Abstract
Purpose
Development of adjacent segment degeneration following anterior cervical decompression and fusion (ACDF) is still controversial, as adjacent-level kinematics is poorly understood. This study reports preliminary data from a high-accuracy 3D analysis technique developed for in vivo cervical kinematics.
Methods
From nine cervical spondylosis patients, four underwent single-level ACDF, and five underwent two-level ACDF using cylindrical titanium cage implant(s). Pre- and post-surgical CT scans were taken in flexion, neutral and extended positions, allowing us to compute segmental ranges of motion for rotation and translation, and 3D disc-height distributions. Differences in segmental motions and disc-height between fused and adjacent levels were analyzed with a Wilcoxon signed-rank test. Results are presented as mean ± SEM.
Results
The flexion/extension angular-ROM at the fusion level decreased after surgery (7.46 ± 1.17° vs. 3.14 ± 0.56°, p < 0.003). The flexion/extension angular-ROM at one caudal adjacent level to the fusion level (3.97 ± 1.29°) tended to be greater post-operatively (6.11 ± 1.44°, p = 0.074). Translation in the anterior-posterior direction during flexion/extension at the fusion level decreased after surgery (1.22 ± 0.20 mm vs. 0.32 ± 0.11 mm, p < 0.01). No differences were found in adjacent-level disc heights between both study time-points.
Conclusions
This study showed increased segmental motion in flexion/extension angular-ROM at one level adjacent to ACDF. However, increases in the rotational angular-ROM were not statistically significant when cranial/caudal adjacent levels were analyzed separately. This preliminary study highlighted the capabilities of a 3D-kinematic analysis method to detect subtle changes in kinematics and disc height at the adjacent levels to ACDF. Thus, reliable evidence related to ACDF’s influence on adjacent-level cervical kinematics can be collected.
Keywords: Cervical spinal fusion, Adjacent level, Kinematics
Introduction
Anterior cervical decompression and fusion (ACDF) is a standard procedure for the treatment of cervical radiculopathy and myelopathy caused by disc herniation and spondylosis. Fusion at the level of pathology has been thought to be especially effective to relieve symptoms caused by spinal instability [1]. A recent metaanalysis of fusion rate of anterior approaches showed that over 89% of ACDF including ACDF with placement of an anterior plate system achieved fusion in the cervical spine [2].
Despite successful clinical outcomes, there have been increasing concerns regarding development of adjacent segment degeneration or disease following ACDF. A clinical study by Hilibrand et al. [3] has shown that as many as 25% of the patients treated with ACDF had new symptomatic disc disease at the adjacent levels within 10 years. Similar studies reported increased rates of disc degeneration at adjacent levels following ACDF [4–7]. Hilibrand et al. [3], however, also reported in the same article that the risk of new disease at the adjacent level was significantly lower following a multilevel arthrodesis than it was following a single-level arthrodesis. Ishihara et al. [8], also reported a lack of relationship between the incidence of adjacent disease and the number of the levels fused. These findings suggest that adjacent segment disease is the result of a continuous process at adjacent levels and is not caused by the spinal fusion itself. The etiology of adjacent segment degeneration is still unclear and it has not been fully established whether adjacent segment degeneration is a consequence of anterior cervical fusion or it represents the natural history of the degenerative cervical process, thus making it a controversial topic [9].
There have been several discussions about the possibility of ACDF altering biomechanical conditions at adjacent segments, therefore resulting in increased loading and excessive motion. While some biomechanical studies using cadaveric cervical spines demonstrated increased segmental motion and disc pressure at the adjacent motion segment to the fusion level [10, 11], other cadaveric studies did not find such increases at the adjacent levels [12, 13]. The limited capacity of cadaveric models was pointed out to model the in vivo biomechanical conditions after anterior cervical fusion [14].
In vivo kinematic studies using flexion/extension plain radiograms have also addressed the effects of anterior cervical fusion on the kinematics at the adjacent levels to fusion [4, 8, 15–22]. Results of these studies were also variable. One of the possible reasons for the inconsistency in the results is that post-fusion changes in kinematics, if existent, are too small to be detected by the methods used in these studies. Recent work has used new imaging techniques using such as three-dimensional (3D) computed tomography (CT) or magnetic resonance (MR) image-based models to measure post-ACDF kinematics [23–25]. In these studies, kinematic data were obtained by fluoroscopy, and the 3D cervical models were virtually moved using a 3D-to-2D registration approach. However, to the best of our knowledge, there is no in vivo 3D kinematic analysis on the changes in kinematics at the adjacent level to ACDF by comparing before and after ACDF conditions in the same patient.
In this preliminary study, we report the initial results from a high accuracy in vivo 3D kinematic analysis system developed to measure lumbar and cervical spine kinematics [26–31]. This method measures lumbar or cervical spine kinematics in a 3D space to allow for true characterization of spinal motion in vivo. The purpose of the current study was to analyze the changes in the cervical segmental motions before and after ACDF using this in vivo 3D analytical method.
Methods
Subjects
Nine patients (two women and seven men) diagnosed with cervical spondylosis were included in the study (IRB approval number H21-01). Informed consent was obtained from all patients. The mean age of the patients was 54.1 years (age range: 36–76 years). Four patients underwent single-level ACDF and five patients underwent two-level ACDF using cylindrical titanium cage implant(s) (m-cage; Ammtec Inc., Tokyo, Japan). Radiograms in flexion, neutral and extended positions were taken before surgery and 12 months after surgery.
CT examination
CT examinations were conducted pre- and post-operatively. The post-operative CT examinations were performed after a mean follow-up period of 12 months and 0.9 days (range: 11 months, 25 days–12 months, 22 days). Patients were scanned in neutral, flexion, and extension positions (0.3–1.0 mm contiguous slices, 120 kV, AEC 10–440 mA, 15 cm field of view, 512 × 512 matrix). For the CT scans in flexed or extended positions, each patient was instructed to flex or extend his/her neck as much as the patient could and cushions were placed under the head or shoulders of the patient so that the maximum flexed or extended position was maintained [29].
Subject-based three-dimensional (3D) CT model creation
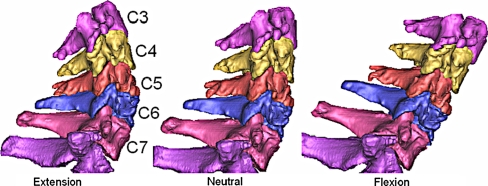
CT image data were segmented with a 3D reconstruction software (Mimics; Materialise Co., Ltd. Yokohama, Japan). A threshold level to define the cortical shell of the vertebral body was selected. The same threshold level was applied to all CT images in flexion, neutral and extension positions in each session (Fig. 1). At the fusion level, the cage and newly formed bone were not included in the vertebral model. A cavity created by the cage and new bone in the original disc space were manually leveled with the surrounding endplate. Two independent investigators created the 3D CT models with the threshold levels blinded to evaluate effects model creation on the following motion analysis. Following segmentation, a point-cloud data set was created of each vertebra including fused level(s) and one cranial and one caudal level to the fused level. Endplate point-cloud data sets and all the 3D motion analyses were created based on the segmentation of the endplate from each vertebra using custom-written programs created in Microsoft Visual C++ with Microsoft Foundation Class (MFC) programming environment (Microsoft Corp., Redmond, WA).
Fig. 1.
Subject-based 3D CT models in extended, neutral and flexed positions
Three-dimensional segmental motion analysis
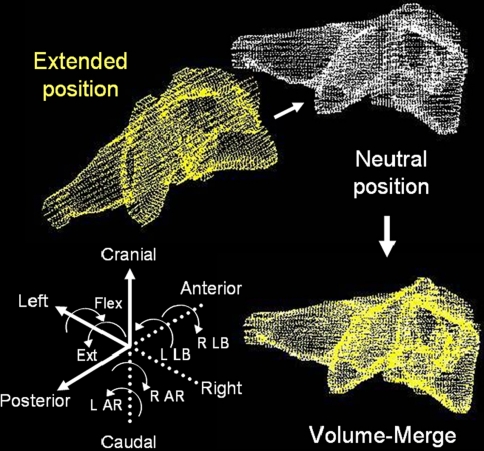
In order to analyze individual vertebral motions during flexion and extension, 3D-3D rigid registration of the CT models was performed. Eigenvectors of each vertebra were calculated to determine the local coordinate of the each vertebra. The centroid of each vertebra was used as an origin of the local coordinate. The vertebra in the rotated position was rotated and translated so that the coordinates in the rotated and neutral vertebrae match. The rotation angles were described by Euler angles. This procedure provides rotation angles and translations during flexion/extension with an accuracy of 1.0° and 1.0 mm [31]. A validated Volume-Merge method was further used to increase accuracy of the 3D-3D registration of the vertebra [26]. In the Volume-Merge method, a vertebral body in a flexed or extended position (the moving vertebra) was virtually rotated and translated towards the same body in a neutral position (the stationary target). These rotations and translations of the vertebral body were conducted with decreasing scales of increments and search ranges. The initial rotations and translations were conducted in 1.6° and 1.6 mm increments within search ranges of ± 3.2° and ± 1.6 mm, respectively. This initial setting was determined considering the accuracy level of the above-mentioned eigenvector method. The percentage of point-cloud merge was calculated at each transformation using an algorithm previously described [26]. The Euler angles and translations, which provided the highest percentage of volume merge, were recorded. The next registration procedure was started from the last orientation and position of the vertebra determined by the Euler angles and translations. This procedure was repeated with decreasing increments and search ranges with a scale factor of 1/2. The final increments in rotations and translations were 0.05° and 0.05 mm, respectively, and the Euler angles and translations providing the highest percentage of volume merge, in this setting, were used for the rotational angles and translations during flexion/extension (Fig. 2). These procedures, including the eigenvector method and the Volume-Merge method, were performed automatically within a custom-made software program written in Visual C++ with a maximum calculation time of approximately 90 s (at the C7 cervical level, with approximately 30,000 points in the point-cloud) using a personal computer (CPU: Intel Core i7-2720QM, 2.2 GHz). The accuracy of the Volume-Merge method is 0.1 mm in translation and 0.2° in rotation, as previously described in Ochia et al. [26].
Fig. 2.
Volume-Merge method for calculation of 3 degree-of-freedom (DOF) rotations. L left, R right, AR axial rotation, LB lateral bending
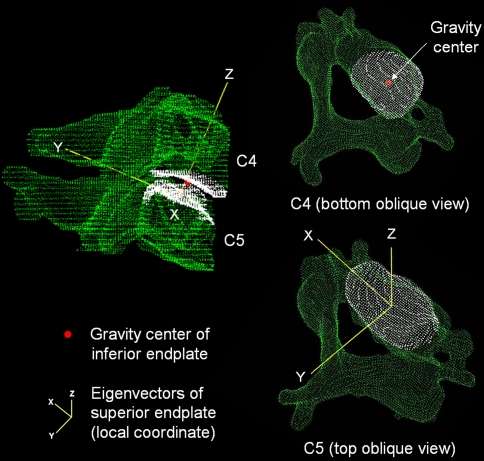
Relative motion between any two vertebral bodies was evaluated by placing local coordinates at the gravity centers of the superior endplate of the caudal vertebrae for each motion segment (Fig. 3). The orientation of the coordinates was defined by endplate eigenvectors calculated by a custom algorithm described elsewhere [32]. Segmental translations were evaluated by the translation of the gravity center of the inferior endplate of the cranial vertebrae in reference to the local coordinate set on the superior endplate of the caudal vertebrae (Fig. 3). The x axis was set in the lateral direction (left side: positive). The y axis was set in the anterior-posterior direction (posterior: positive). The z axis was set in the cranial-caudal direction (cranial: positive) (Figs. 2, 3). The rotations about the x axis in flexion and extension were added and defined as a flexion/extension angular range of motion (ROM). The rotations about the y axis or z axis in flexion and extension were averaged and described as a lateral bending angular rotation or an axial rotation, respectively. The translation distances along the x axis in flexion and extension were averaged and defined as a lateral translation ROM. The translation distances along the y axis in flexion and extension were added and defined as an anterior-posterior (sagittal) translation ROM. The translation distances along the z axis in flexion and extension were averaged and defined as a cranio-caudal (axial) translation ROM.
Fig. 3.
Local coordinate origin set on the caudal vertebral body’s superior endplate to calculate 3 degree-of-freedom translations using the white point-cloud data set for the endplate
Disc height measurement
Disc height distribution was measured using 3D geometric point-cloud data of the inferior and superior endplates segmented from the 3D CT model (Fig. 4). Distances between one point in the point-cloud model of the inferior endplate surface of the disc space (the superior endplate of the caudal vertebral body) and all points in the superior endplate surface of the disc space (the inferior endplate of the cranial vertebral body) were calculated in 3D space using a custom-written program [33]. The inferior endplate of the disc space under analysis was set as the reference frame for a least-distance search directed towards the opposing point-cloud data representing the superior endplate in the disc space. This least-distance at the point in question on the inferior endplate surface of the disc space was defined as the least-distance at the point in the inferior endplate surface of the disc space. This procedure was repeated for all points in the inferior endplate surface of the disc space and a mean least-distance was determined for each disc space (Fig. 4). The disc height at the fusion level after surgery was not evaluated due to surface geometry changes caused by the cage and surgical procedures.
Fig. 4.
Individual Disc height (DH) distribution at cranial, fusion and caudal levels in a preoperative neutral position in a subject using the 3D least distance search algorithm
Statistical analyses
The angular ROM, translation distances, and disc height at the fusion level, one further cranial level and one further caudal level were evaluated. Data was analyzed by grouping all cranial/caudal adjacent cases as a “one adjacent level” category. Since three patients had a fusion at C6-C7 level, caudal adjacent levels were not analyzed in these three patients since only the cranial portion of the Th12 level was scanned in these subjects; therefore, the sample size of the cranial adjacent level was smaller than the number of patients. Differences in segmental motions and disc height before and after surgery at each level were compared in paired fashion by the Wilcoxon signed rank test (α = 0.05). Differences in segmental movements and disc heights between the fused level and the adjacent level were also compared by the Wilcoxon signed rank test. All analyses were conducted using the StatView program (Version 5.0; SAS Institute Inc., Cary, NC). Results are presented as a mean and standard error of the mean (SEM).
Results
Plain radiography
Fusion was confirmed radiographically in all cases. Plain radiograms taken in the flexed, neutral, and extended positions did not show any clear zone in all cases. Not all cases presented with cage subsidence.
Effects of inter-variability of segmentation on 3D-3D registration
The inter-investigator differences in translations measured from 3D CT models segmented by two independent investigators were 0.054 ± 0.006 mm in lateral translation, 0.047 ± 0.004 mm in sagittal translation, and 0.083 ± 0.006 mm in axial translation. When the results were divided by the non-fusion or fusion level, the differences in translations for the non-fusion level and fused level were 0.054 ± 0.008 and 0.054 ± 0.007 mm, respectively, in lateral translation; 0.045 ± 0.004 and 0.055 ± 0.008 mm, respectively, in sagittal translation; and 0.078 ± 0.007 and 0.096 ± 0.013 mm, respectively, in axial translation. There were no statistical differences between the non-fusion and fusion levels.
The differences in Euler angles were 0.195 ± 0.014° in flexion/extension; 0.258 ± 0.038° in lateral bending; and 0.173 ± 0.022° in axial rotation. When the results were divided by the non-fusion or fusion level, the differences of Euler angles in the non-fusion level and fused level were 0.183 ± 0.015° and 0.228 ± 0.037°, respectively, in flexion/extension; 0.258 ± 0.038° and 0.262 ± 0.032°, respectively, in lateral bending; and 0.147 ± 0.023° and 0.243 ± 0.055°, respectively, in axial translation. There were no statistical differences between the non-fusion and fusion levels.
Segmental rotation (Table 1)
Table 1.
Segmental angular ranges of motion at fusion and adjacent levels during flexion/extension (unit: degrees, mean ± SEM)
| Level | Flexion/extension (x axis) | Lateral bending (y axis) | Axial rotation (z axis) | |||
|---|---|---|---|---|---|---|
| Pre-OP | Post-OP | Pre-OP | Post-OP | Pre-OP | Post-OP | |
| Fusion (n = 14) | 7.46 ± 1.17 | 3.14 ± 0.56a | 0.84 ± 0.15 | 0.47 ± 0.12e | 0.81 ± 0.17 | 0.32 ± 0.06f |
| Cranial (n = 9) | 8.58 ± 1.62 | 10.06 ± 1.99b | 1.16 ± 0.28 | 1.08 ± 0.20 | 0.98 ± 0.22 | 0.92 ± 0.21 |
| Caudal (n = 6) | 3.97 ± 1.29 | 6.11 ± 1.44c | 0.53 ± 0.22 | 0.94 ± 0.27 | 0.67 ± 0.24 | 0.66 ± 0.23 |
| Cranial and caudal combined (n = 15) | 6.74 ± 1.22 | 8.48 ± 1.39d | 0.91 ± 0.20 | 1.03 ± 0.16 | 0.86 ± 0.16 | 0.82 ± 0.16 |
n sample size
ap < 0.003 compared to pre-OP
bp = 0.138 compared to pre-OP
cp = 0.074 compared to pre-OP
dp < 0.03 compared to pre-OP
ep = 0.064 compared to pre-OP
fp < 0.04 compared to pre-OP
The flexion/extension angular ROM at the fusion level decreased after surgery (7.46 ± 1.17° pre-operatively and 3.14 ± 0.56° post-operatively, p < 0.003). The flexion/extension angular ROM at one caudal adjacent level to the fusion level (3.97 ± 1.29°) tended to be greater post-operatively (6.11 ± 1.44°, p = 0.074). There were no statistical differences in the flexion/extension angular ROM before and after surgery at one cranial adjacent level (p = 0.138). When the flexion/extension angular ROM at one cranial adjacent level and one caudal adjacent level to the fusion level were combined, the pre-operative angular ROM (6.74 ± 1.22°) increased post-operatively (8.48 ± 1.39°, p < 0.03).
The mean post-operative lateral angular rotation at the fusion level (0.47 ± 0.12°) tended to be lower than the pre-operative mean angular rotation (0.84 ± 0.15°, p = 0.064). The mean lateral angular rotation at the adjacent levels did not show any significant differences before and after surgery.
In axial torsion, the mean post-operative angular rotation at the fusion level (0.32 ± 0.06°) was lower than the pre-operative mean angular ROM (0.81 ± 0.17°, p < 0.04). The mean axial rotation at the adjacent levels did not show any significant differences before and after surgery.
Segmental translation (Table 2)
Table 2.
Segmental translations at fusion and adjacent levels during flexion/extension (unit: mm, mean ± SEM)
| Level | Lateral (x axis) | Anterior-posterior (y axis) | Cranio-caudal (z axis) | |||
|---|---|---|---|---|---|---|
| Pre-OP | Post-OP | Pre-OP | Post-OP | Pre-OP | Post-OP | |
| Fusion (n = 14) | 0.34 ± 0.09 | 0.15 ± 0.04a | 1.22 ± 0.20 | 0.32 ± 0.11b | 0.07 ± 0.09 | 0.07 ± 0.16 |
| Cranial (n = 9) | 0.32 ± 0.09 | 0.28 ± 0.04 | 1.72 ± 0.32 | 1.93 ± 0.38 | −0.01 ± 0.10 | 0.17 ± 0.14 |
| Caudal (n = 6) | 0.24 ± 0.09 | 0.11 ± 0.06 | 0.49 ± 0.24 | 0.74 ± 0.24 | −0.23 ± 0.10 | −0.03 ± 0.06 |
| Cranial and caudal combined (n = 15) | 0.21 ± 0.04 | 0.21 ± 0.04 | 1.22 ± 0.26 | 1.45 ± 0.29 | −0.10 ± 0.07 | 0.09 ± 0.09 |
n sample size
ap = 0.084 compared to pre-OP
bp < 0.01 compared to pre-OP
Translation in the anterior-posterior direction during flexion/extension at the fusion level decreased after surgery (1.22 ± 0.20 mm preoperatively and 0.32 ± 0.11 mm post-operatively, p < 0.01). Anterior-posterior translation at adjacent levels to the fusion level did not show any differences before and after surgery.
Post-operative translation in the lateral direction at the fusion level tended to be lower (0.15 ± 0.04 mm, p = 0.084) compared with the pre-operative value (0.34 ± 0.09 mm). The lateral translation at the adjacent levels to the fusion level did not show any differences before and after surgery. Translation in the axial direction at the fusion and adjacent levels did not show any significant differences before and after surgery.
Disc height (Table 3)
Table 3.
Disc height at fusion and adjacent levels during flexion/extension (unit: mm, mean ± SEM)
| Level | Disc height | |
|---|---|---|
| Pre-OP | Post-OP | |
| Fusion (n = 14) | 1.73 ± 0.15 | – |
| Cranial (n = 9) | 2.27 ± 0.15a | 2.52 ± 0.27 |
| Caudal (n = 6) | 2.56 ± 0.26b | 2.22 ± 0.24 |
| Cranial and caudal combined (n = 15) | 2.39 ± 0.14c | 2.40 ± 0.19 |
n sample size
ap < 0.03 compared to fusion level
bp < 0.04 compared to fusion level
cp < 0.003 compared to fusion level
Disc height at fusion level before surgery was narrower compared to adjacent levels. After surgery, disc height at the fused level did not show any differences with respect to the adjacent levels. The disc heights at adjacent levels did not show any statistical differences before and after surgery.
Discussion
The use of a high-accuracy in vivo 3D kinematic analysis method used in the current study enabled the detection of subtle changes in segmental movement between pre- and post-ACDF conditions at 12 months after ACDF. The results of this study showed increased segmental movements in flexion/extension angular ROM at one level adjacent to ACDF. However, when the adjacent levels were analyzed separately at the cranial and caudal adjacent levels, the increases in the angular ROM were not statistically significant, hinting at a possible limitation of the study due to a small sample size. Previous in vivo kinematic studies on patients who underwent ACDF and/or cervical disc arthroplasty showed controversial results on the changes in flexion/extension angles at the adjacent levels between pre- and post-surgery. Table 4 summarizes the results of the studies which provide absolute values of the rotational ROMs in flexion/extension pre- and post-operatively. Regardless of statistical significance in the post-surgical rotational angular ROM increases, the amount of the increase is small especially in 1- or 2-year follow-up periods reported in the literature and ranges from 0.2° to 1.7° (Table 4). In the current study, anterior-posterior translation, during flexion/extension, did not show any statistical differences following ACDF at the adjacent levels. Limited information is available in the literature regarding changes in translation between pre- and post-ACDF at the adjacent levels. Table 5 summarizes available results of anterior-posterior translation in flexion/extension from both pre- and post-operative conditions. The magnitude of the anterior-posterior translation from full flexion to full extension was as small as 0.3 mm or less (Table 5). Anterior-posterior translation during flexion/extension is an important factor in determining shear strain in the anterior-posterior direction. Matsunaga et al. [18] estimated shear strain at the adjacent levels after ACDF and found increased shear strain at the adjacent to two- or three-level ACDF, while no increases in shear strain were found at the adjacent level to the single-level ACDF. It should be noted that calculation of strain in the intervertebral disc requires even higher accuracy than the measurement of translation in the anterior-posterior direction since strain calculation requires measurement of the translation in the disc height direction at the same time. It is clear that implementation of a high accuracy measurement method will increase the validity of conclusions stemming from a study on kinematics changes at the adjacent level, as pointed out by other authors [34].
Table 4.
In vivo kinematic studies on flexion/extension angles during flexion/extension at adjacent levels to ACDF or cervical disc arthroplasty (CDA) preoperatively and postoperatively
| Authors [Ref.] | Surgery | Period | Level | n | Flexion/extension (degrees) | P | ||
|---|---|---|---|---|---|---|---|---|
| Pre-OP | Post-OP | Diff | ||||||
| Baba et al. [4] | ACDF | 8.5 Y | Cranial | 106 | 8.6 | 13.7 | 5.1 | <0.01 |
| Caudal | 8.8 | 11.6 | 2.8 | <0.05 | ||||
| Wigfield et al. [22] | CDA | 12 M | Cranial | 12 | NA | NA | −0.3a | NA |
| Caudal | NA | NA | −1.1a | NA | ||||
| ACDF | 12 M | Cranial | 13 | NA | NA | 1.5a | NA | |
| Caudal | NA | NA | 0.8a | NA | ||||
| Reitman et al. [34] | ACDF | 13 M | Cranial | 21 | 13.1b | 13.3b | 0.2b | >0.25 |
| Sasso et al. [20] | CDA | 24 M | Cranial | 192 | 8.27 | 9.13 | 0.86 | NS |
| Caudal | 132 | 4.95 | 6.58 | 1.63 | NS | |||
| ACDF | 24 M | Cranial | 242 | 7.83 | NA | NA | NS | |
| Caudal | 5.24 | NA | NA | NS | ||||
| Kim et al. [16] | Single ACDF | 17 M | Cranial | 26 | 9.4 | 10.2 | 0.8 | NA |
| Caudal | 11.4 | 10.8 | −0.6 | NA | ||||
| Single CDA | 18 M | Cranial | 39 | 8.7 | 9.5 | 0.8 | NA | |
| Caudal | 8.3 | 9.2 | 0.9 | NA | ||||
| Double ACDF | 21 M | Cranial | 28 | 7.7 | 4.3 | −3.4 | NA | |
| Caudal | 5.5 | 6.2 | 0.7 | NA | ||||
| Double CDA | 18 M | Cranial | 12 | 9.0 | 9.9 | 0.9 | NA | |
| Caudal | 8.5 | 9.4 | 0.9 | NA | ||||
| Elsawaf et al. [15] | ACDF | 28 M | Cranial | 18 | 13.7c | 15.4c | 1.7c | 0.085c |
| Caudal | 18 | 10.2c | 10.9c | 0.7c | 0.51c | |||
| Park et al. [21] | CDA | 12 M | Cranial | 272 | 9.8 | 10.8 | 1.0 | 0.43 |
| Caudal | 7.3 | 8.0 | 0.7 | 0.368 | ||||
| ACDF | 12 M | Cranial | 182 | 9.6 | 11.0 | 1.4 | 0.003 | |
| Caudal | 7.8 | 8.7 | 0.9 | 0.56 | ||||
Table 5.
In vivo kinematic studies on flexion/extension translations during flexion/extension at adjacent levels to ACDF or cervical disc arthroplasty (CDA) preoperatively and postoperatively
| Authors [Ref.] | Procedure | Period | Level | n | Anterior-posterior (mm) | P | ||
|---|---|---|---|---|---|---|---|---|
| Pre-OP | Post-OP | Diff | ||||||
| Reitman et al. [34] | ACDF | 13 M | Cranial | 21 | 2.0a | 2.3a | 0.3a | >0.25 |
| Park et al. [21] | CDA | 12 M | Cranial | 272 | 1.4 | 1.5 | 0.1 | >0.05 |
| Caudal | 0.7 | 0.8 | 0.1 | 0.35 | ||||
| ACDF | 12 M | Cranial | 182 | 1.3 | 1.5 | 0.2 | 0.09 | |
| Caudal | 0.8 | 0.9 | 0.1 | 0.0929 | ||||
CDA cervical disc arthroplasty, Diff difference between pre-OP and Post-OP, n sample size, Pp value of comparison between Pre-OP and Post-OP
aCalculated from reference [27]
There was no disc height loss associated with disc degeneration during a 1-year period following ACDF in the current study. As with the case of the anterior-posterior translation measurement, there is limited information in the literature about the changes in disc height at the adjacent levels following ACDF (Table 6). Reports by Reitman et al. [34], Park et al. [21] and the current study showed a decrease in disc height of up to 0.34 mm, 12–13 months post-ACDF, in contrast to Kim et al.’s [16] report of a posterior disc height decrease of over 0.7 mm 21 months after ACDF and 19 months after cervical disc arthroplasty (Table 6). This study introduced a high-accuracy method for measuring disc height. While conventional methods based on 2D radiographic images typically use four corner points from the involved vertebral bodies to calculate the disc height, the method presented here measured disc heights at all endplate surface data points in 3D allowing disc height measurement independent of each vertebral body’s 3D orientation, which causes errors in the 2D measurement. It should be noted that the disc height values presented in the current study were smaller than the disc heights measured by conventional methods using 2D radiographic images because our method measures the disc height including Luschka joints, which lowered the mean disc height. It is feasible, however, to measure the disc height without the Luschka joints so that the range of the mean disc height matches with the disc height measured by the conventional methods.
Table 6.
In vivo kinematic studies on disc height changes during flexion/extension at adjacent levels to ACDF or cervical disc arthroplasty (CDA) preoperatively and postoperatively
| Authors [Ref.] | Procedure | Period | Level | n | Cranio-caudal (mm) | P | ||
|---|---|---|---|---|---|---|---|---|
| Pre-OP | Post-OP | Diff | ||||||
| Reitman et al. [34] | ACDF | 13 M | CrA | 21 | 1.7a | 1.6a | 0.1a | >0.25 |
| CrP | 21 | 1.3a | 1.1a | 0.2a | >0.25 | |||
| Kim et al. [16] | Single ACDF | 17 M | CrA | 26 | 4.27 | 3.92 | −0.35 | NA |
| CrP | 2.99 | 2.95 | −0.04 | NA | ||||
| CaA | 4.84 | 4.40 | −0.44 | NA | ||||
| CaP | 2.95 | 2.98 | 0.03 | NA | ||||
| Single CDA | 18 M | CrA | 39 | 4.5 | 4.23 | −0.27 | NA | |
| CrP | 4.12 | 3.74 | −0.38 | NA | ||||
| CaA | 4.77 | 4.57 | −0.20 | NA | ||||
| CaP | 4.24 | 4.59 | 0.35 | NA | ||||
| Double ACDF | 21 M | CrA | 28 | 5.00 | 4.33 | −0.67 | NA | |
| CrP | 4.11 | 3.35 | −0.76 | NA | ||||
| CaA | 4.68 | 4.00 | −0.68 | NA | ||||
| CaP | 4.27 | 3.38 | −0.89 | NA | ||||
| Double CDA | 19 M | CrA | 12 | 4.20 | 4.27 | 0.07 | NA | |
| CrP | 3.53 | 3.48 | −0.05 | NA | ||||
| CaA | 4.03 | 4.01 | −0.02 | NA | ||||
| CaP | 4.07 | 3.31 | −0.76 | NA | ||||
| Park et al. [21] | CDA | 12 M | Cranial | 272 | 4.1 | 4.1 | 0 | >0.9 |
| Caudal | 3.9 | 3.9 | 0 | >0.9 | ||||
| ACDF | 12 M | Cranial | 182 | 4.1 | 4.0 | −0.1 | >0.9 | |
| Caudal | 4.2 | 4.0 | −0.2 | >0.9 | ||||
CDA cervical disc arthroplasty, CaA caudal anterior, CaP caudal posterior, CrA cranial anterior, CrP cranial posterior, Diff difference between pre-OP and Post-OP, n sample size, NA data not available, Pp value of comparison between Pre-OP and Post-OP
aCalculated from reference [27]
Coupled motion during flexion/extension was negligible in the current study as reflected by the minimal absolute values of lateral bending angular (1.16° or less) and axial rotations (0.98° or less) and lateral (0.34 mm or less) and cranio-caudal (0.23 mm or less) translations. Bell et al. [35] used a 6-DOF virtual reality assisted cervical motion tracking device to minimize coupled movements during overall ROM measurements in flexion/extension, lateral bending, and axial rotation. The authors were able to minimize the coupled motion in the overall ROM during flexion/extension but relatively large coupled motions were recorded during lateral bending. Even in the flexion/extension movement, recent studies which measured 6 DOF segmental movements at the adjacent level to ACDF showed over 2° of angular motion [23, 25] and 0.5–1.8 mm translations [23] in the off-axis directions. The coupled segmental motion in flexion/extension could be larger when structural asymmetry exists in the motion segment [28, 29]. Therefore, a 3D measurement able to measure 6-DOF coupled segmental motions is necessary to accurately evaluate subtle alternation of kinematics at the adjacent levels to ACDF. In light of this, the current study used a local coordinate system set at the caudal vertebral body’s superior endplate gravity-center. Thus, measurement of gravity-center translations of the cranial vertebral body’s inferior endplate in three directions along the three axes of the local coordinate system was possible. This measurement allows relative movements parallel to the endplate independent of 3D orientation of the vertebral body. This system is beneficial when the coupled movement occurs during dynamic image examination; for example, anterior direction always directs towards anterior in each vertebral body regardless of any 3D rotations of the vertebral body.
Subject-based CT-derived 3D models were used in the current study. However, CT scanning incurs in an evident disadvantage in terms of exposure to ionizing radiation when compared to MR imaging as a means to create subject-based 3D models. In spite of this, CT scanning is much faster than MR scanning, which aids in flexion/extension positions scanning, especially for cervical disorders patients. In addition, CT images allow evaluation of bone density in the vertebral body and subchondral bone at the facet joints and vertebral endplates. Measurement of the subchondral bone density distribution provides important information on the loading history and osteoarthritic changes at the facet joint by using a CT osteoabsorptiometry technique [36, 37]. Analyses on possible changes in the facet subchondral bone density distribution associated with ACDF and cervical disc arthroplasty at the surgery level and the adjacent level may provide additional information on outcomes of these procedures [38].
Conclusions
The current study showed increased angular ROMs but no increase in translation at one level adjacent to ACDF during flexion/extension 1 year after ACDF using high accuracy 3D kinematic analysis techniques. However, these results are limited due to the small sample size and relatively short follow-up period, as it constitutes a preliminary report. Nevertheless, the present 3D kinematic analysis method was able to detect subtle changes in kinematics and disc height at the adjacent levels to ACDF, which will enable collection of reliable evidence regarding possible ACDF’s influence on cervical kinematics alteration at adjacent levels in a longer follow-up study with a larger sample size.
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Footnotes
Abstracts based on this study have been presented at the 38th Annual Meeting of the Cervical Spine Research Society, held on December 2–4, 2010 in Charlotte, North Carolina and the 2011 Annual Meeting of the Orthopaedic Research Society, January 13–16, 2011, Long Beach, California.
Contributor Information
Nozomu Inoue, Phone: +1-312-9428151, FAX: +1-312-9422040, Email: Nozomu_Inoue@rush.edu.
Alejandro A. Espinoza Orías, Email: Alejandro_Espinoza@rush.edu
Junichi Mizuno, Phone: +81-223-233151, FAX: +81-223-233150, Email: mizuno@minamitohoku.jp.
References
- 1.Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75(9):1298–1307. doi: 10.2106/00004623-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Fraser JF, Hartl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine. 2007;6(4):298–303. doi: 10.3171/spi.2007.6.4.2. [DOI] [PubMed] [Google Scholar]
- 3.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81(4):519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Baba H, Furusawa N, Imura S, Kawahara N, Tsuchiya H, Tomita K. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine. 1993;18(15):2167–2173. doi: 10.1097/00007632-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Goffin J, Geusens E, Vantomme N, Quintens E, Waerzeggers Y, Depreitere B, Calenbergh F, Loon J. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17(2):79–85. doi: 10.1097/00024720-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Gore DR, Sepic SB. Anterior cervical fusion for degenerated or protruded discs. A review of one hundred forty-six patients. Spine. 1984;9(7):667–671. doi: 10.1097/00007632-198410000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto M, Okada E, Ichihara D, Watanabe K, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nishiwaki Y, Iwanami A, Ikegami T, Takahata T, Hashimoto T. Anterior cervical decompression and fusion accelerates adjacent segment degeneration: comparison with asymptomatic volunteers in a ten-year magnetic resonance imaging follow-up study. Spine. 2010;35(1):36–43. doi: 10.1097/BRS.0b013e3181b8a80d. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara H, Kanamori M, Kawaguchi Y, Nakamura H, Kimura T. Adjacent segment disease after anterior cervical interbody fusion. Spine J. 2004;4(6):624–628. doi: 10.1016/j.spinee.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4(6 Suppl):190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Eck JC, Humphreys SC, Lim TH, Jeong ST, Kim JG, Hodges SD, An HS. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine. 2002;27(22):2431–2434. doi: 10.1097/00007632-200211150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Park DH, Ramakrishnan P, Cho TH, Lorenz E, Eck JC, Humphreys SC, Lim TH. Effect of lower two-level anterior cervical fusion on the superior adjacent level. J Neurosurg Spine. 2007;7(3):336–340. doi: 10.3171/SPI-07/09/336. [DOI] [PubMed] [Google Scholar]
- 12.Rao RD, Wang M, McGrady LM, Perlewitz TJ, David KS. Does anterior plating of the cervical spine predispose to adjacent segment changes? Spine. 2005;30(24):2788–2792. doi: 10.1097/01.brs.0000190453.46472.08. [DOI] [PubMed] [Google Scholar]
- 13.Fuller DA, Kirkpatrick JS, Emery SE, Wilber RG, Davy DT. A kinematic study of the cervical spine before and after segmental arthrodesis. Spine. 1998;23(15):1649–1656. doi: 10.1097/00007632-199808010-00006. [DOI] [PubMed] [Google Scholar]
- 14.Rihn JA, Lawrence J, Gates C, Harris E, Hilibrand AS. Adjacent segment disease after cervical spine fusion. Instr Course Lect. 2009;58:747–756. [PubMed] [Google Scholar]
- 15.Elsawaf A, Mastronardi L, Roperto R, Bozzao A, Caroli M, Ferrante L. Effect of cervical dynamics on adjacent segment degeneration after anterior cervical fusion with cages. Neurosurg Rev. 2009;32(2):215–224. doi: 10.1007/s10143-008-0164-2. [DOI] [PubMed] [Google Scholar]
- 16.Kim SW, Limson MA, Kim SB, Arbatin JJ, Chang KY, Park MS, Shin JH, Ju YS. Comparison of radiographic changes after ACDF versus Bryan disc arthroplasty in single and bi-level cases. Eur Spine J. 2009;18(2):218–231. doi: 10.1007/s00586-008-0854-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolstad F, Nygaard OP, Leivseth G. Segmental motion adjacent to anterior cervical arthrodesis: a prospective study. Spine. 2007;32(5):512–517. doi: 10.1097/01.brs.0000256448.04035.bb. [DOI] [PubMed] [Google Scholar]
- 18.Matsunaga S, Kabayama S, Yamamoto T, Yone K, Sakou T, Nakanishi K. Strain on intervertebral discs after anterior cervical decompression and fusion. Spine. 1999;24(7):670–675. doi: 10.1097/00007632-199904010-00011. [DOI] [PubMed] [Google Scholar]
- 19.Sasso RC, Best NM. Cervical kinematics after fusion and bryan disc arthroplasty. J Spinal Disord Tech. 2008;21(1):19–22. doi: 10.1097/BSD.0b013e3180500778. [DOI] [PubMed] [Google Scholar]
- 20.Sasso RC, Best NM, Metcalf NH, Anderson PA. Motion analysis of bryan cervical disc arthroplasty versus anterior discectomy and fusion: results from a prospective, randomized, multicenter, clinical trial. J Spinal Disord Tech. 2008;21(6):393–399. doi: 10.1097/BSD.0b013e318150d121. [DOI] [PubMed] [Google Scholar]
- 21.Park DK, Lin EL, Phillips FM (2011) Index and adjacent level kinematics after cervical disc replacement and anterior fusion: in vivo quantitative radiographic analysis. Spine (Phila Pa 1976) 36(9):721–730 [DOI] [PubMed]
- 22.Wigfield C, Gill S, Nelson R, Langdon I, Metcalf N, Robertson J. Influence of an artificial cervical joint compared with fusion on adjacent-level motion in the treatment of degenerative cervical disc disease. J Neurosurg. 2002;96(1 Suppl):17–21. doi: 10.3171/spi.2002.96.1.0017. [DOI] [PubMed] [Google Scholar]
- 23.Anderst W, Donaldson W, Lee J, Kang J (2010) Fused and adjacent segmental motion in the cervical spine 6 months after anterior cervical discectomy and fusion. In: 56th Annual meeting of the orthopaedic research society, New Orleans. Trans Orthopaedic Research Society, p 146
- 24.Cheng JS, Liu F, Komistek RD, Mahfouz MR, Sharma A, Glaser D. Comparison of cervical spine kinematics using a fluoroscopic model for adjacent segment degeneration. Invited submission from the Joint Section on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2007;7(5):509–513. doi: 10.3171/SPI-07/11/509. [DOI] [PubMed] [Google Scholar]
- 25.McDonald CP, Bachison CC, Chang V, Bartol SW, Bey MJ (2010) 3D in vivo cervical kinematics; preliminary comparison of fusion patients and control subjects. In: 56th annual meeting of the orthopaedic research society, New Orleans. Trans Orthopaedic Research Society, p 147
- 26.Ochia RS, Inoue N, Renner SM, Lorenz EP, Lim TH, Andersson GB, An HS. Three-dimensional in vivo measurement of lumbar spine segmental motion. Spine. 2006;31(18):2073–2078. doi: 10.1097/01.brs.0000231435.55842.9e. [DOI] [PubMed] [Google Scholar]
- 27.Ochia RS, Inoue N, Takatori R, Andersson GB, An HS. In vivo measurements of lumbar segmental motion during axial rotation in asymptomatic and chronic low back pain male subjects. Spine. 2007;32(13):1394–1399. doi: 10.1097/BRS.0b013e318060122b. [DOI] [PubMed] [Google Scholar]
- 28.Takatori R, Tokunaga D, Inoue N, Hase H, Harada T, Suzuki H, Ito H, Nishimura T, An HS, Kubo T. In vivo segmental motion of the cervical spine in rheumatoid arthritis patients with atlantoaxial subluxation. Clin Exp Rheumatol. 2008;26(3):442–448. [PubMed] [Google Scholar]
- 29.Takatori R, Tokunaga D, Hase H, Mikami Y, Ikeda T, Harada T, Imai K, Ito H, Nishimura T, An HS, Inoue N, Kubo T (2010) Three-dimensional morphology and kinematics of the craniovertebral junction in rheumatoid arthritis. Spine (Phila Pa 1976) 35(23):E1278–E1284 [DOI] [PubMed]
- 30.Inoue N, Espinoza Orias AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487–499. doi: 10.1016/j.ocl.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim TH, Eck JC, An HS, McGrady LM, Harris GF, Haughton VM. A noninvasive, three-dimensional spinal motion analysis method. Spine. 1997;22(17):1996–2000. doi: 10.1097/00007632-199709010-00011. [DOI] [PubMed] [Google Scholar]
- 32.Sugisaki K, An HS, Espinoza Orias AA, Rhim R, Andersson GB, Inoue N. In vivo three-dimensional morphometric analysis of the lumbar pedicle isthmus. Spine. 2009;34(24):2599–2604. doi: 10.1097/BRS.0b013e3181b52a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crane B, An HS, Ochia RS, Conrin S, Chen K, Andersson GB, Inoue N (2006) In vivo measurement of changes in lumbar intervertebral disc height distribution during torsion. In: 52nd annual meeting of the orthopaedic research society, Chicago. Trans Orthopaedic Research Society, p 1217
- 34.Reitman CA, Hipp JA, Nguyen L, Esses SI. Changes in segmental intervertebral motion adjacent to cervical arthrodesis: a prospective study. Spine. 2004;29(11):E221–E226. doi: 10.1097/00007632-200406010-00022. [DOI] [PubMed] [Google Scholar]
- 35.Bell KM, Bechara BP, Hartman RA, Shively C, Frazier EC, Lee JY, Kang JD, Donaldson WF (2011) Influence of number of operated levels and postoperative time on active range of motion following anterior cervical decompression and fusion procedures. Spine (Phila Pa 1976) 36(4):263–268 [DOI] [PubMed]
- 36.Wagner S, Weckbach A, Muller-Gerbl M. The influence of posterior instrumentation on adjacent and transfixed facet joints in patients with thoracolumbar spinal injuries: a morphological in vivo study using computerized tomography osteoabsorptiometry. Spine. 2005;30(7):E169–E178. doi: 10.1097/01.brs.0000157431.73969.81. [DOI] [PubMed] [Google Scholar]
- 37.Takatori R, An HS, Ochia RS, Andersson GB, Inoue N (2006) In vivo measurement of lumbar facet joint width and density distribution. In: 52nd annual meeting of the orthopaedic research society, Chicago. Trans Orthopaedic Research Society, p 1285
- 38.Chang UK, Kim DH, Lee MC, Willenberg R, Kim SH, Lim J. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine. 2007;7(1):33–39. doi: 10.3171/SPI-07/07/033. [DOI] [PubMed] [Google Scholar]