Abstract
Introduction
Blunt cerebrovascular injuries (BCVI) of the extra- or intracerebral vessels are frequently observed lesions which may lead to thrombembolic events with focal neurological deficits, stroke or death particularly in patients <60 years. However, a comprehensive standardised clinical algorithm for screening and management of these secondary injuries is still lacking.
Materials and methods
We developed a standardised screening protocol applicable for mild as well as severely injured patients. In this prospective cohort study, we evaluated the feasibility of this diagnostic algorithm in a level 1 trauma centre setting. Trauma patients who met the inclusion criteria underwent a computed tomographic angiography (CTA) as part of standard diagnostic procedure at admission. All suspicions or positive findings were reevaluated by a conventional four-vessel catheter angiography within the first 72 h after trauma. Within this period, anticoagulation with low-dose heparin was started. BCVI confirmation indicated a shift to systemic heparinisation with overlapping phenprocoumon therapy for at least 6 months. All patients were reevaluated after 6 months by another four-vessel angiography. Depending on the diagnostic findings, oral anticoagulation may be discontinued or continued for another 6 months.
Results
A total of 44 patients (8 male, 6 female, age range 19–95 years) were included in the study. 20 BCVIs were detected in 16 patients (36.3%). The most common injuries identified were Biffl Type II (40%) and Type IV lesions (30%). 86.4% of the patients received a CTA upon admission, 93.2% of which were conducted within 12 h posttrauma. None of the patients had a secondary thrombembolic neurological event during the hospital stay or within 3 months postdischarge.
Conclusion
Our results indicate that implementation of the screening protocol can prevent strokes in patients without primary thrombembolic neurological deficits.
Keywords: Blunt cerebrovascular injuries (BCVI), Diagnostic protocol, Trauma care, CT angiography
Introduction
In acute trauma care, cerebrovascular injuries of the extra- or intracerebral vessels (also called blunt cerebrovascular injuries or BCVI) occur in 13–39% of cases following blunt trauma of the neck and the head [5, 21, 29]. If left untreated, these lesions often lead to thrombembolic events with focal neurologic deficits, stroke or death particularly in young patients below 60 years [24]. With prompt and sensitive diagnostics, the incidence of strokes can be reduced through early anticoagulation and if necessary, interventional therapy after detection of the BCVI [17].
Nevertheless, there are currently no consistent recommendations for a standardised diagnostic and therapeutic procedures in level 1 trauma centres with the objective of rapidly and correctly detecting BCVIs and avoiding secondary neurological deficits. Many lesions of the extra- or intracerebral vessels are still missed in trauma patients [5].
Although screening protocols have been introduced in the literature [13, 19, 25, 27], the diagnostic cascade of many hospitals does not focus on BCVIs because the recommended algorithms are not applicable for acute trauma care. To address this limitation, we developed a standardised screening protocol which is also feasible for severely injured patients (ISS > 16 pts.) and can thus be used in level 1 trauma centre. By means of this algorithm, we attempt to identify the major area of the dissected vertebral and carotid arteries without endangering the patient by delaying of the diagnostic or therapeutic cascade of more acute injuries.
The aim of this prospective cohort study is to evaluate the feasibility of this screening protocol and the accompanying diagnostic and therapeutic cascade in the clinical setting. In addition, we also attempted to identify the major part of the dissected vertebral and carotid arteries in this population without endangering the patient by delaying the diagnostic or therapeutic cascade.
Methods
Inclusion criteria
A total of 44 multiple injured patients admitted to the Department of Trauma, Hand, and Reconstructive Surgery of the University Hospital Münster in Germany (a level 1 trauma centre) between September 2007 and February 2009 were included. Inclusion criteria were cervical spine fractures, fractures of the transverse foramen, odontoid fractures, C1 fractures, incomplete or complete atlanto-occipital dislocations, skull base fractures, midface fractures (LeFort type III), seat belt injuries with cervical soft tissue marks, expanding cervical haematoma, cervical bleeding, diffuse axonal injuries with initial Glasgow coma scale <6, neurological deficit without radiological signs and trauma combined with a stroke (Table 1). These tracer injuries for BCVIs are geared to the criteria which were introduced by Biffl and colleagues of Denver [6] and Fabian and colleagues of Memphis [16].
Table 1.
Inclusion criteria
| Indication |
|---|
| Cervical spine fractures |
| Fractures of the transverse foramen |
| Odontoid fractures |
| C1 fractures |
| Atlantooccipital dislocation (complete/ incomplete) |
| Basal skull fracture |
| Midface fractures (LeFort type III) |
| Seabelt injuries with cervical soft tissue marks |
| Diffuse axonal injury with initial GCS < 6 |
| Neurological deficit without radiological signs |
| Stroke at time of trauma |
| Expanding cervical haematoma |
| Cervical bleeding |
GCS Glasgow coma scale
Screening protocol and diagnostic cascade
A computed tomographic angiography (CTA, Siemens SOMATOM Sensation 40-slice CT system, Siemens Medical Somaris/5) was conducted as part of the initial diagnostic protocol at the time of admission. The CTA was performed with primary contrast test bolus of 20 mL with a flow rate of 5 mL/s. When the arterial peak at 100 HU was reached, the circulation time was calculated. A contrast medium of 80 mL was then injected at a flow rate of 5 mL/s. After reaching the calculated delay as before, the CT scan was started with 1 mm slices at a pitch of 0.9 and Acq 40 × 0.6 mm.
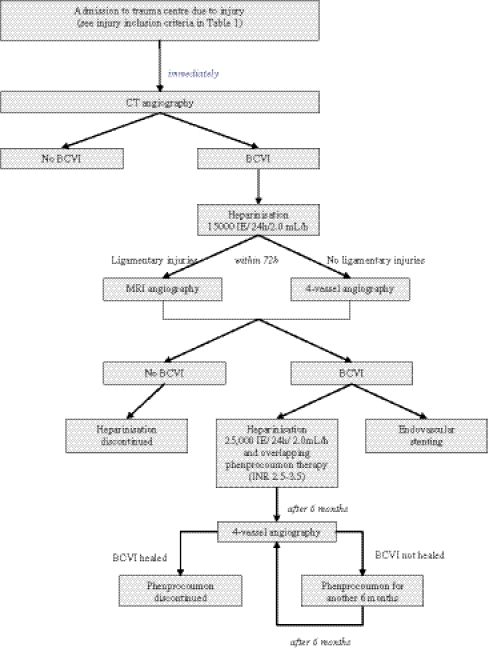
All suspected or positive cases were reevaluated using a conventional four-vessel catheter angiography within 72 h posttrauma to verify the occurrence of BCVI. Within this period, anticoagulation was started with low-dose heparin (15,000 IE/24 h/2.0 mL). In cases of suspected discoligamentary injuries, magnetic resonance imaging (MRI) angiography was performed (Fig. 1).
Fig. 1.
Screening protocol for diagnostics and therapy of blunt cerebrovascular injuries (BCVI). According to the damage control orthopedics concept [23, 28], delaying further diagnostics is acceptable if the treatment of life-threatening injuries takes precedence over the validation of the vessel injury
According to the damage control orthopedics concept [23, 28], delaying further diagnostics is acceptable if the treatment of life-threatening injuries took precedence over the validation of the vessel injury. The four-vessel-angiography was performed as soon as the general condition of the patient permitted.
As soon as BCVI was confirmed, low-dose heparinisation was substituted by systemic heparinisation (25,000 IE, 2 mL/h; desired activated partial thromboplastin time, aPTT = 60–80) and if possible for the type of lesion, therapeutic stenting was performed during the four-vessel angiography. As soon as the concomitant injuries allow, heparin therapy was discontinued after achieving an International Normalised Ratio (INR) of 2.5–3.5 with the oral anticoagulant phenprocoumon (Marcumar®). Phenprocoumon therapy was continued for at least 6 months. Patients who suffered a vertebral or carotid lesion were reevaluated after 6 months by another four-vessel-angiography. Depending on the diagnostic findings, the oral anticoagulation was discontinued or continued for another 6 months. All patients were observed for secondary neurological deficits during a minimum of a 3-month follow-up.
All CTAs and four-vessel-angiographies were analyzed by a radiologist. Aside from the height of the BCVI, every lesion was classified according to the Biffl grading scale [6] (Table 2). Although this scale was originally developed for the classification of blunt injuries of the carotid arteries, we adjusted the grading for the classification of vertebral artery lesions. Statistical descriptive analysis was conducted with SPSS for Windows 17.0 (SPSS Statistics, Chicago, IL, USA).
Table 2.
Blunt arterial injury grading scale according to Biffl et al. [6]
| Injury grade | Description |
|---|---|
| I | Luminal irregularity or dissection with <25% luminal narrowing |
| II | Dissection or intramural haematoma with ≤25% luminal narrowing Intramural thrombus or raised intimal flap |
| III | Pseudoaneurysm |
| IV | Occlusion |
| V | Transection with free extravasation |
Results
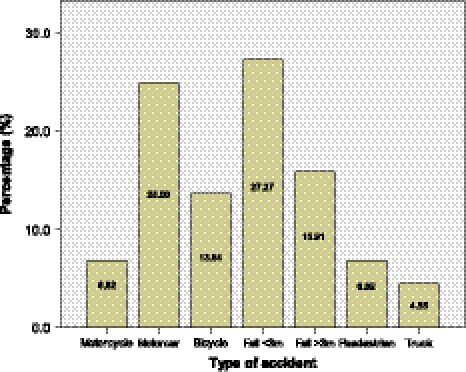
A total of 44 patients (38 male, 6 female) with a mean age of 45 (range 19–95) years were included in this study. The most frequent causes of accidents were falls (43.2%) and motorcar accidents (25%). Other cases involved bicycle (13.6%) motorcycle (6.8%) and truck (4.6%) accidents whereas pedestrian accidents accounted for 6.8% of all cases (Fig. 2).
Fig. 2.
Types of accidents that caused blunt cerebrovascular injuries
About 45% of the patients were severely injured with an injury severity score (ISS) > 16. Two patients died of their injuries within the first 12 h after hospitalisation. In terms of location, 22.7% had lesion of the vertebral arteries and 13.6% were diagnosed with injuries of the carotid arteries. Altogether, 20 cases of traumatic cerebrovascular lesions of the extra- or intracerebral vessels were detected in 16 patients (36.3%). Six BCVIs were localised at the left and six at the right side. A bilateral injury was detected in four patients. 65% of all lesions affected the vertebral and 35% the carotid arteries.
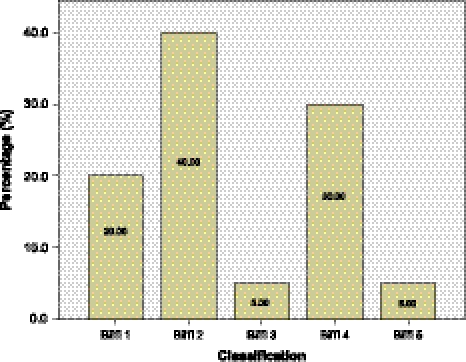
The most common lesion type (40%) detected were dissection or intramural haematomas with ≤25% luminal narrowing intramural thrombus or raised intimal flap (Biffl Type II). An occlusion Biffl Type IV accounted for 30% of lesions observed. Other lesions detected were luminal irregularity or dissection with <25% luminal narrowing Biffl Type I (20%), pseudoaneurysm Biffl Type III (5%) and a transection with free extravasation (5%, Fig. 3).
Fig. 3.
Types of lesions based on classification by Biffl et al. [6]
The vertebral artery was mostly affected at its intraosseous V2 segment (7 vessels). One vessel had an injury at the V1, 3 at the V3 and 2 at the V2 segment. A lesion at the end section of the vertebral artery (V5 segment) proximal to the confluence into the basilar artery was not detected in any of the cases. Three patients suffered from a multisegmental injury of the vertebral artery (Table 3).
Table 3.
Patient demographics and characteristics of BCVI
| Patient characteristics | |
|---|---|
| Number of patients, N | 44 |
| Male, N (%) | 38 (86.4) |
| Female, N (%) | 6 (13.6) |
| Age, years, range (mean) | 19–95 (45.2) |
| ISS > 16, N (%) | 20 (45.5) |
| ISS < 16, N (%) | 24 (54.5) |
| Deaths, N (%) | 2 (4.5) |
| Dissection characteristics and localisation | |
|---|---|
| Total number dissections, N in n patients | 20 dissections in 16 patients |
| Right side dissection, N (%) | 6 (30) |
| Left side dissection, N (%) | 6 (30) |
| Bilateral dissection, N (%) | 4 (20) |
| Primary stroke, N (%) | 1 (5) |
| Secondary stroke, N (%) | 0 (0) |
| Dissection of vertebral artery*, N | 13 |
| V1 | 1 |
| V2 | 7 |
| V3 | 3 |
| V4 | 2 |
| Dissection of carotid arterya, N | 7 |
| C1 | 4 |
| C2 | 2 |
| C3 | 3 |
| C4 | 2 |
| C5 | 2 |
| BCVI type according to Biffl [6] | |
| Biffl Type I, N (%) | 4 (20) |
| Biffl Type II, N (%) | 8 (40) |
| Biffl Type III, N (%) | 1 (5) |
| Biffl Type IV, N (%) | 6 (30) |
| Biffl Type V; N (%) | 1 (5) |
aMultilevel injury possible
ISS Injury severity score
The carotid artery has lesions at the cervical C1 segment in four cases, at the petrous C2 segment in two cases, three times at the lacerum segment C3 and two vessels showed an injury at the cavernous C4 and at the clinoideus C5 segment in each case. Three patients also sustained a multisegmental lesion of the carotid artery (Table 3).
None of the patients had a secondary thrombembolic neurological event during the hospital stay or within at least the next 3 months after discharge. No intraoperative or postoperative bleeding complications occurred. One patient presented with symptoms of a middle cerebral artery stroke already at the time of admission due to a dissection of the right carotid artery. In this case, a spontaneous dissection or a stroke as cause of the accident could not be excluded.
Six patients suffered from an incomplete atlano-occipital dislocation, six patients had an odontoid fracture and four a fracture of the first vertebral body. In three cases, a basal skull fracture and in four patients a midface fracture LeFort type III occurred. The most common injuries were cervical spine fractures or discoligamentary injuries of the cervical spine (18 patients), diffuse axonal injuries (11 patients), and fractures of the transverse foramen (9 patients), and which all indicated CTA. One patient was included in this study because of a cervical contusion caused by a seat belt injury and another patient because of a neurological deficit at time of admission, which was not detected by the cranial CT scan. There were no cases of expanding cervical haematoma or cervical bleeding reported (Table 4).
Table 4.
Types of injuries
| Injury type | N |
|---|---|
| Basal skull fracture | 3 |
| Fracture of transverse foramen | 9 |
| Fracture of cervical spine | 18 |
| Midface fracture Le Fort III | 4 |
| Diffuse axonal injury | 11 |
| Odontoid fracture | 6 |
| Atlanto-occipital dislocation (complete/ incomplete) | 6 |
| C1 Fracture | 4 |
| Stroke at time of trauma | 1 |
| Seat belt injuries | 1 |
| Cervical bleeding | 0 |
| Expanding cervical haematoma | 0 |
| Neurological deficit | 1 |
CTA was performed in 86.4% of the patients upon admission to the hospital. In 93.2% of cases, CTA was done within the first 12 h after the trauma occurred, and all patients were examined within the first 48 h. Time delay was caused by a primary treatment at other hospitals, which did not integrate the CTA into their primary diagnostics. In those cases, the CTA was performed when the patient admitted to our hospital.
Four patients did not have a secondary four-vessel angiography but underwent an MRI angiography. All these patients were suspected to have a discoligamentary injury of the craniocervical junction or the cervical spine. Aside from the evaluation of the vessels, soft tissue injuries could also be verified.
21,850 patients were treated at our hospital between September 2007 and February 2009. Just 16 of them (0.07%) suffered cerebrovascular injuries of the extra- or intracerebral vessels. In 2.75% of all severely injured patients a BCVI occurred. After screening for the inclusion criteria (Table 1) the detection rate improved to 36.3%.
Discussion
Traumatic cerebrovascular injuries of the extra- or intracerebral vessels (BCVI) are often missed in primary diagnostics and leads to neurological deficits in 23–58% of cases [7, 8, 21]. In more than 30% of cases, the diagnostics for BCVI are not started until a symptom caused by the vessel injury appears [20].
Assessing all suffered injuries of the often severely injured patients (45.5%) without major time delay for an early onset of the therapeutic treatment is one of the most difficult but important issue of the diagnostic cascade. Obtaining the diagnosis of all injuries can avoid secondary implication of the sustained injuries, not only concerning the lesion of the vessels.
Because of an incidence of 0.24–1.03% in all casualties of blunt cerebrovascular injuries [5, 7, 12, 20, 21], a feasible screening algorithm which considers on factors and tracer diagnoses that determines a BCVI is strongly required. These factors and tracer diagnoses have been introduced by the Denver [6] and Memphis trauma groups [16] and have been used in many studies [8, 13, 14].
In screening for BCVI, Miller et al. [21] identified lesions of the vertebral artery (VAI) in 33% of patients with cervical spine fractures. 16% of the patients who had neurological deficits which were not visible in brain imaging also had VAIs. In the same group of patients, 31% suffered lesions of the carotid artery (CAI). In patients with basilar skull fracture, a 30% CAI rate was reported.
Edmundson et al. [15] reported a case of a 30-year-old motorcar driver who sustained a dissection of the carotid artery without presenting one of the previously described tracer injuries. Because the inclusion criteria suggested by the Denver and Memphis groups are index injuries for high-velocity traumas, it is not sure, whether the injuries themselves determine the BCVI or if the trauma mechanism does. More precise differentiation should be addressed in future studies with the option to extend the indication for the CTA.
Rogers et al. [26] reported a significant improvement in BCVI detection rates after implementation of CTA into the clinical routine with only marginal loss of time. The CTA can be included into the primary diagnostics without integral retarding effect because most of the patients suffered the characteristic injuries that warrant a CT scan of the cervical spine and the cranium.
In the current study, the proposed screening protocol (Fig. 1) detected BCVI in 2.75% of all severely injured patients and in 0.07% of all patients admitted to our hospital between September 2007 and February 2009. For a feasible and timely implementation of the diagnostic and therapeutic routine into a trauma centre level 1, a sensitive preselection of patients by means of clearly defined inclusion criteria is indispensable. Guidance is given by the factors which were recommended by the Denver [6] and Memphis [16] trauma groups. By using the current screening protocol, our detection rate improved from 0.07 and 2.75–36.3%.
A few years ago, performing a CTA as a screening examination for BCVI was considered controversial. In the meantime, several studies have validated the use of the CTA as a sensitive tool in the diagnostics of BCVIs particularly concerning technical innovation and further development about the resolution of the CT [2, 4, 10, 30]. However, the limitation for the use of a CTA in BCVI diagnostic is the availability of a 16-slice CT system [2, 4, 10, 30]. The efficiency of trauma CT scan of a severely injured patient can be increased by the CTA of the intra- and extracerebral vessels without an essential loss of time. Eastman et al. [14] showed a significant reduction of the time-to-diagnosis of 31.2 ± 41.1 h before introduction of the CTA to 2.65 ± 3.3 h after integration of the CTA into the primary diagnostics. The stroke rate was reduced from 15.2 to 3.8% after introduction of CTA.
MRI angiography has several disadvantages compared to CTA. Despite the fact that the procedure is noninvasive without the need for contrast medium injection, MRI is very time-consuming, expensive and not feasible for severely injured and ventilated patients at the early phase.
Several studies have shown that duplex sonography is also not useful for screening BCVI because of insufficient sensitivity between 38.5% [22] and 86% [11]. Another problem for the diagnosis of the BCVI using the duplex sonography is the localisation of the lesion which is often at the base of the skull or at the intraosseous course of the vertebral artery [9, 17, 21]. The indirect signs of a BCVI which are blood flow disturbances due to pathological narrowing of the vessels similar to arteriosclerosis can lead to incorrect findings, thus BCVI Grade 1 and 3 as well as many Grade 2 lesions can be missed [3]. Therefore, duplex sonography is not a useful tool in the primary diagnostics of BCVI, but maybe can be used during the follow-up in suitable cases.
Seventy-four hours after admission, a conventional four-vessel angiography has to be performed in cases of suspected findings in the primary CTA to validate the BCVI. Although the CTA is an adequate screening procedure for BCVI, its specificity and sensitivity is still lower compared to the conventional angiography without the possibility of primary endovascular stenting [1, 18]. Low-grade injuries like Grade 1 lesions, which cause strokes in 7% of cases and can progress to higher-grade injuries in 9% are missed more often in CTA [9].
In our study, no patient who had an initial CTA without any pathological findings concerning the vertebral or carotid artery had any neurological deficit during the follow-up. Either the CTA did not miss any lesion or the missed lesions did not cause neurological deficits. Thus, a four-vessel angiography in cases of negative CTA findings was not necessary.
If there is a suspicion of an additional discoligamentary injury at the cervical spine, the four-vessel angiography can be substituted for a MRI angiography. If the injuries are serious and transport of the patient is not feasible without endangering the patient, the four-vessel angiography is performed only after stabilisation, as soon the patient’s condition permits.
With the algorithm described here, early therapy which is essential in prevention of neurological deficits can already be started in case of BCVI suspected findings in the CTA immediately after the admission and need not be delayed by waiting time necessary for the organisation of the logistic requirements for the four-vessel angiography or MRI as well as the transport of a severely injured person.
The therapeutic concept has to be planned considering the injuries, the morphology of the vessel lesion, the age of the patient and the comorbidities. After suspect findings in the CTA, a low dose heparinisation has to be started. Depending on the vascular level according to Biffl [6] in single lesions, the endovascular stenting during the four-vessel angiography would be performed. If this is not feasible, a systemic heparinisation (25000 IE/24 h, aPTT 60–80 s) should be started with a subsequent administration of the oral anticoagulant phenprocoumon. After achievement of the therapeutic range (INR 2.5–3.5), the heparinisation can be discontinued but the oral anticoagulation should be continued for 6 months. After 6 months, the BCVI has to be re-evaluated in another four-vessel angiography. Depending on the diagnostic findings, phenprocoumon has to be continued for 6 months.
With the implementation of the described screening protocol, we could prevent any strokes in patients without primary thrombembolic neurological deficits. Thus, the screening protocol and diagnostic cascade we presented here is a feasible and useful tool in the rapid detection of BCVI is seriously injured trauma patients.
References
- 1.Berne JD, Norwood SH, McAuley CE, Villareal DH (2004) Helical computed tomographic angiography: an excellent screening test for blunt cerebrovascular injury. J Trauma 57:11–17 (discussion 17–19) [DOI] [PubMed]
- 2.Berne JD, Reuland KS, Villarreal DH, McGovern TM, Rowe SA, Norwood SH (2006) Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J Trauma 60:1204–1209 (discussion 1209–1210) [DOI] [PubMed]
- 3.Biffl WL. Diagnosis of blunt cerebrovascular injuries. Curr Opin Crit Care. 2003;9:530–534. doi: 10.1097/00075198-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Biffl WL, Egglin T, Benedetto B, Gibbs F, Cioffi WG (2006) Sixteen-slice computed tomographic angiography is a reliable noninvasive screening test for clinically significant blunt cerebrovascular injuries. J Trauma 60:745–751 (discussion 751–752) [DOI] [PubMed]
- 5.Biffl WL, Moore EE, Elliott JP, Ray C, Offner PJ, Franciose RJ, Brega KE, Burch JM. The devastating potential of blunt vertebral arterial injuries. Ann Surg. 2000;231:672–681. doi: 10.1097/00000658-200005000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47:845–853. doi: 10.1097/00005373-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Biffl WL, Moore EE, Ryu RK, Offner PJ, Novak Z, Coldwell DM, Franciose RJ, Burch JM. The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg. 1998;228:462–470. doi: 10.1097/00000658-199810000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biffl WL, Ray CE, Moore EE, Franciose RJ, Aly S, Heyrosa MG, Johnson JL, Burch JM (2002) Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg 235:699–706 (discussion 706–707) [DOI] [PMC free article] [PubMed]
- 9.Biffl WL, Ray CE, Jr, Moore EE, Mestek M, Johnson JL, Burch JM. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma. 2002;53:850–856. doi: 10.1097/00005373-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Borisch I, Boehme T, Butz B, Hamer OW, Feuerbach S, Zorger N. Screening for carotid injury in trauma patients: image quality of 16-detector-row computed tomography angiography. Acta Radiol. 2007;48:798–805. doi: 10.1080/02841850701422104. [DOI] [PubMed] [Google Scholar]
- 11.Cogbill TH, Moore EE, Meissner M, Fischer RP, Hoyt DB, Morris JA, Shackford SR, Wallace JR, Ross SE, Ochsner MG, et al. The spectrum of blunt injury to the carotid artery: a multicentre perspective. J Trauma. 1994;37:473–479. doi: 10.1097/00005373-199409000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Cothren CC, Moore EE, Biffl WL, Ciesla DJ, Ray CE, Jr, Johnson JL, Moore JB, Burch JM (2004) Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg 139:540–545 (discussion 545–546) [DOI] [PubMed]
- 13.Cothren CC, Moore EE, Ray CE, Jr, Ciesla DJ, Johnson JL, Moore JB, Burch JM. Screening for blunt cerebrovascular injuries is cost-effective. Am J Surg. 2005;190:845–849. doi: 10.1016/j.amjsurg.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Eastman AL, Muraliraj V, Sperry JL, and Minei JP (2009) CTA-based screening reduces time to diagnosis and stroke rate in blunt cervical vascular injury. J Trauma 67:551–556 (discussion 555–556) [DOI] [PubMed]
- 15.Edmundson SP, Hirpara KM, Ryan RS, O’Malley T, O’Grady P. Delayed presentation of carotid artery dissection following major orthopaedic trauma resulting in dense hemiparesis. J Bone Joint Surg Br. 2009;91:536–539. doi: 10.1302/0301-620X.91B4.22008. [DOI] [PubMed] [Google Scholar]
- 16.Fabian TC, Patton JH, Jr, Croce MA, Minard G, Kudsk KA, Pritchard FE (1996) Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy. Ann Surg 223:513–522 (discussion 522–525) [DOI] [PMC free article] [PubMed]
- 17.Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21:252–258. doi: 10.1097/BSD.0b013e3180cab162. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin RB, Beery PR, 2nd, Dorbish RJ, Betz JA, Hari JK, Opalek JM, Magee DJ, Hinze SS, Scileppi RM, Franz RW, Williams TD, Jenkins JJ, Suh KI. Computed tomographic angiography versus conventional angiography for the diagnosis of blunt cerebrovascular injury in trauma patients. J Trauma. 2009;67:1046–1050. doi: 10.1097/TA.0b013e3181b83b63. [DOI] [PubMed] [Google Scholar]
- 19.Lewine JD, Davis JT, Bigler ED, Thoma R, Hill D, Funke M, Sloan JH, Hall S, Orrison WW. Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. J Head Trauma Rehabil. 2007;22:141–155. doi: 10.1097/01.HTR.0000271115.29954.27. [DOI] [PubMed] [Google Scholar]
- 20.Miller PR, Fabian TC, Bee TK, Timmons S, Chamsuddin A, Finkle R, Croce MA (2100) Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma 51:279–285 (discussion 285–286) [DOI] [PubMed]
- 21.Miller PR, Fabian TC, Croce MA, Cagiannos C, Williams JS, Vang M, Qaisi WG, Felker RE, Timmons SD (2002) Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg 236:386–393 (discussion 393–395) [DOI] [PMC free article] [PubMed]
- 22.Mutze S, Rademacher G, Matthes G, Hosten N, Stengel D. Blunt cerebrovascular injury in patients with blunt multiple trauma: diagnostic accuracy of duplex Doppler US and early CT angiography. Radiology. 2005;237:884–892. doi: 10.1148/radiol.2373042189. [DOI] [PubMed] [Google Scholar]
- 23.Pape HC, Giannoudis P, Krettek C. The timing of fracture treatment in polytrauma patients: relevance of damage control orthopedic surgery. Am J Surg. 2002;183:622–629. doi: 10.1016/S0002-9610(02)00865-6. [DOI] [PubMed] [Google Scholar]
- 24.Ribbons T, Bell S. Neck pain and minor trauma: normal radiographs do not always exclude serious pathology. Emerg Med J. 2008;25:609–610. doi: 10.1136/emj.2007.050328. [DOI] [PubMed] [Google Scholar]
- 25.Risgaard O, Sugrue M, D’Amours S, Christey G, Smith K, Caldwell E, Lariviere C. Blunt cerebrovascular injury: an evaluation from a major trauma centre. ANZ J Surg. 2007;77:686–689. doi: 10.1111/j.1445-2197.2007.04187.x. [DOI] [PubMed] [Google Scholar]
- 26.Rogers FB, Baker EF, Osler TM, Shackford SR, Wald SL, Vieco P. Computed tomographic angiography as a screening modality for blunt cervical arterial injuries: preliminary results. J Trauma. 1999;46:380–385. doi: 10.1097/00005373-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Scheid R, Zimmer C, Schroeter ML, Ballaschke O, Cramon DY. The clinical spectrum of blunt cerebrovascular injury. Neurologist. 2006;12:255–262. doi: 10.1097/01.nrl.0000243977.17242.ab. [DOI] [PubMed] [Google Scholar]
- 28.Taeger G, Ruchholtz S, Waydhas C, Lewan U, Schmidt B, Nast-Kolb D (2005) Damage control orthopedics in patients with multiple injuries is effective time saving and safe. J Trauma 59:409–416 (discussion 417) [DOI] [PubMed]
- 29.Torina PJ, Flanders AE, Carrino JA, Burns AS, Friedman DP, Harrop JS, Vacarro AR. Incidence of vertebral artery thrombosis in cervical spine trauma: correlation with severity of spinal cord injury. AJNR Am J Neuroradiol. 2005;26:2645–2651. [PMC free article] [PubMed] [Google Scholar]
- 30.Utter GH, Hollingworth W, Hallam DK, Jarvik JG, Jurkovich GJ. Sixteen-slice CT angiography in patients with suspected blunt carotid and vertebral artery injuries. J Am Coll Surg. 2006;203:838–848. doi: 10.1016/j.jamcollsurg.2006.08.003. [DOI] [PubMed] [Google Scholar]