working memory paradigms similar to that used here [Gazzaley et al.,2007; Rissman et al.,2008, 2009; Yoon et al.,2006]. The analysis for this experiment involved using individual regressors (HRF convolved covariates) for each phase of each trial. In this experiment, regressors modeled each phase of the working memory trial (encoding, delay, and response). The analysis generates a beta value for each regressor which can be categorized by trial period and incentive type to form a beta‐series representing the trial‐to‐trial variability associated with each task stage. These analyses were conducted on each subject individually using the functional ROIs from the left and right face‐selective cortex, scene‐selective cortex, and PFC previously described in the prior sections. Notably, the PFC ROIs were separately defined for each successive working memory stage, thus raising the sensitivity to detecting stage‐related PFC connectivity change. When the beta‐series between two ROIs shows a high correlation, these regions are inferred to be functionally interactive. Similarly we compared functional connectivity between the functionally defined PFC regions and the structurally defined ROIs within the amygdala, caudate, putamen, and insula (projected back into native space) for each task phase to evaluate potential interactions between loss‐sensitive areas and the PFC during working memory trials.
In this experiment individual GLM analyses were conducted to produce subject specific beta‐series for the encoding, delay, and response phases. These beta‐series were further divided into loss and safe trials generating six betaseries for each subject. The beta‐series were analyzed for correlations between ROIs in the face‐selective and scene‐selective regions in relation to PFC ROIs. Once R values were obtained for each pair of ROIs, a Fisher's R‐to‐Z transformation [Fisher,1921] was applied to the data to enable statistical tests to determine at a group level whether different task phases resulted in greater correlation and whether the presence of incentive consequences influenced the level of functional connectivity. An alpha level of P < 0.025 (two‐tailed) was used to test for significant differences in functional connectivity. A Bonferroni correction for mutliple comparisons was applied within each ROI pair (e.g., amygdala‐PFC) at each phase. Separate regions were considered to be independent as were separate task phases.
RESULTS
Behavioral Data
Overall accuracy on all trials was relatively high across both trial types (88.07% for loss trials and 86.19% for safe trials; Fig. 2) and during the passive view trials. We had predicted that incentives would potentially lead to higher accuracy and faster response times when a loss was possible in both conditions. To test this prediction means for accuracy and response times were analyzed with paired‐samples t‐tests for each mnemonic condition separately. For the remember faces/ignore scenes condition, loss trials were performed at a higher rate of accuracy (M = 83.95%) than safe trials (M = 77.73%) t(15) = 2.78, P < 0.01. Additionally, there was not a speed‐accuracy tradeoff, as loss trials (M = 696.26 ms) were performed significantly faster than safe trials (M = 724.96 ms) as well t(15)=2.95, P < 0.01. For the remember scenes/ignore faces condition, accuracy between loss vs. safe trials did not differ significantly but the effect of incentives on response times for loss trials (M = 688.97 ms) compared with safe trials (M = 702.09 ms) showed a trend toward significance t(15) = 1.57, P = 0.06. No significant differences between loss vs. safe trials were found for the passive viewing trials.
Figure 2.
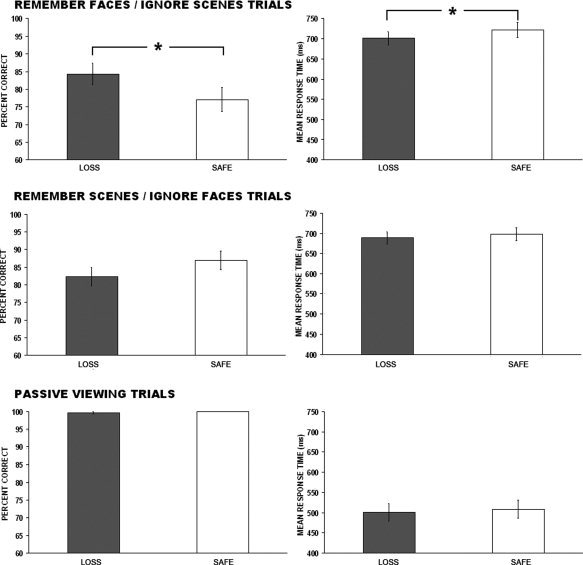
Behavioral data showing accuracy and response time for each behavioral condition. Error bars denote ±SEM.
Functional Imaging Data
Data analyses were carried out on mean parameter estimates extracted from functionally defined face‐selective and scene‐selective cortex, as well as PFC. Initially, each behavioral condition (remember faces/ignore scenes, remember scenes/ignore faces, and passive viewing) was analyzed after collapsing across both incentive levels (loss and safe trials) in order to determine the sensitivity of these regions to attention modulation. Next, more targeted analyses testing for differences between loss and safe trials among the different conditions were performed. These analyses are presented in the following sections organized by task phase (e.g., encoding, delay and response periods) and region of interest.
Encoding period
The encoding period data captures the task period that requires maximal attention toward relevant items and inattention toward irrelevant items. We predicted that this would be the task period most sensitive to top‐down modulation based on prior work with this task [Gazzaley et al.,2005a, b; Krawczyk et al.,2007].
Attentional modulation
Repeated measures ANOVAs conducted on each ROI demonstrated that both left (F(2,30) = 3.90, P < 0.05) and right (F(2,30) = 5.69, P < 0.01) face‐selective and left (F(2,30) = 22.81, P < 0.0001) and right (F(2,30)=19.17, P < 0.0001) scene‐selective ROIs showed significant modulation at the encoding period (see Figs. 3 and 4). Bonferoni corrected post‐hoc tests revealed that the right face‐selective visual cortex showed greater activation in the remember faces/ignore scenes condition (M = 2.73) and remember scenes/ignore faces condition (M = 2.62) compared to the passive viewing condition (M = 1.86) (refer to Fig. 3, left panel). Left and right scene‐selective visual cortex showed greater activation in the remember scenes/ignore faces conditions (left: M = 5.02, right: M = 5.57) than both the passive viewing condition (left: M = 2.92, right: M = 3.63) and the remember faces/ignore scenes condition (left: M = 2.78, right: M = 3.70) (refer to Fig. 4, left panel). Given that bottom‐up input was identical between behavioral conditions, these data demonstrate that the category‐selective visual cortical regions were modulated by top‐down attention during working memory encoding. Additionally, scene‐selective regions were found to exhibit greater categorical selectivity than face‐selective regions.
Figure 3.
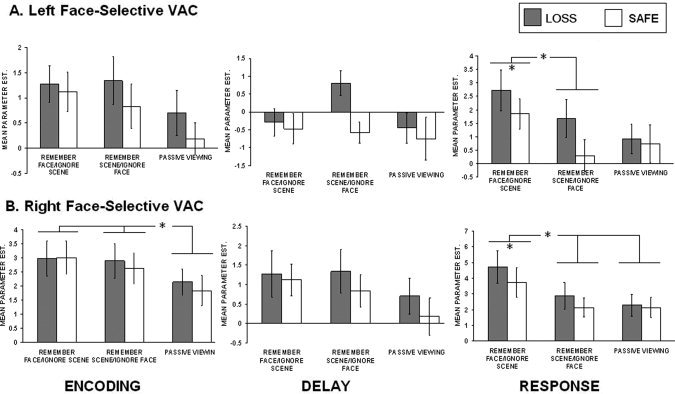
Summary of face‐selective visual association cortex ROI data. A. Mean parameter estimates for loss and safe trials at each condition during the encoding, delay, and response task phases.
Figure 4.
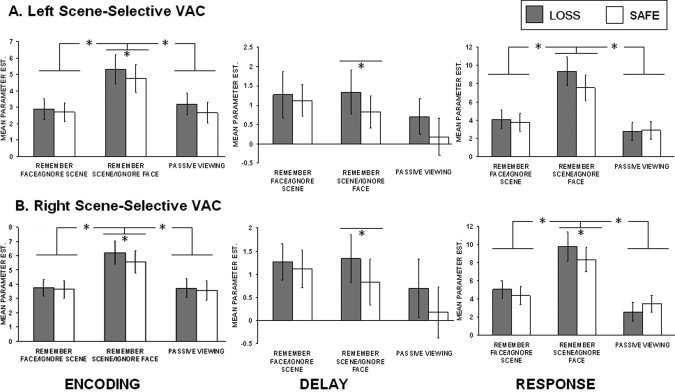
Summary of scene‐selective visual association cortex ROI data. Mean parameter estimates for risk and safe trials at each attention condition are presented.
The PFC also showed evidence of attention modulation, demonstrating significantly more activity during remember conditions compared to the passive viewing condition (left: F(2, 45)=16.54, P < 0.001; right: F(2, 45) = 13.08, P < 0.001). Bonferroni corrected post‐hoc tests in the left PFC revealed that both the remember faces/ignore scenes (M = 2.15) and remember scenes/ignore faces trials (M = 2.75) were more active than the passive view condition (M = 0.54). Additionally, the remember scenes/ignore faces condition was significantly more active than the remember faces/ignore scenes condition. In the right PFC there was greater activation for the remember faces/ignore scenes condition (M = 2.22) over the passive view condition (M = 0.99), and for the remember scenes/ignore faces (2.76) over both the remember faces/ignore scenes condition and the passive view condition.
Incentive modulation
We next conducted analyses to investigate whether loss trials resulted in enhanced top‐down modulation in visual association cortex and PFC. These analyses were conducted using paired‐samples t‐tests on the category of trials most associated with each category‐selective ROI (e.g., remember faces/ignore scenes trials in the face‐selective visual cortex). No significant effects were found in face‐selective cortex but both left (t(15) = 2.06, P < 0.05) and right (t(15) = 3.02, P < 0.005) scene‐selective visual cortical regions showed significantly increased activity in loss (left: M = 5.29, right: M = 6.21) as compared with safe trials (left: M = 4.75, right: M = 5.57) during the remember scene/ignore face condition (see Figs. 3 and 4, left panels). We also performed separate analyses of the magnitude of enhancement and suppression compared to baseline (e.g., passive view condition) for each incentive category. We calculated enhancement and suppression indices by subtracting passive viewing activation from each mnemonic condition within the ROI congruent with this class of stimuli (enhancement) and subtracting the to be ignored class of items from passive view baseline (suppression). These analyses failed to reach significance in any visual cortex ROIs.
Similar analyses were conducted to test for modulation by incentives in the PFC. Significant incentive modulation was found with loss trials (left: M = 2.95, right: M = 3.11) showing greater activation than safe trials (left: M = 2.55, right: M = 2.40) for the remember scenes/ignore faces condition for both the left (t(15) = 2.62, P < 0.01) and right (t(15) = 4.55, P < 0.001) PFC ROIs (Fig. 5, left panel). The remember faces/ignore scenes condition failed to reach significance, but showed similar patterns.
Figure 5.
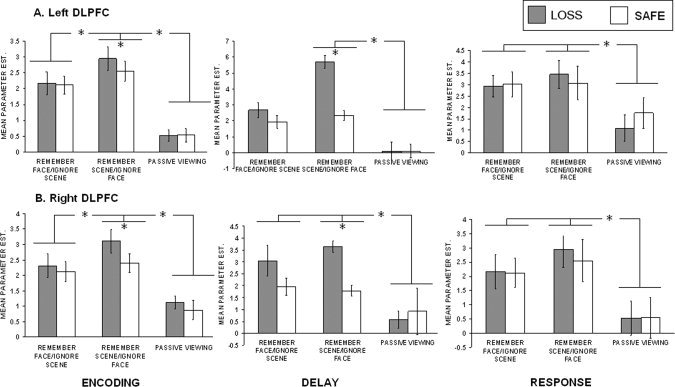
Summary of lateral PFC ROI data. Mean parameter estimates for loss and safe trials at each attention condition are presented.
Delay period
This task period is associated with maintenance of information. This task phase was predicted to show robust incentive modulation with increases in activation in loss trials over safe within PFC, as prior electrophysiological studies have identified this phase as sensitive to enhancement of delay period PFC neural firing by incentives [Hikosaka and Watanabe,2000; Leon and Shadlen,1999; Tsujimoto and Sawaguchi,2004].
Attentional modulation
In our previous study using this paradigm, the delay period did not show top‐down modulation effects in visual association cortex [Krawczyk et al.,2007]. Consistent with these findings, none of the visual cortex ROIs (collapsed across incentive conditions) showed reliably greater activity during the remember trials (for each relevant stimulus) as compared with the passive view condition (see Figs. 3 and 4, middle panels). Greater PFC activity during the remember trials (for each relevant stimulus) as compared to the passive view condition (see Fig. 5, middle panels) for both the left (F(2, 42) = 17.72, P < 0.001) and right (F(2, 33) = 6.78, P < 0.005) ROIs. Corrected post‐hoc tests revealed that the remember scenes/ignore faces condition showed greater PFC delay period activation (left: M = 4.01, right: M = 2.71) relative to the passive view condition (left: M = 1.85, right: M = 0.74) (P < 0.001). Similarly the remember faces/ignore scenes condition showed greater right PFC delay period activation (M = 4.53) relative to the passive view condition (P < 0.001).
Incentive modulation
In the remember scenes condition, loss trials showed greater delay period activity (left: M = 0.08, right: M = 0.84) than safe trials (left: M = −0.63, right: M = −0.01) for both the left t(15) = 2.52, P < 0.05) and right t(15) = 2.52, P < 0.05) scene‐selective ROIs; Fig. 4, middle panel. We observed significant enhancement above passive view baseline (requiring no mnemonic maintenance) for loss trials (M = 1.38) as compared with safe trials (M = −0.26) in the right scene‐selective visual cortex t(15) = 1.94, P < 0.05. No similar significant effects were found in the face‐selective ROIs. Significant incentive modulation was also found for both the left PFC (loss: M = 3.33, safe M = 2.10) (t(14) = 3.49, P < 0.005) and the right PFC (loss: M = 3.64, safe M = 1.78) (t(11) = 7.00, P < 0.0001) ROIs in the remember scenes/ignore faces conditions (Fig. 5, middle panel).
Response period
We predicted greater activation for the congruent category‐specific visual regions (e.g., faces in a face‐selective region) during judgment of whether the probe item was a match or not during the probe period.
Attentional modulation
Repeated measures ANOVAs collapsed across incentive conditions revealed significant differences among the three conditions in left (F(2,30) = 4.80, P < 0.05) and right (F(2,30) = 8.03, P < 0.01) face‐selective visual cortex (Fig. 3, right panel) and left (F(2,30) = 21.50, P < 0.0001) and right (F(2,30) =2 2.62, P < 0.0001) scene‐selective visual cortex (Fig. 4, right panel). Bonferroni corrected post‐hoc tests revealed that the left face‐selective ROI was significantly more active in the remember faces/ignore scenes condition (M = 2.86) than the remember scenes/ignore faces condition (M = 1.57). The right face‐selective ROI was significantly more active in the remember faces/ignore scenes condition (M = 3.38) than both the remember scenes/ignore faces condition (M = 2.87) and the passive viewing condition (M = 2.00). The left scene‐selective regions were significantly more active in the remember scenes/ignore faces condition (M = 1.50) than both the remember faces/ignore scenes condition (M = 0.95) the passive view condition (M = 0.86). Finally, the right scene‐selective region was also more active in the remember faces/ignore scenes condition (M = 1.46) compared to the remember faces/ignore scenes condition (M = 0.93) and the passive view condition (M = 0.77).
Analyses of the PFC ROIs revealed greater sensitivity to the remember trials over the passive viewing trials (Fig. 5, right panel). In the left PFC ROI, an ANOVA across the three conditions reached marginal significance (F(2, 36)=3.05, P < 0.06). Corrected post‐hoc tests revealed that this ROI was significantly more active for the remember faces/signore scenes condition (M = 2.97) and the remember scenes/ignore faces condition (M = 3.26) than for the passive viewing condition (M = 1.43). Similar results were found to be robust in the right PFC ROI as well (F(2, 42) = 3.84, P < 0.05). Post‐hoc tests showed that both conditions (Remember faces/ignore scenes, M = 4.19; Remember scenes/ignore faces, M = 4.28) were again more active than the passive view condition (M = 2.22).
Incentive modulation
We next tested for incentive modulation at the response period using paired samples t‐tests on condition specific comparisons and computations of the enhancement and suppression indices. Right (t(14) = 1.94, P < 0.05) and left face‐selective visual cortex (t(15)=4.40, P < 0.001) exhibited significant incentive modulation with loss trials showing greater activation (left: M = 2.36, right: M = 3.69) than safe trials (left: M = 2.16, right: M = 3.36) during the relevant memory condition (Figs. 3 and 4, right panels). The right scene‐selective visual cortex showed this same pattern (t(15) = 2.78, P < 0.01) with loss trials showing greater activation (M = 9.79) than safe trials (M = 8.35). The left scene‐selective visual cortex ROI showed marginal significance for this same comparison (loss trials greater than scene trials) (t(9) = 1.52, P = 0.08). Additionally, the enhancement index measures showing increased activation for the remember conditions over the passive view conditions were significantly greater for loss trials relative to safe trials in the remember scenes/ignore faces condition for the left (t(15) = 1.95, P < 0.05) (loss: M = 4.92, safe: M = 3.69) and right (t(15) = 1.94, P < 0.05) scene‐selective visual regions (loss: M = 5.26, safe: M = 3.44).
General trends toward significant incentive modulation showing greater activation of loss trials relative to safe trials was observed for both PFC ROIs under most conditions with the exception of the left PFC in the remember faces/ignore scenes condition (Fig. 5, right panel).
Structural ROI analyses
Structurally defined ROIs within the amygdala, insula, caudate, and putamen were tested in order to assess the effects of incentive‐related regions during this task. Comparisons of activity of loss to safe trials during the cue period prior to the encoding of stimuli (collapsed across all conditions) revealed robust incentive modulation in the amydgala (left: t(15) = 2.17, P < 0.05) (loss: M = 0.87, safe: M = −0.49)) (right: t(15) = 3.35, P < 0.01) (loss: M = 0.93, safe: M = 0.28)), caudate (left: t(15) = 2.01, P < 0.05) (loss: M = 0.65, safe: M = −0.12)) (right: t(15) = 1.75, P < 0.05) (loss: M = 0.77, safe: M = 0.11)), and putamen (left: t(15)=1.98, P < 0.05) (loss: M = 0.72, safe: M = −0.29)) (right: t(15) = 4.25, P < 0.001) (loss: M = 0.85, safe: M = 0.28)) bilaterally but not in insular cortex. No such effects were found during the trial outcome period.
Bivariate beta‐series analyses
The single subject‐derived beta‐series correlations between task phase‐specific PFC regions and their associated visual regions were grouped and analyzed using within‐subjects 3 (phase) × 2 (incentive) ANOVAs. These were carried out independently for eight different combinations (e.g., right PFC with right face‐selective cortex, right PFC with left face‐selective cortex, etc.). A significant effect of task phase was found for the right PFC to left PPA pairing F(2, 24) = 3.42, P < 0.05. Bonferroni corrected post‐hoc analyses revealed that the PFC‐PPA correlations were significantly greater during the encoding phase than the delay phase. Additionally, there was a significant quadratic effect present in this ROI pairing F(2, 24) = 6.80, P < 0.05 (refer to Fig. 6) indicating that the delay period showed somewhat less correlation than the response period between these ROIs. This comparison approached significance in a post hoc test (P = 0.09).
Figure 6.
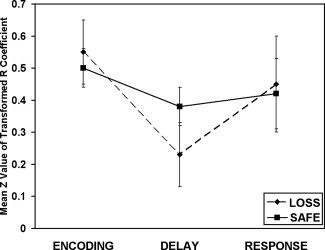
Functional connectivity analysis showing the correlations between the right PFC and left scene‐selective region. The encoding phase showed reliably greater connectivity between these two regions than was observed at the delay period. Additionally, the response period showed a marginal trend toward greater connectivity than the delay period.
Additional tests were carried out using t‐tests and bivariate correlations at each task phase to test for differences between the incentive conditions for the PFC and visual areas (as indexed by t‐value). No differential correlations were observed at any task phase between the loss and safe trials.
The bivariate correlations between PFC areas and loss‐related ROIs within the amygdala, caudate, putamen, and insula were analyzed to test whether there was differential connectivity between loss and safe conditions. This analysis was carried out using paired t‐tests at each task phase (encoding, delay, and response) corrected for multiple comparisons within each ROI pair (e.g., amygdala‐PFC) at each task phase. We observed significantly greater connectivity between the left PFC and the left insula for loss (M = 0.50) over safe (M = 0.24) conditions at the delay period (t(14) = 2.96, P = 0.01).
DISCUSSION
In summary, we have demonstrated significant modulation of working memory processes through threats of potential monetary losses. Behavioral performance was influenced by loss‐threatening incentives in the form of faster and more accurate performance. This performance variation was most strongly demonstrated in the remember scenes condition. Loss‐threatening incentives also influenced magnitude of activity and functional connectivity of task‐related brain regions across different phases of the working memory task. Specifically, enhanced activity in working memory‐related regions was robust throughout all stages of processing and occurs in the direction of amplifying attention toward items to be recalled, rather than suppressing the activity toward items to be ignored. Moreover, brain regions involved in incentive cueing, including the striatum and amygdala, showed greater activation during loss trials. These findings complement prior studies of enhancement through monetary gain potential [Krawczyk et al.,2007; Pochon et al., 2003; Small et al., 2006; Taylor et al.,2004], as well as nonhuman primate electrophysiology studies that have shown neuronal firing enhancement when motivation is present [Kobayashi, 2003; Leon and Shadlen, 2001]. Finally, we report evidence of greater functional connectivity for loss trials over safe trials between the left PFC and left insula at the delay period suggesting that the insula may modulate PFC‐driven working memory processes.
Incentives and Behavioral Performance
Both accuracy and speed of processing during the working memory task was enhanced when incentives were present. Compared with a prior study with the same working memory task but using only incentive gains, we observed higher overall performance in accuracy and RT in this study [Krawczyk et al.,2007]. Also, there were fewer overall differences between incentive and nonincentive conditions. These different outcomes between studies may be due to the greater psychological impact that monetary losses have relative to gains [Dreher,2007]. These results suggest that task difficulty leads to differential effects due to loss or gain incentives, as indicated by a task‐switching study reported by Locke and Braver [2008] who reported fastest performance for rewards and slowest for trials with threatened penalties. Brain activation results also varied between our reward study and the current penalty study in that robust suppression effects were observed with rewards, but not with threatened penalties. The higher salience of potential losses may have led to higher overall attention levels throughout all trial types within this task, in which case suppression of items to be ignored may have been more difficult. Further studies directly comparing monetary gains and losses in working memory studies will be needed to more directly compare differences between incentive types.
High accuracy and fast responses in both penalty and nonpenalty trials is consistent with increases in vigilance and heightened attention that has an influence across the task. Similar results were reported in a recent fMRI study of primary reinforcers [Savine et al.,2010] that compared incentivized working memory performance using a delayed item‐recognition task. In that study aversive and pleasant liquid incentives that were delivered after each trial led to equivalent performance enhancement through faster responses.
Effect of Incentives on Component Processes of Working Memory
Similar to prior findings [Gazzaley et al.,2005a, b; Krawczyk et al.,2007] we demonstrated that category‐specific visual regions exhibit greater activation during memory encoding of relevant stimuli, as compared with a passive viewing condition, consistent with top‐down modulation since the bottom‐up input was identical across conditions. These top‐down effects were amplified during loss as compared to safe trials. Unlike our prior study with incentives [Krawczyk et al.,2007], these effects extended into the delay and response phases of the task. The PFC also showed greater activation when losses were possible throughout all phases of the task but most robustly at the delay period. Finally, although our functional connectivity analyses revealed interactions between PFC and visual association cortex during working memory processes, there were no differences found between the loss and safe trials between visual areas. The lack of strong connectivity modulation between these areas may be explained by the fact that performance was relatively fast and accurate on all trials and both conditions resulted in the hypothesized attention modulations in prefrontal and ventral visual regions. Another possibility is that we simply did not have the sensitivity in this experiment, through trial numbers or other aspects of the design in order to observe potential differences that occur between the working memory areas when losses were present over safe trials. The observed differences in magnitude of activation are consistent with cortical areas increasing in sensitivity leading to overall amplification of attention when incentives are present. All of the top‐down effects were most robust in scene‐selective visual association cortex, consistent with prior studies [Gazzaley et al.,2005b; Krawczyk et al.,2007; Rissman et al.,2008;2009). This may be due to the stimulus qualities of faces. Human faces are highly salient, difficult to ignore and possess a high degree of spatial and featural similarity. These aspects of face processing may have made the task more difficult when faces needed to be ignored potentially leading to higher levels of stimulus specific activation regardless of the presence of incentives. There are potentially many reasons that faces may operate differently as memory stimuli. Another relevant point is that the scene memory conditions were evaluated using the scene‐selective visual cortex, while the face memory condition used the more posterior and superior face sensitive areas within fusiform cortex.
In contrast to prior studies [Gazzaley et al.,2005a, b; Krawczyk et al.,2007], we did not observe robust suppression in stimulus‐selective ROIs when the nonpreferred stimulus was attended to. This difference may be due to the fact that we had included lower exposure times for each item and longer ISIs than were originally presented in the study by Gazzaley et al. Another possibility is that threatened monetary losses, relative to potential gains, may lead to greater overall activation of visual regions which could limit their ability to be further suppressed in incentive conditions. It is important to note that this difference may also be attributable to the incentive types, with losses affecting the task differently than gains.
These findings extend our understanding of the effects of incentives on cognitive performance. Initial fMRI studies of the effects of incentives established activation enhancements of the midbrain dopaminergic regions [Breiter et al., 2000; Elliott et al.,1999, 2000]. While prior studies of working memory and motivation focused on reward‐related effects [Beck et al.,2010; Krawczyk et al.,2007; Pochon et al,2002; Savine et al.,2010; Taylor et al; 2003], we demonstrate influences of monetary penalties on selective attention, encoding, and maintenance along with modulation of subcortical areas associated with detection of incentives when they are cued prior to the trials. Loss‐related incentives influenced neural systems involved in working memory‐related processes in prefrontal and visual region depending on the task phase. While recent evidence from Rowe et al. [2008] reported that PFC and visual cortex connectivity increases with reward expectancy, we observed the most extensive modulations in univariate ROI responses. The face and scene‐sensitive areas showed modulation at the encoding, delay, and response phases. Meanwhile, the PFC showed incentive modulation at the encoding and delay periods primarily. This suggests that the PFC areas have a top‐down influence on attention toward relevant stimuli in order to encode the appropriate information, but less direct influence at the time of response. The enhancement of attention in the visual association areas extended to the response period as well, indicating that the working memory‐related enhancement is sustained until the response period with the relevant region (scene‐related or face‐related) maximally engaged at the appropriate time to screen the response item. Furthermore, the scene‐selective areas proved to show greater incentive‐related enhancement relative to the face‐selective areas suggesting a functional difference between these ROIs and emphasizing that the type of stimuli affect the task performance and neural activation.
Processing Motivational Cues
Participants were cued about the potential consequences of their performance prior to each trial and we predicted that this would engage areas associated with processing anticipation of financial loss [Breiter et al., 2002; Elliott et al.,2000; Knutson et al.,2001, 2003]. The amygdala and striatum showed significantly greater activation when money was at loss on the trial indicating that these regions play similar roles in processing potential value during a working memory task as with other tasks. Similar regions have been shown to be modulated by a diversity of rewards [Breiter et al., 2001; O'Doherty et al., 2001; Small et al., 2004] and penalties in the form of financial losses in prior studies with random gain or loss consequences that were not tied to a specific cognitive task [Breiter et al., 2000; Elliott et al.,1999, 2000; Ramnani and Miall, 2004]. These findings also complement results reported by Neuwenhuis et al. [2005] indicating that context influences responses to possible outcome possibilities. They reported several regions that showed greater activation when the lowest possible loss was cued, including the PFC and striatum. Our results suggests that the amygdala and striatum are involved in an initial transient signal of incentive value that influences regions involved in cognitive control, while the insula showed evidence of higher functional connectivity with task‐related PFC regions when losses were possible. These results expand upon the growing literature linking incentive processing regions in the limbic system to cognitive control regions including the PFC relevant to working memory function.
Acknowledgements
The authors thank Ben Bendig, Raman Muhar, and Rahul Modi for their assistance. They also thank Suzanne Baker, Charlotte Boettiger, Christian Fiebach, and members of the Despolab and NCBIP at the UCSF Gallo Center for their helpful comments and suggestions.
REFERENCES
- Amemori K, Sawaguchi T ( 2006): Contrasting effects of reward expectation on sensory and motor memories in primate prefrontal neurons. Cereb Cortex 7: 1002–1015. [DOI] [PubMed] [Google Scholar]
- Beck, SM , Locke HS, Savine AC, Jimura K, Braver TS ( 2010): Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PLoS One 5: 9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC ( 2007) Sensitivity of the brain to loss aversion during risky gambles. Trends Cogn Sci 11: 270–272. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rees G, Dolan RJ ( 1999): Ventromedial prefrontal cortex mediates guessing. Neuropsychologia 37: 403–11. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ ( 2000): Dissociable neural responses in human reward systems. J Neurosci 20: 6159–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA ( 1921): On the ‘probable error’ of a coefficient of correlation deduced from a small sample. Metron 1: 3–32. [Google Scholar]
- Fuster JM, Bauer RH, Jervey JP ( 1985): Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res 330: 299–307. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney J, McEvoy K, Knight RT, D'Esposito M ( 2005a): Top‐down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci 17: 507–517. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M ( 2005b): Top‐down suppression deficit underlies working memory impairment in normal aging. Nature Neurosci 8: 1298–1300. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney JW, Rutman A, Seibert T, Clapp W, D'Esposito, M ( 2007): Age‐related deficits in component processes of working memory. Cereb Cortex 17: 125–135. [Google Scholar]
- Gilbert AM, Fiez JA ( 2004): Integrating rewards and cognition in the frontal cortex. Cogn Affect Behav Neurosci 4: 540–552. [DOI] [PubMed] [Google Scholar]
- Heitz RP, Schrock JC, Payne TW, Engle RW ( 2007): Effects of incentive on working memory capacity: Behavioral and pupillometric data. Psychophysiology 45: 119–129. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M ( 2000): Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex 10: 263–271. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD ( 2009a): Encoding of reward and space during a working memory task in the orbitofrontal cortex and anterior cingulate sulcus. J Neurophysiol 102: 3352–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD ( 2009b): Reward‐dependent modulation of working memory in lateral prefrontal cortex. J Neurosci 29: 3259–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D ( 2000): FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12: 20–27. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D ( 2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong, GW , Bennett, SM , Adams CS, Hommer D ( 2003): A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event‐related FMRI. NeuroImage 18: 263–272. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G ( 2005): Distributed neural representation of expected value. J Neurosci 25: 4806–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Lauwereyns J, Koizumi M, Sakagami M, Hikosaka O ( 2002): Influence of reward expectation on visuospatial processing in macaque lateral prefrontal cortex. J Neurophysiol 87: 1488–1498. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D'Esposito M ( 2007): Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res 1141: 168–177. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN ( 1999): Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron 24: 415–425. [DOI] [PubMed] [Google Scholar]
- Locke HS, Braver TS ( 2008): Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cogn Affective Behav Neurosci 8: 99–112. [DOI] [PubMed] [Google Scholar]
- Miller BT, D'Esposito M ( 2005): Searching for “the top” in top‐down control. Neuron 48: 535–538. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Hayashi T ( 2000): Neural representation of visual objects: encoding and top‐down activation. Curr Opin Neurobiol 10: 187–194. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Mars RB, Holroyd CB, Yeung N ( 2005): Activity in human reward‐sensitive brain areas is strongly context dependent. Neuroimage 25: 1302–1309. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD ( 2006): Dopamine‐dependent prediction errors underpin reward‐seeking behaviour in humans. Nature 442: 1042–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihon D, Dubois B ( 2002): The neural system that bridges reward and cognition in humans: An fMRI study. Proc Natl Acad Sci USA 99: 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D'Esposito M ( 2000): Using event‐related fMRI to assess delay‐period activity during performance of spatial and nonspatial working memory tasks. Brain Res Brain Res Protoc 5: 57–66. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Miall RC ( 2003): Instructed delay activity in the human prefrontal cortex is modulated by monetary reward expectation. Cereb Cortex 13: 318–327. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Degutis J, D'Esposito M ( 2004): Category‐specific modulation of inferior temporal activity during working memory encoding and maintenance. Cogn Brain Res 20: 37–45. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M ( 2004): Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23: 752–763. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M ( 2008): Dynamic adjustments in frontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cereb Cortex 18: 1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M ( 2009): The effect of non‐visual working memory load on top‐down modulation of visual processing. Neuropsychologia 47: 1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Eckstein D, Braver T, Owen AM ( 2008): How does reward expectation influence cognition in the human brain? J Cogn Neurosci 20: 1980–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, Beck SM, Edwards BG, Chiew KS, Braver TS ( 2010): Enhancement of cognitive control by approach and avoidance motivational states. Cognition Emotion 24: 338–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulaum MM ( 2005): Monetary incentives enhance processing in brain regions mediating top‐down control of attention. Cereb Cortex 15: 1855–1865. [DOI] [PubMed] [Google Scholar]
- Spiridon M, Fischl B, Kanwisher N ( 2006): Location and spatial profile of category‐specific regions in human extrastriate cortex. Hum Brain Mapp 27: 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Welsch RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ ( 2004): A tional neuroimaging study of motivation and executive function. Neuroimage 21: 1045–1054. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Sawaguchi T ( 2004): Properties of delay‐period neuronal activity in the primate prefrontal cortex during memory‐ and sensory‐guided saccade tasks. Eur J Neurosci 19: 447–457. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D ( 1981): The framing of decisions and the psychology of choice. Science 211: 453–458. [DOI] [PubMed] [Google Scholar]
- Watanabe M ( 2008): Motivational control of learning in the prefrontal cortex. Brain Nerve 60: 815–824. [PubMed] [Google Scholar]
- Watanabe M, Cromwell HC, Tremblay L, Hollerman JR, Hikosaka K, Schultz W ( 2002): Behavioral reactions reflecting differential reward expectations in monkeys. Exp Brain Res 140: 511–518. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hikosaka K, Sakagami M, Shirakawa S ( 2002): Coding and monitoring of motivational context in the primate prefrontal cortex. J Neurosci 22: 2391–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler EZ, Fellows LK ( 2008): The human ventromedial frontal lobe is critical for learning from negative feedback. Brain 131: 1323–1331. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Curtis CE, D'Esposito M ( 2006): Differential effects of distraction during working memory on delay‐period activity in the prefrontal cortex and the visual association cortex. Neuroimage 29: 1117–1126. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D'Esposito M ( 1997): A trial‐based experimental design for fMRI. Neuroimage 6: 122–138. [DOI] [PubMed] [Google Scholar]


