Abstract
Purpose
To investigate the effect of treatment of multiple myeloma (MM)-associated spinal fracture with percutaneous vertebroplasty (PVP) and chemotherapy.
Methods
Patients with MM-associated spinal fracture were randomly divided into combined (PVP and chemotherapy) treatment group (n = 38) and single chemotherapy group (n = 38). For the combined treatment group, bone cement was injected into vertebral body via DSA guided-percutaneous puncture. M2 scheme was used for both groups. And a 5-year follow-up was conducted for the study.
Results
At the 1-year follow-up visits, PVP combined with chemotherapy achieved complete remission (CR) in six patients (15.8%); near complete remission (nCR) in ten patients (26.30%); partial remission (PR) in nine patients (23.7%); minimal response (MR) in three patients (7.9%); no change (NC) in four patients (10.5%), and disease progression (DP) in five patients (13.2%). Only chemotherapy alone resulted in 3 CR (7.9%); 8 nCR (26.30%); 19 PR (77.5%); 4 MR (17.5%); 4 NC (17.5%), and 2 DP (5.0%). While the overall response rate (ORR) in the combined treatment group (65.8%) and the single chemotherapy group (50.0%) were significantly different, their visual analog pain scales (3.01 ± 0.62 and 5.97 ± 0.40, respectively) and Karnofsky performance scores (89.4 ± 6.3 and 80.3 ± 7.2, respectively) were significantly improved after treatment (P = 0.032 and P = 0.002, respectively). And the ORR between the two groups were significantly different (P = 0.001).
Conclusion
Percutaneous vertebroplasty is a minimally invasive surgery for MM-associated pathologic fracture. PVP had the characteristics of minimal trauma, easy operation and less complication. PVP can achieve long-term analgesic effect, and enhance the spinal stability.
Keywords: Percutaneous puncture, Spine, Multiple myeloma, Fracture, Bone cement
Introduction
Multiple myeloma (MM) is a blood disease characterized by malignant proliferation of monoclonal immunoglobulin-producing abnormal plasma cells. The disease caused osteolytic lesions and (or) diffuse osteoporosis. Approximately 1/3–2/3 MM patients have bone damages at the time of diagnosis [1]. Chemotherapy was the main treatment for MM. Myeloma cells and the M proteins secreted by myeloma cells can infiltrate and damage the vertebral bodies and attachments. Spine is an important part bearing the gravity. Thus, pathological compression fracture of vertebral bodies can easily occur, which results in decreased spinal stability, back pain, neurological dysfunction, depression, irritability and a series of psychiatric symptoms. Consequently, the life quality of the patients was severely affected. Currently, there are many approaches for the treatment of MM-associated spinal fracture, including chemotherapy, radiotherapy, isotope therapy, bisphosphonate therapy, pain treatment and palliative surgical treatment. The main shortcoming of conservative treatment is that it cannot solve the spinal instability caused by tumor damages. More importantly, conservative treatment may increase the risk of spinal collapse and nerve compression [2]. Surgery is suitable for patients with spinal cord compression, but it has large areas of trauma, high incidence of complications and cannot be applied to patients with fractures in multiple vertebral bodies.
With the rapid development of minimally invasive spinal surgical intervention techniques, percutaneous vertebroplasty (PVP) has attracted attentions of more and more spinal surgeons [3–8]. PVP is a recently developed minimally invasive surgical technique for the treatment of spinal osteolytic damage and pathological compression fractures [3, 4]. PVP relieves pain and prevents vertebral body collapse by increasing the spinal stability. From February 2003 to July 2005, our hospital adopted PVP combined with chemotherapy and bisphosphonate treatment for 38 MM patients with pathological compression fractures in vertebral bodies, and satisfactory efficacy was achieved. We also compared the efficacy of combined treatment (PVP and chemotherapy) with that of conservative treatment (chemotherapy only).
Materials and methods
Clinical information
A total of 76 patients with MM-associated spinal fracture (confirmed by bone marrow biopsy or pathological examination) were randomly divided into PVP and chemotherapy combined treatment group (n = 38) and single chemotherapy group (n = 38). The random principle aimed to make all index to be almost equal between two groups, including gender, age, fracture of spinal segments and other physical conditions, each patient had the same probability of being assigned to any particular treatment. All the patients complied to the following conditions: (1) the patients did not have severe heart, lung or brain diseases and can lie on stomach for a continuous of 1–2 h; (2) thoracic and lumbar fractures were confirmed by preoperative physical examination combined with imaging and there were no symptoms associated with spinal cord or nerve root damages; (3) pain continuously existed and cannot be improved by drug or physical therapy. The two treatment groups were identical in terms of all baseline data before treatment, including age, visual analog pain scale (VAS), KPS, numbers of spinal lesions, and the height of diseased vertebral bodies (P > 0.05 for all values), indicating a good randomization. The patients in both groups received M2 plan and α-INF chemotherapy for 6 weeks. Regular dosage and methods were used for treatment. During the intermittent period between chemotherapies, the patients were provided with bisphosphonate treatment. All the patients had been screened by CT and X-ray pre-operatively, only ten cases were performed with both MRI and CT. The images of post-operation were also screened by CT. Although MRI has advantage for analyzing spinal cord compression, CT has the same effect on analyzing vertebral body collapse, osteolytic damage, the integrity of the posterior wall of vertebral body and bone cement filling condition as MRI, which was the main index we observed in this study. According to our clinical experience, CT and X-ray can provide a reliable judgment for preoperative examination; CT also can reduce the medical expense for patients (Table 1).
Table 1.
Clinical information of 76 patients with MM-associated spinal fractures
| Parameters | Combine-treated group | Single chemotherapy-treated group | p value |
|---|---|---|---|
| Age | 58.91 ± 4.32 | 59.63 ± 6.20 | 0.352 |
| Gender | |||
| Male | 20 | 19 | |
| Female | 18 | 19 | 0.693 |
| Typing | |||
| IgD type | 2 | 3 | |
| IgA type | 12 | 11 | |
| IgG type | 20 | 19 | |
| Untypable | 4 | 5 | 0.892 |
| Spinal segment | |||
| 1 segment | 3 | 4 | |
| 2 segments | 15 | 13 | |
| ≥3 segments | 20 | 21 | 0.909 |
| Height of vertebral body (mm) | |||
| Anterior | 15.71 ± 0.70 | 16.03 ± 0.63 | |
| Center line | 13.65 ± 0.59 | 14.20 ± 0.67 | |
| Posterior | 23.67 ± 0.92 | 24.02 ± 0.89 | 0.936 |
| Average VAS | 8.95 ± 1.03 | 8.83 ± 0.96 | |
| Average KPS | 64.3 ± 6.7 | 67.4 ± 7.2 | 0.820 |
Equipment and drugs
Percutaneous vertebroplasty equipments including needle, syringe and pressure devices were provided by Shandong Guanlong Company. Needle diameters were 2.5 and 3.2 mm with a length of 100–150 mm. The needles were used for vertebral body puncture and injection of methyl methacrylate (Polymethylmethacrylate, PMMA). Rotary compression devices contained a 10-ml-syringe for the injection of PMMA. PMMA was produced by Tianjin Synthetic Materials Research Institute (Tianjin, China). We added 75% diatrizoate in order to increase the imaging of PMMA under X-ray. The ratio of powder (g)/liquid (ml)/contrast agent (ml) was 3:2:1.
Surgical methods
Preoperative X-ray, CT or MRI were conducted to determine the location and the number of vertebral bodies involved, the degree of vertebral body collapse, osteolytic damage, the integrity of the posterior wall of vertebral body, invasion degree of pedicle root, and the spinal cord compression. Routine examinations including cardiopulmonary function, blood sugar, PT, liver and kidney function tests and iodine allergy tests were also performed. Analgesic treatment was performed 15 min before surgery. PVP was completed under the guidance of DSA machine.
Patients were in prone position and pedicle pathway was adopted. The tilt angle of pedicle was determined. The spinous process distance of the puncture points and the depth from the puncture point skin to the pedicle were measured. The puncture point was located in 2–3 cm of the spinous process. Lidocaine (1%) was used for local anesthesia. In orthotopic perspective, when the needle reached the depth of cortical bone, the needle did not exceed the leading edge of the pedicle, the needle should be located within the shadow (bull’s-eye sign) of pedicle. When the needle penetrate the bone cortex into the vertebral bodies, surgical hammer was used under the surveillance of the lateral fluoroscopy to slowly hammer the puncture needle to the anterior 1/3 site. At this point, the tip point of the puncture needle has crossed over the inner edge of the pedicle. The puncture needle with bevel point surface was recommended. The direction of the needle can be adjusted as necessary. When puncture is completed, the needle core was removed and 5 ml of contrast agent was injected. DSA records dispersion situation of the contrast agent in the vertebral bodies and venous flow. The residual contrast agent and blood in the vertebral body were absorbed with negative pressure in order to reduce the pressure in the vertebral body.
Bone cement (PMMA added with non-ionic contrast agent) is pumped into the syringe after mixing. The whole process of injection was monitored by lateral fluoroscopy (Fig. 1). Bone cement leakage into the outside of the vertebral body was strictly prevented. During the injection, the needle was rotated to achieve a good filling of the cement. At the end of the injection, the puncture needle was retracted to the cortical bone, and the needle core was inserted. The puncture needle was rotated to prevent the sticking of the needle by the cement. The needle was taken out before solidification of the cement. After 15–20 min of injection until the polymerization of the cement, CT scanning was conducted. The volume of the injected cement ranged from 3 to 9.5 ml (average 4.5 ml for thoracic vertebral body, and 6.2 ml for lumbar vertebral body). PVP was performed in 1 segment for 3 cases, 2 segments for 15 cases and 3 segments for 20 cases (Fig. 2). A total of 62 thoracic vertebral bodies and 51 lumbar vertebral bodies were included in this study. Single side puncture was performed for 90 vertebral bodies, and both sides puncture was performed for 23 vertebral bodies.
Fig. 1.
An image on lateral fluoroscopy position showed that the needle tip was in 1/3 of the front edge of the vertebral body
Fig. 2.
DSA images for compression fractures of vertebral bodies (Segments T8, 10 and 12) in patients with multiple melanoma during surgery (a) and after surgery (b)
Evaluation of the therapeutic efficacy
If the bone cement was safely injected into the spinal target, and after 1 day, no serious complication was found, the PVP surgery was defined success. European Blood and Marrow Transplantation (EBMT) standard was used to evaluate the therapeutic efficacy [9]. Complete remission (CR), near complete remission (nCR), partial remission (PR), minor response (MR), no change (NC) and disease progression (DP) were used in the evaluation. Overall response rate (ORR) was calculated by using the following formula: ORR = CR + nCR + PR.
Statistical analysis
The student’s t test was used for quantitative analysis, and the Chi-square test was used for qualitative analysis. Kaplan–Meier analysis was used for survival comparisons.
Results
Surgery-related complications
Percutaneous vertebroplasty was performed in 38 patients with MM-associated spinal fracture. The number of vertebral bodies ranged from 1 to 9 in each case. Blood pressure, oxygen saturation and pressure were decreased, and thromboxane level was increased in three patients. After oxygen uptake and intravenous injection of dexamethasone, all the parameters were recovered to normal level. Infections did not occur in the injection and puncture sites of vertebral body. Postoperative CT examination showed that bone cement in the vertebral bodies was distributed in a point- and sheet-manner. Leakage of the bone cement to the anterior or lateral side of the vertebral body occurred in 20 patients. However, spinal cord or nerve root compression symptoms did not occur. The average height of anterior vertebral body after surgery was 16.61 ± 0.67 mm, which was significantly higher than that before surgery (15.71 ± 0.70) (P = 0.002). The average height of center line of vertebral body after surgery was 14.52 ± 0.85 mm, which was significantly higher than that before surgery (13.65 ± 0.59 mm) (P = 0.001). The average height of posterior vertebral body after surgery was 23.70 ± 0.97 mm, which was not significantly different from that before surgery (23.67 ± 0.92 mm) (P = 0.120).
Follow-up at 1-year after treatment
All patients were followed up every 2 months after treatment. At 1-year follow-up, CR, nCR, PR, MR, NC and DP were observed in 18.4%, 26.3% (10/38), 23.7% (9/38), 7.9% (3/38), 10.5% (4/38) and 13.2% (4/38), respectively, of the patients treated with combined PVP and chemotherapy. The overall response rate (ORR) in the combined treatment group was 65.8%. In the single chemotherapy treatment group, CR, nCR, PR, MR, NC and DP were observed in 7.9 (3/38), 21.1% (8/38), 21.1% (8/38), 10.6% (4/38), 13.2% (5/38) and 26.3% (10/38), respectively, of the patients. The ORR in the single chemotherapy treatment group was 50%. The ORR in the combined treatment group was significantly higher than that in the chemotherapy only treatment group (P = 0.001). Pain score and VAS values of the combined treatment group and the single chemotherapy group were 3.01 ± 0.62 and 5.97 ± 0.40, respectively, both of which was significantly decreased compared to their respective baseline values(58.91 ± 4.32 for combine-treated group and 59.63 ± 6.20 for single treated group). The VAS values of the two groups at this time point were significantly different (P = 0.032). KPS of the combined treatment group and the single chemotherapy group was 89.4 ± 6.3 and 80.3 ± 7.2, respectively, and the value of both groups was increased compared with their base line values before operation. There was a significant improvement of the average KPS in the combined treatment group compared with that of the single chemotherapy treatment group (P = 0.002).
Follow-up at 3-year after treatment
All the survival patients returned for 3-year follow up. In the combined treatment group, the analgesic effect of the treatment and the functional recovery of the spine were sustained without recurrence of the spinal pain. There was no occurrence of paraplegia and the 3-year survival rate was 73.7% (28/38). In the single chemotherapy group, there were two cases of paraplegia and the 3-year survival rate was 52.6% (22/38). The 3-year survival rate between the two groups was significantly different. The VAS and KPS scores between the two groups were significantly different (P = 0.000 and P = 0.000, respectively) (Table 2).
Table 2.
Survival patient and tumor data after 3-year follow-up
| Parameters | Combine-treated group | Single chemotherapy-treated group | p value |
|---|---|---|---|
| Age | 59.46 ± 2.78 | 58.36 ± 4.27 | 0.539 |
| Gender | |||
| Male | 17 | 12 | |
| Female | 15 | 13 | 0.659 |
| Typing | |||
| IgD type | 1 | 1 | |
| IgA type | 11 | 10 | |
| IgG type | 19 | 17 | |
| Untypable | 2 | 2 | 0.736 |
| Spinal segment | |||
| 1 segment | 3 | 2 | |
| 2 segments | 14 | 10 | |
| ≥3 segments | 15 | 13 | 0.528 |
| Height of vertebral body (mm) | |||
| Anterior | 16.36 ± 0.54 | 15.93 ± 0.52 | |
| Center line | 14.28 ± 0.78 | 13.70 ± 0.42 | |
| Posterior | 23.40 ± 0.85 | 23.01 ± 0.76 | 0.824 |
| Average VAS | 3.01 ± 0.62 | 5.97 ± 0.40 | |
| Average KPS | 89.4 ± 6.3 | 80.3 ± 7.2 | 0.000 |
Follow-up at 5-year after treatment
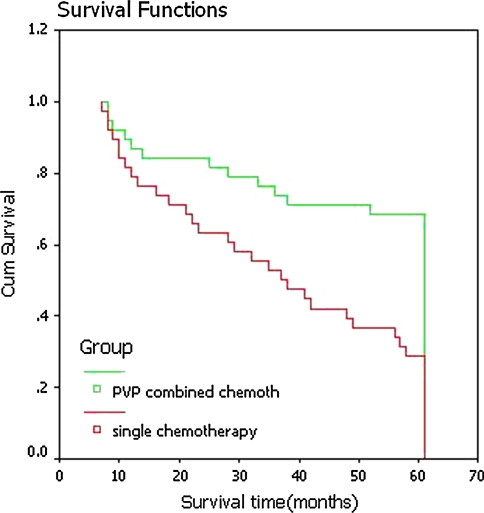
Figure 3 showed that patients in the combined treatment group had a better survival than those in the single chemotherapy group. In the combined treatment group, 5-year survival rate was 68.4% (26/38), which was significantly higher than that in the single chemotherapy group (42.1%, 16/38). One case had T7, 8, 11, 12, L1, 2, 3, 5 pathologic vertebral compression fractures (Fig. 4a, b). PVP was performed for three times within 2 weeks for this patient. During the operation, tissue of MM was collected for pathological examination. CT examination after operation showed that bone cement filled each vertebral body well (Fig. 4c). Two days after each PVP operation, the vertebral body pain was relieved at a certain level. Two days after the completion of all PVP operations, the patient was able to walk out of the bed by themselves with a VAS score of 1.5. M2 chemotherapy was performed for the patient after PVP operation for 6 weeks, and back pain was completely relieved. This patient has been followed up for 6.5 years, and the function of the spine was stable. The patient can walk freely without any difficulties. X-ray and CT scan showed no changes of vertebral displacement and no further vertebral compression (Fig. 4d).
Fig. 3.
Comparison of survival rate of patients with multiple myeloma using Kaplan–Meier survival analysis
Fig. 4.
The imaging examination of spinal pathologic fractures in patients with multiple myeloma. a Preoperative lateral X-ray film; b preoperative CT scan; c CT scan after cement injection showed that the cement filled the focus of vertebral body; d Multi-slice Spiral CT three dimensional reconstruction of T7, 8, 11, 12, L1, 2, 3, 5 in the follow-ups after years of PVP
Discussion
Traditional treatments of MM are more concentrated in MM themselves, but paying less attention to the bone diseases, which is a mistaken idea of treating MM. MM-associated bone disease also need active treatment. Utilization of bisphosphonates can inhibit protein isopentenylation and osteoclast activity, induce apoptosis, relieve bone pain, prevent pathologic fracture and reduce the incidence of hypercalcemia. However, for those patients with pathologic vertebral compression fractures, the vertebral body is severely damaged by tumors, and the risk of spinal cord compression caused by uneven pressure on the vertebral body is increased.
Chemotherapy and bisphosphonate therapy cannot stabilize the spine and effectively relieve the pain. Thus, surgical operation is normally adopted to treat vertebral compression fractures. However, surgical operation has a large area of trauma and high incidence of complications, and requires a long period of recovery, which inevitably affect the implementation of chemotherapy. In addition, open surgical operation is not suitable for non-adjacent multiple vertebral fractures [8].
We applied PVP to treat spinal tumors, which achieved positive effect in alleviating the pain and improving life qualities. Cortet et al. [3] applied PVP to treat MM and achieved a 68.5% of complete pain-relieving rate and a 30% of partial pain-relieving. We applied PVP for the treatment of pathological vertebral fractures caused by MM, and achieved positive effect in alleviating pain and improving life qualities. In combination with chemotherapy, a 100% efficiency rate was achieved. There are several proposed analgesic mechanisms involved in the treatment. Firstly, PMMA monomer is cytotoxic, resulting in tumor cell dehydration, solidify and finally apoptosis [10–12]. Secondly, stabilization of the small fractures prevents stimulation of the pain nerve endings caused by squeezing up and down and left–right frictions. Thirdly, polymerization of bone cement can release heat up to 72–78°C, which is sufficient to induce necrosis in tumor tissues and vertebral pain nerve endings [13–16]. Fourthly, solidification of bone cement can cut off blood supply to induce tumor necrosis. Lastly, hardened cement increased vertebral bone support force, and maintained spinal function [17–21]. In this study, the anterior, central line and posterior heights of vertebral body after treatment were significantly different from those before treatment. Follow-up examination did not identify any displacement of treated vertebral bodies.
The original changes of physiological curvature, angular and slipping did not progress further. Further compression of vertebral bodies, spinal cord and nerve endings did not occur. There was no reoccurrence of treated vertebral bodies. These results suggest that after PVP the spine is well stabilized, and the tumors in the vertebral bodies are evenly filled with bone cement. The cement solidifies the lesion areas, provides structural replacement and prevents further damage and collapse of the spine and compression of spinal cord [20]. In this study, we found that leakage of PMMA to the anterior or lateral side of the vertebral bodies occurred in 21 patients, but there were no symptoms and other complications, indicating that PVP is a relatively safe minimally invasive operation.
In this study, the ORR in the combined treatment group was 65.8%, which was significantly higher than that in the single chemotherapy treatment group. After treatment, VAS score was significantly decreased and KPS score was significantly increased in both groups. However, degree of the VAS and KPS score change in combined treatment group was larger than that in the single chemotherapy treatment group. In addition, combined treatment can reduce the occurrence of paraplegia. The most prominent feature of this technology (combination of PVP and chemotherapy) is the treatment of intractable pain caused by pathological vertebral fractures, increase in the spine stability and improvement in the life quality. Digital subtraction angiography (DSA) machine can reflect the leakage of contrast medium into the vertebral tube and the spinal vertebral venous flow, which increases the safety of the operation [22–25]. Repetitive aspiration of substances from the vertebral bodies can effectively reduce the pressure within the vertebral bodies, and achieve a good filling of the bone cement. Applications of needle tip with bevel surface can well control the direction of the needle tip. Continuous adjustment of the needle direction makes the filling of bone cement more efficient, and reduces the incidence of cement leakage [26]. At last, the disadvantages of PVP operation must be discussed, although the bone cement can solidify the target segments, the solid target segments can cause adjacent segment fractures [27], and induce the new fractures; meanwhile, the most common risk is bone cement leakage, sometimes bone cement can leak into spinal canal, cause spinal cord or nerve root compression symptoms. In this present study, although leakage occurrence was found in 21 patients, it didn’t leak into spinal canal to harm the nerves, and avoided the serious complications. When PVP operation is performed, the whole process of injection must be monitored carefully to prevent the leakage occurrence as much as possible [4].
In summary, PVP can effectively alleviate the pain caused by the osteolytic damage of vertebral body, increase vertebral body strength and improve the spinal stability. Operation of PVP is simple and safe without significant damages and systemic side effects. PVP is suitable for patients with multiple vertebral fractures. Therefore, PVP is an effective minimally invasive treatment for MM-associated vertebral fractures. In combination with chemotherapy and other comprehensive treatment, long-term effect is more satisfactory.
Conflict of interest
None.
Footnotes
The authors ZuozhangYang, Jing Tan, Yongqing Xu and Hongpu Sun contributed equally to this work and each is considered as first author.
References
- 1.Fonseca R, Trendle MC, Leong T, et al. Prognostic value of serum markers of bone metabolism in untreated multiple myeloma patients. Br J Haematol. 2000;109(1):24–29. doi: 10.1046/j.1365-2141.2000.01960.x. [DOI] [PubMed] [Google Scholar]
- 2.Garmatis CJ, Chu F. The effectiveness of radiation therapy in the treatment of bone metastases from breast cancer. Radiology. 1978;126(1):235–237. doi: 10.1148/126.1.235. [DOI] [PubMed] [Google Scholar]
- 3.Cortet B, Cotton A, Boutry N, et al. Percutaneous vertebroplasty in patients with osteolytic metastases or multiple myeloma. Rev Rhum Engl Ed. 1997;64(3):177–183. [PubMed] [Google Scholar]
- 4.Cotton A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myleoma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200(2):525–530. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- 5.Galibert P, Deramond H, Rosat P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33(2):166–168. [PubMed] [Google Scholar]
- 6.Hoffmann RT, Jakobs TF, Wallnofer A, et al. Percutaneous vertebroplasty (pv): indications, contraindications, and technique. Radiologe. 2003;43(9):709–717. doi: 10.1007/s00117-003-0947-y. [DOI] [PubMed] [Google Scholar]
- 7.Truumees E, Hilibrand A, Vaccaro AR. Percutaneous vertebral augmentation. Spine J. 2004;4(2):218–229. doi: 10.1016/j.spinee.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 8.Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(1 Suppl):21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 9.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation Myeloma Subcommitte for the EBMT. Br J Haematol. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 10.Gough JE, Downes S. Osteoblast cell death on methacrylate polymers involves apoptosis. J Biomed Mater Res. 2001;57(4):497–505. doi: 10.1002/1097-4636(20011215)57:4<497::AID-JBM1195>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Gangi A, Buy X. Percutaneous bone tumor management semin intervent. Radiology. 2010;27(2):124–136. doi: 10.1055/s-0030-1253511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urrutia J, Bono CM, Mery P, et al. Early histologic changes following polymethylmethacrylate injection (vertebroplasty) in rabbit lumbar vertebrae. Spine (Phila Pa 1976) 2008;33(8):877–882. doi: 10.1097/BRS.0b013e31816b46a5. [DOI] [PubMed] [Google Scholar]
- 13.Ruíz DSM, Burkhardt K, Jean B, et al. Pathology findings with acrylic implants. Bone. 1999;25(Suppl 2):85–90. doi: 10.1016/S8756-3282(99)00140-4. [DOI] [PubMed] [Google Scholar]
- 14.Jefferiss CD, Lee AJC, Ling RS. Thermal aspects of self-curing polymethylmethacrylate. J Bone Joint Surg Br. 1975;57:511–518. [PubMed] [Google Scholar]
- 15.Deramond H, Wright NT, Belkoff SM. Temperature elevation caused by bone cement polymerization during vertebroplasty. Bone. 1999;25(2 Suppl):17S–21S. doi: 10.1016/S8756-3282(99)00127-1. [DOI] [PubMed] [Google Scholar]
- 16.Vrind HH, Wondergem J, Haveman J. Hyperthermia-induced damage to rat sciatic nerve assessed in vivo with functional methodsand with electrophysiology. J Neurosci Methods. 1992;45:65–74. doi: 10.1016/0165-0270(92)90073-m. [DOI] [PubMed] [Google Scholar]
- 17.Sun G, Cong Y, Xie Z, et al. Percutaneous vertebroplasty using instruments and drugs made in China for vertebral metastases. Chin Med J (Engl) 2003;116(8):1207–1212. [PubMed] [Google Scholar]
- 18.Weill A, Chiras J, Simon JM, et al. Spinal metastases: indicaion for and results of percutaneous injection of acylic surgical cement. Radiology. 1999;199(1):241–247. doi: 10.1148/radiology.199.1.8633152. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Xu J, Sang C. Clinical studies on treatment of patients with malignant spinal tumors by percutaneous vertebroplasty under guidance of digital subtraction angiography. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(10):999–1003. [PubMed] [Google Scholar]
- 20.Yang ZZ, Xu JB, Yuan T, et al. Treating metastatic vertebral tumor with percutaneous vertebroplasty:a report of 28 cases. Chin J Cancer. 2005;24(2):194–198. [PubMed] [Google Scholar]
- 21.Andreula C, Muto M, Leonardi M. Interventional spinal procedures. Eur J Radiol. 2004;50(2):112–119. doi: 10.1016/j.ejrad.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Mathis JM, Barr JD, Belkoff SM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. Am J Neuroradiol. 2001;22(2):373–381. [PMC free article] [PubMed] [Google Scholar]
- 23.Mathis JM, Ortiz AO, Zoarski GH. Vertebroplasty versus kyphoplasty: a comparison and contrast. AJNR Am J Neuroradiol. 2004;25(5):840–845. [PMC free article] [PubMed] [Google Scholar]
- 24.Layton KF, Thielen KR, Koch CA, et al. Vertebroplasty, first 1,000 levels of a single center: evaluation of the outcomes and complications. AJNR Am J Neuroradiol. 2007;28(4):683–689. [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald RJ, Trout AT, Gray LA, et al. Vertebroplasty in multiple myeloma: outcomes in a large patient series. AJNR Am J Neuroradiol. 2008;29(4):642–648. doi: 10.3174/ajnr.A0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Yang D, Xie L, et al. Treatment of metastatic spinal tumors by percutaneous vertebroplasty versus percutaneous vertebroplasty combined with interstitial implantation of 125I seeds. Acta Radiol. 2009;50(10):1141–1148. doi: 10.3109/02841850903229133. [DOI] [PubMed] [Google Scholar]
- 27.Astolfi S, Scaramuzzo L, Logroscino CA. A minimally invasive surgical treatment possibility of osteolytic vertebral collapse in multiple myeloma. Eur Spine J. 2009;18(Suppl 1):115–121. doi: 10.1007/s00586-009-0977-x. [DOI] [PMC free article] [PubMed] [Google Scholar]