Abstract
Background and objectives
To summarise our experience treating patients with spinal malignant peripheral nerve sheath tumours (MPNSTs).
Methods
We retrospectively reviewed the records of patients diagnosed with spinal MPNSTs who received surgical treatment from January 1998 to December 2009.
Results
Postoperative follow-up data were available for 14/16 patients with spinal MPNSTs (7 men, 7 women; median age = 44 years [range: 23–68 years]). Eight of 14 (57.1%) patients had primary and 6/14 (42.9%) recurrent MPNSTs. A total of 12/14 (85.7%) patients underwent total tumour resection, whereas 2/14 (14.3%) patients underwent subtotal tumour resection. Malignancies were graded low in 4 (28.6%) and high in 10 (71.1%) cases. A total of 12/14 (85.7%) patients experienced tumour recurrence and 10/14 (71.4%) patients died during the course of follow-up. The 0.5- 1-, 3-, and 5-year survival rates were 64.3, 48.2, 32.1, and 21.4%, respectively. Overall survival was significantly associated with tumour malignant degree (P = 0.012).
Conclusion
Diagnosis of spinal MPNSTs should be made with reference to clinical, radiological, and pathological findings. Surgical resection is the best available option for treating spinal MPNST; however, postoperative prognosis is poor.
Keywords: Diagnosis, Malignant peripheral nerve sheath tumours, Prognosis, Spinal tumour, Treatment
Introduction
Malignant peripheral nerve sheath tumours (MPNSTs) are a rare soft tissue sarcoma, often originating from Schwann cells, which account for 3–10% of all soft tissue sarcomas [1]. The most common locations for MPNSTs are the trunk, limbs, and head and neck [2]. Spinal MPNSTs have rarely been reported [3] and indeed to our knowledge there has been no systemic study of spinal MPNSTs involving a relatively large number of patients. Reports in the literature comprise either individual case reports or small case series’. Given that MPNSTs are highly malignant and that the associated survival rate is very low [4], we believe that sharing information regarding the diagnosis, treatment, and prognosis of patients with spinal MPNSTs is imperative to improving treatment outcomes.
In the present report, we summarise our experience treating a relatively large number (N = 16) of patients with spinal MPNST over approximately 10 years. More specifically, we describe the clinical and imaging features of spinal MPNST, including the classification of MPNST based on axial CT findings, the surgical treatment strategies, and the factors influencing prognosis.
Materials and methods
Patients
We retrospectively reviewed the records of all patients who were diagnosed with spinal MPNST (malignant schwannoma or neurofibrosarcoma) at the Third Hospital of Peking University from January 1998 to December 2009. The study inclusion criteria were as follows: a diagnosis of MPNST as confirmed by two pathologists after review of pathological sections; no evidence of MPNST metastasis; and surgical treatment for MPNST.
The following details were obtained from each patient’s medical records: demographic details, history of disease, radiological manifestations, tumour pathology, surgical approach used and operative details, and postoperative tumour recurrence and survival. After surgery, patients were followed up at 3, 6, and 12 months and then once every 6 months, during which computed tomography (CT) and/or magnetic resonance imaging (MRI) scans were performed.
The study was approved by the ethics committee of the Third Hospital of Peking University.
Radiological manifestations of MPNSTs and tumour classification
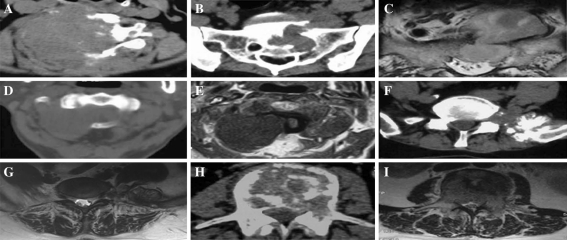
Tumours were classified by examining CT and MRI images of the tumour and surrounding bone and/or soft tissue. The images obtained were also used for surgical planning. We initially established the MPNSTs classification as Type I (soft tissue) or Type II (intraosseous). Type I tumours were characterised by primary lesions involving the soft tissue and a secondary lesion involving the vertebral structure. Type I tumours were further subclassified as Type IA (dumbbell) or Type IB (paravertebral). Type IA tumours were characterised by a dumbbell shaped lesion expanding out of the intervertebral foramina, whereas Type IB tumours were characterised by paravertebral lesion without intraspinal involvement. Type II tumours were characterised by extensive vertebral body and posterior bony elements destruction without soft tissue involvement (Fig. 1a).
Fig. 1.
Computed tomographic and magnetic resonance images of spinal malignant peripheral nerve sheath tumours (MPNSTs). a Recurrent spinal MPNST. There was no clear border between the tumour and the surrounding soft tissues. The anatomic structure was difficult to identify. b, c Type IA “dumbbell type” tumour. The tumour was located inside and outside the sacral canal (S1-2) and had an unclear border. Bone erosion of sacrum was evident. d, e Type IA tumour. The tumour was located in the C1-2 intervertebral foramen and was derived from the C2 nerve root. The bone was intact without obvious destruction, but was mildly depressed. The tumour was initially suspected to be a benign schwannoma; however, postoperative pathological examination confirmed the diagnosis of MPNST. f, g Type IB paravertebral tumour. The tumour was located on the left side of the L5 vertebral body and posterior bony elements. The sacroiliac joint was involved with an unclear border. h, i Type II “intraosseous type” tumour. The tumour was located in the L3 vertebral body, which was extensively eroded. The bone cortex was destroyed and the paravertebral soft tissues and spinal canal were involved
Pathology and diagnosis
Preoperative biopsies were obtained from most patients under the guidance of CT, and histology and immunohistochemical staining of S-100, SMA, and EMA were examined by two independent, experienced pathologists. Pathological grading of MPNSTs was performed in accordance with the criteria described by Ducatman et al. [2], which considers nuclear atypia, cellularity, nuclear enlargement, hyperchromasia, mitotic rate, and necrosis. Tumours were classified as high grade or low grade. High-grade tumours are characterised by fasciculated cells with hyperchromatic nuclei and frequent mitotic figures. High-grade tumours typically have >4 mitoses per 10 high power fields and areas of geographic necrosis with or without pseudopalisading. Low-grade tumours are characterised by decreased cellularity and fewer hyperchromatic cells (distributed in a variably collagenised stroma) compared with high-grade tumours. Further, mitotic figures and tumour necrosis are rare in low-grade tumours [5].
Statistical analysis
The associations between postoperative survival and prognostic factors were assessed by univariate Cox regression model analysis and log-rank test. The postoperative survival rate was compared by surgical method, pathology grading, and tumour condition using Fisher’s exact test. All statistical assessments were considered significantly if P < 0.05. Statistical analyses were performed using SPSS 15.0 statistical software (SPSS Inc., Chicago, IL).
Results
Demographic and clinical characteristics
A total of 16 patients (9 men and 7 women) met the inclusion criteria. The mean patient age was 43 ± 15 years (range: 17–68 years), while the mean history of disease before diagnosis was 4.9 months (range: 1.0–12.0 months). There were 10 cases of primary spinal MPNST and 6 cases of recurrent spinal MPNST. Spinal MPNST locations included the thoracic vertebra (n = 6), lower cervical vertebra (n = 3), lumbar vertebra (n = 3), sacral vertebra (n = 3), and upper cervical vertebra (n = 1) (Table 1). No patient had a history of local radiotherapy and only one patient had associated neurofibromatosis type I.
Table 1.
Demographic, clinical, and follow-up characteristics of patients with spinal malignant peripheral nerve sheath tumours
| Patient | Sex | Age (years) | Disease history (months) | Level | Malignancy grade | Tumour occurrence | Surgical procedure | Length of follow-up (months) | Alivea | Frequency of recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 42 | 2 | L1 | High | Primary | Subtotal resection | 5 | No | 1 |
| 2 | F | 41 | 1 | L3 | High | Primary | Total resection | 11 | No | 1 |
| 3 | F | 41 | 3 | S1-2 | High | Primary | Subtotal resection | 21 | No | 1 |
| 4 | F | 68 | 3 | C4-6 | High | Recurrent | Total resection | 3 | No | 1 |
| 5 | F | 26 | 12 | T11-12 | Low | Primary | Total resection | 91 | Yes | 5 |
| 6 | F | 33 | 6 | T8-10 | High | Recurrent | Total resection | 4 | No | 1 |
| 7 | F | 60 | 1 | L5/sacroiliac joint | High | Primary | Total resection | 26 | Yes | 0 |
| 8 | M | 23 | 12 | L5-S1 | Low | Primary | Total resection | 92 | Yes | 1 |
| 9 | M | 33 | 2 | C2 | High | Recurrent | Total resection | 2 | No | 1 |
| 10 | M | 44 | 6 | T4-6 | Low | Recurrent | Total resection | 43 | No | 4 |
| 11 | M | 27 | 5 | T9-10 | High | Recurrent | Total resection | 19 | No | 2 |
| 12 | M | 65 | 5 | T10-12 | High | Recurrent | Total resection | 8 | No | 1 |
| 13 | M | 65 | 4 | C1-2 | Low | Primary | Total resection | 6 | Yes | 0 |
| 14 | M | 51 | 6 | C6-T1 | High | Primary | Total resection | 3 | No | 1 |
F female, M male
aAlive at most recent follow-up
Radiological manifestations and tumour classification
All six patients with recurrent MPNST were excluded from tumour classification because of the variable surgical methods used and differing degrees of extensive soft tissue lesions and bone erosion (Fig. 1a).
A total of six patients had Type I tumours (Fig. 1b–g), whereas four patients had Type II tumours (Fig. 1h, i). Of the patients with Type I tumours, five had Type IA (Fig. 1b–e) tumours and one had a Type IB tumour (Fig. 1f, g).
Pathology and diagnosis
Of the ten patients with primary MPNSTs, eight had clear evidence of bone structure destruction. Preoperative biopsy and subsequent pathological examination confirmed the diagnosis of MPNST in these eight patients. For the remaining two patients with Type 1A tumours, imaging revealed a clear boundary in each case and no evidence of significant bone destruction. Preoperative biopsies were not obtained in these patients because of the risk of tumour capsule damage and reducing surgical resection integrity. Of the six patients with recurrent MPNSTs, five had received a previous pathological diagnosis of MPNST and one had haemangioendothelioma.
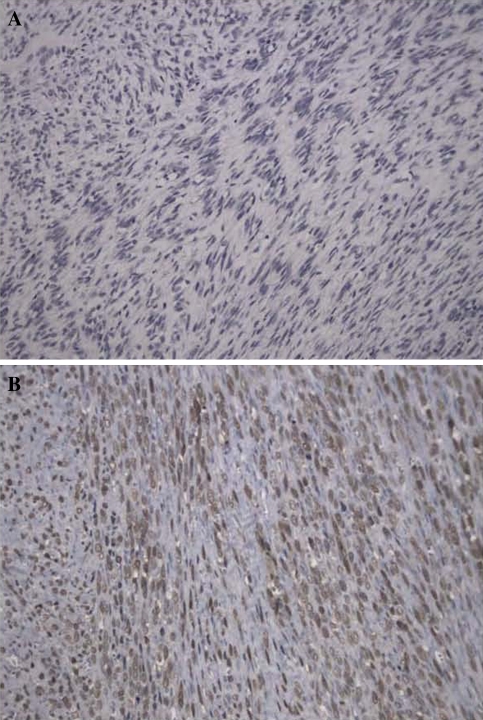
Tumour cells obtained from all patients were symmetrically tapered spindle cells with irregular buckled nuclei, marked nuclear atypia, increased cellularity with nuclear enlargement, and hyperchromasia (Fig. 2a).
Fig. 2.
Representative hematoxylin and eosin stained spinal malignant peripheral nerve sheath tumour samples (magnification = ×20). a Tumour cells were spindle shaped with irregular buckled nuclei, marked nuclear atypia, increased cellularity with nuclear enlargement, and hyperchromasia. b A total of 12/16 samples were positive for S-100
Immunohistochemical study of biopsy samples revealed that 75% (12/16), 38% (3/8), and 29% (2/7) of samples were positive for S-100 (Fig. 2b), SMA, and EMA, respectively.
Histological and immunohistochemical examination findings confirmed the diagnosis of MPNST in all patients. Malignancies were graded as low in 4 cases and high in 12 cases.
Surgery
A total of 14 patients received total resection and 2 patients received subtotal resection. En bloc tumour resection was carried out in one patient, piecemeal resection in eight patients, and curettage in seven patients.
Type I tumours
For the six patients with Type I tumours, the initial plan was extra-capsule dissection of the tumour and then en bloc resection of tumour. However, due to the close proximity of the spinal dura mater, the dissection of intraspinal tumours could only be performed along the capsule. As the capsule is thin and attached to the dura mater without a clear border, the tumours had to be cut into blocks then removed. There was only one case of en bloc resection in which the surgical margin was considered positive because the capsule was broken during resection.
For sclerotin invading tumours (4/6 patients), en bloc chiselling was initiated from the unaffected region. A total of five patients received total resection and one patient received subtotal tumour resection because of significant tumour adhesions to peritoneal structures. Unilateral total joint removal was performed in two patients. Both of these patients underwent internal fixation (with pedicle screws) for stabilisation. Anterior-lateral intervertebral fusion with titanium mesh was performed in one patient because of the loss of a significant portion of the vertebral body during tumour resection. The mean blood loss during surgery in patients with Type I MPNSTs was 1,728 ± 328 mL (range: 400–5,000 mL).
Type II tumours
For the four patients with Type II tumours, piecemeal removal of whole vertebrae was performed. A total of three patients with thoracolumbar involvement underwent one-stage spondylectomy using a posterior and anterior-lateral approach followed piecemeal resection, and stability reconstruction via a combination of anterior-lateral and posterior internal fixation (nail-stick system) and intervertebral fusion with titanium mesh. Of these three patients, two underwent total tumour resection and one subtotal resection. The patient who underwent subtotal resection presented with significant tumour adhesion to the large blood vessels in the peritoneal cavity, which made tumour resection difficult. As a result, residual tumour was suspected after surgery and the procedure was categorised as subtotal resection. The fourth patient with a Type II tumour exhibited sacral involvement (S1-2) and received posterior curettage surgery followed by posterior internal fixation (nail-stick system). The mean blood loss during surgery in patients with Type I MPNSTs was 4,000 ± 544 mL (range: 2,600–6,000 mL).
Recurrent tumours
For the six patients with recurrent tumours, the surgical strategy was to completely remove the tumour, release the depressed spinal cord, and rebuild spinal stability. A tumour curettage procedure was performed in each case because of anatomical structural alterations with no apparent tumour envelope around the tumour. The tumour was completely removed in all six patients with recurrent tumours. The mean blood loss during surgery in patients with recurrent MPNSTs was 2,533 ± 462 mL.
Postoperative treatment and follow-up
Chemotherapy and radiotherapy were not routine adjuvant treatments. Only one patient underwent postoperative radiotherapy; however, this treatment was performed in another hospital and the exact radiotherapy dosage is not known. No patients underwent chemotherapy.
Follow-up data were available for 14 of 16 patients, 13 of who were followed-up for more than 1 year (Table 1). The 14 patients comprised 7 men and 7 women, with a mean age of 44.2 years (range: 23–68 years) and a mean disease history of 4.9 months (range: 1.0–12.0 months). Malignancies were graded low in 4 (28.6%) cases and high in 10 (71.4%) cases. Of the 14 patients, 12 (85.7%) underwent total resection and 2 (14.3%) underwent subtotal resection. A total of 12 of 14 (85.7%) patients experienced tumour recurrence, while 10 of 14 (71.4%) patients died during the course of follow-up (Table 1). The median duration of postoperative survival was 11 months (95% confidence interval [CI]: 0–29.5 months). The 2 patients who had no tumour recurrence were followed-up for 26 and 6 months, respectively. One of the four patients with low-grade malignancies and 9 of the 10 patients with high-grade malignancies died after surgery. The median survival time for patients with high-grade malignancies was 5 months (95% CI: 0.0–11.2 months). However, the survival rate in patients with low grade was greater than 50% (1 of 3 patients with low-grade malignancies died). One patient with tumour recurrence who underwent a second surgery did not present with any new tumour recurrence during the last follow-up and had (at that time) survived 41 months after the second operation (case 8). One patient with multiple tumour recurrence underwent multiple surgeries. This patient had survived 91 months postoperatively with tumour recurrence at the time of last follow-up and had not undergone any further surgery (case 5, Fig. 3). The 0.5- 1-, 3-, and 5-year survival rates were 64.3, 48.2, 32.1, and 21.4%, respectively.
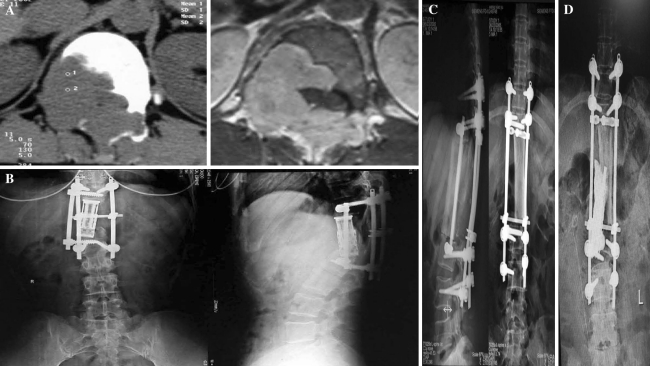
Fig. 3.
Preoperative and postoperative radiographs of a patient (case 5) with a Type II intraosseous tumour. a The left preoperative cross-sectional computed tomographic (CT) image of the T11 segment shows that the right half of the T11 vertebral body and the right pedicle of the vertebral arch (and accessories) were completely destroyed by the tumour; however, the boundary between the tumour and the paravertebral soft tissue remained clear. The right cross-sectional magnetic resonance image at the same level shows a clear boundary between the tumour and the spinal cord. b The patient underwent en bloc resection involving T11-12 vertebral body partition and resection, artificial vertebral body implantation between T10 and L1, and internal fixation. Follow-up revealed tumour recurrence 30 months surgery. The tumour affected the L1 vertebral body and right accessory. Internal fixation at the posterior site was ruptured. CT-guided biopsy suggested a malignant peripheral nerve sheath tumour of the L1 lamina vertebra. The X-ray images show L1 vertebral body collapse, artificial vertebral body displacement, and rupture of the original pedicle screw at L1. c Combined anterior-posterior en bloc resection of the L1 vertebra was performed via the previous surgical incision (partition and resection). A tibial allograft was implanted between T10 and L2 via the anterior approach, and pedicle screw internal fixation of T9-L3 was performed via the posterior approach. Postoperative X-ray images are shown. d A second recurrence was detected 80 months after the initial surgery; the implanted artificial tibial allograft was invaded. Posterior palliative curettage of the tumour was performed. Bone cement and a titanium rod were inserted at the artificial tibial defect to provide anterior support after curettage. A postoperative X-ray image is shown. At most recent follow-up (after a total of 5 surgical procedures), the patient was alive (with tumour) with Frankel Grade E nerve function
Univariate Cox regression analysis revealed that overall survival was associated with tumour malignant degree (P = 0.012); however, overall survival was not associated with resection type or initial tumour condition or other factors.
The analysis of prognostic factors for postoperative survival indicated that postoperative survival did not vary by tumour occurrence (primary vs. recurrent), surgical resection type (total resection vs. subtotal resection), or the need for a second surgical procedure after recurrence (yes vs. no) at any time point (Table 2). The 3-year postoperative survival rate was significantly higher in patients with low-grade as compared with high-grade malignancies (75 vs. 0%, P = 0.011).
Table 2.
Summary of survival rates by prognostic factors in patients with spinal malignant peripheral nerve sheath tumours
| Prognostic factor | 0.5-year survival rate (%) | P value | 1-year survival rate (%) | P value | 3-year survival rate (%) | P value |
|---|---|---|---|---|---|---|
| Tumour occurrence | ||||||
| Primary (n = 8) | 75 | 0.580 | 50 | 0.627 | 25 | 1.000 |
| Recurrent (n = 6) | 50 | 33 | 17 | |||
| Surgical resection | ||||||
| Total (n = 12) | 67 | 1.000 | 42 | 1.000 | 25 | 1.000 |
| Subtotal (n = 2) | 50 | 50 | 0 | |||
| Malignancy grade | ||||||
| High (n = 10) | 50 | 0.221 | 30 | 0.245 | 0 | 0.011* |
| Low (n = 4) | 100 | 75 | 75 | |||
| Surgery after recurrence | ||||||
| Yes (n = 12) | 58 | 0.505 | 42 | 1.000 | 25 | 1.000 |
| No (n = 2) | 100 | 50 | 0 | |||
Survival rates are summarised as percentages and were compared between prognostic factors using Fisher’s exact test
* Indicates statistical significance (P < 0.05)
Discussion
Herein we have retrospectively reviewed the clinical findings regarding diagnosis, surgical treatment, and outcomes for 16 cases of spinal MPNSTs from a single institution over the past decade. To our knowledge, our report is the largest single centre report on spinal MPNSTs and hence, we believe is a valuable contribution to the existing literature on this rare form of MPNST.
Malignant peripheral nerve sheath tumours can arise from multiple cell types [6]. Further, the presentation of MPNSTs can vary greatly, making diagnosis challenging [7]. Magnetic resonance imaging can be particularly useful for determining the features of a malignancy such as ill-defined margins, heterogeneity, and peripheral reactive edema surrounding the lesion. Computed tomographic examination can allow for the assessment of bone degradation by the tumour i.e., in spinal MPNSTs, erosive destruction of bone structures in the vertebra. In our experience, although spinal MPNSTs lack consistent/specific radiological manifestations, the combination of clinical, radiological, and pathological assessments facilitate the diagnosis of MPNST. A preoperative diagnosis of MPNST should be confirmed by immunological and histological analysis of biopsy specimens obtained under CT guidance.
In the present report, we have described a classification system for Spinal MPNSTs, which may be used as a guide for surgical treatment. Specifically, we classified spinal MPNSTs as Type I (soft tissue) or Type II (intraosseous). We further subclassified Type I tumours as IA (dumbbell) or IB (paravertebral). The majority of spinal tumours described in our report were Type I tumours; interestingly, however, there were several Type II (intraosseous) spinal tumours. Intraosseous spinal tumours are rare and only a small number of cases have been described in the literature [6, 8–10]. Some authors [11, 12] have suggested that these tumours may arise from the terminal nerve fibres, which accompany the nutrient vessel of the vertebral body.
Surgical resection is the treatment of choice for MPNSTs [7]. As with other soft tissue sarcomas, en bloc resection is often ideal for MPNSTs. Wong et al. [5] reported that the rate of en bloc resection was 83% in 128 patients with (non-spinal) MPNSTs and that only 48% of patients had negative surgical margins. However, in cases of spinal MPNST, en bloc resection is often very difficult/impractical because of the surrounding spinal cord, dura mater, and large blood vessels, the complexity of the vertebrae, and the large volume of blood typically lost during spinal tumour surgery. Indeed, en bloc resection was possible for only one of the patients in our cohort. We typically used a piecemeal approach for tumour resection. Quite clearly, residual tumour cells will remain on the surrounding dura mater and large blood vessel walls in some cases and lead to recurrence. A further obvious cause of recurrence in our cohort is subtotal resection. In both cases in which subtotal resection was performed, the preoperative diagnosis was not definite; hence, blood loss was significant during surgery and the procedure was limited to curettage or subtotal resection. In cases such as these, any additional surgery because of recurrence is likely to be complicated due to the presence of tumour and dural adhesions, the lack of normal anatomical structure, and tumour bleeding.
With regards to adjuvant therapy for MPNSTs, many authors have suggested that auxiliary radiotherapy may enhance the local control of MPNSTs [2, 5, 13]. Wong et al. [5] suggested that radiotherapy using a dose higher than 60 Gy is effective for local MPNST control. Although radiotherapy may be an effect adjuvant for patients with non-spinal MPNSTs, local high dose radiation may induce radiation myelopathy in patients with spinal MPNSTs. Indeed, in our cohort, only one patient received postoperative radiotherapy. The effectiveness of adjuvant chemotherapy for MPNST remains controversial [7]. However, it is worth noting that relatively high overall response rates (45%) have been found in paediatric patients with MPSNTs who received adjuvant chemotherapy [14]. Nevertheless, the efficacy of adjuvant chemotherapy in the treatment of MPNSTs warrants further investigation in a large-scale, multicenter, prospective trial.
The prognosis for patients with MPNST is relatively poor, with recurrence rates estimated to range from 20 to 40% [7] and 5-year survival rates ranging from 34 to 52% [2, 5]. A number of factors are known to influence prognosis including tumour size, location, and histological grade, whether removal is en bloc (or not), resection margin, the presence of recurrence, and metastasis [7]. Of these, having a negative surgical resection margin is thought to be the most significant prognostic factor for survival [5]. Other factors reported to influence MPNST prognosis include neurofibromatosis type I, nuclear expression of p53, and S-100 [5, 15, 16]. Consistent with previous reports, and unsurprisingly, we found that tumour malignant degree was significantly associated with postoperative survival [7]. In contrast with previous reports [7], we did not find any other factors to be associated with postoperative survival. This is surprising, but reflects the fact that our analyses only included a small number of patients.
In our cohort of patients with spinal MPNSTs, the rate of tumour recurrence was 85.7% and the 5-year survival rate was only 21.4%. Conti et al. [17] and Mazel et al. [18] have reported similar rates of survival after surgery in a small number of patients with spinal MPNSTs. We believe that our high recurrence and low survival rates can be explained by incomplete tumour removal. Indeed, complete resection of soft tissue type MPNSTs with tumour-free margins is extremely challenging due to the likelihood of residual tumour cells remaining on surrounding dura mater and large blood vessels. With current approaches, we believe that survival and recurrence can be optimised by making a clear preoperative plan detailing the embolisation of tumour blood vessels and the scope of surgical resection. Particular emphasis should be paid to the tumour vascular boundary and dural border during surgery. Further, the resection field should be soaked with cisplatin and then distilled water after resection.
We found that postoperative survival rates did not differ by surgical approach or by tumour occurrence (primary or recurrent). The most likely explanation for these findings is that only a small number of patients were included in the analysis, thus limiting statistical power. Further, MPNSTs were removed using a piecemeal approach, which, although considered total resection, does not exclude the possibility of residual tumour remaining.
Conclusions
In summary, we have retrospectively described our experience treating a relatively large number of patients with spinal MPNSTs over a period of approximately 10 years. The diagnosis of spinal MPNSTs should be made with reference to clinical, radiological, and pathological examination findings. Spinal MPNSTs should be treated with surgical resection; however, en bloc resection is challenging because of the surrounding anatomical structures. As a consequence, postoperative rates of recurrence are high and survival low. Quite clearly more effective approaches are needed for treating spinal MPSNTs. As the molecular pathways involved in the pathogenesis of MPSNTs are elucidated [19–22], specific treatments targeting these pathways will hopefully be developed. Further, the use of more sophisticated radiotherapy approaches such as intensity-modulated radiation therapy may also prove to decrease recurrence and increase survival in patients with spinal MPSNTs.
Conflict of interest
None.
References
- 1.Weiss SW, Goldblum JR. Enzinger and Weiss’s soft tissue tumours. St Louis: Mosby Inc; 2001. [Google Scholar]
- 2.Ducatman BS, Scheithauer BW, Piepgras DG, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::AID-CNCR2820571022>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Chamoun RB, Whitehead WE, Dauser RC, et al. Primary disseminated intradural malignant peripheral nerve sheath tumor of the spine in a child: case report and review of the literature. Pediatr Neurosurg. 2009;45:230–236. doi: 10.1159/000224621. [DOI] [PubMed] [Google Scholar]
- 4.Gupta G, Maniker A. Malignant peripheral nerve sheath tumors. Neurosurg Focus. 2007;22:E12. doi: 10.3171/foc.2007.22.6.13. [DOI] [PubMed] [Google Scholar]
- 5.Wong WW, Hirose T, Scheithauer BW, et al. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys. 1998;42:351–360. doi: 10.1016/S0360-3016(98)00223-5. [DOI] [PubMed] [Google Scholar]
- 6.Moon SJ, Lee JK, Seo BR, et al. An intraosseous malignant peripheral nerve sheath tumor of the cervical spine: a case report and review of the literature. Spine (Phila Pa 1976) 2008;33:E712–E716. doi: 10.1097/BRS.0b013e31817e6995. [DOI] [PubMed] [Google Scholar]
- 7.Grobmyer SR, Reith JD, Shahlaee A, et al. Malignant peripheral nerve sheath tumor: molecular pathogenesis and current management considerations. J Surg Oncol. 2008;97:340–349. doi: 10.1002/jso.20971. [DOI] [PubMed] [Google Scholar]
- 8.Gnanalingham K, Bhattacharjee S, O’Neill K. Intraosseous malignant peripheral nerve sheath tumor (MPNST) of the thoracic spine: a rare cause of spinal cord compression. Spine (Phila Pa 1976) 2004;29:E402–E405. doi: 10.1097/01.brs.0000138410.28657.ee. [DOI] [PubMed] [Google Scholar]
- 9.Miyakoshi N, Nishikawa Y, Shimada Y, et al. Intraosseous malignant peripheral nerve sheath tumor with focal epithelioid differentiation of the thoracic spine. Neurol India. 2007;55:64–66. doi: 10.4103/0028-3886.30431. [DOI] [PubMed] [Google Scholar]
- 10.Khan RJ, Asgher J, Sohail MT, et al. Primary intraosseous malignant peripheral nerve sheath tumor: a case report and review of the literature. Pathology. 1998;30:237–241. doi: 10.1080/00313029800169376. [DOI] [PubMed] [Google Scholar]
- 11.Nannapaneni R, Sinar EJ. Intraosseous schwannoma of the cervical spine. Br J Neurosurg. 2005;19:244–247. doi: 10.1080/02688690500207546. [DOI] [PubMed] [Google Scholar]
- 12.Chang CJ, Huang JS, Wang YC, et al. Intraosseous schwannoma of the fourth lumbar vertebra: case report. Neurosurgery. 1998;43:1219–1222. doi: 10.1097/00006123-199811000-00120. [DOI] [PubMed] [Google Scholar]
- 13.Vauthey JN, Woodruff JM, Brennan MF. Extremity malignant peripheral nerve sheath tumors (neurogenic sarcomas): a 10-year experience. Ann Surg Oncol. 1995;2:126–131. doi: 10.1007/BF02303627. [DOI] [PubMed] [Google Scholar]
- 14.Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23:8422–8430. doi: 10.1200/JCO.2005.01.4886. [DOI] [PubMed] [Google Scholar]
- 15.Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 16.Brekke HR, Kolberg M, Skotheim RI, et al. Identification of p53 as a strong predictor of survival for patients with malignant peripheral nerve sheath tumors. Neuro Oncol. 2009;11:514–528. doi: 10.1215/15228517-2008-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conti P, Pansini G, Mouchaty H, et al. Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. 2004;61:34–43. doi: 10.1016/S0090-3019(03)00537-8. [DOI] [PubMed] [Google Scholar]
- 18.Mazel C, Topouchian V, Grunenwald D. Effectiveness of radical resections in malignant dumbbell tumors of the thoracic spine: review of three cases. J Spinal Disord Tech. 2002;15:507–512. doi: 10.1097/00024720-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Lopez G, Torres K, Liu J, et al. Autophagic survival in resistance to histone deacetylase inhibitors: novel strategies to treat malignant peripheral nerve sheath tumors. Cancer Res. 2011;71:185–196. doi: 10.1158/0008-5472.CAN-10-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byer SJ, Eckert JM, Brossier NM, et al. Tamoxifen inhibits malignant peripheral nerve sheath tumor growth in an estrogen receptor-independent manner. Neuro Oncol. 2011;13:28–41. doi: 10.1093/neuonc/noq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtkamp N, Malzer E, Zietsch J, et al. EGFR and erbB2 in malignant peripheral nerve sheath tumors and implications for targeted therapy. Neuro Oncol. 2008;10:946–957. doi: 10.1215/15228517-2008-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahller YY, Vaikunth SS, Currier MA, et al. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol Ther. 2007;15:279–286. doi: 10.1038/sj.mt.6300038. [DOI] [PubMed] [Google Scholar]