Abstract
Introduction
Percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP) are effective procedures to alleviate pain caused by osteoporotic vertebral compression fractures (VCFs). New vertebral compression fracture (NVCF) has been noted as a potential late sequela of the procedures. The incidence of NVCFs and affecting risk factors were investigated.
Materials and methods
The authors retrospectively analyzed the occurrence of NVCFs in 147 patients treated with PVP or PKP for osteoporotic VCFs. Possible risk factors, such as age, gender, body mass index, bone mineral density (BMD), location of treated vertebra, treatment modality, amount of bone cement injected, anterior–posterior ratio of the fractured vertebra, cement leakage into the disc space, and pattern of cement distribution, were assessed.
Results
Twenty-seven patients (18.4%) had subsequent symptomatic NVCFs with a median time to new fracture was of 70 days. The 1-year symptomatic fracture-free rate was 85.0% by the Kaplan–Meier estimate. Eighteen (66.7%) of the 27 patients had an NVCF on the adjacent vertebra. Significant differences (P < 0.05) were found between the NVCF and control groups in regard to age, treatment modality, BMD, and the proportion of cement leakage into the disc space. Discal cement leakage and low BMD affected on adjacent NVCFs.
Conclusion
The most important risk factors affecting NVCFs were osteoporosis and intervertebral discal cement leakage.
Keywords: Osteoporotic compression fracture, Vertebroplasty, Kyphoplasty, BMD, Discal leakage
Introduction
The minimally invasive vertebral augmentation techniques of percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP) are safe and efficient procedures for controlling pain caused by osteoporotic vertebral compression fractures (VCFs) [3, 19]. Since Galiebert first described the procedure of bone cement injection in 1987, PVP and PKP are widely accepted treatments for VCFs of various causes [4, 5]. These procedures are associated with a decrease in the morbidity rates after VCFs; however, complications also have been reported [1, 16, 19].
A possible increase in the risk of new vertebral compression fractures (NVCFs) at non-treated vertebra following augmentation is of concern, especially in osteoporotic patients [13, 24]. There is still controversy about whether new vertebral body fractures are simply a result of the natural progression of osteoporosis or whether they should be regarded as a consequence of augmentation. Several studies have reported an increase in the incidence of NVCF after bone cement augmentation, compared with conservative treatment [6, 10, 25]. The proposed risk factors include the amount of injected bone cement, intradiscal leakage, compact and solid cement pattern, and greater kyphosis correction [7, 9, 11, 13–15, 20–23].
The purpose of this study was to quantify symptomatic NVCFs in patients who underwent PVP or PKP and to investigate factors that could contribute to de novo fractures.
Materials and methods
Patients
We retrospectively reviewed 162 patients with VCFs who were treated with PVP or PKP between March 2005 and February 2010. The patients had osteoporotic VCFs and were followed-up for at least 1 year. The exclusion criteria were loss to the follow-up in less than 1 year (six patients) and the presence of pathologic compression fractures (metastasis, multiple myeloma, etc.; nine patients). A total of 147 patients (197 vertebrae) met the above criteria and were enrolled in the study.
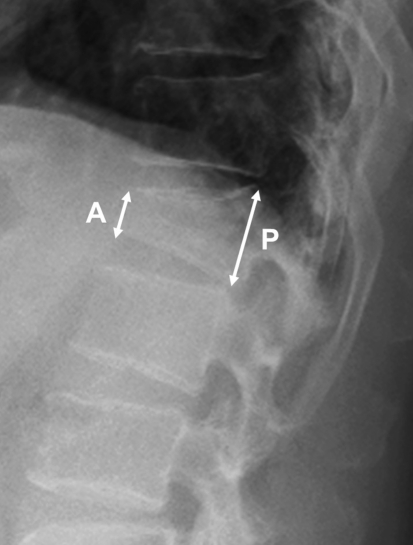
Percutaneous vertebroplasty or PKP was performed in patients who had relatively severe pain (VAS > 7) despite undergoing conservative management for 2 weeks. The procedures were performed as soon as possible without conservative management in patients aged over 80 years or with underlying diseases such as pneumonia, thrombotic phlebitis, and diabetes that is not controlled well, because prolonged bed rest could worsen their medical condition. PKP was performed when the compression fracture showed 40 to 80% wedge deformities by the anterior–posterior (AP) ratio (Beck Index, Fig. 1), when restoration of kyphosis was necessary, or when minimal involvement of fracture in the posterior wall was observed. Both PVP and PKP were considered in compression fractures with a Beck Index of 30–40%.
Fig. 1.
The anterior–posterior ratio of the fractured vertebra was calculated as the height of the anterior wall (A) divided by that of the posterior wall (P). The smaller AP ratio implies a larger degree of wedge deformity
Osteoporotic VCFs were diagnosed primarily on the basis of clinical features and simple AP and lateral radiographs of the vertebrae. Magnetic resonance image (MRI) was performed in all patients to confirm recent VCFs. Bone mineral density (BMD) was measured in all patients, and some patients underwent computed tomography scan or Tc-99 m methylene diphosphonate whole-body bone scan, if needed. Acute/subacute fractures were defined by the presence of marrow edema or an acute fracture line on the MRI (spin-echo T1-weighted, T2-weighted, and especially fat suppression T2-weighted images).
Vertebroplasty and kyphoplasty procedures
All PVPs and PKPs were performed by one surgeon (W.J.C.), with a routine bipedicular approach in most cases. With the administration of local anesthesia, two 11-G needles were advanced into the vertebral body under fluoroscopic guidance. A polymethylmethacrylate (PMMA) Spine-Fix® Biomimetic Bone Cement (TEKNIMED SA, France) was used as the bone filler, which was injected using 1 mL syringes during the PVP. In the PKPs, Kyphon® balloon tamps (Kyphon Inc., Sunnyvale, CA) were used. The PMMA injection was terminated when adequate filling of the vertebral body was achieved or if leakage occurred. If leakage occurred, the needle was repositioned, and additional PMMA was injected to fill the remaining part of the bone. In patients with multiple VCFs, all the vertebral segments showing marrow edema on MRI were treated. Patients were encouraged to ambulate 3 h after the procedure.
Follow-up and new symptomatic VCFs
Patients were followed-up regularly at 2 weeks, 1 month and every 3 months during the first year after the procedure, and then on a yearly basis. All patients were prescribed alendronate medication for at least 1 year. BMD was measured annually, and the medication was continued if the T-score <−3.0. In the case of new-onset back pain, physical examination and radiologic work-up were performed to confirm the presence of de novo fractures. Symptomatic NVCF was diagnosed in the case of acute back pain and tenderness with (1) a definite decrease in the height of the vertebral body on plain radiograph and (2) bone marrow edema on MRI at the corresponding anatomic level. When symptomatic NVCF was present, repeated PVPs or PKPs were performed if the pain persisted after 2 weeks of conservative care.
Asymptomatic incidental fractures were defined by a change in vertebral height on follow-up radiography, which is not accompanied by acute aggravated pain. This type of fractures was not considered in this study because of the ambiguity of the analysis.
Review of patient data
Possible risk factors that could affect symptomatic NVCFs were retrospectively reviewed: age, gender, body mass index (BMI), lumbar spine BMD, level and number of treated vertebrae, maximal amount of PMMA injected per vertebral body, leakage of cement to the adjacent disc, and preoperative AP body height ratio of the fractured vertebra. The AP ratio was calculated to assess the degree of wedge deformation of the fractured vertebral body (Fig. 1). When multiple lesions were present, the mean of the AP ratios of each fractured vertebra was calculated. According to the site of VCF occurrence, we categorized the cases into the “T–L junction” or “non-T–L junction” groups.
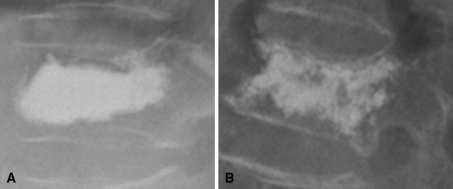
Cement distribution was classified as follows: (1) compact type—vertebrae with compact and solid filling, and (2) trabecular type—vertebrae with sponge-like filling [22] (Fig. 2). In patients with multiple augmentations, distribution patterns in both ends of the continuously treated vertebrae were considered. If treated vertebrae were separated or had a different distribution type at either end, with at least one compact pattern, they were classified as “compact type”.
Fig. 2.
Cement distribution was classified into two groups. A simple radiograph showing solid and compact cement filling in the treated vertebra was defined as compact type (a), while sponge-like filling pattern was defined as the trabecular type (b)
Patients who developed symptomatic NVCFs during the follow-up were grouped into the “NVCF” group. The others, who did not experience further symptomatic VCFs, were considered as the control group. The NVCF group was divided further into “adjacent” and “remote” VCF groups.
All statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) with the significance level set at level P = 0.05. Independent t test was used for comparison of continuous variables and cross-table analysis for nominal data. To evaluate the annual rate of NVCF, the Kaplan–Meier estimate was adopted, and multivariate analysis was performed using logistic regression analysis.
Results
One hundred forty-seven patients, accounting for a total of 197 vertebrae, managed with PVP or PKP were enrolled in this study. There were 45 male and 102 female patients. The mean age of patients was 70 (range 49–93) years and the mean follow-up duration was 35.5 (range 12–73) months. Six patients died of medical disease after 1 year of follow-up (mean survival 32 months).
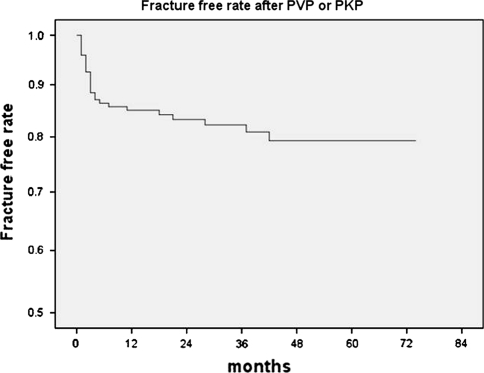
There was no major complication attributed directly to the PVP or PKP procedures. The mean number of initially treated VCFs was 1.34 (range 1–5). Twenty-seven patients (18.4%) were proved to have subsequent symptomatic NVCFs (Table 1). Five patients responded to conservative management and did not undergo further surgical treatments. The other 22 patients underwent secondary vertebroplasty for NVCFs. The median time to de novo fracture was 70 (range 3–1,275) days, and 74% of the NVCFs developed within 6 months of the procedures. The 1-year symptomatic fracture free rate was 85.0% according to the Kaplan–Meier estimate (Fig. 3). Eighteen (66.7%) of 27 patients had an NVCF on the adjacent vertebra. Asymptomatic decreases in vertebral height were observed in three patients during routine radiographic follow-up, which were all “remote”; however, further evaluations were not performed.
Table 1.
Summary of clinical features of 27 patients who developed new vertebral compression fracture(s) after percutaneous vertebroplasty or kyphoplasty
| No. | Age (years) | Gender | Initial treatmenta | Symptom-free interval (days)b | New vertebral fracture(s) | AP ratio | Discal cement leakage | Volume of PMMA | BMI (kg/m²) | BMD (T-score) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 83 | M | T12 | 3 | L1 | 0.48 | (−) | 8 | 23.6 | −3.1 |
| 2 | 84 | F | T12 | 27 | T11 | 0.67 | (+) | 8 | 21.8 | −3.8 |
| 3 | 58 | M | T7, 10, L1 | 845 | T4, 5, 6, L2 | 0.56 | (+) | 8/2/3 | 19.7 | −3.9 |
| 4 | 82 | M | T11 | 49 | L1 | 0.55 | (−) | 5 | 21.8 | −3.3 |
| 5 | 71 | M | L1, 2 | 19 | T11, 12 | 0.73 | (+) | 9/8 | 17.3 | −4.0 |
| 6 | 89 | F | T12 | 188 | L1, 2 | 0.63 | (−) | 6 | 21.6 | −4.3 |
| 7 | 79 | F | T11, L2 | 12 | T12, L1 | 0.84 | (+) | 7/8 | 22.4 | −4.1 |
| 8 | 53 | M | T11, L1 | 30 | T9, 10, 12, L2 | 0.65 | (−) | 6.5/6 | 20.9 | −4.2 |
| 9 | 70 | F | L5 | 47 | L2, 3, 4 | 1.04 | (−) | 10 | 22.9 | −3.7 |
| 10 | 81 | F | L1 | 317 | T12 | 0.68 | (−) | 9 | 19.1 | −5.2 |
| 11 | 70 | M | T12 | 5 | L1 | 0.64 | (+) | 6 | 19.1 | −3.6 |
| 12 | 74 | F | L3, 4, 5 | 47 | L2 | 0.72 | (+) | 6/4/5 | 22.2 | −5.6 |
| 13 | 60 | F | L2, 4 | 70 | L1 | 0.67 | (+) | 8/6 | 32.1 | −3.9 |
| 14 | 72 | F | L1, 3 | 77 | T12, L1 | 0.72 | (−) | 4/3 | 32.6 | −3.3 |
| 15 | 76 | F | L1 | 1,121 | T12 | 0.57 | (+) | 9 | 26.9 | −4.2 |
| 16 | 84 | F | L1 | 68 | T12 | 0.71 | (−) | 8 | 22.0 | −2.9 |
| 17 | 92 | F | T12, L1 | 147 | T6 | 0.76 | (−) | 4.5/6 | 19.2 | −5.4 |
| 18 | 65 | F | T11, 12 | 56 | L2, 4 | 0.68 | (+) | 5/2 | 24.9 | −4.3 |
| 19 | 65 | F | L2 | 76 | L1 | 0.81 | (−) | 11 | 20.6 | −4.7 |
| 20 | 72 | F | L1 | 111 | T8 | 0.67 | (−) | 7 | 19.1 | −3.9 |
| 21 | 80 | M | L1 | 70 | T12 | 0.69 | (−) | 8 | 20.6 | −3.8 |
| 22 | 70 | F | T7 | 1,265 | L1 | 0.63 | (−) | 5 | 18.2 | −3.3 |
| 23 | 82 | M | L2, 3 | 45 | L5 | 0.84 | (+) | 6 | 19.4 | −5.9 |
| 24 | 70 | F | T5 | 120 | T6, 8 | 0.69 | (+) | 5 | 28.9 | −4.1 |
| 25 | 84 | M | L3 | 71 | L2 | 0.78 | (+) | 6 | 19.2 | −3.5 |
| 26 | 73 | F | T12 | 520 | L2 | 0.65 | (–) | 7 | 32.39 | −4.3 |
| 27 | 75 | F | T12 | 635 | L2 | 0.65 | (−) | 6 | 18.25 | −4.2 |
PMMA polymethylmethacrylate, BMI body mass index, BMD bone mineral density
aVertebral level(s) initially treated with PVP or PKP
bTime from initial percutaneous vertebroplasty or PKP to development of new symptomatic fracture(s)
Fig. 3.
The Kaplan–Meier survival curve shows the estimated fracture-free rate of vertebrae in the vicinity of the cemented vertebra at 1 year after vertebroplasty or kyphoplasty to be 85.0%
Significant differences (P < 0.05) were found between the NVCF and control groups with regard to age, treatment modality (PKP, PVP, or PKP + PVP), BMD, and cement leakage into the disc space by univariate analysis. BMI was lower in the NVCF group compared with the controls but the difference was not statistically significant (P = 0.08). BMD was the only significant factor determined by multivariate analysis (P = 0.02; Table 2).
Table 2.
Summary of variables between the control and new vertebral compression fracture group following vertebroplasty or kyphoplasty for compression fractures
| Variable | Control group (120) | New fracture group (27) | P value | |
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Age (years) | 69.0 ± 12.5 | 74.6 ± 9.4 | 0.03** | 0.53 |
| Gender (M:F) | 36:84 | 9:18 | 0.73 | 0.16 |
| PKP:PVP:PKP + PVP | 52:59:9 | 5:19:3 | 0.04** | 0.12 |
| BMI (kg/m²) | 24.1 ± 3.0 | 22.5 ± 4.4 | 0.08 | 0.39 |
| BMD (T-score) | −3.54 ± 0.97 | −4.01 ± 0.79 | 0.01** | 0.01** |
| T–L junctiona | 72.5% | 85.2% | 0.22 | 0.14 |
| Multiple level treated | 22.5% | 37.0% | 0.11 | 0.95 |
| PMMA (cc) | 7.1 ± 1.8 | 7.3 ± 1.6 | 0.72 | 0.90 |
| Discal cement leakage | 25% | 44% | 0.04** | 0.49 |
| A-P ratio (mean) | 0.67 ± 0.12 | 0.69 ± 0.11 | 0.54 | 0.65 |
| Pattern of cementb | 60:60 | 13:14 | 0.86 | 0.63 |
PMMA polymethylmethacrylate, BMI body mass index, BMD bone mineral density, PVP percutaneous vertebroplasty, PKP percutaneous kyphoplasty
** P < 0.05
aT11-L2 vertebrae
bCompact: trabecular
To evaluate a direct and more precise effect of the procedures on untreated vertebrae, the NVCF group was subdivided into adjacent and remote fracture groups. The treatment modality was only significant difference between two subgroups (P = 0.02; Table 3).
Table 3.
Characteristics of patients in the adjacent new vertebral compression fracture (NVCF) group and remote NVCF group
| Variable | Adjacent NVCFs (18) | Remote NVCFs (9) | P value |
|---|---|---|---|
| Age (years) | 74.1 ± 10.1 | 75.7 ± 8.2 | 0.68 |
| Gender (M:F) | 7:11 | 2:7 | 0.67 |
| PKP:PVP:PKP + PVP | 5:10:3 | 0:9:0 | 0.02 |
| BMI (kg/m²) | 22.8 ± 4.4 | 21.8 ± 4.6 | 0.58 |
| BMD (T-score) | −4.0 ± 0.74 | −4.2 ± 0.89 | 0.47 |
| T–L junctiona | 88.9% | 77.8% | 0.58 |
| Multiple level treated | 38.9% | 33.3% | 0.13 |
| PMMA (cc) | 7.4 ± 1.7 | 7.0 ± 1.5 | 0.55 |
| Discal cement leakage | 55.6% | 22.2% | 0.01** |
| A-P ratio | 0.68 ± 0.08 | 0.71 ± 0.14 | 0.93 |
| Pattern of cementb | 10:8 | 3:6 | 0.28 |
| Symptom free interval (m) | 5.8 | 10.5 | 0.32 |
PMMA polymethylmethacrylate, BMI body mass index, BMD bone mineral density, PVP percutaneous vertebroplasty, PKP percutaneous kyphoplasty
** P < 0.05
aT11-L2 vertebrae
bCompact: trabecular
When comparing the control group and the adjacent NVCF group, discal cement leakage was the only significant factor that affected the adjacent NVCF in univariate analysis (P = 0.01). BMD was significantly lower in the adjacent NVCF group, compared with the control group, from the results of multivariate analysis (P = 0.02; Table 4).
Table 4.
Statistical analysis of risk factors between control and ‘adjacent’ new vertebral compression fracture group
| Variable | P value | Relative risk odds ratio 95% CI | |
|---|---|---|---|
| Univariate | Multivariate | ||
| Age (years) | 0.10 | 0.42 | |
| Gender (M:F) | 0.45 | 0.06 | |
| PKP:PVP | 0.30 | 0.74 | 1.29 (0.48–3.49) |
| BMI (kg/m²) | 0.13 | 0.92 | |
| BMD (T-score) | 0.08 | 0.02** | |
| TL junctiona | 0.16 | 0.08 | 3.03 (0.66–13.92) |
| Multiple level treated | 0.13 | 0.49 | 2.19 (0.78–6.20) |
| PMMA (cc) | 0.55 | 0.87 | |
| Discal cement leakage | 0.01** | 0.27 | 3.75 (1.36–10.37)** |
| A-P ratio (mean) | 0.93 | 0.78 | |
| Pattern of cementb | 0.66 | 0.97 | 0.80 (0.30–2.17) |
CI confidence interval, PMMA polymethylmethacrylate, BMI body mass index, BMD bone mineral density, PVP percutaneous vertebroplasty, PKP percutaneous kyphoplasty
** P < 0.05
aT11-L2 vertebrae
bCompact: trabecular
Discussion
Percutaneous vertebroplasty and PKP are safe and effective techniques for alleviating pain, and they allow patients to resume their normal daily life earlier. Several clinical studies have focused on the effects of PVP or PKP on the development of NVCFs. Naturally, the initial osteoporotic VCF itself is known to increase the risk of adjacent fractures by 2- to 12.6-fold during the initial year [8, 12, 21, 25]. Lindsay [12] reported the incidence of a NVCF in the subsequent year following an osteoporotic fracture to be 19.2%. Various studies have reported the 1-year NVCF rate after PVP or PKP to be 20.5% (Lin et al. [14]), 21.7% (Syed et al. [21], symptomatic), 7.8% (Lee et al. [11], symptomatic), 7.9% (Kim et al. [7]), and 15.5% (Moon et al. [15]). In our study, which is larger in case population, the NVCF-free rate of 85.0% over 1 year is comparable to these data; however, our study was confined to only symptomatic subsequent NVCFs.
In this study, 67% of the NVCFs involved the vertebra adjacent to the previously treated one(s), which is similar to other studies [10, 23]. Grados and Legroux-Gérot [6, 10] showed increased vertebral fracture in the vicinity of a cement-augmented vertebra. It is probable that PVP or PKP may increase the risk of adjacent compression fractures by imposing greater stress on the untreated levels. The increased stiffness of the augmented vertebra alters the biomechanics of load transfer to the adjacent vertebra by the “stress-riser” effect [2]. This is still only a theory, however, and it is not clear whether there truly is an increased risk associated with vertebroplasty. Our results did not show an increased refracture rate, compared with reports of the natural course, which suggest that NVCFs that develop after vertebroplastic intervention might be the result of the osteoporosis itself, and not due to the intervention, as previous reports have pointed [11, 21, 25].
As for the risk of subsequent fracture after vertebroplasty, various causative factors have been proposed [7, 11, 13–15, 20, 21, 24]. In our study, when comparing the control and NVCF groups, age, treatment modality, BMD, and discal leakage were found to be the significant factors.
Age, BMD, and BMI may all reflect the consistency of untreated vertebrae [17]. BMD tends to decrease with increasing age because of progressive bone resorption. BMI is positively associated with estrogen activity, and estrogen stimulates osteoblasts to increase bone mass through increased secretion of osteoid. Thus, a high BMI would have protective effects against bone loss [14, 18]. Although BMI was not statistically significant, BMD and age were found to be significant, and BMD was the only significant factor according to multivariate logistic regression. We postulate that the most important risk factor for additional fracture is the osteoporosis itself. All patients who underwent PVP or PKP have anti-osteoporotic medication after the procedure, which might affect the occurrences of NVCFs.
We observed more frequent NVCFs in patients who underwent PVP rather than PKP. As for the occurrence of NVCFs, our search of the literature found no report on the difference between PVP and PKP. Greater kyphosis correction and greater degree of height restoration were thought to be related to NVCF [7, 11, 14]. Restoration of the collapsed vertebral body height might aggravate tension, leading to increased loading on the other vertebrae, particularly those adjacent to the original fracture. Our results are contrary to previous hypotheses. In the PVP group, the preoperative AP ratio was larger (0.71 vs. 0.59, respectively; P < 0.01), the PMMA amount was smaller (6.83 vs. 7.53 cc, respectively; P = 0.02), BMI was relatively small (23.3 vs. 24.1 kg/m²; P = 0.18), and BMD was lower (−3.8 vs. −3.5; P = 0.09). The exact cause is unknown, but the plausible explanations are as follows: (1) in the PKP group, wedge deformity was not corrected enough, and the larger AP ratio found in PVP patients might reflect lower tension on uncemented vertebrae than expected. (2) The surgeon might prefer PVP for patients with poor general condition or with medical problems, because of the short operation time and relatively simple procedure.
New vertebral compression fractures were more frequently observed in patients with discal leakage (odds ratio, 2.4; 95% C.I., 1.02–5.68). The only difference between the adjacent and remote NVCF groups was the discal cement leakage. Furthermore, when comparing the control and adjacent NVCF groups, discal leakage was the only significant factor on univariate analysis. BMD was the significant factor found on performing multivariate logistic regression between these two groups. It is obvious that both cement leakage into the intervertebral disc space and osteoporosis play important roles in the onset of NVCFs following augmentation. In patients with both discal leakage and low BMD (T score <−3.6), the relative risk of adjacent NVCF was 1.65, and prophylactic PVP may be considered. However, cost versus risk analysis with a larger patient group is necessary to confirm prophylaxis.
Extra-osseous leakage is the most frequent complication of vertebroplasty, and it occurs on the point with the lowest resistance [1]. Cement leakage into the intervertebral disc space is known to be frequent in patients with a low AP ratio or at an acute stage of the disease [9, 13]. Hard bone cement that has leaked into the disc space may concentrate stress and weaken the endplate of adjacent vertebrae mechanically. Discal cement leakage was observed in 28.5% of patients in this study. We could not observe any differences in BMD, AP ratio or PMMA amount in patients with discal cement leakage (P = 0.57, P = 0.66, P = 0.87).
Other risk factors, especially the amount of bone cement, multiple treatments, AP ratio, location of the fracture, and cement distribution pattern did not influence the onset of NVCFs. Similar to previous studies, the amount of PMMA itself was irrelevant to the development of NVCFs. [9, 11, 14, 15]. Treatment of multiple vertebrae at initial presentation and compression fractures at the T–L junction may stimulate untreated vertebrae biomechanically, which was not proven in our study [7, 9, 11]. The distribution pattern of the cement may relate to cement amount and height restoration, and can affect the tension on the adjacent vertebrae [22]. This effect was not observed even though cement volume was significantly higher in compact type (P < 0.01).
This study has several limitations. This is a retrospective study. We considered symptomatic fractures only, and the actual re-fracture rate would be higher than the observed rate. To evaluate true incidence and causative factors of NVCFs, asymptomatic MRI-proven fractures should be included in the study. NVCFs were managed as a single event and the cumulative effect of multiple osteoporotic VCFs with time was not considered as a risk factor. Also, studies dealing with vertebral refracture, either after untreated osteoporotic fractures or after PVP/PKP, may differ in design and outcome measure, and caution is necessary when comparing the results.
Conclusion
Symptomatic NVCFs occurred in 15% of the patients during the year following PVP or PKP. The factors affecting NVCFs are old age, PVP procedure, low BMD, and discal cement leakage. The most predictive factors were cement leakage into the intervertebral disc space and osteoporosis. A prospective study involving a large number of patients with long-term follow-up is necessary to confirm the results of our study.
Conflict of interest
None.
References
- 1.Baumann C, Fuchs H, Kiwit J, Westphalen K, Hierholzer J. Complications in percutaneous vertebroplasty associated with puncture or cement leakage. CardioVasc Interv Radiol. 2007;30:161–168. doi: 10.1007/s00270-006-0133-5. [DOI] [PubMed] [Google Scholar]
- 2.Berlemann U, Ferguson SJ, Nolte LP, Heini PF. Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J Bone Joint Surg Br. 2002;84:748–752. doi: 10.1302/0301-620X.84B5.11841. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JE, Lylyk P, Ceratto R, Kaplan L, Umanskyt F, Gomori JM. Percutaneous vertebroplasty: technique and results in 192 procedures. Neurol Res. 2004;26:41–49. doi: 10.1179/016164104773026516. [DOI] [PubMed] [Google Scholar]
- 4.Fourney DR, Schomer DF, Nader R, Chlan-Fourney J, Suki D, Ahrar K, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21–30. doi: 10.3171/jns.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 5.Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- 6.Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 2000;39:1410–1414. doi: 10.1093/rheumatology/39.12.1410. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Kang HS, Choi JA, Ahn JM. Risk factors of new compression fractures in adjacent vertebrae after percutaneous vertebroplasty. Acta Radiol. 2004;45:440–445. doi: 10.1080/02841850410005615. [DOI] [PubMed] [Google Scholar]
- 8.Klotzbuecher CM, Ross PD, Landsmen PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 9.Komemushi A, Tanigawa N, Kariya S, Kojima H, Shomura Y, Komemushi S, et al. Percutaneous vertebroplasty for osteoporotic compression fractures: multivariate study of predictors of new vertebral body fracture. Cardiovasc intervent Radiol. 2006;29:580–585. doi: 10.1007/s00270-005-0138-5. [DOI] [PubMed] [Google Scholar]
- 10.Legroux-Gérot I, Lormeau C, Boutry N, Cotten A, Duquesnoy B, Cortet B. Long-term follow-up of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Clin Rheumatol. 2004;23:310–317. doi: 10.1007/s10067-004-0914-7. [DOI] [PubMed] [Google Scholar]
- 11.Lee WS, Sung KH, Jeong HT, Sung YS, Hyun YI, Choi JY, et al. Risk factors of developing new symptomatic vertebral compression fractures after percutaneous vertebroplasty in osteoporotic patients. Eur Spine J. 2006;15:1777–1783. doi: 10.1007/s00586-006-0151-7. [DOI] [PubMed] [Google Scholar]
- 12.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fractures in the year following a fracture. JAMA. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 13.Lin EP, Ekholm S, Hiwatashi A, Westesson PL. Vertebroplasty cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR. 2004;25:175–180. [PMC free article] [PubMed] [Google Scholar]
- 14.Lin WC, Cheng TT, Lee YC, Wang TN, Cheng YF, Lui CC, et al. New vertebral osteoporotic compression fractures after percutaneous vertebroplasty: retrospective analysis of risk factors. J Vasc Interv Radiol. 2008;19:225–232. doi: 10.1016/j.jvir.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Moon ES, Kim HS, Park JO, Moon SH, Lee HM, Shin DE, et al. The incidence of new vertebral compression fractures in women after kyphoplasty and factors involved. Yonsei Medical J. 2007;48:645–652. doi: 10.3349/ymj.2007.48.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano M, Hirano N, Ishihara H, Kawaguchi Y, Matsuura K. Calcium phosphate cement leakage after percutaneous vertebroplasty for osteoporotic vertebral fractures: risk factor analysis for cement leakage. J Neurosurg Spine. 2005;2:27–33. doi: 10.3171/spi.2005.2.1.0027. [DOI] [PubMed] [Google Scholar]
- 17.Nevitt MC, Thompson DE, Black DM, Rubin SR, Ensrud K, Yates AJ. Effect of alendronate on limited activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Arch Intern Med. 2000;160:77–85. doi: 10.1001/archinte.160.1.77. [DOI] [PubMed] [Google Scholar]
- 18.Ooms ME, Lips P, Lingen A, Valkenburg HA. Determinants of bone mineral density and risk factors for osteoporosis in healthy elderly women. J Bone Miner Res. 1993;8:669–675. doi: 10.1002/jbmr.5650080604. [DOI] [PubMed] [Google Scholar]
- 19.Peh WC, Gilula LA, Peck DD. Percutaneous vertebroplasty for severe osteoporotic vertebral body compression fractures. Radiology. 2002;223:121–126. doi: 10.1148/radiol.2231010234. [DOI] [PubMed] [Google Scholar]
- 20.Ryu KS, Park CK, Kim MC, Kang JK. Dose-dependent epidural leakage of polymethylmethacrylate after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. J Neurosurg. 2002;96:56–61. doi: 10.3171/spi.2002.96.1.0056. [DOI] [PubMed] [Google Scholar]
- 21.Syed MI, Patel NA, Jan S, Harron MS, Morar K, Shaikh A. New symptomatic vertebral compression fractures within a year following vertebroplasty in osteoporotic women. AJNR. 2005;26:1601–1604. [PMC free article] [PubMed] [Google Scholar]
- 22.Tanigawa N, Komemushi A, Kariya S, Kojima H, Shomura Y, Naoto O, et al. Relationship between cement distribution pattern and new compression fracture after percutaneous vertebroplasty. AJR. 2007;189:W348–W352. doi: 10.2214/AJR.07.2186. [DOI] [PubMed] [Google Scholar]
- 23.Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR. 2006;27:217–223. [PMC free article] [PubMed] [Google Scholar]
- 24.Uppin AA, Hirsch JA, Centenera LV, Pfiefer BA, Pazianos AG, Choi IS. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–124. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 25.Voormolen MH, Lohle PN, Juttmann JR, van der Graaf Y, Fransen H, Lampmann LE. The risk of new osteoporotic vertebral compression fractures in the year after percutaneous vertebroplasty. J Vasc Interv Radiol. 2006;17:71–76. doi: 10.1097/01.RVI.0000190910.43602.3C. [DOI] [PubMed] [Google Scholar]