Abstract
Compared with isometric and dynamic velocity-constrained (isokinetic) tasks, less is known regarding velocity-dependent (isotonic) muscle power and recovery in older adults following repeated fatiguing lengthening contractions. We investigated voluntary and evoked neuromuscular properties of the dorsiflexors in nine old (68.3 ± 6.1 years) and nine young women (25.1 ± 1.3 years) during and following 150 lengthening contractions for up to 30 min of recovery. At baseline, the old were ~21% weaker for maximum isometric voluntary contraction (MVC) torque (P < 0.05), ~21% slower for peak loaded shortening velocity (P < 0.05), and ~39% less powerful compared with the young (P < 0.05). Following the task, MVC torque was depressed equally (~28%) for both groups (P < 0.05), but power was reduced ~19% in the old and only ~8% in the young (P < 0.05). Both measures remained depressed during the 30-min recovery period. Peak twitch torque (Pt) was ~50% lower in the old at task termination, whereas the young were unchanged. However, by 5 min of recovery, Pt was reduced similarly (~50%) in both groups, and neither recovered by 30 min. The old were affected more by low-frequency torque depression than the young, as shown by the ~40% and ~20% decreases in the stimulated 10:50 Hz ratio at task termination respectively, whereas both groups were affected similarly (~50%) 5 min into recovery, and neither recovered by 30 min. Thus, the coexistence of fatigue and muscle damage induced by the repetitive lengthening contractions impaired excitation–contraction coupling and cross-bridge function to a greater extent in the old, leading to a more pronounced initial loss of power than the young for up to 10 min following the exercise However, power remained blunted in both groups during the 30-min recovery period. These results indicate that older women are more susceptible to power loss than young following lengthening contractions, likely owing to a greater impairment in calcium kinetics.
Keywords: Eccentric contraction, Shortening velocity, Muscle damage, Aging, Fatigue
Keywords: Life Sciences, Molecular Medicine, Geriatrics/Gerontology, Cell Biology
Introduction
Much research on age-related muscle fatigue has focused primarily on isometric and shortening contractions. Far less is known in older adults regarding neuromuscular function and short-term recovery following repeated high-intensity lengthening contractions which can provoke long-lasting impairments in neuromuscular performance (Byrne et al. 2004; Power et al. 2010). Furthermore, we are interested in the velocity component (i.e., voluntary maximal shortening velocity) of power following lengthening contractions. This contraction mode in which the load is fixed and the velocity of movement is unconstrained allows alterations in shortening velocity to be elucidated, which, in older adults, already is impaired and is a strong indicator of age-related fatigability (Dalton et al. 2010; McNeil et al. 2007; Petrella et al. 2005).
By the eighth decade of life, the senescent adult has undergone alterations to both the structure and function of the neuromuscular system that lead to impaired muscle performance (Christou and Enoka 2011; Narici et al. 2003; Vandervoort 2002). These include muscle atrophy (preferentially type II muscle fibers) and the death and remodeling of motor units (MUs), resulting in a greater relative composition of slow-type muscle fibers (Vandervoort 2002) and architectural changes to the muscle and musculotendinous unit (Narici et al. 2003). Additionally, neural changes can include greater antagonist co-activation (Klein et al. 2001) and lower maximal MU discharge rates (Connelly et al. 1999). The combined consequence of these are a slowing of intrinsic muscle contractile properties (Vandervoort and McComas 1986), lower rates of torque development, and reduced cross-bridge kinetics (Aagaard et al. 2010). Hence, older adults exhibit impairments in maximal voluntary shortening velocity, torque production, and especially muscle power (McNeil et al. 2007). Despite these negative implications, there is a relative preservation of eccentric strength (Hortobagyi et al. 1995; Porter et al. 1997; Vandervoort et al. 1992). Although older adults can experience similar (Clarkson and Dedrick 1988), less (Lavender and Nosaka 2006), or more (Faulkner et al. 2007) muscle damage than young adults, it is unknown whether maintained eccentric strength is advantageous for effective neuromuscular performance during and following a bout of repeated lengthening contractions.
It seems well established that older adults are more fatigue-resistant than young adults during isometric tasks (Kent-Braun 2009), yet the fatigue response during and following dynamic shortening contractions is equivocal and depends upon the task. Older adults can experience less (Lanza et al. 2004; Rawson 2010), similar (Callahan et al. 2009; Laforest et al. 1990), or more (Baudry et al. 2007; McNeil and Rice 2007; Petrella et al. 2005) fatigue than young. However, tasks which are performed with a fast unconstrained velocity component (i.e., velocity-dependent) always yield a greater fatigue response in older adults than young (Dalton et al. 2010; McNeil and Rice 2007; Petrella et al. 2005). The only study investigating age-related fatigability following lengthening contractions (Baudry et al. 2007) reported that the reduction in maximum voluntary isometric contraction (MVC) torque did not differ between old and young adults during or following repeated isokinetic (60°/s) lengthening contractions. However, isokinetic torque loss during the lengthening contractions was greater in older adults than the young. Power was not assessed following that protocol, and thus, it is unknown whether repetitive lengthening contractions affect concentric power differently in old and young adults and which component of power (torque or shortening velocity) is more compromised.
Voluntary shortening velocity recovers rapidly (<5 min) in young adults after isometric and concentric fatigue tasks (Cheng and Rice 2005, 2010). However, lengthening contractions result in muscle damage which can take multiple days to recover (Clarkson and Hubal 2002; Proske and Allen 2005). It is unclear how damage may affect velocity-dependent power during short-term recovery (up to 30 min) in older adults. Impaired torque production following lengthening contractions can be attributed to a mechanical disruption of the sarcoplasmic reticulum, impairing calcium release (Ingalls et al. 1998; Warren et al. 2001), and the redistribution of sarcomere lengths (Morgan and Proske 2004), resulting in a length–tension relationship shift to longer muscle lengths of optimal torque production. As well, dynamic performance is impaired (Byrne et al. 2004; Sargeant and Dolan 1987), although the mechanisms are not entirely understood. Recently, we (Power et al. 2010) reported that MVC torque and velocity-dependent power did not recover fully up to 30 min following 150 lengthening contractions in young men and women. Because excitation–contraction (E–C) coupling is compromised in older adults (Payne and Delbono 2004) and maximal shortening velocity is slower (Dalton et al. 2010; McNeil et al. 2007; Petrella et al. 2005) compared with young adults, the old may be energetically disadvantaged during this task. Thus, older adults may experience a greater perturbation in ATP homeostasis, consequently exacerbating their fatigue response, resulting in a greater reduction in velocity-dependent power than young.
Therefore, the purpose was to investigate the effect of repeated high-intensity lengthening contractions on neuromuscular function in old and young women with a particular emphasis on the short-term recovery of velocity-dependent power. As a result of equivalent muscle damage, MVC torque will be reduced similarly in both old and young women and remain blunted throughout a 30-min recovery period. However, when tested under dynamic conditions, we hypothesize that the older women will have a larger reduction in velocity-dependent power than the young owing to a greater impairment in shortening velocity and impairments in E–C coupling, which are known to be compromised in older adults and may not be observable during isometric testing. As a result of muscle damage, neither group will recover by 30 min.
Materials and methods
Participants
Nine old (68.3 ± 6.1 years) and nine young women (25.1 ± 1.3 years) from the university population and local community groups, who were free from musculoskeletal disorders, volunteered for this study. All participants were recreationally active. The mean height and mass of the old and young women were 162.0 ± 7.3 cm and 67.7 ± 8.5 kg, and 167.1 ± 7.0 cm and 63.7 ± 10.4 kg, respectively. All participants were asked to refrain from strenuous exercise 1 day prior to testing and to not consume caffeine on the testing day. This study was approved by the local University’s Review Board for Health Sciences Research Involving Human Subjects and conformed to the Declaration of Helsinki. Informed oral and written consent was obtained from all participants prior to testing.
Experimental arrangement
All testing was conducted on a Biodex multi-joint dynamometer (System 3, Biodex Medical Systems, Shirley, New York). For a detailed explanation and experimental timeline of the testing setup and procedures, please refer to Power et al. (2010). The right foot was strapped tightly to the Biodex ankle attachment footplate, aligning the lateral malleolus with the rotational axis of the dynamometer. Extraneous movements were minimized using non-elastic shoulder, waist, and thigh straps. Participants sat in a slightly reclined position with the hip, knee, and ankle angles set at ~110°, ~140°, and ~30° plantar flexion, respectively. All voluntary and evoked isometric dorsiflexion contractions were performed at an ankle joint angle of 30° of plantar flexion. Shortening contractions began from the plantar flexed position of 30° and ended at the neutral ankle angle (0°). The lengthening contractions commenced at the neutral ankle angle (0°) and ended at 30° plantar flexion; thus, both dynamic actions moved through a 30° range of motion. All dynamic contractions were performed using the isotonic mode of the Biodex. However, due to inherent mechanical limitations of the dynamometer (unable to maintain an exact constant external load throughout an entire range of motion), these contractions are neither purely isotonic nor iso-inertial as the load is fixed (mechanically) and not influenced by gravity but rather the braking of the dynamometer. And therefore, we refer to these contractions as “velocity-dependent.” A velocity-dependent movement is characterized by a participant producing a dynamic contraction as fast as possible in which the angular velocity is unconstrained while the load or resistance is fixed at a predetermined value (i.e., 20% MVC).
Surface electromyography (EMG) was collected from the tibialis anterior and soleus muscles using self-adhering Ag–AgCl electrodes (1.5 × 1 cm; Kendall, Mansfield, MA). The skin was cleaned forcefully with an alcohol swab prior to the application of the electrodes. A monopolar electrode configuration was used with the active electrode positioned on the proximal portion of the tibialis anterior over the innervation zone (~7 cm distal to the tibial tuberosity and ~2 cm lateral to the tibial anterior border) and a reference electrode placed over the distal tendinous portion of the tibialis anterior at the malleoli. The active electrode for the soleus was positioned ~2 cm distal to the lower border of the medial head of the gastrocnemius and a reference placed over the calcaneal tendon.
Stimulated contractions of the dorsiflexors were evoked electrically using a bar electrode held distal to the fibular head over the deep branch of the common peroneal nerve. A computer-triggered stimulator (model DS7AH, Digitimer, Welwyn Garden City, Hertfordshire, UK) set at 400 V provided the electrical stimulation using a pulse width of 50–100 μs.
Experimental procedures
Peak twitch torque (Pt) was determined by increasing the current until a plateau in dorsiflexor Pt, and tibialis anterior M-wave amplitude were reached, and then the current was further increased by 10–15% to ensure the activation of all motoneurons via supramaximal stimulation. This stimulation intensity was used for the evoked doublet (Pd, two pulses at a 10-ms interpulse interval) and to assess voluntary activation. Next, a 1-s train at 50 Hz was delivered to assess peak tetanic torque by increasing the current until there was a plateau in the evoked torque. The tetanic evoked contractions were tolerated by all young women and four of the older women.
Three isometric MVCs were then performed, each of 3- to 5-s duration. Three minutes of rest was given between all contractions. To ensure that MVC attempts were maximal, participants were provided visual feedback of the torque tracing on a computer monitor and exhorted verbally during all voluntary efforts; voluntary activation was assessed using the modified interpolated twitch technique (Gandevia 2001). The amplitude of the interpolated torque evoked during the peak plateau of the MVC was compared with a resting Pd, evoked ~1 s following the MVC when the muscles were relaxed fully. Percent voluntary activation was calculated as voluntary activation (%) = [1 − interpolated Pd/resting Pd] × 100. Values from the MVC with the highest peak torque were used for data analysis. Next, 10 and 50 pulses were delivered over a 1-s period to determine a 10- to 50-Hz relationship in all nine young and four old participants using the current required to evoke peak 50-Hz torque.
Once MVC torque was determined, the dynamometer was switched from the isometric to isotonic mode and a load equal to 20% MVC was programmed to allow for the determination of maximal shortening velocity with this load and velocity-dependent power. The 20% MVC resistance was chosen because it represents a moderate load for the fast shortening contractions, and one that all subjects could perform through the entire range of motion even after a bout of repeated high-intensity lengthening contractions. Before the footplate moved during the velocity-dependent shortening contractions, participants had to overcome the pre-programmed resistance. The dynamometer absorbs this increase in applied torque, resulting in a directly proportional increase in angular velocity. This is a helpful feature to explore the effect of damaging lengthening contractions on alterations in the velocity of unconstrained movement and power. The dynamometer was programmed to allow the footplate to return to 30° of plantar flexion at the end of each shortening voluntary contraction while the participant relaxed fully. Familiarization with these “fast” shortening contractions involved participants performing several (typically five) velocity-dependent shortening contractions until a stable value was obtained (no change in maximal shortening velocity). To ensure a maximal effort (peak velocity) contraction, all participants were instructed to move the load “as hard and as fast as possible throughout the entire range of motion.” To assist participants in reaching their maximal shortening velocity, visual feedback of the velocity profile was provided via a computer monitor, and a horizontal cursor was positioned at the previous plateau in peak velocity. Participants rested for 3 min and then performed two consecutive contractions which were used to establish baseline values for maximum shortening velocity and peak power.
Fatigue and recovery protocol
With a load of 80% MVC, participants performed five sets of 30 eccentric dorsiflexion contractions, with each set separated by 30 s of rest. Participants were provided visual feedback of the torque and instructed to resist the lowering of the footplate through the 30° range of motion over a 1-s period. After the lengthening contraction, the foot was returned to the neutral ankle starting position by the investigator over a 2-s (15°/s) period while the participant relaxed fully. The participant was then instructed to resist the lowering of the footplate immediately again until the protocol was complete. The voluntary and electrically evoked responses of the dorsiflexors were recorded at baseline, during the fatigue protocol immediately following each of the five sets, and during recovery at 0.5, 2, 5, 10, 15, 20, and 30 min after task termination. Measures following the fatigue protocol included and were performed in the following order: (1) maximum evoked twitch properties, (2) assessment of MVC and voluntary activation, (3) post-activation twitch and twitch doublet, (4) measure of low-frequency torque depression (10:50 Hz ratio), and (5) velocity-dependent shortening power.
Data reduction and analysis
Torque, position, and velocity data were sampled at a rate of 100 Hz. All data were converted to digital format using a 12-bit analog-to-digital converter (model 1401 Plus, Cambridge Electronic Design, Cambridge, UK). Surface EMG signals were pre-amplified (×100), amplified (×2) and band-pass filtered (10–1,000 Hz), and sampled online at 2,500 Hz using Spike 2 software (version 6.10, Cambridge Electronic Design Ltd.). Surface EMG from the MVC was expressed as root mean squared (RMS) and values were obtained from a 1-s time period about the peak torque. All subsequent MVC RMS values were normalized to the level obtained during baseline. Peak RMS values of the raw surface EMG during the fast shortening contractions was calculated through the 30° range of motion from the onset of movement to the end of the range of motion and then normalized to the fastest baseline contraction. Power was calculated as the product of torque (newton meter) and the peak shortening velocity (radian per second) of the faster of two contraction attempts. Post-activation potentiation was determined by calculating the ratio between the amplitude of the peak twitch torque recorded before and following the isometric MVC. Spike 2 software was used off-line to determine M-wave amplitude, area, duration, the peak twitch torque (Pt), peak doublet torque (Pd), doublet time to peak twitch (DTPT), half-relaxation time (DHRT) of the doublet, and doublet rate of torque development (DMRTD). Low-frequency torque depression was calculated using a ratio of peak 10 Hz to peak 50 Hz evoked torques (10:50 Hz).
Statistical analysis
Using SPSS software (version 16, SPSS Inc. Chicago, IL) a two-way (age × time) repeated measures analysis of variance was performed to assess all neuromuscular data. Because voluntary activation values are not normally distributed, a Mann–Whitney U test was employed and an unpaired t test was used for subject characteristics and baseline measures to assess group differences. The level of significance was set at P < 0.05. When a significant main effect or interaction was present, post hoc analysis using unpaired t tests was performed with a Bonferroni correction factor to determine where significant differences existed. Effect sizes (ES) were calculated using the partial eta-squared method to explore the magnitude of apparent statistical effects. Due to the small sample size of old women for low-frequency fatigue (LFF, n = 4), unpaired t tests were performed for this parameter. The table is presented as means ± SD and figures as means ± SE values, normalized to baseline (pretest).
Results
Baseline measures
As shown in Table 1, the old women as compared with the young women were ~21% weaker for MVC torque (P = 0.021, ES = 0.292) despite similar high voluntary activation (~95%, P = 0.682, ES = 0.012). Peak loaded shortening velocity (Fig. 1) was ~21% slower for the old women than the young (P < 0.001, ES = 0.522), which led to power (calculated as the product of peak loaded shortening velocity at 20% MVC) being ~39% less in the old compared with the young women (P = 0.006, ES = 0.383). Both groups had a similar Pd (P = 0.685, ES = 0.011), while DTPT was ~16% slower (P = 0.023, ES = 0.284) and DHRT was ~33% longer in old compared with young, respectively (P = 0.012, ES = 0.337). Despite similar Pt (P = 0.735, ES = 0.007) for the old (3.9 ± 1.6 N m) and young (4.0 ± 0.9 N m) women, the older adults had a reduced capacity for potentiation (105.8 ± 6.0%) compared with the young (124.6 ± 17.2%) (P = 0.023, ES = 0.339).
Table 1.
Voluntary and evoked participant baseline characteristics
| Group(n = 9) | Twitch doublet properties | |||||||
|---|---|---|---|---|---|---|---|---|
| Pd (N m) | TPT (ms) | HRT (ms) | MRTD (s−1) | MVC (N m) | VA (%) | Velocity (deg/s) | Power (W) | |
| Young | 9.1 ± 2.7 | 148.2 ± 24.8 | 80.0 ± 15.2 | 11.3 ± 1.2 | 34.0 ± 4.6 | 99.2 ± 0.9 | 134.3 ± 9.0 | 15.8 ± 3.3 |
| Old | 8.6 ± 2.3 | 173.3 ± 21.9a | 101.0 ± 23.1a | 10.0 ± 1.1a | 28.2 ± 4.9a | 98.9 ± 2.1 | 109.1 ± 16.1a | 11.2 ± 2.7a |
Old women had slower absolute evoked doublet twitch torque (Pd) contractile properties for time to peak twitch (DTPT; P = 0.023, ES = 0.284), half-relaxation time (DHRT; P = 0.012, ES = 0.337), and maximum rate of torque development (DMRTD; P = 0.031, ES = 0.258) compared with young. MVC torque (P = 0.021, ES = 0.292), maximum shortening velocity (P = 0.001, ES = 0.522), and peak power (P = 0.006, ES = 0.383) were lower in the old than young women. Voluntary activation (VA) (0.682, ES = 0.012) and doublet twitch torque (P = 0.685, ES = 0.011) were not significantly different between groups
aSignificant difference between old and young women
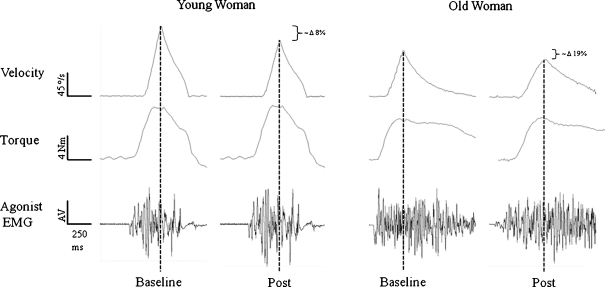
Fig. 1.
Representative unprocessed data from a young and older woman performing a fast velocity-dependent shortening contraction at baseline and 30 s following (Post) the lengthening contraction task. The EMG amplitude is presented with arbitrary values (AV). The dashed vertical line indicates peak velocity
Fatigue and recovery measures
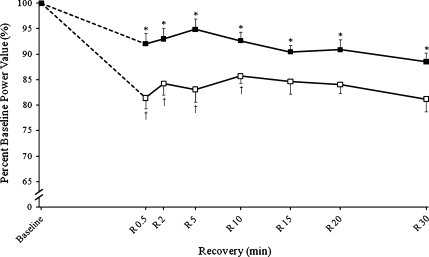
All participants were capable of completing all contractions, although as reported previously using this contraction mode some subjects had difficulty lowering the footplate at a steady pace for the last few contractions of each set (Power et al. 2010). This failure to maintain a constant velocity resulted in increased eccentric velocities which ranged from 36° to 42°/s. Despite the variation in velocity, the duty cycles were similar (P = 0.295, ES = 0.680) between old and young women, 0.33 ± 0.07. For the velocity-dependent shortening contractions, all participants were capable of completing the 30° range of motion during baseline measures and following the lengthening contraction task. Neuromuscular fatigue measures were analyzed with regard to relative changes over time. For maximum loaded shortening velocity and subsequently peak power (Fig. 2), there were main effects for time (P < 0.001, ES = 0.681) and age (P = 0.007, ES = 0.396) and an interaction (P = 0.004, ES = 0.244). Thus, at task termination, the old women had a greater loss of power (~19%) than the young (~8%). This difference persisted until 10 min of recovery and did not recover by 30 min post-intervention.
Fig. 2.
Velocity-dependent power short-term recovery of velocity-dependent power calculated at 20% MVC and maximal shortening velocity normalized to 100% of baseline values for old (open symbols) and young women (solid symbols). The dashed lines represent the lengthening contraction intervention, during which time only isometric measures were obtained. Significant effects for time (*P < 0.05) and age (†P < 0.05). Values are the means±SE
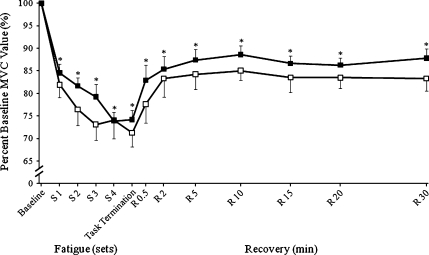
For dorsiflexor MVC torque, there was only a significant effect for time (P < 0.001, ES = 0.696). Isometric MVC torque decreased similarly in the old and young by ~19% following the first set of 30 eccentric contractions, and following each successive set, it continued to decrease until it was reduced by ~28% immediately following task termination. By the end of the 30-min recovery period, the MVC regained 9%, but was still significantly less than baseline (Fig. 3). Voluntary activation was maintained >95% at baseline and did not change (P = 0.910, ES = 0.022) throughout fatigue and recovery. The incomplete recovery of MVC by 30 min post-intervention suggests that similar muscle damage had occurred in young and old women.
Fig. 3.
Maximum voluntary isometric contraction (MVC). Maximal voluntary isometric strength during and following lengthening contractions normalized to 100% of baseline values for old (open symbols) and young women (solid symbols). Significant effects for time (*P < 0.05). Values are the means±SE
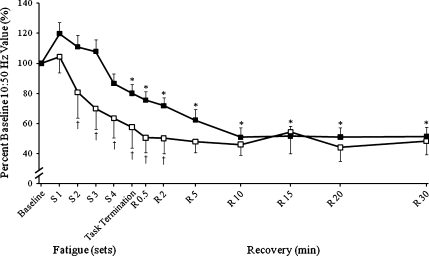
Low-frequency torque depression (10:50 Hz) presented a significant effect for time (P < 0.001, ES = 0.960) and age (P = 0.032, ES = 0.225). Over time, the alterations in the 10:50 Hz ratio were manifested by the greater reduction in 10-Hz evoked torque compared with the 50 Hz. This indicated that there was significant low-frequency torque depression following the lengthening contractions for both groups. Low-frequency torque depression persisted in both groups throughout the 30-min recovery period. However, at task termination, the 10:50 Hz ratio was reduced by 40% in the four old, but only 20% in the young, suggesting that there was an initial greater impairment in E–C coupling in the old women. This age-related difference was present up to 10 min in the recovery period (Fig. 4), at which time both groups were reduced by 50% and did not change during the final 20 min of the recovery period.
Fig. 4.
Low-frequency torque depression (10:50 Hz). Low-frequency torque depression during and following lengthening contractions normalized to 100% of baseline values for old (n = 4, open symbols) and young (n = 9) women (solid symbols). The decrease in the 10:50 Hz ratio was driven primarily by the progressive decline in 10-Hz torque (40% decrease at task termination and 60% decrease by 30 min of recovery) with a minimal decrease in 50 Hz (20% decrease at task termination and throughout recovery). Significant effects for time (*P < 0.05) and age (†P < 0.05). Values are the means±SE
There were main effects for time (P < 0.001, ES = 0.646) and age (P = 0.005, ES = 0.437) and an interaction (P < 0.001, ES = 0.409) for Pt (Fig. 5). Twitch torque decreased by ~21% in the old women following the first set of 30 lengthening contractions, while the young women had a potentiation of Pt, which increased to ~130% of baseline following set 1. At task termination, the values for the old women were reduced by 50%, whereas Pt for the young women was not different from baseline values. Once the potentiating effects of the fatigue protocol were mitigated in the young, both groups were reduced similarly (~50%) 5 min into recovery. For the Pd torque, there was only a significant effect for time (P < 0.001, ES = 0.648). Pd continued to decrease (Fig. 5) during the lengthening contractions and remained blunted in both groups by ~40% throughout the 30-min recovery period. For doublet twitch contractile speeds, there were only main effects for time for DTPT (P < 0.001, ES = 0.544) and DHRT (P < 0.001, ES = 0.475), meaning doublet twitch contractile properties slowed similarly in both groups by ~15–20%. However, there was a main effect of time (P < 0.001, ES = 0.356) and age (P = 0.003, ES = 0.429) and an interaction (P = 0.05, ES = 0.118) for the DMRTD, which was reduced ~15% greater in old women than young women at task termination but was no longer significantly different between groups 30 s later.
Fig. 5.
Peak twitch torque (Pt). Peak twitch torque during and following lengthening contractions normalized to 100% of baseline values for old (open symbols) and young women (solid symbols). Significant effects for time (*P < 0.05) and age (†P < 0.05)
Tibialis anterior M-wave properties, including peak-to-peak amplitude, duration, and area, showed a main effect for time (P = 0.004, ES = 0.190), meaning M-wave properties were reduced similarly in both old and young women by ~10–15% until task termination and returned to baseline value by the end of the 30-min recovery period. For both the old and young women, there were no significant changes from baseline for RMS EMG (P = 0.064, ES = 0.151) of the agonist tibialis anterior during MVCs throughout the protocol. As well, RMS EMG of the soleus muscle did not differ for time or age from baseline (P = 0.222, ES = 0.0125). During the velocity-dependent shortening contractions, RMS EMG of the agonist tibialis anterior or antagonist soleus showed no effect for time (P = 0.135, ES = 0.125) or age (P = 0.426, ES = 0.070), meaning there was no difference in “neural drive” from baseline contractions.
Discussion
This investigation tested the hypothesis that neuromuscular function of the dorsiflexors following repeated lengthening contractions would be impaired more in the old women than young. Specifically, velocity-dependent power would be reduced more in the old than young and would remain depressed throughout the 30-min period of recovery following task termination. Indeed, peak power was reduced by 19% for the older women after the lengthening contractions, whereas the young women only incurred an 8% decrement at task termination; for both groups, full recovery had not been achieved by 30 min after task termination. In contrast, isometric MVC torque was reduced similarly (28%) in both the old and young and did not recover fully during this time period. Despite similar muscle damage as indicated by incomplete recovery of MVC torque by 30 min (Warren et al. 1999), these findings suggest that old women have greater decrements in velocity-dependent power than their younger counterparts following repeated lengthening contractions. Therefore, the greater power loss in the old than young women is driven more by fatigue mechanisms influencing impairments in whole muscle loaded shortening velocity following lengthening contractions than those affecting torque generation per se.
Baseline
The old women in this study were weaker and slower (Table 1) for whole muscle shortening velocity, leading to a greater reduction in power when compared with young women. The 39% reduction in velocity-dependent power compared with the young is greater than that reported previously for velocity-dependent contractions of the dorsiflexors (25%) of old men (McNeil et al. 2007) and similar to the plantar flexors (38%) of old men (Dalton et al. 2010) and elbow flexors (41%; Valour et al. 2003) and knee extensors (45%; Petrella et al. 2005) of old women. As well, older women rely more on the velocity component of power than torque production when compared with old men and younger adults (Valour et al. 2003). Valour et al. (2003) reported that when peak muscle power was compared among various loads (i.e., % MVC), older women reached peak power at a lower percentage of MVC torque than older men and women. In the current study, we used a relative load of 20% MVC which relies strongly on the velocity component of power (Power et al. 2010). Factors discussed below that impair whole muscle shortening velocity in older women may greatly impair their ability to generate power more so than older men and younger men and women.
Lengthening contraction intervention
In the current study, following 150 high-intensity lengthening contractions, the old women incurred (up to 10 min) a greater loss of velocity-dependent power (19%) than the young (8%) following task termination, while both the old and young women experienced similar reductions in isometric MVC torque at task termination (28%). This is similar to findings from isovelocity fatigue studies in which older adults incur a greater decline in eccentric isokinetic torque than young while still maintaining isometric strength (Baudry et al. 2007). Interestingly, the reduction in MVC torque at 30-min recovery (~19%) is similar to the reduction following the first 30 lengthening contractions (Fig. 3), suggesting that the primary insult of muscle damage occurred during the first set of contractions and the further decrease in MVC torque to task termination can be attributed to fatigue processes (Choi and Widrick 2009; Morgan et al. 2004). Despite similar reductions in isometric MVC torque following the lengthening contractions, low-frequency torque depression was greater in the old than the young women (~25% difference) following the second set of lengthening contractions and for up to 5 min into recovery; neither recovered during the 30-min period of recovery .
The development of fatigue can manifest through central or peripheral mechanisms (Allen et al. 2008; Gandevia 2001), or both. In the current study, voluntary activation and RMS EMG amplitude of the tibialis anterior during the isometric MVCs was not reduced from baseline and did not differ between age groups. In accord with previous investigations utilizing velocity-dependent contractions, RMS EMG amplitude of the agonist tibialis anterior during velocity-dependent shortening contractions did not differ throughout the study (Power et al. 2010) or between young and old. Hence, the main site of fatigue is likely peripheral in nature. Voluntary activation failure can account for torque loss following muscle damage in other limb muscles (Prasartwuth et al. 2005); however, maintained voluntary activation to the tibialis anterior is a common finding following lengthening contractions (Baudry et al. 2007; Pasquet et al. 2000; Power et al. 2010). Furthermore, in the present study, M-wave parameters (i.e., p-p amplitude, area, duration) were reduced similarly in old and young, indicating that muscle damage may have disturbed sarcolemmal excitability in both age groups equally. However, findings are equivocal; some studies show a decrease in M-wave properties (Hedayatpour et al. 2009) while others using similar lengthening contraction protocols do not (Pasquet et al. 2000; Power et al. 2010). The reason for this disparity among studies is unclear, but it may be related to rest intervals between contractions or because of different aged populations tested.
Fatigue and muscle damage
Although lengthening contractions are less energetically demanding than isometric and dynamic shortening contractions (Abbott et al. 1952; Ryschon et al. 1997), they are known to induce muscle fatigue in addition to muscle damage (Baudry et al. 2007; Choi and Widrick 2009; Morgan et al. 2004; Pasquet et al. 2000). A commonly accepted indirect measure of muscle damage is the reduction and incomplete recovery of isometric MVC torque (Armstrong et al. 1991; Clarkson and Hubal 2002; Warren et al. 1999). The concomitant existence of fatigue and damage may account for the greater initial decline in MVC torque than either factor alone; however, because MVC torque did not recover fully, this observation may represent muscle weakness (Gandevia 2001) and suggest that muscle damage occurred. The long-term deficits in force production may be due to damage-induced impairments in E–C coupling (Ingalls et al. 1998; Warren et al. 2001). In the present study, it seems that the old had an initial greater perturbation in E–C coupling, as shown by the reduced twitch torque and greater low-frequency torque depression compared with the young (Figs. 4 and 5). As well, following lengthening contractions, a shift to longer muscle lengths for optimal torque production represents an increase in series compliance of the muscle (Gregory et al. 2007; Widrick and Barker 2006). The presence of overstretched, disrupted sarcomeres in series with still functional sarcomeres results in an immediate shift in optimum length and is considered to be a reliable indicator of muscle damage as it relates to the number of overstretched sarcomeres (Brockett et al. 2002; Chen et al. 2007). An immediate shift in muscle length for optimal torque production following 120 lengthening contractions has been previously observed in the ankle dorsiflexors (Lee et al. 2010). With our study design utilizing a velocity-dependent contraction task, we were not able to record optimal muscle torque–length; however, based on the same muscle tested and a similar protocol of repeated lengthening contractions, we would expect a similar shift to longer muscle lengths in the optimal muscle length–tension relationship as is known to be induced by muscle damage.
The mechanisms responsible for force loss that occurs following muscle damage have been reviewed extensively (Allen 2001; Clarkson et al. 1992), whereas the processes responsible for impairments in shortening velocity have received little attention (Choi and Widrick 2009; Morgan et al. 2004; Widrick and Barker 2006). Data from our study highlight that the effects of fatigue on loaded shortening velocity are independent of muscle damage and that the coexistence of fatigue and damage is evident by the time course of the transient effects of fatigue and long-lasting effects of damage. Hence, the combined effects of fatigue and muscle damage more greatly affect the production of shortening velocity and subsequently power than either variable alone following this task. Indeed, voluntary maximal shortening velocity is known to recover rapidly (<5 min) in young adults after isometric and concentric fatigue tasks (Cheng and Rice 2005, 2010). Interestingly, following repeated lengthening contractions, the velocity component of power does not recover fully (Power et al. 2010; Widrick and Barker 2006). In a recent study of young men and women, following repeated lengthening contractions, power remained blunted for up to 30 min following task termination (Power et al. 2010). Therefore, muscle damage appears to limit power production (Byrne et al. 2001; Power et al. 2010) following lengthening contractions 30 min into recovery.
Both the old and young women possibly incurred a similar amount of muscle damage (i.e., prolonged reduction in isometric MVC), yet the old were more fatigable than the young, as indicated by the greater power loss up to 10 min into the recovery period. Once the transient effects of fatigue were recovered, both groups had a similar reduced power, and for this reason, we can argue that both groups experienced similar impairments in muscle function owing to muscle damage. However, the old women incurred more fatigue than the young women, which can account for the greater power loss immediately following the lengthening contractions. The loss of power in the old women in the current study following 150 lengthening contractions is less than that observed in studies using protocols of shortening contractions (Baudry et al. 2007; Dalton et al. 2010; McNeil and Rice 2007). For example, in older men, McNeil and Rice (2007) found a 20% loss of power for the dorsiflexors following 25 fast shortening contractions, and Dalton et al. (2010) found a 26% reduction following 50 fast shortening plantar flexion contractions. The greater mechanochemical efficiency for lengthening compared with isometric and shortening contractions result in less perturbation of intracellular high-energy phosphate (Pi) energetics (Choi and Widrick 2009; Ryschon et al. 1997). Thus, despite the greater number of contractions in this study than the others, the disparate results can be explained by the task-dependent nature of fatigue (Enoka and Duchateau 2008).
Young vs. old metabolic (dis)advantage
It is well known that older adults are more fatigue-resistant than young when performing isometric tasks, owing to their slower contracting muscle and lower MU discharge rates required to reach fused tetanus as indicated by a shift to the left in the force–frequency relationship (Allman and Rice 2004). That is to say that under isometric conditions, the lower glycolytic flux in old compared with young is less energetically costly (lower ATP required) with a greater energy turnover through oxidative processes (Lanza et al. 2007), resulting in less metabolic acidosis and accumulation of inorganic phosphates, thus mitigating the reduction in isometric MVC torque (Kent-Braun 2009). However, when “stressed” with repeated dynamic shortening contractions, this apparent fatigue resistance in older adults is abolished, and in some situations, older adults are more fatigable than young (Callahan et al. 2009). This is found exclusively during tasks which allow velocity to be unconstrained (i.e., velocity-dependent; Dalton et al. 2010; McNeil and Rice 2007; Petrella et al. 2005). Furthermore, it appears based on the greater power loss incurred by the old women in this study we now show that older adults may be “energetically” disadvantaged following repeated lengthening contractions, thus further exacerbating fatigue mechanisms related to whole muscle shortening velocity and the subsequent generation of power. The greater accumulation of muscle metabolites during the lengthening contraction protocol in older women impairs E–C coupling and may limit cross-bridge function while performing a subsequent fast shortening contraction.
A greater initial impairment in E–C coupling is supported further by the reduced 10:50 Hz ratio in the four old women tested and, coupled with an impaired capacity for potentiation, may have disadvantaged the older adults for the performance of subsequent “fast” velocity-dependent contractions (Fig. 2) compared with the young. In contrast, the young had a greater twitch potentiation and were less influenced by LFF in the first 5 min into recovery (Figs. 4 and 5). Post-activation potentiation, due to myosin light-chain phosphorylation, can compensate for impaired E–C coupling by increasing myofibrillar calcium sensitivity in spite of the presence of LFF (Green and Jones 1989; Rassier and Macintosh 2000). In our current study, this suggests that the young had less of a reduction in myofibrillar calcium sensitivity (Grange and Houston 1991) compared with old, meaning they were less adversely affected by cellular mechanisms of fatigue. This could include increased H+ and Pi, which directly reduce force output, and can result in a decline in the number and/or force per unit of the strongly bound cross bridges (Fitts 2008) as well as impaired ADP dissociation from the myosin head (Fitts 2008), limiting peak shortening velocity. Following lengthening contractions, a failure of the dihydropyridine receptors to stimulate sarcoplasmic reticulum Ca2+ release (Ingalls et al. 1998) and reduced myofibrillar Ca2+ sensitivity together with minimal potentiation capability might have heightened the effects of the “potentially greater” metabolite accumulation in older adults effect on the impaired generation of velocity-dependent power (Fitts 2008; Westerblad et al. 1998; Westerblad and Lannergren 1994). Whereas velocity-dependent power in both the old and young women reached a similar value by 10 min into recovery, the greater potentiation in young may have helped offset the initial perturbations in E–C coupling, thus mitigating the reduction in shortening velocity (Baudry and Duchateau 2007) and power at task termination. The greater power loss in older women is likely a result of greater LFF and E–C coupling failure in the muscles of older compared with young women, as this is also supported by our observation of a greater reduction in doublet twitch rate of torque development in the old women than the young at task termination.
In summary, the damaging lengthening contractions impaired shortening velocity and thus power in both the old and young women, with a greater reduction in the old for up to 10 min into recovery, at which time subsequently both remained blunted for the duration of the 30-min recovery period. The observations were not related to neural drive changes but to peripheral alterations primarily affecting E–C coupling. The mechanisms responsible for the reduction in shortening velocity following muscle damage may include decreases in the number of functioning sarcomeres in series, Ca2+ kinetics, and myofibular Ca2+ sensitivity. The greater fatigue in older women can be attributed to their blunted potentiation, a factor in the young which may have helped offset initial fatigue-induced impairments in shortening velocity. Furthermore, our findings highlight the value of investigating changes in the velocity component of power (i.e., shortening velocity) following perturbations to the neuromuscular system.
Acknowledgments
We would like to thank all those who participated in the study. As well, we are grateful for the assistance of Dr. Arthur Cheng in the final preparation of this manuscript. This research is supported by funding from The Newfoundland and Labrador Center for Applied Health Research (NLCAHR) and The Natural Sciences and Engineering Research Council of Canada (NSERC)
References
- Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- Abbott BC, Bigland B, Ritchie JM. The physiological cost of negative work. J Physiol. 1952;117:380–390. doi: 10.1113/jphysiol.1952.sp004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG. Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol Scand. 2001;171:311–319. doi: 10.1046/j.1365-201x.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Allman BL, Rice CL. An age-related shift in the force–frequency relationship affects quadriceps fatigability in old adults. J Appl Physiol. 2004;96:1026–1032. doi: 10.1152/japplphysiol.00991.2003. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- Baudry S, Duchateau J. Postactivation potentiation in a human muscle: effect on the load-velocity relation of tetanic and voluntary shortening contractions. J Appl Physiol. 2007;103:1318–1325. doi: 10.1152/japplphysiol.00403.2007. [DOI] [PubMed] [Google Scholar]
- Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–525. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- Brockett CL, Morgan DL, Gregory JE, Proske U. Damage to different motor units from active lengthening of the medial gastrocnemius muscle of the cat. J Appl Physiol. 2002;92:1104–1110. doi: 10.1152/japplphysiol.00479.2001. [DOI] [PubMed] [Google Scholar]
- Byrne C, Eston RG, Edwards RH. Characteristics of isometric and dynamic strength loss following eccentric exercise-induced muscle damage. Scand J Med Sci Sports. 2001;11:134–140. doi: 10.1046/j.1524-4725.2001.110302.x. [DOI] [PubMed] [Google Scholar]
- Byrne C, Twist C, Eston R. Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med. 2004;34:49–69. doi: 10.2165/00007256-200434010-00005. [DOI] [PubMed] [Google Scholar]
- Callahan DM, Foulis SA, Kent-Braun JA. Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve. 2009;39:692–702. doi: 10.1002/mus.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TC, Nosaka K, Sacco P. Intensity of eccentric exercise, shift of optimum angle, and the magnitude of repeated-bout effect. J Appl Physiol. 2007;102:992–999. doi: 10.1152/japplphysiol.00425.2006. [DOI] [PubMed] [Google Scholar]
- Cheng AJ, Rice CL. Fatigue and recovery of power and isometric torque following isotonic knee extensions. J Appl Physiol. 2005;99:1446–1452. doi: 10.1152/japplphysiol.00452.2005. [DOI] [PubMed] [Google Scholar]
- Cheng AJ, Rice CL. Fatigue-induced reductions of torque and shortening velocity are muscle-dependent. Med Sci Sports Exerc. 2010;42:1651–1659. doi: 10.1249/MSS.0b013e3181d6c5b5. [DOI] [PubMed] [Google Scholar]
- Choi S, Widrick JJ. Combined effects of fatigue and eccentric damage on muscle power. J Appl Physiol. 2009;107:1156–1164. doi: 10.1152/japplphysiol.00403.2009. [DOI] [PubMed] [Google Scholar]
- Christou EA, Enoka RM (2011) Aging and movement errors when lifting and lowering light loads. Age (Dordr). doi:10.1007/s11357-010-9190-4 [DOI] [PMC free article] [PubMed]
- Clarkson PM, Dedrick ME. Exercise-induced muscle damage, repair, and adaptation in old and young subjects. J Gerontol. 1988;43:M91–M96. doi: 10.1093/geronj/43.4.m91. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24:512–520. [PubMed] [Google Scholar]
- Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol. 1999;87:843–852. doi: 10.1152/jappl.1999.87.2.843. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Power GA, Vandervoort AA, Rice CL. Power loss is greater in old men than young men during fast plantar flexion contractions. J Appl Physiol. 2010;109:1441–1447. doi: 10.1152/japplphysiol.00335.2010. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586:11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34:1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol. 2008;104:551–558. doi: 10.1152/japplphysiol.01200.2007. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Grange RW, Houston ME. Simultaneous potentiation and fatigue in quadriceps after a 60-second maximal voluntary isometric contraction. J Appl Physiol. 1991;70:726–731. doi: 10.1152/jappl.1991.70.2.726. [DOI] [PubMed] [Google Scholar]
- Green HJ, Jones SR. Does post-tetanic potentiation compensate for low frequency fatigue? Clin Physiol. 1989;9:499–514. doi: 10.1111/j.1475-097X.1989.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Allen TJ, Proske U. The shift in muscle’s length-tension relation after exercise attributed to increased series compliance. Eur J Appl Physiol. 2007;99:431–441. doi: 10.1007/s00421-006-0363-x. [DOI] [PubMed] [Google Scholar]
- Hedayatpour N, Falla D, Arendt-Nielsen L, Vila-Cha C, Farina D. Motor unit conduction velocity during sustained contraction after eccentric exercise. Med Sci Sports Exerc. 2009;41:1927–1933. doi: 10.1249/MSS.0b013e3181a3a505. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol A Biol Sci Med Sci. 1995;50:B399–B406. doi: 10.1093/gerona/50A.6.B399. [DOI] [PubMed] [Google Scholar]
- Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E–C coupling failure in mouse EDL muscle after in vivo eccentric contractions. J Appl Physiol. 1998;85:58–67. doi: 10.1152/jappl.1998.85.1.58. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev. 2009;37:3–9. doi: 10.1097/JES.0b013e318190ea2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol. 2001;91:1341–1349. doi: 10.1152/jappl.2001.91.3.1341. [DOI] [PubMed] [Google Scholar]
- Laforest S, St-Pierre DM, Cyr J, Gayton D. Effects of age and regular exercise on muscle strength and endurance. Eur J Appl Physiol Occup Physiol. 1990;60:104–111. doi: 10.1007/BF00846029. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol. 2004;97:967–975. doi: 10.1152/japplphysiol.01351.2003. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol. 2007;583:1093–1105. doi: 10.1113/jphysiol.2007.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender AP, Nosaka K. Comparison between old and young men for changes in makers of muscle damage following voluntary eccentric exercise of the elbow flexors. Appl Physiol Nutr Metab. 2006;31:218–225. doi: 10.1139/h05-028. [DOI] [PubMed] [Google Scholar]
- Lee HD, Kim JS, Lee DY, Kurihara T, Lee YS, Kawakami Y. Shift in optimal joint angle of the ankle dorsiflexors following eccentric exercise. Exp Mech. 2010;50:661–666. doi: 10.1007/s11340-009-9245-6. [DOI] [Google Scholar]
- McNeil CJ, Rice CL. Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci. 2007;62:624–629. doi: 10.1093/gerona/62.6.624. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Vandervoort AA, Rice CL. Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J Appl Physiol. 2007;102:1962–1968. doi: 10.1152/japplphysiol.01166.2006. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Proske U. Popping sarcomere hypothesis explains stretch-induced muscle damage. Clin Exp Pharmacol Physiol. 2004;31:541–545. doi: 10.1111/j.1440-1681.2004.04029.x. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Gregory JE, Proske U. The influence of fatigue on damage from eccentric contractions in the gastrocnemius muscle of the cat. J Physiol. 2004;561:841–850. doi: 10.1113/jphysiol.2004.069948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. J Appl Physiol. 2003;95:2229–2234. doi: 10.1152/japplphysiol.00433.2003. [DOI] [PubMed] [Google Scholar]
- Pasquet B, Carpentier A, Duchateau J, Hainaut K. Muscle fatigue during concentric and eccentric contractions. Muscle Nerve. 2000;23:1727–1735. doi: 10.1002/1097-4598(200011)23:11<1727::AID-MUS9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Payne AM, Delbono O. Neurogenesis of excitation–contraction uncoupling in aging skeletal muscle. Exerc Sport Sci Rev. 2004;32:36–40. doi: 10.1097/00003677-200401000-00008. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol. 2005;98:211–220. doi: 10.1152/japplphysiol.00294.2004. [DOI] [PubMed] [Google Scholar]
- Porter MM, Vandervoort AA, Kramer JF. Eccentric peak torque of the plantar and dorsiflexors is maintained in older women. J Gerontol A Biol Sci Med Sci. 1997;52:B125–B131. doi: 10.1093/gerona/52A.2.B125. [DOI] [PubMed] [Google Scholar]
- Power GA, Dalton BH, Rice CL, Vandervoort AA. Delayed recovery of velocity-dependent power loss following eccentric actions of the ankle dorsiflexors. J Appl Physiol. 2010;109:669–676. doi: 10.1152/japplphysiol.01254.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasartwuth O, Taylor JL, Gandevia SC. Maximal force, voluntary activation and muscle soreness after eccentric damage to human elbow flexor muscles. J Physiol. 2005;567:337–348. doi: 10.1113/jphysiol.2005.087767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Allen TJ. Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev. 2005;33:98–104. doi: 10.1097/00003677-200504000-00007. [DOI] [PubMed] [Google Scholar]
- Rassier DE, Macintosh BR. Coexistence of potentiation and fatigue in skeletal muscle. Braz J Med Biol Res. 2000;33:499–508. doi: 10.1590/S0100-879X2000000500003. [DOI] [PubMed] [Google Scholar]
- Rawson ES. Enhanced fatigue resistance in older adults during repeated sets of intermittent contractions. J Strength Cond Res. 2010;24:251–256. doi: 10.1519/JSC.0b013e3181a8f7cf. [DOI] [PubMed] [Google Scholar]
- Ryschon TW, Fowler MD, Wysong RE, Anthony A, Balaban RS. Efficiency of human skeletal muscle in vivo: comparison of isometric, concentric, and eccentric muscle action. J Appl Physiol. 1997;83:867–874. doi: 10.1152/jappl.1997.83.3.867. [DOI] [PubMed] [Google Scholar]
- Sargeant AJ, Dolan P. Human muscle function following prolonged eccentric exercise. Eur J Appl Physiol Occup Physiol. 1987;56:704–711. doi: 10.1007/BF00424814. [DOI] [PubMed] [Google Scholar]
- Valour D, Ochala J, Ballay Y, Pousson M. The influence of ageing on the force–velocity–power characteristics of human elbow flexor muscles. Exp Gerontol. 2003;38:387–395. doi: 10.1016/S0531-5565(02)00265-6. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol. 1986;61:361–367. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, Chesworth BM, Cunningham DA, Paterson DH, Rechnitzer PA, Koval JJ. Age and sex effects on mobility of the human ankle. J Gerontol. 1992;47:M17–M21. doi: 10.1093/geronj/47.1.m17. [DOI] [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27:43–59. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]
- Warren GL, Ingalls CP, Lowe DA, Armstrong RB. Excitation–contraction uncoupling: major role in contraction-induced muscle injury. Exerc Sport Sci Rev. 2001;29:82–87. doi: 10.1097/00003677-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Lannergren J. Changes of the force–velocity relation, isometric tension and relaxation rate during fatigue in intact, single fibres of Xenopus skeletal muscle. J Muscle Res Cell Motil. 1994;15:287–298. doi: 10.1007/BF00123481. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Dahlstedt AJ, Lannergren J. Mechanisms underlying reduced maximum shortening velocity during fatigue of intact, single fibres of mouse muscle. J Physiol. 1998;510(Pt 1):269–277. doi: 10.1111/j.1469-7793.1998.269bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widrick JJ, Barker T. Peak power of muscles injured by lengthening contractions. Muscle Nerve. 2006;34:470–477. doi: 10.1002/mus.20608. [DOI] [PubMed] [Google Scholar]