Abstract
In contrast to that in the middle-aged, higher body mass index (BMI) in older people is associated with higher survival rates. Yet, BMI makes no distinction between fat elsewhere and abdominal fat, the latter being metabolically more harmful. We hypothesized that overall adiposity might be protective in old age, but that central fat might offset that benefit and remained harmful as in the middle-aged. Three thousand nine hundred seventy-eight Chinese elderly ≥65 years had demographics, medical conditions, physical activity, and body composition by DXA recorded at baseline. Overall adiposity was measured as whole body fat%, and abdominal adiposity as waist circumference, waist–hip ratio, and relative abdominal fat (RAF) (relative abdominal fat = abdominal fat according to anatomical landmarks/whole body fat). Deaths within 1 year from baseline were excluded from analysis. All-cause and cardiovascular mortality were analyzed using Cox regression, adjusted for covariates. The lowest quintile of adiposity measurements was used for comparison. After a mean follow-up of 72.3 months, 13.7% men and 4.5% women had died. In men, the highest two quintiles of whole body fat % and the upper four quintiles of RAF were associated with significantly lower all-cause mortality, and adjusted hazard ratio (95% CI) in ascending quintiles of RAF compared with the lowest quintile was 0.62 (0.43–0.89), 0.58 (0.4–0.85), 0.52 (0.36–0.77), and 0.67 (0.47–0.96). No relationship was found between abdominal adiposity and cardiovascular mortality in both genders. Higher whole body fat % as well as higher proportion of abdominal fat was associated with lower all-cause mortality in men. No such relation was found in women.
Keywords: Central adiposity, Obesity, Elderly, Mortality
Keywords: Life Sciences, Molecular Medicine, Geriatrics/Gerontology, Cell Biology
Introduction
Higher body mass index (BMI) in older people is associated with higher survival rates, in contrast to middle-aged people (Adams et al. 2006; Al Snih et al. 2007; Reis et al. 2009; Auyeung et al. 2010; Janssen 2007; Tamakoshi et al. 2010). This has led to the postulation that obesity might be less harmful or even protective in old age (Al Snih et al. 2007; Reis et al. 2009; Auyeung et al. 2010; Lea et al. 2009). Yet, BMI is a measure of overall adiposity, which makes no distinction between fat elsewhere and abdominal fat, the latter being metabolically more harmful (Lapidus et al. 1994; Chang et al. 2000; Snijder et al. 2006). Some have demonstrated that overall adiposity and abdominal adiposity act in different directions regarding the risk of metabolic diseases such as diabetes or cardiovascular diseases (Lapidus et al. 1994; Lafortuna et al. 2006). The effect of higher central adiposity on survival in older adults however was controversial (Reis et al. 2009; Auyeung et al. 2010; Visscher et al. 2001; Woo et al. 2002; Lindqvist et al. 2006). Many have reported that central adiposity was more related to cardiovascular diseases and health risk, independent of BMI (Zamboni et al. 2005), and that peripheral fat such as that in the hips was independently protective (Lissner et al. 2001).
Previous studies have often used waist circumference and the waist–hip ratio (WHR) as a measurement of abdominal adiposity. However, the relationship between mortality, and waist circumference and WHR is controversial among the older population (Reis et al. 2009; Visscher et al. 2001; Woo et al. 2002; Price et al. 2006; Pischon et al. 2008; Jacobs et al. 2010). While some found a more linear relationship between WHR and mortality (Lindqvist et al. 2006; Price et al. 2006; Zhang et al. 2007), others were more in favor of the waist circumference (Visscher et al. 2001; Woo et al. 2002; Pischon et al. 2008; Jacobs et al. 2010). Dual-energy X-ray absorptiometry (DXA) can provide both accurate measurements of overall and regional adiposity (Snijder et al. 2006) and thus may help to elucidate the relationship between different measurements of abdominal fat, fat distribution, and mortality.
Trunk adiposity includes the breasts in women and in obese men. This might have led to overestimation of central or abdominal adiposity (Auyeung et al. 2010) when it is used as a measurement of the latter, confounding the true effect of abdominal obesity on health outcomes. This might also have obscured the relationship between abdominal adiposity and mortality, particularly in women. In the present study, we used DXA-measured abdominal adiposity, delineated according to specific anatomical landmarks, to examine its effect on survival. We hypothesized that overall adiposity might be protective in old age, but that abdominal adiposity might offset that benefit and remain harmful as in middle-aged people. To study that effect, we examined the relationship between how abdominal adiposity, as a proportion of overall adiposity measured by DXA, as waist circumference or as WHR, affects mortality in a cohort of older adults.
Methods
Four thousand community-living Chinese men and women aged 65 years or over were recruited for a cohort study on osteoporosis and general health in Hong Kong between August 2001 and December 2003. Recruitment was by notices in senior social centers and housing estates as a large proportion of the elderly population resides in housing estates and attends senior social centers. Talks were given to explain the purpose, procedures, and investigations to be carried out. We excluded those who (1) were unable to walk independently; (2) had had bilateral hip replacements; (3) were not competent to give informed consent; and (4) had medical conditions (in the judgment of the study physicians) which made it unlikely that they would survive the follow-up period of 4 years for the osteoporosis study. The sample was stratified so that approximately 33% were in each of the age groups: 65–69, 70–74, and 75 or above. The study was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong. All participants gave written consent to allow their personal, psychosocial, and physical data thus obtained to be used for research purposes, prior to undergoing a health check in the School of Public Health in the Chinese University of Hong Kong.
The questionnaire
A questionnaire containing information regarding demographics, lifestyle, physical activity level, and medical history was administered by trained interviewers. Smokers were classified by having ever smoked more than five packs of cigarettes in the past, smoking currently, or never smoked. Physical activity level was assessed using the Physical Activity Scale of the Elderly (PASE) (Washburn et al. 1993), which measured the hours spent per day in leisure, household, and occupational physical activities over the previous 7 days. A summary score reflected the daily physical activity level. Cognitive function was measured by the Mini-Mental State Examination (MMSE), a cognitive test including items on time and place orientation, registration and delayed recall, calculation and concentration, language, and praxis. The maximum score was 30 (Folstein et al. 1975). The cut-off for suspected cognitive impairment for the Hong Kong population was set at 18 for those with no formal education, and 22 for those with elementary education (Chiu et al. 1994). Socioeconomic status was recorded using the social economic status ladder—Hong Kong. Participants were asked to mark their position on the picture of a ladder with ten rungs, with the explanation that the top rung represented people with the most money, most education, and the most respected jobs, and the bottom rung represented the other end of the spectrum. The ladder represented the individual's perception of his/her own status in the community with respect to income, education, and occupation. This subjective measure of socioeconomic status has been associated with health outcomes in various populations including that of Hong Kong (Adler et al. 2000; Cheng et al. 2002; Singh-Manoux et al. 2005).
Medical diagnoses and medications
Medical diagnoses were based on the subjects' report of their physician's diagnoses, supplemented by medications brought to the interviewers. Diabetes, heart disease, and cancer were defined by self-reporting (ever being told to have the condition by a physician). Heart disease included coronary heart disease, heart failure, and myocardial infarction. Self-report diseases have been recognized as a valid method for collecting medical diagnosis in large-scale studies (Bourdel-Marchasson et al. 1997; McGuire et al. 2006). The number of medications was taken as the total number of medications the participants was taking at the time of the assessment and which they brought to the place of the assessment.
Physical measurements
Body weight was measured, with subjects wearing a light dressing gown, by the Physician Balance Beam Scale (Health-O-Meter, Arlington Heights, IL, USA). Height was measured by the Holtain Harpenden stadiometer (Holtain Ltd, Crosswell, UK). Waist circumferences (the circumference around the trunk midway between the rib cage and the pelvis) and hip circumferences (the maximum circumference around the buttock posteriorly and the pubis symphysis anteriorly) were measured with a flexible measuring tape. Only one measurement was taken. Four staff were involved in the measurement of the waist and the hip. The inter-rater reliability intra-class correlation using 15 subjects was 0.985 and 0.879 for waist and hip, respectively. Body weight at age 25 was obtained by recall of the subjects. Weight change since age 25 was calculated by the formula: body weight at the physical assessment minus recalled weight at age 25.
Body composition
We measured body fat by DXA using Hologic Delphi W4500 (Hologic Delphi, auto whole body version 12.4, Hologic Inc, Bedford, Massachusetts, USA) at baseline. The upper border of the abdominal region was defined by a horizontal line drawn through the lower one third of the vertical height between the left midpoint acromion and the external end of left iliac crest. The lower border of the abdominal region was defined by a horizontal line through the external ends of the iliac crests. The method was adopted from that of Bertin et al. (2000). The abdominal height was reduced to the lower one third instead of the lower half as in the report by Bertin et al. because the latter method would have included the lungs and heart due to the smaller body size in the Chinese population. We were not able to use the method of measuring abdominal fat as defined by the region between the L1 and L4 vertebrae because many subjects had scoliosis and low bone mass, making the delineation of the upper or lower borders of these vertebrae difficult from a whole-body DXA scan. The relative abdominal fat (RAF) was calculated as the proportion of abdominal fat within whole body fat (RAF = abdominal fat/whole body fat × 100%). The Hologic Body composition step phantom was scanned daily to ensure proper calibration for fat and nonfat compartments. The maximum coefficient of variation for fat is 1.47%.
Follow-up procedure and mortality data
Follow-up was done every 4-monthly by phone then every 2-yearly by a mailed reminder for a follow-up body check appointment until the end of 4 years (a total of three follow-up visits) or death, whichever occurred earlier. Phone reminders were given again close to the appointment dates, and defaulters were given a second appointment to enhance attendance rates. Mortality status was confirmed by annual reports from the death registry in the Department of Health in Hong Kong. Cardiovascular causes of death were identified by the cause of death reported on the death certificate, and classified according to the International Classification of Disease (ICD) version 10 codes as those ranging from 100 to 199.
Statistical methods
Data analysis was performed by using SPSS version 17.0 (IBM Corp, Somers, NY, USA). As body composition differs with gender, all statistical tests were done separately for men and women. Characteristics of decedents and survivors were compared. Unpaired t tests were used for continuous variables and chi-square tests for categorical variables. Crude mortality was plotted against quintiles of different adiposity measurements. All-cause and cardiovascular mortality as on February 28, 2009 were analyzed using Cox regression, adjusted for covariates that were relevant to mortality in older individuals (age, physical activity, smoker status, history of cancer, diabetes, heart disease, measures of socioecomonic status and medications. To adjust for the effect of lifelong obesity, recalled weight change since early adulthood at 25 years of age was further adjusted for. The lowest quintile of all adiposity measures was used as the comparison group. Early deaths within 12 months of the baseline assessment were excluded to avoid bias due to reverse causality. All tests were two-sided, and a p value of <0.05 was taken as statistically significant.
Results
After a mean follow-up of 72.3 ± 11.7 months (median 74.4 months), 286 (14.3%) men and 97 (4.9%) women had died. Those who died within the first year after the baseline visit were excluded from the analysis (15 men and 7 women) to eliminate bias in body fat changes close to the time of death (reverse causality). Decedents in both men (n = 271) and women (n = 90) were older, had lower MMSE scores, and were more likely to have a past history of cancer at baseline. In men, those who died also had lower physical activity scores and were more likely to have diabetes (Table 1).
Table 1.
Characteristics of decedents and survivors
| Men | Women | |||
|---|---|---|---|---|
| Decedents (n = 271) | Survivors (n = 1,714) | Decedents (n = 90) | Survivors (n = 1,903) | |
| Age | 75. (5.4) | 71.9 (4.8)* | 74.9 (6.2) | 72.5 (5.3)* |
| Physical activity score | 84.0 (45.2) | 99.5 (50.8)* | 85.2 (33.2) | 85.4 (33.2) |
| Smoker status (%)**,a | ||||
| Never | 24.7 | 37.9 | 77.8 | 91.1 |
| Ex-smoker | 60.9 | 50.5 | 16.7 | 7.2 |
| Current smoker | 14.4 | 11.6 | 5.6 | 1.7 |
| Number of medicationsb | 1 (0–7) | 1 (0–7)* | 1 (0–6) | 1 (0–7) |
| Social economic status ladder—Hong Kong | 4.2 (2.0) | 4.5 (1.8)* | 4.5 (2.0) | 4.6 (1.9) |
| MMSE | 26.2 (3.7) | 27.1 (2.6)* | 23.3 (4.1) | 24.3 (3.9)* |
| Diabetes (%)a | 21.8 | 13.4* | 15.6 | 14.2 |
| Heart disease (%)a | 20.7 | 17.8 | 15.6 | 16.6 |
| History of cancer (%)a | 6.6 | 3.9* | 8.9 | 4.3* |
| Adiposity measurements | ||||
| BMI | 22.8 (3.5) | 23.6 (3.0)* | 24.0 (4.1) | 23.9 (3.4) |
| Whole body fat % | 23.6 (5.3) | 24.5 (4.9)* | 33.8 (7.0) | 34.6 (5.2) |
| Waist (cm) | 87.1 (13.0) | 87.4 (8.9) | 87.1 (10.2) | 85.6 (9.4) |
| Waist–hip ratio | 0.93 (0.07) | 0.92 (0.07) | 0.93 (0.09) | 0.92 (0.08) |
| Relative abdominal fat (%) | 14.7 (2.7) | 15.2 (2.4)* | 14.0 (2.1) | 14.0 (2.0) |
MMSE Mini-Mental State Examination score, BMI body mass index
Numbers are expressed as mean (SD) unless stated otherwise. All comparisons were by t test unless stated otherwise
*p < 0.05; **p for trend <0.05
aComparison by chi-square test
bNumber expressed as median (range)
Men who survived had higher BMI, higher whole body fat %, and higher relative abdominal fat. There was no difference in the waist circumference and waist–hip ratio between the survivors and the decedents (Table 1). In women, no difference in any of the adiposity measurements was observed between survivors and decedents.
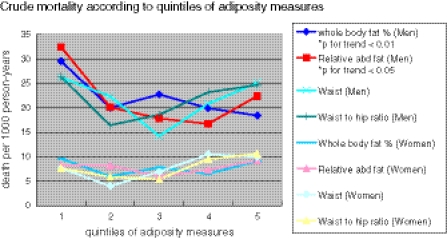
Figure 1 (or Table 6) showed the crude mortality rates of both men and women across the quintiles of four obesity measures (whole body fat % for general adiposity, waist circumference, WHR, and relative abdominal fat for abdominal adiposity). Higher quintiles of both whole body fat % and RAF were associated with lower crude mortality in men (whole body fat %, p for trend <0.01; relative abdominal fat, p for trend <0.05). Waist circumference and WHR had a U-shaped relationship with mortality with the lowest rate at the third quintile and second quintile, respectively. None of the obesity measures bore any significant relationship with mortality in women.
Fig. 1.
Relationship between crude mortality rate and quintiles of adiposity measurements in older men and women
Table 6.
Relationship between crude mortality rate and quintiles of adiposity measurements in older men and women
| Quintiles | Death per 1,000 person-years | ||||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | |
| Men | |||||
| Whole body fat %* | 29.5 | 20.0 | 22.7 | 19.9 | 18.4 |
| Relative abdominal fat** | 32.4 | 20.1 | 17.8 | 16.7 | 22.4 |
| Waist | 26.2 | 22.3 | 14.2 | 20.8 | 25.1 |
| Waist to hip ratio | 26.3 | 16.4 | 18.6 | 23.2 | 24.6 |
| Women | |||||
| Whole body fat % | 9.6 | 6.0 | 7.8 | 6.5 | 9.1 |
| Relative abdominal fat | 8.2 | 8.2 | 6.0 | 7.3 | 9.2 |
| Waist | 7.6 | 4.0 | 6.9 | 10.5 | 10.0 |
| Waist to hip ratio | 7.6 | 5.9 | 5.4 | 9.5 | 10.6 |
*p for trend <0.01; **p for trend <0.05
Table 2 shows the hazard ratios (HR) of all-cause and cardiovascular mortality according to quintiles of whole body fat %, relative abdominal fat, waist circumference, and WHR. In men, each of the four upper quintiles of RAF was associated with a significantly lower HR for all-cause mortality after adjustment for age, physical activity, history of cancer, diabetes, heart disease and smoker status. Only the highest two quintiles of whole body fat % showed similar protective effect. The p for trend was significant for the HRs of all-cause mortality across quintiles of whole body fat % (p for trend <0.001) and relative abdominal fat (p for trend = 0.011) in men, indicating a tendency for those with higher fatness to have lower mortality, after adjustment for age, physical activity, smoker status, history of cancer, diabetes, and heart disease. Waist circumference and WHR quintiles however were not related to all-cause mortality. No relationship between these measures of obesity and all-cause of mortality was found in women, nor was there any relationship found between any of the three abdominal adiposity measurements and cardiovascular mortality in both genders.
Table 2.
Hazard ratios of all-cause and cardiovascular mortality according to adiposity measurement quintiles
| Quintile | Whole body fat % | Relative abdominal fat | Waist circumference | Waist–hip ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | All-cause mortality | Cardiovascular mortality | n | All-cause mortality | Cardiovascular mortality | n | All-cause mortality | Cardiovascular mortality | n | All-cause mortality | Cardiovascular mortality | |
| Men | ||||||||||||
| 1st | 373 | 1.00 | 1.00 | 396 | 1.00 | 1.00 | 407 | 1.00 | 1.00 | 396 | 1.00 | 1.00 |
| 2nd | 396 | 0.72 (0.50, 1.04) | 1.33 (0.57, 3.09) | 397 | 0.62 (0.43, 0.89)** | 0.82 (0.37, 1.80) | 402 | 0.85 (0.59, 1.21) | 0.97 (0.38, 2.44) | 395 | 0.68 (0.46, 1.00) | 1.25 (0.54, 2.91) |
| 3rd | 336 | 0.81 (0.56, 1.18) | 1.48 (0.61, 3.45) | 399 | 0.58 (0.40, 0.85)** | 0.72 (0.32, 1.66) | 371 | 0.58 (0.38, 0.88)* | 1.82 (0.80, 4.16) | 399 | 0.78 (0.53, 1.14) | 1.02 (0.42, 2.48) |
| 4th | 352 | 0.63 (0.43, 0.92)* | 0.91 (0.37, 2.26) | 396 | 0.52 (0.36, 0.77)** | 0.58 (0.25, 1.38) | 406 | 0.83 (0.57, 1.19) | 1.40 (0.59, 3.30) | 395 | 0.92 (0.64, 1.33) | 1.27 (0.55, 2.93) |
| 5th | 528 | 0.54 (0.38, 0.78)** | 1.09 (0.49, 2.43) | 397 | 0.67 (0.47, 0.96)* | 1.31 (0.64, 2.68) | 398 | 0.87 (0.60, 1.25) | 1.54 (0.67, 3.53) | 400 | 0.86 (0.60, 1.23) | 1.56 (0.71, 3.42) |
| p value for trend | <0.001 | 0.692 | 0.011 | 0.443 | 0.196 | 0.296 | 0.811 | 0.417 | ||||
| Women | ||||||||||||
| 1st | 396 | 1.00 | 1.00 | 398 | 1.00 | 1.00 | 387 | 1.00 | 1.00 | 404 | 1.00 | 1.00 |
| 2nd | 399 | 0.72 (0.37, 1.42) | 0.48 (0.09, 2.50) | 398 | 1.10 (0.58, 2.09) | 0.45 (0.09, 2.33) | 384 | 0.55 (0.24, 1.23) | 0.79 (0.13, 4.77) | 378 | 0.76 (0.37, 1.55) | 0.77 (0.17, 3.46) |
| 3rd | 399 | 1.05 (0.56, 1.98) | 0.57 (0.11, 3.05) | 400 | 0.75 (0.38, 1.50) | 0.22 (0.03, 1.92) | 426 | 1.00 (0.51, 1.97) | 1.52 (0.33, 6.93) | 416 | 0.74 (0.36, 1.52) | 0.83 (0.19, 3.73) |
| 4th | 399 | 0.83 (0.43, 1.62) | 1.04 (0.27, 3.98) | 400 | 0.94 (0.49, 1.82) | 0.66 (0.16, 2.78) | 361 | 1.44 (0.76, 2.71) | 2.06 (0.49, 8.70) | 398 | 1.12 (0.60, 2.10) | 0.67 (0.15, 3.03) |
| 5th | 400 | 1.22 (0.66, 2.25) | 0.55 (0.10, 2.91) | 397 | 1.20 (0.65, 2.24) | 0.93 (0.25, 3.50) | 434 | 1.31 (0.70, 2.44) | 0.33 (0.03, 3.14) | 396 | 1.18 (0.63, 2.21) | 0.42 (0.07, 2.32) |
| p value for trend | 0.896 | 0.504 | 0.783 | 0.831 | 0.096 | 0.700 | 0.124 | 0.549 | ||||
By Cox regression, adjusted for age, physical activity, smoker status, history of cancer, diabetes, and heart disease
*p < 0.05; **p < 0.01
In men, there was a mean increase in weight since age 25 among decedents (4.78 ± 10.13 kg) and survivors (7.26 ± 9.02 kg), p < 0.000, 95% CI 1.17 to 3.81. Among decedents, the weight gain from age 25 of those died within the first year of follow-up was not different from those who survived beyond the first year: 3.23 ± 11.99 kg vs. 4.78 ± 10.13 kg, p > 0.05, 95% CI −7.07 to 3.99. Further adjustment for weight change since the age of 25 slightly attenuated the protective effect of the highest two quintiles of whole body fat % and the second to fourth quintiles of relative abdominal fat %, but the relationship remained significant. The highest quintile in relative abdominal fat % ceased to be protective after this adjustment (Table 3).
Table 3.
Hazard ratios of all-cause mortality according to adiposity measurement quintiles in men, further adjusted for weight changes since age 25, in addition to age, physical activity, smoker status, history of cancer, diabetes, and heart disease
| Quintile | Whole body fat % | Relative abdominal fat | Waist circumference | Waist–hip ratio | ||||
|---|---|---|---|---|---|---|---|---|
| n | HR (95% CI) | n | HR (95% CI) | n | HR (95% CI) | n | HR (95% CI) | |
| 1st | 373 | 1.00 | 396 | 1.00 | 407 | 1.00 | 396 | 1.00 |
| 2nd | 396 | 0.73 (0.50, 1.08) | 397 | 0.65 (0.44, 0.95)* | 402 | 0.94 (0.65, 1.38) | 395 | 0.74 (0.49, 1.11) |
| 3rd | 336 | 0.86 (0.57, 1.29) | 399 | 0.62 (0.41, 0.92)* | 371 | 0.69 (0.44, 1.07) | 399 | 0.87 (0.58, 1.31) |
| 4th | 352 | 0.65 (0.42, 1.00)* | 396 | 0.59 (0.39, 0.89)* | 406 | 1.11 (0.73, 1.68) | 395 | 1.13 (0.77, 1.67) |
| 5th | 528 | 0.58 (0.38, 0.90)* | 397 | 0.74 (0.49, 1.11) | 398 | 1.16 (0.73, 1.85) | 400 | 1.01 (0.68, 1.51) |
| p value for trend | 0.010 | 0.175 | 0.772 | 0.379 | ||||
*p < 0.05
The relationship between all-cause mortality and quintiles of fat measurements remained essentially unchanged after further adjustment of the model in Table 2 for the number of medications and self-rank socioeconomic status (Table 4). The p for trend for relative abdominal fat became more significant with the adjustment (p for trend increased from 0.011 to 0.005).
Table 4.
Hazard ratios of all-cause mortality according to adiposity measurement quintiles in men, further adjusted for number of medications and self-rated socioeconomic status, in addition to age, physical activity, smoker status, history of cancer, diabetes, and heart disease
| Quintile | Whole body fat % | Relative abdominal fat | ||
|---|---|---|---|---|
| n | HR (95% CI) | n | HR (95% CI) | |
| 1st | 373 | 1.00 | 396 | 1.00 |
| 2nd | 396 | 0.77 (0.53, 1.11) | 397 | 0.65 (0.45, 0.92)* |
| 3rd | 336 | 0.86 (0.60, 1.25) | 399 | 0.59 (0.41, 0.86)* |
| 4th | 352 | 0.65 (0.44, 0.96)* | 396 | 0.51 (0.35, 0.75)* |
| 5th | 528 | 0.51 (0.35, 0.73)* | 397 | 0.63 (0.43, 0.90)* |
| p value for trend | <0.001 | 0.005 | ||
*p < 0.05
The relative abdominal fat quintile with the lowest adjusted HR for all-cause mortality was the fourth quintile. Common obesity measurements of the five quintiles in relative abdominal fat (men) were shown for clinical reference (Table 5). The corresponding waist circumference in the fourth quintile of relative abdominal fat was 90.53 cm, the BMI 24.6, the waist–hip ratio 0.94, and whole body fat % 26.4%.
Table 5.
Clinical anthropometric measurements of men according to quintiles of relative abdominal fat
| Quintiles of relative abdominal fat | Waist (cm) | Body mass index | Waist–hip ratio | Whole body fat % | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| 1st | 407 | 77.2 (7.5) | 393 | 20.2 (2.6) | 396 | 0.87 (0.06) | 373 | 18.9 (4.9) |
| 2nd | 402 | 86.3 (6.5) | 398 | 22.9 (2.3) | 395 | 0.91 (0.05) | 396 | 22.9 (2.3) |
| 3rd | 371 | 88.8 (9.1) | 398 | 23.9 (2.4) | 399 | 0.93 (0.05) | 336 | 25.4 (3.6) |
| 4tha | 406 | 90.5 (6.8) | 398 | 24.6 (2.6) | 395 | 0.94 (0.06) | 352 | 26.4 (3.6) |
| 5th | 398 | 94.6 (7.3) | 398 | 25.7 (2.8) | 400 | 0.97 (0.06) | 528 | 27.3 (3.7) |
Numbers are expressed as mean (SD)
aThe fourth quintile of relative abdominal fat had the lowest all-cause mortality
Discussion
We found that higher abdominal fat proportion was associated with lower all-cause mortality in older men. Mortality did not increase with increase in abdominal obesity in older men. This finding is in line with that of previous authors (Reis et al. 2009; Auyeung et al. 2010) which showed higher waist circumference or waist–hip ratio conferred survival benefits, but in contrary with those of others (Lindqvist et al. 2006; Price et al. 2006; Pischon et al. 2008; Jacobs et al. 2010). Waist circumference does not take into consideration the distribution of fat, as it only measures the waist; a large waist circumference could imply central fat accumulation, overall fatness, or a large body size. The waist–hip ratio does not take into account the loss of gluteal muscles with aging, to the effect that a stable waist circumference with a decreasing hip circumference due to gluteal muscle loss would result in an increasing waist–hip ratio. On the other hand, DXA-measured abdominal fat describes more accurately fat distribution and hence may be more suitable to address the question on the effect of central fat versus peripheral fat. We and others have shown that adiposity (Adams et al. 2006; Flicker et al. 2010) and truncal adiposity (Auyeung et al. 2010) may be beneficial for survival in old age, even for those with history of cardiovascular disease (Lea et al. 2009). Our present results showed that in contrary to findings in middle-aged adults, higher proportion of abdominal fat may also be beneficial for survival in men older than 65 years of age.
With adjustment, both whole body fat % and relative abdominal fat still maintained a linear trend with all-cause mortality (Table 2, p for trend <0.001 and equals to 0.011, respectively). This highlights the need to review current nutrition guidelines for older adults that advocate weight control or reduction. In fact, the negative impact of obesity should be reviewed when the individual reaches the age of 65. The waist circumference cutoffs for metabolic risks in midlife may not be applicable to old age, when overall mortality within a shorter life expectancy is considered.
As quintiles of relative abdominal fat showed a reverse relationship with all-cause mortality in older men (Table 6), we further described the phenotype of the men having the amount of relative abdominal fat associated with lowest mortality. According to the WHO classification of BMI (World Health Organization Western Pacific Region 2000; Ko et al. 2005), and criteria for metabolic syndrome in Asian populations, these men were in a group at risk of metabolic diseases: mean waist 90.53 cm (>90 cm cutoff for obesity), mean BMI 24.6 (>23.5, in overweight range), mean waist–hip ratio 0.94 (>0.9 for obesity), and mean whole body fat 26.38% (≥25%, in high body fat range). According to the BMI and the waist circumference, these men should be considered to have moderate metabolic risk, yet our results showed that instead of having adverse effects, older men having these markers of obesity have a lower HR for mortality. Obesity criteria have been mostly developed using younger adult data. Heim et al. have shown that waist circumference cutoff for disability outcomes could be higher in older adults (Heim et al. 2010).
The relationship between obesity and mortality may be altered by age or by frailty (Kopple 2005). In older, healthy individuals without chronic diseases, the risk of obesity may remain similar to that in middle-aged adults (Adams et al. 2006; Schooling et al. 2006). However, the level of fitness might modulate the mortality risk of obesity. In fact, among those within the same strata of low, moderate, or high cardiorespiratory fitness, overweight and obese men consistently had lower mortality than normal weight men, in groups of middle-aged and older male veterans (McAuley et al. 2010, 2009). This is consistent with our results which showed that beyond the age of 65, in a cohort of high functioning older men and women, obesity operated in a different direction regarding longevity than in midlife. Possible explanations are that in older or frail individuals, infections and acute illnesses become significant causes of death, and those with greater fat or energy reserve tend to survive acute illnesses better. Indeed, weight loss in old age might be a marker of risk of mortality (Newman et al. 2001). In contrary, those with end-stage chronic diseases tend to be more cachexic; therefore, those with more adiposity should be the ones with less severe chronic diseases. In old age, therefore, the more fat an elderly individual, the better the survival, and thus, overweight or obese people might rank high regarding life expectation. It is important to note however that despite significant trends for linearity showing the higher the fat, the better the survival, those in the highest quintile of relative abdominal adiposity in our cohort had a mean BMI of 25.7, which was at the lower end of the obesity range for Asians, and had a mean whole body fat % of 27.3% only. The fatness–survival relationship might be different for those in the upper end of the obesity range.
Another possible explanation for the negative relationship between fat mass and all-cause mortality in the older population could be that traditional cardiovascular risk predictors such as those used in the Framingham risk score (systolic blood pressure, total and high density lipoprotein cholesterol, diabetes mellitus, smoking, and electrocardiogram-based left ventricular hypertrophy) and the International Diabetes Federation (IDF) criteria (high waist circumference, high blood pressure, high blood sugar or diabetes, high triglyceride, and low high density lipoprotein) (Alberti et al. 2005) were less predictive of cardiovascular risks in the elderly (de Ruijter et al. 2009; Motta et al. 2009).
Lifelong weight trajectories might have a role in modifying the effects of obesity in old age. In a cohort of healthy men, those who were overweight in midlife but had normal weight in late life had the highest mortality (Strandberg et al. 2009). We did not have the weight in midlife; therefore, we attempted to use the recalled weight change since the age of 25 to estimate the weight trajectories of our subjects, and adjusted for that in our model to assess the relationship between different obesity measurements and mortality. Although recalled weight changes did not differ between survivors and decedents, we did find that it attenuated the protective effect of obesity in late life. Nevertheless, the obesity paradox remained robust in our model: higher overall fatness (whole body fat %) and abdominal adiposity (relative abdominal fat) remained protective against mortality. More importantly, all adiposity measurements, both general and abdominal, did not affect cardiovascular mortality, even with adjustment for recalled weight change in adulthood.
The lack of associations in women might be explained by the small numbers of deaths during follow-up, or that mortality in older women might genuinely be independent of central adiposity. Our previous report using trunk fat did not observe any relationship between that and mortality in women, which could have been due to the inclusion of breast fat (Auyeung et al. 2010). Using a more defined abdominal region in the present study, we confirmed the absence of relationship even with the exclusion of breast fat. It has been reported that women have twice as much subcutaneous fat than men, but their amount of intra-abdominal fat and liver fat is similar. However, as only liver fat, but not subcutaneous or intra-abdominal fat is independently associated with markers on insulin resistance, the same amount of general abdominal fat and whole body fat % as measured by DXA would have implied different levels of insulin resistance or cardiovascular risks in men and women. This might be one of the explanations for the gender differences seen in the associations between fat measures and mortality in our cohort (Westerbacka et al. 2004).
Since the number of cardiovascular death outcomes was small, this might account for the difference in results between all-cause mortality and cardiovascular deaths (lack of association with the latter). A longer follow-up duration with more cardiovascular deaths is needed to demonstrate more clearly whether it is a genuine lack of association or that fat may even be protective towards cardiovascular deaths in the elderly.
There are limitations in this study. DXA could not differentiate between abdominal subcutaneous fat from visceral fat in contrast to CT measurements. Nevertheless, trunk fat as determined by DXA was found to correlate well with insulin resistance and dyslipidemia, and survival (Auyeung et al. 2010; Hamdy et al. 2006). Only one measurement of the waist and hip was taken. We used a novel method of defining abdominal adiposity as it was difficult to delineate margins of the vertebral bodies in older participants due to scoliosis and low bone mass. Our results may reflect the phenomenon of selective survival in which only middle-aged persons more resistive to the hazard of central adiposity survived into old age and were thus included in the present study. Those with serious complications of abdominal obesity or its related metabolic disorders might have premature death prior to age 65 or have become too disabled to be included in our cohort. Our cohort is from a population with relatively little morbid obesity; therefore, findings should not be generalized into populations with a high proportion of obesity. Our cohort is more educated and more physically active than the general elderly population in Hong Kong; therefore, the results might not be generalized to those who are institutionalized or frailer, or with lower education level. Caution should be exercised in the interpretation of lifelong weight trajectory using just weights at two points in time. Someone who was overweight both at age 25 and when baseline measures were taken would have a weight change of zero, while another person who had normal weight or was underweight at both these time points could have the same weight change value. In addition, our result could not take into account the weight fluctuations in between these time points. Using recalled weight at age 25 may be prone to bias, and the accuracy depends on age, sex, lapsed time, current body mass index, and cognitive function (Perry et al. 1995; Stevens et al. 1990). The number of cardiovascular deaths was small, especially among women; therefore, the power of analysis in demonstrating a genuine lack of relationship between fatness and cardiovascular deaths, or even whether fat might be protective as in all-cause mortality, might be limited. A longer follow-up period with higher number of cardiovascular deaths might be able to provide a more definitive answer. Nevertheless, the all-cause mortality in women did not show any association with any of the four adiposity measurements.
Conclusion
Higher abdominal adiposity in addition to whole body fat might be beneficial for survival in older men. Waist circumference, whole body fat %, and body mass index cutoffs corresponding to the quintile of abdominal adiposity with the lowest mortality were higher than those recommended for middle-aged adults.
Acknowledgments
This study was funded by the Hong Kong Research Grant Council Grant (CUHK4101/02M). We thank The S H Ho Centre for Gerontology and Geriatrics, Faculty of Medicine, The Chinese University of Hong Kong for personnel support in data collection.
Contributor Information
Jenny Shun Wah Lee, Phone: +86-852-26367688, FAX: +86-852-26351037, Email: jennylee@cuhk.edu.hk.
Tung Wai Auyeung, Email: auyeungtw@cuhk.edu.hk.
References
- Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Eng J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- Adler NE, Epil ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19:586–592. doi: 10.1037/0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Snih S, Ottenbacher KJ, Markides KS, Kuo Y-F, Eschbach K, Goodwin JS. The effect of obesity on disability vs mortality in older Americans. Arch Intern Med. 2007;167:774–780. doi: 10.1001/archinte.167.8.774. [DOI] [PubMed] [Google Scholar]
- Alberti KGMM, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group The metabolic syndrome—a new world-wide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Auyeung TW, Lee JS, Leung J, Kwok T, Leung PC, Woo J. Survival in older men may benefit from being slightly overweight and centrally obese—a 5-year follow-up study in 4,000 older adults using DXA. J Gerontol A Biol Sci Med Sci. 2010;65:99–104. doi: 10.1093/gerona/glp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin E, Marcus C, Ruiz JC, Eschard JP, Leutenegger M. Measurement of visceral adipose tissue by DXA combined with anthropometry in obese humans. Int J Obes Relat Metab Disord. 2000;24:263–270. doi: 10.1038/sj.ijo.0801121. [DOI] [PubMed] [Google Scholar]
- Bourdel-Marchasson I, Dubroca B, Manciet G, Decamps A, Emeriau JP, Dartigues JF. Prevalence of diabetes and effect on quality of life in older French living in the community: the PAQUID Epidemiological Survey. J Am Geriatr Soc. 1997;45:295–301. doi: 10.1111/j.1532-5415.1997.tb00943.x. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Wu CH, Yao WJ, Yang YC, Wu JS, Lu FH. Relationships of age, menopause and central obesity on cardiovascular disease risk factors in Chinese women. In J Obes Relat Metab Disord. 2000;24:1699–1704. doi: 10.1038/sj.ijo.0801457. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Chi I, Boey KW, Ko LS, Chou KL. Self-rated economic condition and the health of elderly persons in Hong Kong. Soc Sci Med. 2002;55:1415–1424. doi: 10.1016/S0277-9536(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Chiu HFK, Lee HC, Chung WS, Kwong PK. Reliability and validity of the Cantonese version of the Mini-Mental State Examination—a preliminary study. J Hong Kong Coll Psych. 1994;4(SP2):25–28. [Google Scholar]
- Ruijter W, Westendorp RG, Assendelft WJ, Elzen WP, Craen AJ, Cessie S, Gussekloo J. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker L, McCaul KA, Hankey GJ, et al. Body mass index and survival in men and women aged 70 to 75. J Am Geriatr Soc. 2010;58:234–241. doi: 10.1111/j.1532-5415.2009.02677.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diab Rev. 2006;2:367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- Heim N, Snijder MB, Heymans MW, Deeg DJH, Seidell JC, Visser M. Exploring cut-off values for large waist circumference in older adults: a new methodological approach. J Nutr Health Aging. 2010;14:272–277. doi: 10.1007/s12603-010-0060-7. [DOI] [PubMed] [Google Scholar]
- Jacobs EJ, Newton CC, Wang Y, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170:1293–1301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity (Silver Spring) 2007;15:1827–1840. doi: 10.1038/oby.2007.217. [DOI] [PubMed] [Google Scholar]
- Ko GTC, Cockram CS, Chow CC, Yeung V, Chan WB, So WY, Chan NN, Chan JCN. High prevalence of metabolic syndrome in Hong Kong Chinese—comparison of three diagnostic criteria. Diab Res Clin Prac. 2005;69:160–168. doi: 10.1016/j.diabres.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Kopple JD. The phenomenon of altered risk factor patterns or reverse epidemiology in persons with advanced chronic kidney failure. Am J Clin Nutr. 2005;81:1257–1266. doi: 10.1093/ajcn/81.6.1257. [DOI] [PubMed] [Google Scholar]
- Lafortuna CL, Agosti F, Proietti M, Adorni F, Sartorio A. The combined effect of adiposity, fat distribution and age on cardiovascular risk factors and motor disability in a cohort of obese women (aged 18–83) J Endocrinol Invest. 2006;29:905–912. doi: 10.1007/BF03349195. [DOI] [PubMed] [Google Scholar]
- Lapidus L, Bengtsson C, Björntorp P. The quantitative relationship between “the metabolic syndrome” and abdominal obesity in women. Obes Res. 1994;2:372–377. doi: 10.1002/j.1550-8528.1994.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Lea JP, Crenshaw DO, Onufrak SJ, Newsome BB, McClellan WM. Obesity, end-stage renal disease, and survival in an elderly cohort with cardiovascular disease. Obesity (Silver Spring) 2009;17:2216–2222. doi: 10.1038/oby.2009.70. [DOI] [PubMed] [Google Scholar]
- Lindqvist P, Andersson K, Sundh V, Lissner L, Björkelund C, Bengtsson C. Concurrent and separate effects of body mass index and waist-to-hip ratio on 24-year mortality in the Population Study of Women in Gothenburg: evidence of age-dependency. Eur J Epidemiol. 2006;21:789–794. doi: 10.1007/s10654-006-9074-1. [DOI] [PubMed] [Google Scholar]
- Lissner L, Björkelund C, Heitmann BL, Seidell JC, Bengtsson C. Larger hip circumference independently predicts health and longevity in a Swedish female cohort. Obes Res. 2001;9:644–646. doi: 10.1038/oby.2001.85. [DOI] [PubMed] [Google Scholar]
- McAuley P, Pittsley J, Myers J, Abella J, Froelicher VF. Fitness and fatness as mortality predictors in healthy older men: the Veterans Exercise Testing Study. J Gerontol A Biol Sci Med Sci. 2009;64A:695–699. doi: 10.1093/gerona/gln039. [DOI] [PubMed] [Google Scholar]
- McAuley PA, Kokkinos PF, Oliveira RB, Emerson BT, Myers JN. Obesity paradox and cardiorespiratory fitness in 12,417 male veterans aged 40 to 70 years. Mayo Clinic Proceedings. 2010;85:115–121. doi: 10.4065/mcp.2009.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire LC, Ford ES, Ajani UA. The impact of cognitive functioning on mortality and the development of functional disability in older adults with diabetes: the second longitudinal study on aging. BMC Geriatrics. 2006;6:8–14. doi: 10.1186/1471-2318-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta M, Bennati E, Cardillo E, Passamonte M, Ferlito L, Malaguarnera M. The metabolic syndrome (MS) in the elderly: considerations on the diagnostic criteria of the International Diabetes Federation (IDF) and some proposed modifications. Arch Gerontol Geriatr. 2009;48:380–384. doi: 10.1016/j.archger.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP, Cardiovascular Study Research Group Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6:61–66. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–460. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL. Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity. 2009;17:1232–1239. doi: 10.1038/oby.2008.664. [DOI] [PubMed] [Google Scholar]
- Schooling CM, Lam TH, Li ZB, et al. Obesity, physical activity and mortality in a prospective Chinese elderly cohort. Arch Intern Med. 2006;166:1498–1504. doi: 10.1001/archinte.166.14.1498. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom Med. 2005;67:855–861. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Snijder MB, Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- Stevens J, Keil JE, Waid LR, Gazes PC. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;132:1156–1163. doi: 10.1093/oxfordjournals.aje.a115758. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Strandberg AY, Salomaa VV, et al. Explaining the obesity paradox: cardiovascular risk, weight change, and mortality during long-term follow-up in men. Eur Heart J. 2009;30:1720–1727. doi: 10.1093/eurheartj/ehp162. [DOI] [PubMed] [Google Scholar]
- Tamakoshi A, Yatsuya H, Lin Y, JACC Study Group et al. BMI and all-cause mortality among Japanese older adults: findings from the Japan collaborative cohort study. Obesity (Silver Spring) 2010;18:362–369. doi: 10.1038/oby.2009.190. [DOI] [PubMed] [Google Scholar]
- Visscher TL, Seidell JC, Molarius A, Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001;25:1730–1735. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janny CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Westerbacka J, Cornér A, Tiikkainen M, Tamminen M, Vehkavaara S, Häkkinen A-M. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–1369. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- Woo J, Ho SC, Yu ALM, Sham A. Is waist circumference a useful measure in predicting health outcomes in the elderly? Int J Obes. 2002;26:1349–1355. doi: 10.1038/sj.ijo.0802080. [DOI] [PubMed] [Google Scholar]
- World Health Organization Western Pacific Region (2000) International Association for the Study of Obesity and the International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia Pty Limited, Australia
- Zamboni M, Mazzali G, Zoico E, Harris TB, Meigs JB, Francesco V, Fantin F, Bissoli L, Bosello O. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes. 2005;29:1011–1029. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shu XO, Yang G, et al. Abdominal adiposity and mortality in Chinese women. Arch Intern Med. 2007;167:886–892. doi: 10.1001/archinte.167.9.886. [DOI] [PubMed] [Google Scholar]