Abstract
Introduction
Previous studies have shown the existence of either cellular or humoral MBP-reactive elements up to 5 years after spinal cord injury (SCI), but not the presence of both after 10 years.
Materials and methods
Twelve SCI patients, with more than 10 years of evolution, and 18 healthy blood donors were studied. Lymphocyte proliferation (colorimetric-BrdU ELISA assay) and antibody titers against MBP (ELISA Human IgG MBP-specific assay) were assessed.
Results
SCI patients presented a significant T-cell proliferation against MBP (lymphocyte proliferation index: 3.7 ± 1.5, mean ± SD) compared to control individuals (0.7 ± 0.3; P < 0.001). Humoral response analysis yielded a significant difference (P < 0.0001) between the antibody titers of controls and SCI patients. A significant correlation between cellular and humoral responses was observed. Finally, patients with an ASIA B presented the highest immune responses.
Conclusion
This work demonstrates, for the first time, the existence of both cellular and humoral responses against MBP in the chronic stages (>10 years) of injury.
Keywords: Humoral response, IgG, Spinal cord injury, T-cell response
Introduction
Spinal cord injury (SCI) has different causes, 80% of the cases are caused by traumatic injury such as motor vehicle collisions, trauma sustained from extreme sports or falls and violence (e.g. gunshot wounds) among others. The other 20% are caused by malformations, bone degenerative compressions, infections or invading tumors [1]. SCI pathophysiology is quite complex and involves a number of secondary events that are triggered immediately after injury. These harmful phenomena significantly exacerbate the damage caused by the injury and consequently, impair neurological function. Few hours after injury, ischemia and infarction are the first secondary events to develop, these cause clinical features, such as, the lack of sensitivity and mobility. Other well-studied detrimental mechanisms that are involved in secondary damage are: lipid peroxidation, excitotoxicity, activation of the apoptotic cascade, ionic dysregulation, inflammation and the activation of an autoreactive response against neural constituents [2, 3]. Regarding the latter, it has been shown that SCI induces the stimulation of immune components against neural self-antigens. The development of autoreactive responses against myelin basic protein (MBP) or other neural constituents [3] has specifically been described in murine species [4]. In SCI humans, a significant hyperactivity of MBP-reactive T cells has been reported, it is even comparable to that found in patients with multiple sclerosis (MS) [5]. In spite of these findings, research on this topic is scarce and inconclusive. The few existing studies have not assessed the humoral and cellular responses in the same group of patients. None of the studies have reported the persistence of a self-reactive response, specifically against MBP, in patients with more than 10 years of injury [5–7]. The present work, explored for the first time, the humoral and cellular immune responses against MBP in the same group of individuals. This study also reports a MBP-specific response in patients with a longstanding injury to the spinal cord. The study of this autoreactive response is of relevance since the pathology of other diseases like MS is determined by the action of lymphocytes and antibodies against MBP.
Methods
An analytical cross-sectional trial was performed on twelve patients with clinically confirmed SCI. Injuries varied in location on the spinal cord and severity. Requirements of eligibility for entry into the study were: an evolution of more than 10 years, a level of injury between C4 and T12 and between 22 and 46 years of age. Patients had a controlled general state of health: no history of current or recent (<20 days) infections, no pressure ulcers, no autoimmune disease and no treatment with antibiotics in the last month previous to the study. Eighteen healthy blood donors were recruited from the family of SCI patients or from the medical clinic at the National Medical Center and were used as a control group. All control subjects ranged between 23 and 45 years in age and were screened for the absence of recent infection as well as for their general health status. Consent forms were obtained from all studied individuals after explaining the experimental procedures. The protocol was approved by the Human Subjects Committee at Anahuac University and at Siglo XXI National Medical Center.
Blood samples were drawn through standard venipuncture. Each whole blood sample was collected aseptically by a trained phlebotomist into two Vacutainer brand collection tubes; one of which contained EDTA and the other contained no additives.
Lymphocyte proliferation assay
A 5 mL blood sample anticoagulated with EDTA was diluted with sterile phosphate buffered saline (PBS) 2:1 and layered over 5 mL of Lymphoprep (Axis-Shield) in a 15 mL conical tube; the sample was later centrifuged at 800×g for 20 min. The interface layer containing the lymphocytes was extracted using a Pasteur pipette, and the cells resuspended in 10 mL of sterile PBS in order to wash them. The cell suspension was centrifuged at 250×g for 10 min after which the supernatant was decanted leaving the cell pellet. Two additional washes were done with the final suspension of cells resuspended in 5 mL of 20% fetal bovine serum (FBS) and 2% antibiotic–antimycotic (GIBCO) fortified RPMI cell culture medium (Invitrogen). Cells were then counted and the viability was assessed by 0.04% trypan blue dye exclusion. Only lots with >93% of the viable cells were used. A 96-well flat bottom cell culture microplate (NUNC) was prepared with quintuplicates containing 100 μl of: (a) 2.5 μg/mL concanavalin A (ConA) (Sigma-Aldrich) (b) 10 μg/mL ovalbumin (Ova; Sigma-Aldrich), (c) 5 μg/mL MBP (Sigma-Aldrich), (d) 10 μg/mL MBP, and (e) 20 μg/mL MBP. Cell suspensions of 100,000 cells/100 μL were added to all wells. Control wells contained no antigen or mitogen. The microplate was then incubated at 37.0°C and 5% CO2 for 72 h after which 10 μL of BrdU labeling solution [Cell Proliferation ELISA, BrdU (colorimetric), Roche] was added to all wells. The microplate was then incubated for an additional 10 h. The cells in all wells were resuspended by gentle aspiration and later centrifuged at 300×g for 10 min to cause the cells to pelletise uniformly at the bottom of the wells. The supernatant was collected for later analysis and the microplate dried at 60°C for 60 min. Microplates were later processed as instructed in the Cell Proliferation ELISA, BrdU (colorimetric) kit (Roche). The microplates were then read using a Multiskan Spectrum Microplate Photometer (Thermo) at 370 nm. The lymphocyte stimulation index (LSI) was calculated by dividing the mean absorbance of experimental wells by the mean absorbance of the cells cultured in medium alone.
Enzyme-linked immunosorbent assay
To detect anti-MBP IgG antibodies, a 5 mL sample of whole blood was left to clot without additives. After the sample coagulated, it was refrigerated for 30 min to cause clot contraction. After removing the clot, the resultant serum was centrifuged at 400×g for 10 min. The serum was then aliquoted in 0.5 mL test tubes and stored at –20°C for later use. High affinity microplates for ELISA (Maxisorb, NUNC) were prepared by adding 5 μg/mL of MBP in 100 μL of carbonate buffer 0.5 M, pH 9.6 and incubated for 4 h at ~37°C and subsequently overnight at 4°C. Afterwards, all wells were washed with 300 μL of 50 mM Tris, 0.14 M NaCl, 0.05% Tween 20, pH 8.0. Subsequently, wells were then blocked using 300 μL of standard blocking reagent (ELISA Blocking Reagent, Roche) and the plates were further incubated at ~37°C for 4 h and again overnight at 4°C. Afterwards, 100 μL of the collected human sera were added to each well at 1:160, 1:320, 1:640 and 1:1,280 dilutions in 50 mM Tris, 0.14 M NaCl, 1% BSA, 0.05% Tween 20, pH 8.0. After incubation, wells were washed using the previously mentioned method and 100 μL of 1:10,000 secondary antibody (goat anti-Human IgG-HRP conjugate, Bethyl) dissolved in 50 mM Tris, 0.14 M NaCl, 1% BSA, 0.05% Tween 20, pH 8.0 was added to each well. After three additional washes, 100 μL of substrate (ABTS, Roche) was added to all wells. Finally, plates were read at 415 nm using a Multiskan Spectrum Microplate Photometer (Thermo).
Statistical analysis
Data were analyzed using the GraphPad Prism 3.0 software. Results related to lymphocyte proliferation assays were compared using the Mann–Whitney U test. Two-factor ANOVA for repeated measures was used to determine statistical significance of differences among data of humoral response. Correlation between cellular and humoral responses was analyzed using Pearson’s correlation test. Student’s t test was used to compare cellular and humoral responses between ASIA A and ASIA B patients. Statistical significance was considered relevant when P ≤ 0.05.
Results
The average age of control subjects was of 32 ± 5 years, while SCI individuals 33 ± 6 years old (mean ± SD). The cause of SCI included motor vehicle collision (66.66%), traumatic falls (25.0%) and gunshot wounds (8.33%). The level at which the injury was sustained ranged from the cervical (41.66%) to thoracic (58.34%) regions of the spinal cord. The average time of SCI evolution was 19.55 ± 8.4 (mean ± SD) years. The neurological recovery of patients was assessed using the ASIA scale classification and corresponded to A (50.0%) and B (50.0%). No changes in impairment were observed between the first and last clinical evaluations. Tables 1 and 2 show the general characteristics of the whole group.
Table 1.
Clinical characterization of SCI patients
| Code | Gender | Age (years) | SCI level | Mechanism of lesion/diagnosis | ASIA | Years of evolution |
|---|---|---|---|---|---|---|
| MANA | M | 33 | C4–C5 | Traumatic fall/spine compression | A | 13 |
| MVR | M | 46 | T9–T10 | Traumatic fall/spine compression | A | 37 |
| ACNO | M | 26 | C6–C7 | Vehicle collision/spine compression | B | 17 |
| AACDP | M | 28 | T7–T8 | Vehicle collision/spine compression | B | 16 |
| JLCP | M | 22 | T5–T6 | Vehicle collision/spine compression | B | 12 |
| JECL | M | 34 | C7–T1 | Gunshot/spine transection | A | 22 |
| RLD | M | 37 | T7–T8 | Vehicle collision/spine compression | B | 13 |
| JAMP | M | 36 | C4–C5 | Vehicle collision/spine compression | B | 17 |
| RHL | F | 35 | T3–T4 | Vehicle collision/spine compression | A | 29 |
| CCP | F | 31 | T8–T9 | Vehicle collision/spine compression | B | 20 |
| MAJM | F | 35 | T4–T5 | Traumatic fall/Spine compression | A | 17 |
| LVA | F | 34 | C4–C5 | Vehicle collision/spine compression | A | 18 |
Table 2.
First and last neurological evaluations
| Code | 1st evaluation | Last evaluation (10 years later) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Motor force | Sensitive level | TBI | SC | Paraesthesias | Motor force | Sensitive level | SC | Paraesthesias | |
| MANA | 24/100 | C6 | – | – | + | 24/100 | C6 | – | + |
| MVR | 53/100 | T9 | – | – | + | 53/100 | T9 | – | + |
| ACNO | 25/100 | C6 | + | – | + | 25/100 | C6 | – | + |
| AACDP | 56/100 | T7 | – | – | + | 56/100 | T7 | – | + |
| JLCP | 54/100 | T5 | + | – | + | 54/100 | T5 | – | + |
| JECL | 37/100 | C7 | – | – | + | 37/100 | C7 | – | + |
| RLD | 52/100 | T6 | – | – | + | 52/100 | T6 | – | + |
| JAMP | 13/100 | C4 | – | – | + | 13/100 | C4 | – | + |
| RHL | 50/100 | T2 | – | – | + | 50/100 | T2 | – | + |
| CCP | 55/100 | T8 | – | – | + | 55/100 | T8 | – | + |
| MAJM | 50/100 | T5 | – | – | + | 50/100 | T5 | – | + |
| LVA | 21/100 | C6 | – | – | + | 21/100 | C6 | – | + |
TBI Traumatic brain injury, SC sphincter contraction
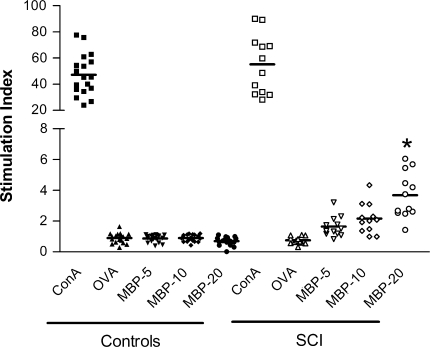
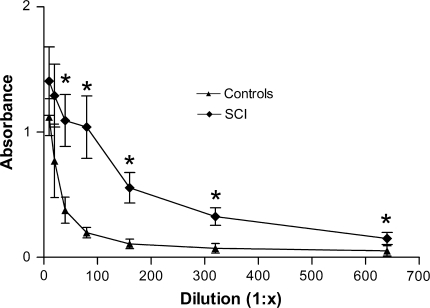
SCI patients presented a significant T-cell proliferation against MBP [20 μg/mL; lymphocyte stimulation index (LSI): 3.7 ± 1.5 (mean ± SD)], compared to control individuals (0.7 ± 0.3; P < 0.001). The stimulation of lymphocytes with 20 μg/mL of MBP induced a proliferative response in SCI patients that, at least, corresponded to double the one presented by cells from control patients under the same conditions (minimum LSI in SCI patients was 1.4). Eleven SCI patients presented a LSI above 2.0 (Fig. 1). In the analysis of the humoral response, there was a significant difference (P < 0.0001) between the antibody levels of controls and SCI patients (see Fig. 2) regardless of the serum dilution. Even at higher dilutions, SCI patients presented higher absorbance values compared to control individuals. The latter indicates the considerable amount of anti-MBP antibodies in SCI patients.
Fig. 1.
Proliferative response of MBP-reactive lymphocytes obtained from patients with chronic SCI or control subjects. Horizontal line represents the mean of the values. *Represents a statistically significant difference from MBP-20 in control patients (P < 0.001, Mann–Whitney U test). MBP-reactive lymphocytes were significantly increased in all patients with chronic SCI. ConA concanavalin A, OVA ovalbumin, MBP myelin basic protein, SCI spinal cord injury. MBP 5, 10, 20: each concentration in μg/mL
Fig. 2.
Serum levels of anti-MBP IgG antibodies shown in patients with chronic SCI and control subjects. Data are presented as a mean of nine (SCI) or eighteen (control) subjects and compared by two-factor ANOVA for repeated measures. *Indicates a statistically significant difference from control subjects (P < 0.05). The levels of IgG antibodies against MBP were significantly increased in patients with chronic SCI compared to healthy controls. SCI spinal cord injury, 1:x corresponds to ascendant dilutions
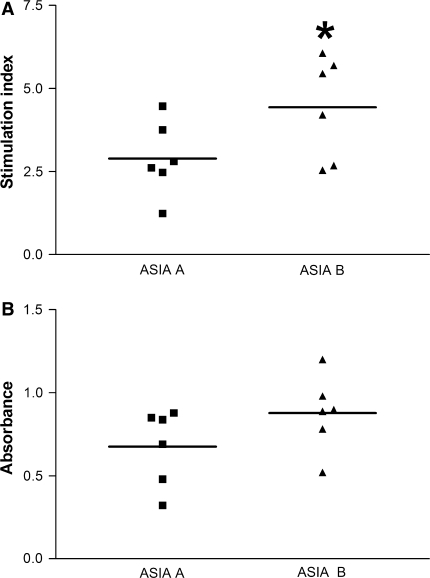
Noteworthy, a significant correlation between cellular and humoral responses in SCI patients was observed (r = 0.90; P = 0.001). Finally, we analyzed the cellular (20 μg/mL, best response) and humoral (dilution 1:160, as an example since all other dilutions showed a similar pattern) responses in SCI patients according to the ASIA impairment scale and found that patients with an ASIA B presented a higher immune reaction (Fig. 3). The proliferative response against MBP was significantly higher (P = 0.04) in patients with incomplete impairment (ASIA B; LSI = 4.44 ± 1.5, mean ± SD) compared to those with complete impairment (ASIA A; LSI = 2.89 ± 1.1). Although not significant (P = 0.07), humoral response against MBP was also higher in the group with incomplete impairment (ASIA B: 0.87 ± 0.22; ASIA A: 0.62 ± 0.33).
Fig. 3.
Cellular and humoral responses observed in patients with complete or incomplete impairment. Lymphocyte proliferation (a) and IgG antibody response (b) against MBP were analyzed. Horizontal line represents the mean of the values. *Different from ASIA A (P = 0.04, Student’s t test). Patients with ASIA B impairment showed an elevated response against MBP. ASIA American Spinal Injury Association
Discussion
In the last years, the self-reactive response developed after SCI has become a relevant topic. This is due to the need of conclusive research that clarifies the exact role the immunological cells play after spinal cord trauma. At the moment, some studies suggest that immune cells contribute in expanding tissue damage after injury [8, 9]; other findings provide significant evidence on the participation of immune cells in promoting neuroprotection and neuroregeneration [10–12]. Collectively, these findings provide interesting elements to hypothesize a conciliatory scenario where these autoreactive responses could behave as a harmful or beneficial phenomenon. Autoreactivity could be influenced by diverse factors (e.g. genetic predisposition, severity of injury, amount of antigen released, etc), and then it becomes an uncontrolled immune response that attacks the CNS, this could perpetuate tissue damage. This is what we observe in autoimmune diseases like MS where anti-MBP lymphocytes and antibodies participate in CNS damage, under these conditions, the uncontrolled autoreactive reaction causes tissue destruction and aggravates the neurological impairment of patients. On the other hand, the modulation of autoreactive responses has shown to improve morphological and functional outcomes in MS and acute neurodegenerative diseases such as traumatic brain injury (TBI) or SCI [13–15]. Accordingly, if the reaction is immediately modulated (by immunizing with neural-derived antigens), even in individuals with the aforementioned factors, the autoreactive response is capable of providing beneficial effects. In this way, the prevalence of autoreactive components at chronic phases of injury offers the possibility of two different scenarios: neurodegeneration or neurorestoration. According to the study reported by Beck et al. [16], immune cells serve as a reparative factor in chronic stages of lesion since, the blockade of these, reduced motor recovery and myelination in the injured spinal cord. In acute SCI, the protective and restorative actions of immune cells have also been widely demonstrated [11, 12, 15]. The findings of the present manuscript confirm the presence of MBP-reactive elements which could be beneficial in promoting restorative processes in patients with chronic SCI. Kil et al. [5] had already reported similar results. They demonstrated hyperactivity of MBP-reactive T cells in patients with chronic SCI; however, the best frequencies of these cells were found in patients with <5 years of evolution. Interestingly, their patients with chronic SCI (>10 years, n = 8) presented values that were quite similar to those observed in normal subjects. In contrast with these results, the present work showed that patients with chronic injuries (>10 years) present not only a cellular but also a humoral autoreactive response against MBP. The apparent disparity in data could be related to the magnitude of injury. The patients evaluated in this work sustained severe injuries (verified by several diagnostic criteria) causing a minimal recovery posterior to injury (ASIA A and B). This could provoke a massive and constant immune stimulation (at least until the blood–spinal cord barrier closes); maintaining autoreactivity until the late chronic stages of SCI. Unfortunately, the study reported by Kil and co-workers does not provide information about the magnitude or the clinical recovery of the patients, making it impossible to confirm or reject this theory. Other factors involved in data disparity are level of injury and genetic background; both should be considered and studied in the future.
Michal Schwartz’s group at the Weizmann Institute of Science provided the first evidence on autoreactive humoral responses against MBP in chronic stages of SCI [6]. They found IgG antibodies against MBP in 68% of the patients with SCI. Unfortunately, the report does not provide the exact time of SCI evolution; thus, it is not possible to determine if the chronicity of those patients is comparable with the one reported in the present work. In any case, more than half of the patients reported by Schwartz’s laboratory presented MBP-reactive antibodies in chronic stages of injury, which means that this response is more frequent than would be expected in chronic stages. These results suggest that this topic deserves further research.
To our knowledge this is the first study that reports the presence, and significant correlation, of cellular and humoral responses against MBP in chronic stages of SCI in the same group of patients. The exact contribution of this response to the pathophysiology of SCI should be studied in detail. Previous studies have shown that MBP-reactive T cells of patients with SCI are similar to those found in patients with MS. These similarities are due to the T cells’ recognition of the same regions of MBP; this observation speaks about the harmful potential of these cells. Nevertheless, the functional differences observed in the cytokine production between the two MBP-reactive T cell lines suggest that those derived from MS patients have a much higher inflammatory potential [5]. Therefore, the autoreactive response observed in patients with SCI could not necessarily be harmful. Autoreactivity against MBP has also been reported after TBI. In this case, both clinical and experimental studies did not find neurological impairments; moreover, the neurological evaluations suggested that autoreactive phenomena improved the neurological outcome [14, 17]. In the present study, two SCI patients additionally suffered from TBI; however, immune reactions and neurological outcome of these patients were very similar to the ones observed in the rest of the patients (data not shown).
In this work, no significant change in neurological impairment was evident in SCI patients. This observation correlates with the one reported by Beck and co-workers [16]. In SCI animals, they observed that the second phase of the immune response (chronic stage of injury) did not coincide with changes in motor performance; however, the elimination of immune cells at this stage of injury was associated with a significant loss of motor skills. These observations suggest that the presence of immune cells and perhaps of the autoreactive response at these stages is necessary to maintain the neurological function, avoiding a possible increase in impairment. Therefore, the role of autoreactive phenomena after SCI could be related more to neurorestoration than neurodegeneration.
It is important to mention that the response to MBP was higher in patients with incomplete impairment (ASIA B) than those with complete impairment (ASIA A). Regarding this, we have previously demonstrated that the immune response after injury depends on the intensity of the injury. Severe injuries significantly reduce the immune system response, even in chronic stages of injury [18], this could be the reason of the difference between ASIA A and ASIA B patients.
At the moment, there are no studies evaluating both cellular and humoral responses in patients with a long SCI evolution. Our results are overwhelming since 12 out of 12 SCI patients (100%) showed an immune response to MBP. Further studies are needed to determine the exact role of this autoreactive phenomena in chronic stages of SCI.
Conclusion
This work demonstrates, for the first time, the existence of both cellular and humoral responses against MBP in the chronic stages of SCI (>10 years) in the same group of patients. The exact contribution of this response to the pathophysiology of SCI should be determined.
Acknowledgments
This work was partially supported by the National Council of Science and Technology of Mexico (CONACYT), grant no. 57204. We would like to thank M.Sc. Fela Mendlovic for providing technical assistance.
Conflict of interest
None
References
- 1.Chiu WT, Lin HC, Lam C, et al. Epidemiology of traumatic spinal cord injury: comparisons between developed and developing countries. Asia Pac J Public Health. 2010;22:9–18. doi: 10.1177/1010539509355470. [DOI] [PubMed] [Google Scholar]
- 2.Rowland JW, Hawryluk GW, Kwon B, et al. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 3.Ibarra A, Correa D, Willms K, et al. Effects of cyclosporin-A on immune response, tissue protection and motor function of rats subjected to spinal cord injury. Brain Res. 2003;979:165–178. doi: 10.1016/S0006-8993(03)02898-1. [DOI] [PubMed] [Google Scholar]
- 4.Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45:349–363. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Kil K, Zang YCQ, Yang D, et al. T cell response to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J Neuroimmunol. 1999;98:201–207. doi: 10.1016/S0165-5728(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 6.Mizrachi Y, Ohry A, Aviel A, et al. Systemic humoral factors participating in the course of spinal cord injury. Paraplegia. 1983;21:287–293. doi: 10.1038/sc.1983.48. [DOI] [PubMed] [Google Scholar]
- 7.Hayes KC, Hull TC, Delaney GA, et al. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma. 2002;19:753–761. doi: 10.1089/08977150260139129. [DOI] [PubMed] [Google Scholar]
- 8.Popovich PG, Guan Z, McGaughy V, et al. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- 9.Jones TB, Basso DM, Sodhi A, et al. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shechter R, London A, Varol C, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:el000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibarra A, García E, Flores N, et al. Immunization with neural-derived antigens inhibits lipid peroxidation after spinal cord injury. Neurosci Lett. 2010;476:62–65. doi: 10.1016/j.neulet.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Ziv Y, Avidan H, Pluchino S, et al. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. PNAS. 2006;103:13174–13179. doi: 10.1073/pnas.0603747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racke MK, Lovett-Racke AE. Glatiramer acetate treatment of multiple sclerosis: an immunological perspective. J Immunol. 2011;186:1887–1890. doi: 10.4049/jimmunol.1090138. [DOI] [PubMed] [Google Scholar]
- 14.Kipnis J, Nevo U, Panikashvili D, et al. Therapeutic vaccination for closed head injury. J Neurotrauma. 2003;20:559–569. doi: 10.1089/089771503767168483. [DOI] [PubMed] [Google Scholar]
- 15.Martiñon S, Garcia E, Flores N, et al. Vaccination with a neural-derived peptide plus administration of glutathione improves the performance of paraplegic rats. Eur J Neurosci. 2007;26:403–412. doi: 10.1111/j.1460-9568.2007.05650.x. [DOI] [PubMed] [Google Scholar]
- 16.Beck KD, Nguyen HX, Galvan MD, et al. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox AL, Coles AJ, Nortje J, et al. An investigation of auto-reactivity after head injury. J Neuroimmunol. 2006;174:180–186. doi: 10.1016/j.jneuroim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Ibarra A, Jiménez A, Cortes C, et al. Influence of the intensity, level and phase of spinal cord injury on the proliferation of T cells and T-cell-dependent antibody reactions in rats. Spinal Cord. 2007;45:380–386. doi: 10.1038/sj.sc.3101972. [DOI] [PubMed] [Google Scholar]