Abstract
Purpose
Numerous posterior non-fusion systems have been developed within the past decade to resolve the disadvantages of rigid instrumentations and preserve spinal motion. The aim of this study was to investigate the effect of a new dynamic stabilization device, to measure the screw anchorage after flexibility testing and compare it with data reported in the literature.
Methods
Six human lumbar spine motion segments (L2–5) were loaded in a spine tester with pure moments of 7.5 Nm in lateral bending, flexion/extension and axial rotation. Specimens were tested intact, after instrumentation of the intact segment, after destabilization by a nucleotomy and after instrumentation of the destabilised segment with the new non-fusion device (Elaspine). After flexibility testing all screws were subjected to a pull-out test.
Results
Instrumentation of the intact segment significantly reduced the RoM (p < 0.002) in flexion, extension and lateral bending to 49.7, 44.6 and 53% of the intact state, respectively. In axial rotation, the instrumentation resulted in a non-significant RoM reduction to 95% of the intact state. Compared to the intact segment, instrumentation of the destabilized segment significantly (p < 0.05) reduced the RoM to 69.8, 62.3 and 79.1% in flexion, extension and lateral bending, respectively. In axial rotation, the instrumented segment showed a significantly higher RoM than the intact segment (137.6% of the intact state (p < 0.01)). The pull-out test showed a maximum pull-out force of 855.1 N (±334) with a displacement of 6.1 mm (±2.8) at maximum pull-out force.
Conclusions
The effect of the investigated motion preservation device on the RoM of treated segments is in the range of other devices reported in the literature. Compared to the most implanted and investigated device, the Dynesys, the Elaspine has a less pronounced motion restricting effect in lateral bending and flexion/extension, while being less effective in limiting axial rotation. The pull-out force of the pedicle screws demonstrated anchorage comparable to other screw designs reported in the literature.
Keywords: Posterior dynamic stabilization, Kinematics, Biomechanics, Lumbar spine
Introduction
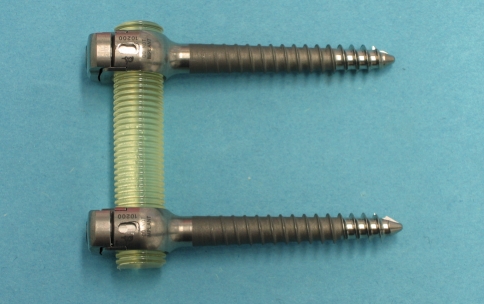
In industrialized countries, epidemiological studies state the percentage of people suffering from low back pain at some point of their life with 70–85% [1]. Once conservative treatment fails, spinal fusion, sometimes accompanied with neural decompression, is still considered the standard treatment [2, 3] for higher degrees of segmental degeneration and instability. However, spinal fusion supported by rigid instrumentation can have undesired side effects, such as accelerated degeneration of adjacent segments [4, 5]. It is hypothesized, that fusion of a motion segment leads to an overload and hypermobility of adjacent segments. Non-fusion, also known as dynamic stabilization or motion preservation devices aim to maintain or moderately reduce the mobility of a motion segment and thereby decrease or eliminate adjacent level degeneration. Generally, these devices can be divided into anterior and posterior implant concepts. Posterior devices can be further grouped into interspinous spacers, facet joint replacements and pedicle-screw-based implant concepts [6]. The latter either consists of a non-rigid connecting element between the pedicle screws, or a joint between the pedicle screw/rod connection allowing a hinged or polyaxial motion. There are various design concepts of non-rigid connection elements ranging from flexible springs to more sophisticated damping elements with direction dependable stiffness in tension, compression and bending. Undoubtedly, the Dynesys (Zimmer GmbH, Winterthur, Switzerland) is the most widespread and popular posterior dynamic stabilization device. It is built up of a polycarbonate urethane (PCU) spacer surrounding a polyethylene-therephtalate (PET) cord, which is connected to the pedicle screws. Flexion movements resulting in tensile forces in the device are limited by the PET cords connected to the pedicle screws. Extension movements causing compression of the device are restricted by the PCU spacer between the pedicle screws. However, biomechanical studies have shown, that the Dynesys device has a more motion restricting effect in flexion than in extension [7, 8]. The newly developed Elaspine device (Spinelab AG, Winterthur, Switzerland) investigated in the present study is comprised of pedicle screws and a locking clip mechanism with a 360° form-fit connecting to an elastic PCU rod (Fig. 1). This design is intended to allow a relatively homogenous load transmission in all directions. The aim of the study was to evaluate the effect of Elaspine on the range of motion (RoM) of an intact instrumented and surgically destabilized motion segment and to compare it with published data of clinically established non-fusion devices. Furthermore, the anchorage of the pedicle screws was tested in a pull-out test and further compared to other pedicle screw designs published in the literature.
Fig. 1.
Investigated new pedicle screw based motion preservation implant (Elaspine, Spinelab AG, Winterthur, Switzerland) comprised of pedicle screws and a locking clip mechanism with a 360° form-fit connecting to an elastic PCU rod
Methods
Specimen preparation
For biomechanical testing, six fresh frozen human lumbar spines (L2–5) with a mean age of 62.2 years (SD 12.7) were used. Prior to testing a quantitative computed tomography (qCT) scan (GE Lightspeed 16, GE Medical Systems, Waukesha, WI, USA) including European Forearm Phantom calibration (EFP; QRM GmbH, Möhrendorf, Germany) was carried out to determine bone mineral density (BMD). Specimens with previous spinal surgery, posttraumatic abnormalities and structural disorders were excluded. Mean measured trabecular BMD of the instrumented vertebrae was 108.2 mg/cm3 (SD 21.9). Specimens were vacuum-sealed in double plastic bags and kept frozen at −30°C. Prior to testing, specimens were thawed overnight at 6°C and prepared at room temperature right before testing. All muscular tissue was dissected while ligaments, joint capsules and discs were kept intact. For fixation of the specimens in a spine tester, the cranial half of L2 and the caudal half of L5 were embedded in PMMA cement (polymethyl-methacrylate, Technovit 3040, Heraeus Kulzer, Wehrheim, Germany) and two flanges were mounted on the PMMA blocks. Screws for fixation of a 3D motion analysis system (Winbiomechanics, Zebris, Isny, Germany) were fixed to the anterior side of each vertebra.
Surgical procedure
Prior to the flexibility tests, Elaspine pedicle screws (Spinelab AG, Winterthur, Switzerland, Fig. 1) were implanted in L3 and L4 under fluoroscopic control. All pedicle screws were 6.5 mm in diameter and 45 mm long. To verify pedicle screw positioning, anterior-posterior and lateral radiographs were taken (BV 25, Philips, Eindhoven, Netherlands). The following states of the specimens were tested:
intact
after instrumentation of the intact specimens with an elastic spacer in L3–4
after destabilization of L3–4
after instrumentation of the destabilized segment with an elastic spacer. The destabilisation consisted of a nucleotomy with removal of approximately 2 g of nucleus to mechanically simulate additional surgical intervention and/or a possible instability caused by disc degeneration.
Biomechanical testing
Flexibility testing of the specimens was conducted in a six-degree-of-freedom spine tester allowing pure moment loading [9]. The caudal end, where specimens are fixed to the load frame allows two translations in the transverse plane (x–y). At the cranial end, the bending moment is induced by cable pulleys via a stepper motor. The design of the cranial fixation allows free rotation in all three axes and translation in the longitudinal (z) axis. The mass of the cranial fixation is counterbalanced by dead weights. Moments and forces induced at the cranial end of the specimen were continuously recorded using a force/moment sensor (Schunk FT Delta SI 660-60, Lauffen/Neckar, Germany). Intersegmental motion was measured using an ultrasound-based motion analysis system (Winbiomechanics, Zebris, Isny, Germany, resolution 0.1°). The flexibility tests were conducted according to the recommendations for testing of spinal implants [10, 11] in the three main motion planes using pure moments of 7.5 Nm. In order to minimize viscoelastic effects and allow for preconditioning, only the third load cycle was used to determine the range of motion (RoM) and neutral zone (NZ) from the hysteresis curves.
After flexibility tests the discs were dissected, the L3 and L4 vertebrae were isolated and cleaned of all soft tissue and subjected to a pull-out test. Vertebrae were fixed in a bench vice with a ball-socket-joint and mounted in a servohydraulic material testing machine (858 MiniBionix II, MTS, MN, USA). Care was taken to align each pedicle in the axis of the testing machine actuator to ensure a loading of the pedicle screws in its axis. The pull-out test was performed with a preload of 20 N and a constant axial displacement rate of 5 mm/min until the pedicle screws were completely extracted. Maximum pull-out forces and corresponding displacement as well as work to failure were determined from force/displacement graphs of the recorded data. Stiffness values of pedicle screw anchorage were determined for selected load ranges. Maximum pull-out force was compared to data of pedicle screws with other thread designs available in the literature.
Statistical analysis was performed using the SPSS software package (Microsoft Windows release 17.0; SPSS Inc., Chicago, IL, USA). All reported results represent the mean and standard deviation. To analyse for differences between the four tested states, repeated measures analysis of variance (ANOVA) with Bonferroni post hoc analysis was carried out. Significance was specified for a p value lower than 0.05.
Results
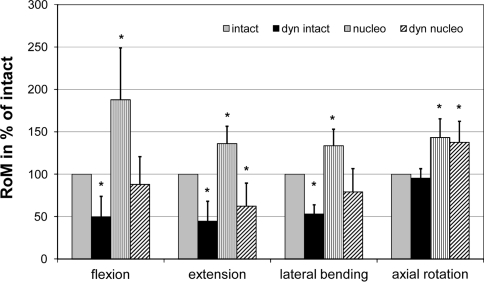
RoM and NZ of the instrumented segment in degrees are listed in Table 1. Changes in RoM normalized to the intact segment are presented in Fig. 2. Instrumentation of the intact motion segment caused a significant reduction in RoM in flexion, extension and lateral bending to 49.7, 44.5 and 53.5% of the intact specimen (Fig. 2). In axial rotation, the instrumentation of the intact segment had the least motion restricting effect (95% of intact). Destabilization of the specimens by a nucleotomy resulted in a significant increase in RoM in flexion, extension lateral bending and axial rotation to 187.8, 136.0, 133.7 and 143.3% of the intact, respectively. Instrumentation of the nucleotomized specimens reduced the RoM in flexion (87.9%) and lateral bending (79.2%) to values below those of intact specimens. In extension, the RoM was significantly reduced (62.3%), while in axial rotation the increase in RoM caused by the nucleotomy remained significantly higher than the intact RoM (137.6%).
Table 1.
Mean and standard deviation of RoM and NZ of the tested states in degrees
| Parameter and loading direction | Intact (°) | Dyn intact (°) | Nucleotomy (°) | Dyn nucleotomy (°) |
|---|---|---|---|---|
| RoM | ||||
| Flexion/extension | 6.74 (±1.73) | 2.45 (±0.31) | 9.34 (±2.72) | 4.17 (±0.85) |
| Lateral bending | 8.19 (±2.22) | 4.32 (±1.40) | 11.07 (±3.85) | 6.29 (±2.47) |
| Axial rotation | 3.65 (±1.54) | 3.49 (±1.64) | 5.25 (±2.49) | 4.91 (±2.08) |
| NZ | ||||
| Flexion/extension | 0.71 (±0.12) | 0.42 (±0.30) | 2.92 (±1.51) | 1.03 (±0.52) |
| Lateral bending | 1.08 (±0.34) | 0.95 (±0.29) | 3.49 (±1.90) | 1.74 (±0.97) |
| Axial rotation | 0.51 (±0.42) | 0.48 (±0.25) | 1.17 (±0.55) | 0.97 (±0.52) |
Fig. 2.
RoM normalized to the intact specimens for the intact instrumented specimen (dyn intact), the destabilized specimen (nucleo) and the destabilized instrumented specimen (dyn nucleo). Significant differences to the intact state are indicated by an asterisk
The pull-out test of the pedicle screws, subsequent to the flexibility test, showed a maximum pull-out force of 855.1 N (±334) with a displacement of 6.1 mm (±2.8) at maximum pull-out force. There was no correlation between BMD and maximum pull-out force (R = 0.140, p = 0.664), whereas the pull-out forces of the left and right pedicle screws of the same vertebra were highly correlated (r = 0.805, p = 0.005). The mean work to failure was 3036.1 Nmm (±2162). Stiffness values were highest for small loads and showed a non-linear decrease with increasing load magnitude (Table 2).
Table 2.
Stiffness determined for low and high load magnitudes in the pull-out test of the pedicle screws
| Range used for calculating stiffness | 20–35 N | 35–50 N | 50–100 N | 100–200 N | 200–350 N |
|---|---|---|---|---|---|
| Stiffness in N/mm | 336.1 | 303.7 | 255.3 | 214.2 | 213.9 |
| Standard deviation | 158.9 | 224.1 | 233.19 | 182.3 | 132.1 |
Discussion
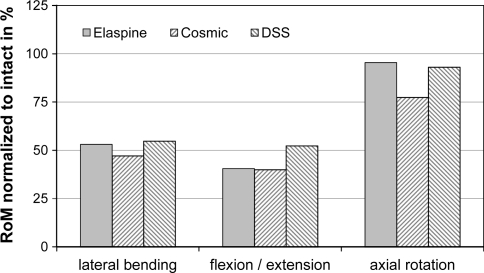
The present study investigated the effect of a newly introduced posterior motion preservation device on the stiffness of a motion segment, with and without the presence of a surgical induced structural defect of the intervertebral disc. Instrumentation of an intact segment without additional surgical intervention resulted in a reduction in RoM of approximately 50% in flexion, extension and lateral bending. In axial rotation, the Elaspine device showed the least motion restricting effect. This is comparable with two published in vitro results of other non-fusion devices on intact segments (Fig. 3) also showing the least motion restricting effect in axial rotation. After instrumentation of an intact segment with the DSS implant (Paradigm Spine, Wurmlingen, Germany), Wilke et al. [12] reported motion reductions to 52, 55 and 93% of the intact values in flexion/extension, lateral bending and axial rotation, respectively. Similar results of intact segment instrumentation were reported for a hinged non-fusion device (Cosmic, Ulrich Medical, Ulm, Germany) in flexion/extension and lateral bending, while in axial rotation the hinged design restricted the motion to 77% of the intact [13].
Fig. 3.
Comparison of RoM for intact instrumented segments with data published in the literature (cosmic—Schmoelz et al. [13]; DSS—Wilke et al. [12])
Clinically, motion preservation is aimed to fill the gap between segmental reconstruction techniques and rigid fusions. While allowing different magnitudes of motion a protection of segmental structures is aimed to be achieved by various load and motion controlling mechanisms. However, different biomechanical studies highlighted the limitations of such devices. While a motion restriction is achieved to a certain extent in flexion/extension and lateral bending—in intact segments as well as in defect situations [7, 12–18]—in axial rotation the motion restriction seems to be limited in most of the implant designs [7, 8, 12, 14, 17, 18]. Therefore, the clinical indication should be limited to early degeneration stages in the absence of a high-grade instability. However, matching of the individual grade of segmental instability with an ideal motion restriction provided by a certain device seems to be an unsolved problem.
Recently, for one of the clinically most used motion preservation system an increased incidence of pedicle screw loosening and/or failure was reported [19–21]. This might be associated with an altered screw loading reported for motion preservation devices in experimental and Finite Element studies [22, 23]. Although pull-out tests of pedicle screws apply a non-physiological load vector, they are a generally accepted tool to evaluate pedicle screw anchorage. In this study, we were able to show that the pedicle screws of the Elaspine device are able to resist pull-out forces well comparable to values reported in the literature.
Direct comparison of the effect of different non-fusion devices on the RoM after an additional surgical intervention to other published in vitro studies is difficult. This is due to the differences in surgical intervention and different extents of decompressions/defects performed in the in vitro studies, causing different magnitudes of instabilities. Depending on surgical indications and surgeons preferences the instabilities and interventions carried out in in vitro studies range from isolated instabilities of the disc, instabilities of the disc combined with posterior decompression and transection of posterior ligaments to isolated posterior decompression with transection of posterior ligaments [7, 8, 12, 13, 16–18, 24, 25]. These surgical interventions cause different magnitudes of RoM increase and hence pose different demands on the stabilizing capability of a device.
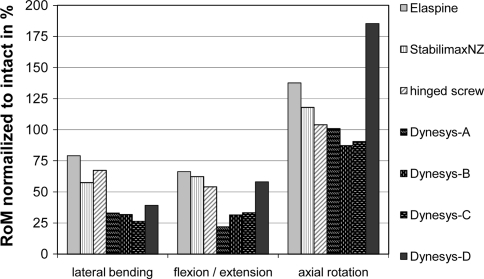
To allow a comparison to other studies that investigated non-fusion devices, all published RoM data was normalized to the reported intact state of each study (Fig. 4). Following surgical destabilisation, the Elaspine device investigated in this paper showed comparable results to the StabilimaxNZ [17] (Applied Spine Technology, new haven, CT, USA) and a hinged pedicle screw system [24] (Safinaz, Algorithma AS, Turkey) in lateral bending (60–79% of intact) and flexion/extension (55–66% of intact). In axial rotation, the Elaspine, the StabilimaxNZ and the hinged pedicle screw did not reduce the RoM of previously surgically destabilized level below intact values.
Fig. 4.
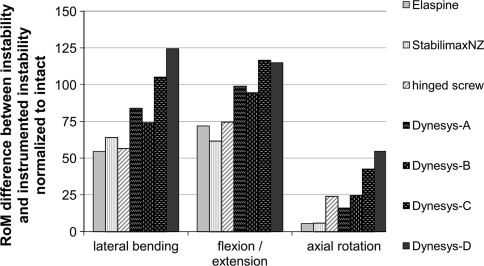
Comparison of RoM for destabilized instrumented segments with data published in the literature (StabilimaxNZ—Panjabi et al. [17]; hinged screw—Bozkus et al. [15]; Dynesys-A—Gedet et al. [7]; Dynesys-B—Schulte et al. [18]; Dynesys-C—Niosi et al. [16]; Dynesys-D—Schmoelz et al. [8])
In summarizing the results of the Dynesys that was investigated in several in vitro experiments, the majority of studies showed a RoM in the range between 20 and 40% of the intact specimen [7, 16, 18] in lateral bending and flexion/extension. In axial rotation, the Dynesys resulted in a RoM of 90–101% of the intact. Opposing these results, Schmoelz et al. [8] reported a less motion restricting effect for the Dynesys. However, this might be due to a larger increase in RoM caused by the simulated surgical intervention. In order to more clearly assess the effect of a device after surgical intervention, one should also look at the difference between the instability caused by surgical intervention and the RoM after instrumentation of the instability. This difference compensates the effect of different surgical interventions to some extent and highlights the effect of a device more clearly (Fig. 5).
Fig. 5.
Comparison of RoM adjusted for differences in the simulated instability. Bars indicate the difference between the instability and the instrumented instability for data published in the literature (StabilimaxNZ—Panjabi et al. [17]; hinged screw—Bozkus et al. [15]; Dynesys-A—Gedet et al. [7]; Dynesys-B—Schulte et al. [18]; Dynesys-C—Niosi et al. [16]; Dynesys-D—Schmoelz et al. [8])
This difference shows comparable effects in lateral bending and flexion/extension for the Elaspine, StabilimaxNZ and the hinged pedicle screw design, while all studies investigating the Dynesys restricted the motion to a higher degree. In axial rotation, the Elaspine and StabilimaxNZ had a relatively small effect, while the hinged pedicle screw was in the lower range of the motion restriction reported for the Dynesys.
Maximum values of the measured pull-out forces for the Elaspine pedicle screws can be compared to other screw designs in the literature and to control groups of pull-out experiments investigating the effect of screw augmentation. Bullmann et al. [26] reported a lower maximum pull-out force (632.7 N) with a higher displacement at maximum force (6.8 mm) compared to the present study (855.1 N and 6.1 mm). This might be due to the older specimens (69 years) and the lower BMD (62.4 mg/cm3) in their control group. Masaki et al. [27] also used older specimens (78.3 years) and reported similar pull-out strength (647 N) as Bullmann et al. In the present study we did not find a correlation between BMD and maximum pull-out force. This might be due to the fact, that screw anchorage is not only affected by the trabecular structure within the vertebral body (where BMD was measured), but also on pedicle geometry and whether the screw is anchored only in the trabecular structure of the pedicle or has also cortical thread purchase in the pedicle. The higher stiffness for lower loads could due to the initial anchorage of the screw in the trabecular structure of the vertebra and the pedicle. For higher load magnitudes, first trabeculae might have been already fractured resulting in a decrease in pull-out stiffness.
The present study has some limitations as well. As in all biomechanical experiments, the number of specimens is limited and due to large inter-specimen variation the statistical power is limited. The present study only assessed the immediate post-operative state and did not take any long-term effects, such as bony ingrowth of the pedicle screws, into consideration. This could be an important factor concerning the pedicle screw pull-out test. The conducted pull-out tests only assessed the initial screw purchase. For assessment of screw loosening a repetitive loading in flexion/extension would be required. Therefore, it is not possible to draw any conclusion on long-term outcome and pedicle screw loosening.
Conclusion
The effect of the motion preservation device Elaspine investigated in the present study on the RoM of treated segments is in the range of other devices reported in the literature and currently under investigation in clinical trials. As compared with the most implanted and published device, the Dynesys, Elaspine is more flexible and allows a more physiological motion closer to the intact functional spinal unit in lateral bending and flexion extension. On the other hand, the less stiff device is also less effective in limiting motion in axial rotation. The pull-out force of the pedicle screws of the new device is comparable to other screw designs reported in the literature. While varying degrees of motion restriction were demonstrated for different devices, the decision for an ideal clinical application still widely discussed.
Acknowledgments
The study was supported by institutional funds of Spinelab AG, Winterthur, Switzerland. None of the authors received personal funding.
Conflict of interest
None.
References
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Fritzell P, Hagg O, Wessberg P, Nordwall A (2001) Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976) 26 (23):2521–2532; discussion 2532–2524 [DOI] [PubMed]
- 3.Gibson JN, Waddell G (2005) Surgery for degenerative lumbar spondylosis: updated Cochrane Review. Spine (Phila Pa 1976) 30 (20):2312–2320 [DOI] [PubMed]
- 4.Disch AC, Schmoelz W, Matziolis G, Schneider SV, Knop C, Putzier M. Higher risk of adjacent segment degeneration after floating fusions: long-term outcome after low lumbar spine fusions. J Spinal Disord Tech. 2008;21(2):79–85. doi: 10.1097/BSD.0b013e3180577259. [DOI] [PubMed] [Google Scholar]
- 5.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4(6 Suppl):190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Khoueir P, Kim KA, Wang MY. Classification of posterior dynamic stabilization devices. Neurosurg Focus. 2007;22(1):E3. doi: 10.3171/foc.2007.22.1.3. [DOI] [PubMed] [Google Scholar]
- 7.Gedet P, Haschtmann D, Thistlethwaite PA, Ferguson SJ. Comparative biomechanical investigation of a modular dynamic lumbar stabilization system and the Dynesys system. Eur Spine J. 2009;18(10):1504–1511. doi: 10.1007/s00586-009-1077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmoelz W, Huber JF, Nydegger T, Dipl I, Claes L, Wilke HJ. Dynamic stabilization of the lumbar spine and its effects on adjacent segments: an in vitro experiment. J Spinal Disord Tech. 2003;16(4):418–423. doi: 10.1097/00024720-200308000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Knop C, Lange U, Bastian L, Blauth M. Three-dimensional motion analysis with Synex. Comparative biomechanical test series with a new vertebral body replacement for the thoracolumbar spine. Eur Spine J. 2000;9(6):472–485. doi: 10.1007/s005860000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panjabi MM. Biomechanical evaluation of spinal fixation devices: I. A conceptual framework. Spine. 1988;13(10):1129–1134. doi: 10.1097/00007632-198810000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Wilke HJ, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7(2):148–154. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilke HJ, Heuer F, Schmidt H (2009) Prospective design delineation and subsequent in vitro evaluation of a new posterior dynamic stabilization system. Spine (Phila Pa 1976) 34 (3):255–261 [DOI] [PubMed]
- 13.Schmoelz W, Onder U, Martin A, Strempel A. Non-fusion instrumentation of the lumbar spine with a hinged pedicle screw rod system: an in vitro experiment. Eur Spine J. 2009;18(10):1478–1485. doi: 10.1007/s00586-009-1052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schilling C, Kruger S, Grupp TM, Duda GN, Blomer W, Rohlmann A. The effect of design parameters of dynamic pedicle screw systems on kinematics and load bearing: an in vitro study. Eur Spine J. 2010;20:297–307. doi: 10.1007/s00586-010-1620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozkus H, Senoglu M, Baek S, Sawa AG, Ozer AF, Sonntag VK, Crawford NR. Dynamic lumbar pedicle screw-rod stabilization: in vitro biomechanical comparison with standard rigid pedicle screw-rod stabilization. J Neurosurg Spine. 2010;12(2):183–189. doi: 10.3171/2009.9.SPINE0951. [DOI] [PubMed] [Google Scholar]
- 16.Niosi CA, Zhu QA, Wilson DC, Keynan O, Wilson DR, Oxland TR. Biomechanical characterization of the three-dimensional kinematic behaviour of the Dynesys dynamic stabilization system: an in vitro study. Eur Spine J. 2006;15(6):913–922. doi: 10.1007/s00586-005-0948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panjabi MM, Henderson G, James Y, Timm JP. StabilimaxNZ) versus simulated fusion: evaluation of adjacent-level effects. Eur Spine J. 2007;16(12):2159–2165. doi: 10.1007/s00586-007-0444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulte TL, Hurschler C, Haversath M, Liljenqvist U, Bullmann V, Filler TJ, Osada N, Fallenberg EM, Hackenberg L. The effect of dynamic, semi-rigid implants on the range of motion of lumbar motion segments after decompression. Eur Spine J. 2008;17(8):1057–1065. doi: 10.1007/s00586-008-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham BW, Dawson JM, Hu N, Kim SW, McAfee PC, Griffith SL. Preclinical evaluation of the Dynesys posterior spinal stabilization system: a nonhuman primate model. Spine J. 2010;10(9):775–783. doi: 10.1016/j.spinee.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Ko CC, Tsai HW, Huang WC, Wu JC, Chen YC, Shih YH, Chen HC, Wu CL, Cheng H. Screw loosening in the Dynesys stabilization system: radiographic evidence and effect on outcomes. Neurosurg Focus. 2010;28(6):E10. doi: 10.3171/2010.3.FOCUS1052. [DOI] [PubMed] [Google Scholar]
- 21.Ianuzzi A, Kurtz SM, Kane W, Shah P, Siskey R, van Ooij A, Bindal R, Ross R, Lanman T, Buttner-Janz K, Isaza J (2010) In vivo deformation, surface damage, and biostability of retrieved Dynesys systems. Spine (Phila Pa 1976) 35 (23):E1310–E1316 [DOI] [PubMed]
- 22.Liu CL, Zhong ZC, Shih SL, Hung C, Lee YE, Chen CS. Influence of Dynesys system screw profile on adjacent segment and screw. J Spinal Disord Tech. 2010;23(6):410–417. doi: 10.1097/BSD.0b013e3181b63d89. [DOI] [PubMed] [Google Scholar]
- 23.Meyers K, Tauber M, Sudin Y, Fleischer S, Arnin U, Girardi F, Wright T. Use of instrumented pedicle screws to evaluate load sharing in posterior dynamic stabilization systems. Spine J. 2008;8(6):926–932. doi: 10.1016/j.spinee.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Bozkus H, Senoglu M, Baek S, Sawa AG, Ozer AF, Sonntag VK, Crawford NR (2010) Dynamic lumbar pedicle screw-rod stabilization: in vitro biomechanical comparison with standard rigid pedicle screw-rod stabilization. J Neurosurg Spine 12 (2):183–189 [DOI] [PubMed]
- 25.Scifert JL, Sairyo K, Goel VK, Grobler LJ, Grosland NM, Spratt KF, Chesmel KD (1999) Stability analysis of an enhanced load sharing posterior fixation device and its equivalent conventional device in a calf spine model. Spine (Phila Pa 1976) 24 (21):2206–2213 [DOI] [PubMed]
- 26.Bullmann V, Schmoelz W, Richter M, Grathwohl C, Schulte TL (2010) Revision of cannulated and perforated cement-augmented pedicle screws: a biomechanical study in human cadavers. Spine (Phila Pa 1976) 35:E932–E939 [DOI] [PubMed]
- 27.Masaki T, Sasao Y, Miura T, Torii Y, Kojima A, Aoki H, Beppu M (2009) An experimental study on initial fixation strength in transpedicular screwing augmented with calcium phosphate cement. Spine (Phila Pa 1976) 34 (20):E724–E728 [DOI] [PubMed]