Abstract
Study design
Blinded radiographic analysis of CT scans reformatted for precise lumbar spinous process (LSP) measurement.
Objective
To investigate the effect of ageing on LSP morphology and influence of LSP morphology on lumbar spine sagittal alignment.
Summary of background data
There is little data reporting the influence of ageing on spinous process size. There is data describing the increase in size of other body parts with age, such as the femur, ears, vertebral body, and nose. Several old cadaveric and radiographic studies have reported the formation of osseous spurs within the supraspinous and interspinous ligaments.
Method
200 abdominal CT scans taken for trauma and vascular investigation were reformatted to allow precise bony measurement of the lumbar spine. Two observers were blinded from the age and demographics of the patients. Sagittal and coronal plane projections were used to measure the height and width of the spinous processes (L1–L5), respectively. The relationship between spinous process size, age, and supine lordosis was investigated.
Results
LSP height increases by 0.03–0.07 mm/year (p < 10−3 to 10−8) and width by 0.05–0.06 mm/year (p < 10−11 to 10−15). Lumbar lordosis decreases with increasing LSP height (p < 0.0004) but is not related to increasing LSP width (p = 0.195). Supine lordosis increases by 0.1°/year (p = 0.004).
Conclusions
This study demonstrates that the dimensions of the LSP change with age. Increases in LSP height and even more impressive increases in LSP width occur with advancing age. There is an inverse relationship between lumbar lordosis and LSP height.
Keywords: Spinous process, Ageing, Sagittal alignment, Lumbar lordosis
Introduction
The lumbar spinous processes (LSP) have an important anatomical and biomechanical function protecting the neural structures in the spinal canal, and as an anchor for the interspinous and supraspinous ligaments, and the intersegmental paraspinal muscles. They also influence access to the spinal canal for neural decompressive surgical procedures. More recently, the LSP have attracted increased interest as a site for surgical device attachment (Wallis [1], XStop [2], Diam [3]) in an attempt to both decrease the symptoms of spinal stenosis, and as a site for inter-segmental stabilization without formal fusion. There is evidence that anatomical structures such as the femur [4], and vertebral body [5] have altered morphology with ageing. Several cadaveric and radiographic studies [6, 7] report the formation of osseous spurs forming within the spinous process ligaments with ageing. Abutment of the LSP or ‘kissing spines’ is well recognised in the elderly [8]. Along with the potential to be a pain source LSP abutment may block further lordosis contributing to a loss of sagittal plane alignment. It has been our observation that the LSP increases in size with age in both vertical and axial dimensions. The increase in size is not limited to the site of attachment of ligaments but appears to be a global increase involving both height and width. Vertical dimension increases of the LSP have the potential to reduce the space available for the supraspinous ligaments, the interspinous ligaments, may reduce extension range of motion, and may contribute to a loss of lumbar lordosis. Axial increased dimensions of the LSP reduces the space available for access to the spinal canal in parasagittal laminotomy approaches. This study aims to clarify the effect of ageing on LSP morphology.
Materials and methods
We accessed 200 abdominal CT scans performed in a 1-month period in our hospital on a storage server ‘Webspace’. The original indication for CT scanning was either trauma or vascular investigation. Scans were excluded if there was any indication for investigation of spinal pathology, if the LSP were not completely seen between L1 and L5, or there was obvious spinal pathology such as fracture, degenerative or isthmic spondylolisthesis, scoliosis, or evidence of previous spinal surgery.
The original scans had been acquired using a Siemens SOMATOM Sensation 64 scanner producing images with 1–1.5 mm slice thickness and 512 × 512 pixel resolution. The images had been reconstructed in true axial, coronal and sagittal planes adjusting for slight differences in patient position on the scanning table. The scans were reformatted with bone windows in sagittal and coronal planes. Osirix software used for viewing the scans had inbuilt electronic tools for measurement of length and angulation.
Measurements were made by two observers and a preset was applied to blind the observer from the patients’ name, age, and gender. The scans were subsequently unmasked to allow recording of the patients’ age and gender. The patients’ identity remained anonymous throughout and from the recorded data it was not possible for a third party to identify a patient.
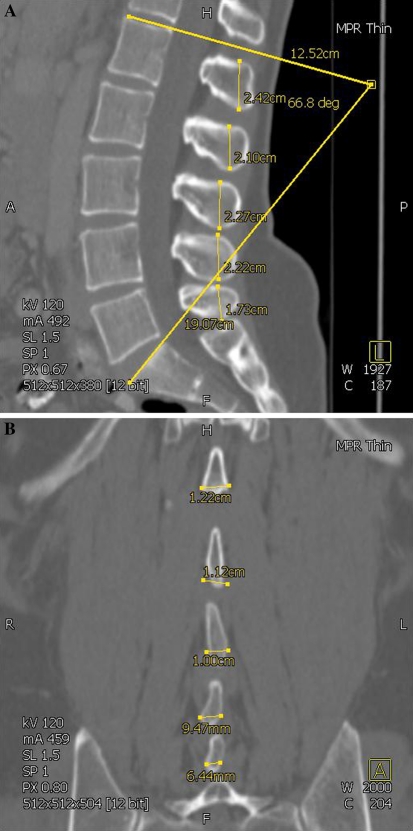
The height (sagittal images) and width (coronal images) of the LSP from L1 to L5 was recorded in millimetres (Fig. 1a, b). Measurement points were placed at the most superior, inferior and widest points of the LSP as shown in Fig. 1a, b producing a measurement line that was within a few degrees of the vertical and horizontal, respectively. As shown in these figures due to the different positions of the LSP in 3D space not all height or width measurements for one spine are captured on one specific CT slice.
Lumbar lordosis was measured between the superior endplate of L1 and the sacral dome (Fig. 1a).
In 15 patients, CT measurements were made by both observers allowing a test of inter-observer reliability.
Data were analysed by an independent statistician using statistical software R [9]. Linear regression models were applied using the Im function in R. This returns p values on whether the fitted coefficients could be equal to zero.
Fig. 1.
a CT image (sagittal plane) showing measurement of lumbar spinous process height and lumbar lordosis. b CT image (coronal plane) showing measurement of lumbar spinous process width
Results
Of the 200 subjects (mean age 54, range 16–91, SD 21), 111 were male (mean age 53, range 16–91, SD 20) and 89 female (mean age 56, range 16–89, SD 22). No significant difference in measurement was seen between the two observers with an average difference of −0.036 CI [−0.28, 0.20] (p = 0.77, paired t test). Table 1 shows the height and width of the LSP in males and females. The highest and widest spinous processes were at L1, L2 and L3. The smallest spinous process was at L5. The male spinous process was on average 2–3 mm higher and 1 mm wider than the female spinous process at all levels in the lumbar spine.
Table 1.
Spinous process dimensions (mm) of males and females from L1 to L5
| Spinous process | Size range (mm) | Mean size (mm) | SD | |
|---|---|---|---|---|
| Male | Female | |||
| Height | ||||
| L1 | 16–36 | 26 | 23 | 4 |
| L2 | 18–35 | 27 | 24 | 3 |
| L3 | 18–38 | 27 | 24 | 3 |
| L4 | 14–32 | 24 | 21 | 3 |
| L5 | 10–34 | 20 | 18 | 4 |
| Width | ||||
| L1 | 6–16 | 11 | 10 | 2 |
| L2 | 6–16 | 11 | 10 | 2 |
| L3 | 5–17 | 11 | 10 | 2 |
| L4 | 4–18 | 9 | 8 | 2 |
| L5 | 3–15 | 9 | 8 | 2 |
The mean supine lordosis for males was 46° (range 26–73°, SD 11) and for females 49° (range 21–80°, SD 12).
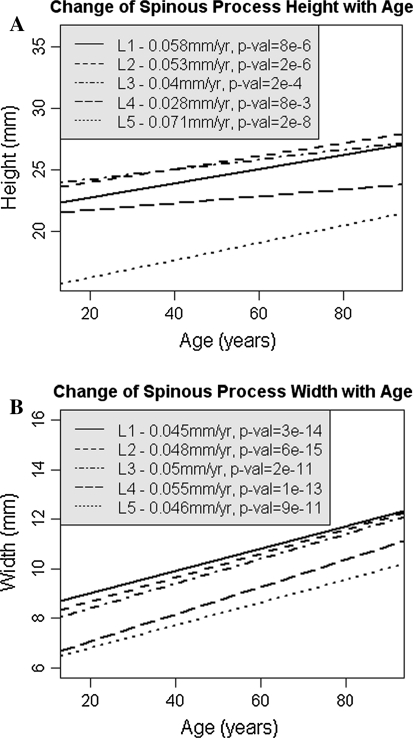
There was a significant increase in spinous process height and width with age (Fig. 2a, b). Height increased by 2–5 mm between the ages of 20 and 85 years with a 31% increase in height at L5 (p < 10−8). Width increased proportionally more, by 3–4 mm or greater than 50% at each lumbar level (p < 10−11). Similar morphological changes were seen in males and females when considered separately.
Fig. 2.
a Change in lumbar spinous process height (L1–L5) with age. b Change in lumbar spinous process width (L1–L5) with age
The rate of increase in LSP dimensions for height and width was 0.03–0.07 and 0.05–0.06 mm/year, respectively.
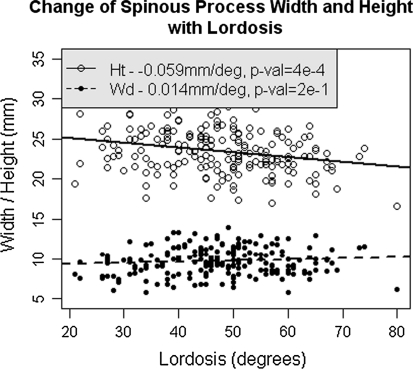
Increased spinous process height is significantly associated with a decrease in lumbar lordosis (p < 10−4). Lordosis decreases by 1° on average for each additional millimetre in average height. Increased spinous process width, however, is not related to lumbar lordosis (p = 0.2) (Fig. 3).
Fig. 3.
The relationship between increasing lumbar spinous process height and decreasing lumbar lordosis. Lumbar lordosis is not related to changes in spinous process width
Discussion
This study set out to address the observation that the lumbar spinous processes get bigger with age. This question is not answered through a search of the available literature. The present study confirms that the LSP do indeed enlarge with age. Furthermore, this enlargement goes along with a loss of sagittal alignment.
The study is not truly longitudinal in that the same subjects were not followed up over 60 years, however, the large numbers in the study likely make the conclusions valid. The difference in LSP size between males and females probably reflects the difference in average physical size between genders.
The margins of the LSP in younger spines normally appeared smooth with a slight posterior facing convexity. Older spinous processes were more irregular and less rounded, often appearing ‘squared off’ in the sagittal plane. Osseous excrescences were at times seen projecting from either the posterosuperior or posteroinferior corner of the LSP. Histologically this corresponds to a process of gradual, progressive ossification of the ligament and tendon attachments (enthesitis ossificans) [7]. Fibrocartilagenous metaplasia within the infraspinous but more so the supraspinous ligament and subsequent osteogenesis leads to a reported increase in height of the LSP with age [7]. Despite the irregularity of the LSP it was straightforward to accurately and consistently measure the cranio-caudal dimension of the LSP. This measurement reflected the global height of the spinous process rather than a high point over the tip of an osseous spur. High measurement concordance was seen between the two observers.
The width of the lumbar spinous process was measured from the coronal images. Moving from the posterior tip of the spinous process towards the lamina, the spinous process initially widens more inferiorly, often taking on the shape of a ‘bowling skittle’. The width of the spinous process appears greatest at this level. The LSP then narrows before flaring, to blend with the laminar. With age, the LSP becomes more bulbous with a loss of this ‘bowling skittle’ shape, medullary osteopenia and cortical thinning. This uniform enlargement of spinous process width made measurement straightforward as reflected by high levels of concordance between observers.
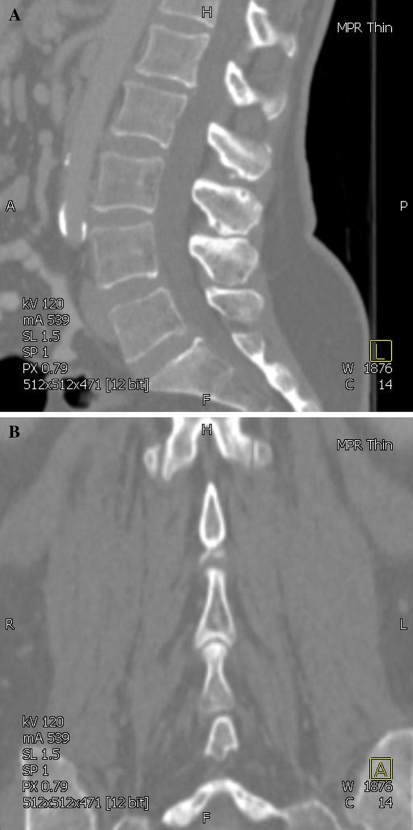
Numerous radiographic changes were observed in the spinous processes and these were more often seen in the older spine. Bony abutment was commonly seen between the inferior edge of the spinous process and the superior edge of the caudal process. Bony contact occurred along the length of the process rather than just at the posterior margin. Bony sclerosis, osteophytes, interspinous and intraspinous cysts, and occasionally a ‘ball and socket’ articulation were seen in association with this bony contact (Fig. 4a, b). Several cadaveric and histological studies [6, 7] describe eburnation, interspinous bursae formation and synovial metaplasia, which supports the radiographic appearances observed in this study. Degenerative changes in the LSP have been termed Baastrup’s phenomenon/disease [8, 10].
Fig. 4.
a CT image (sagittal plane) in older subject showing bony abutment and intraspinous cysts. b CT image (coronal plane) in older subject showing abutment and ‘ball and socket’ type articulation between lumbar spinous processes
Why do the LSP enlarge with age? Is this a primary event (degenerative process or enthesopathy) or a secondary adaptive response to changes in the mechanical environment? This is a ‘chicken and egg’ type problem which we cannot answer based on the results of this study. In a non-degenerate spine approximately 20% of the axial compressive load goes through the posterior elements. This proportion increases with disc degeneration, particularly where there is bone on bone contact either between adjacent facet joints or presumably spinous processes [11]. In simple terms, Wolf’s Law states that bone remodels to best resist the forces applied to it. We also know that the material properties of bone decline with ageing. To offset this bone has the potential to remodel its geometry increasing inner and outer cortical diameters away from a neutral axis, thereby increasing the area moment of inertia and decreasing bone’s bending stresses [11].
Lumbar lordosis arises from the lumbar intervertebral disc. Disc degeneration leads to a loss of disc height and generally a decrease in lordosis. This is the first study that makes a direct link between increasing spinous process height and reduced lumbar lordosis. The observation that decreasing lumbar lordosis was not related to either a change in spinous process width or increasing age per se gives credence to the relationship between lordosis and LSP height. In addition, the lumbar spinous processes were often seen to abut each other with no interspinous gap. This has the effect of blocking lumbar extension or lordosis. It seems likely therefore that superoinferior enlargement of the LSP with age may be a causative factor that contributes to a loss of lordosis and sagittal alignment in the elderly. Alternatively it is possible that the increase in vertical height of the spinous process is a response to change in sagittal alignment caused by disc height loss, reducing lordosis at the respective segment, and increasing interspinous ‘space’ facilitating further increase in spinous process height. The rate in increase in height (0.03–0.07 mm/year) and width (0.05–0.06 mm/year) is similar, yet the magnitude of width increase is much greater considering the smaller initial dimension. Presumably this is because there is no physical restriction to width increase, yet height increase is obstructed by the adjacent LSP. To be certain which of disc space height loss and lumbar spinous process height gain contributes most to the genesis of lumbar lordosis will require prospective longitudinal study with monitoring of the progression of associated disc degeneration.
The measurement of lumbar lordosis in this study was based on CT scan measurements performed in the supine position. Normally alignment is measured on erect X-rays, so this difference warrants scrutiny. Supine CT scans would likely under estimate any reduced lordosis once axial load was applied in the upright position. As axial load has a proven effect on reducing intervertebral disc height [12, 13], and this study has shown that LSP height enlargement leads to LSP abutment, the supine CT will likely underestimate the lordosis reduction that might be seen on erect X-rays, thus underestimating the deleterious effect that increased LSP height with age has upon reduction of lumbar lordosis, i.e. standing position increases disc load which will narrow disc space leading to a narrowed gap between the LSP. Consequently the enlarged LSP are more likely to abut and are therefore more likely to impact on standing alignment.
Enlargement of the LSP is clinically relevant for a number of reasons. Posterior decompression in the lumbar spine may be limited by bulky wider spinous processes particularly if attempts are made to preserve the midline or a percutaneous surgical approach is employed. If an ipsilateral surgical approach is used to decompress, for example, lateral recess stenosis a widened spinous process may obstruct surgical access forcing a kerrison away from the midline, impairing the ‘attack angle’ and therefore the ability to undercut the facet. As a consequence adequate decompression may necessitate greater sacrifice of the facet joint. An operation that is possible in a young patient may become impossible in an older patient with a spinous process which has become 3–4 mm wider.
Spinal fusions aim to reproduce some lumbar lordosis and avoid a ‘flat back’ syndrome. Increasing LSP height may narrow or eliminate the interspinous gap making it impossible to lordose a lumbar segment across a pedicle screw/rod construct without removing the spinous process. Decompression for spinal stenosis that removes the midline LSP over several levels may facilitate increased lordosis and the frequently seen patient perception of improved postoperative posture.
Enlarged LSP encroach more on the midline making spinal/epidural injections harder to perform. An oblique or parasagittal approach for injection may be more successful by avoiding the crowded midline.
The increase in height and width, with the observation of reduced cortical thickness, means that the older LSP may provide a greater repository of ‘local bone’ for fusion procedures. The value of this local bone is proven in outcome studies of fusion using this technique [14, 15].
In conclusion, this study has demonstrated that there are very clear changes in the morphology of the LSP as the population ages. This has implications for surgical techniques that involve decompression and fusion in the lumbar spine, along with therapeutic interventional techniques involving intra-spinal injection. There are implications for interspinous implant design that may vary dependent on the age cohorts considered for treatment. Finally there is a clear evidence that the change in LSP morphology may be a significant factor in the reduction of lumbar lordosis that is seen with ageing, and thus contribute to flat back and the postural changes seen with advancing age.
Conflict of interest
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drugs(s).
Contributor Information
Caspar Edward William Aylott, Phone: +44-7884-004198, Email: caspar@doctors.net.uk.
Peter Alexander Robertson, Phone: +64-96300214, Email: p.a.robertson@xtra.co.nz.
References
- 1.Senegas J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: the Wallis system. Eur Spine J. 2002;11(Suppl 2):164–169. doi: 10.1007/s00586-002-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zucherman JF, Hsu KY, Hartjen CA, et al. A multicenter, prospective, randomized trial evaluating the XSTOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine. 2005;30(24):2846–2847. doi: 10.1097/01.brs.0000166618.42749.d1. [DOI] [PubMed] [Google Scholar]
- 3.Sobottke R, Schluter-Brust K, Kaulhausen T, et al. Interspinous implants (XStop, Wallis, Diam) for the treatment of lumbar spinal stenosis: is there a correlation between radiological parameters and clinical outcome? Eur Spine J. 2009;18(10):1494–1503. doi: 10.1007/s00586-009-1081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi S, Kageyama I, Awatake T, et al. Enlargement of the femoral marrow cavity with ageing. Kaibogaku Zasshi. 1998;73(3):259–264. [PubMed] [Google Scholar]
- 5.Israel H. Progressive enlargement of the vertebral body as part of the process of human skeletal ageing. Age Ageing. 1973;2:71–79. doi: 10.1093/ageing/2.2.71. [DOI] [PubMed] [Google Scholar]
- 6.Sartoris DJ, Resnick D, Tyson R, et al. Age-related alterations in the vertebral spinous processes and intervening soft tissues: radiologic-pathologic correlation. AJR. 1985;145:1025–1030. doi: 10.2214/ajr.145.5.1025. [DOI] [PubMed] [Google Scholar]
- 7.Scapinelli R. Morphological and functional changes of the lumbar spinous processes in the elderly. Surg Radiol Anat. 1989;11:129–133. doi: 10.1007/BF02096469. [DOI] [PubMed] [Google Scholar]
- 8.Baastrup CI. On the spinous processes of the lumbar vertebrae and the soft tissues between them, and on pathological changes in that region. Acta Radiol (Stockh) 1983;14:52–54. doi: 10.3109/00016923309132353. [DOI] [Google Scholar]
- 9.R Development Core Team (computer program) (2008) Version 2.8.1. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. ISBN 3-900051-07-0
- 10.Jinkins JR. Acquired degenerative changes of the intervertebral segments at and suprajacent to the lumbosacral junction. A radioanatomic analysis of the nondiskal structures of the spinal column and perispinal soft tissues. Radiol Clin N Am. 2001;39(1):73–99. doi: 10.1016/S0033-8389(05)70264-5. [DOI] [PubMed] [Google Scholar]
- 11.Adams M, Bogduk N, Burton K, Dolan P (2002) The biomechanics of back pain, 1st edn, Chaps 4 and 10. Churchill Livingstone, London
- 12.Adams MA, Dolan P, Hutton WC, Porter RW. Diurnal changes in spinal mechanics and their clinical significance. J Bone Joint Surg Br Mar. 1990;72(2):266–270. doi: 10.1302/0301-620X.72B2.2138156. [DOI] [PubMed] [Google Scholar]
- 13.Keller TS, Nathan M. Height change caused by creep in intervertebral discs: a sagittal plane model. J Spinal Disord. 1999;12(4):313–324. doi: 10.1097/00002517-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Lee SC, Chen JF, Wu CT, Lee ST. In situ local autograft for instrumented lower lumbar or lumbosacral posterolateral fusion. J Clin Neurosci. 2009;16(1):37–43. doi: 10.1016/j.jocn.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta DK, Truumees E, Patel CK, Kazmierczak C, Hughes B, Elders G, Herkowitz HN. Outcome of local bone versus autogenous iliac crest bone graft in the instrumented posterolateral fusion of the lumbar spine. Spine (Phila Pa 1976) 2006;31(9):985–991. doi: 10.1097/01.brs.0000215048.51237.3c. [DOI] [PubMed] [Google Scholar]