Abstract
Arterial and venous thrombosis have always been regarded as different pathologies and epidemiological studies have examined the association between venous thrombosis and indicators of atherosclerosis and/or arterial thromboembolic events. We measured the flow-mediated dilation (FMD), a well-known marker of arterial endothelial dysfunction, in young–middle-aged and old-aged patients with and without unprovoked deep venous thrombosis (DVT). The aim of this study was to investigate whether DVT was a significant predictor for impaired FMD, considering all the patients and young–middle-aged (age < 65 years) and old-aged (age ≥ 65 years) patients separately. FMD was measured in the brachial artery on a population of 120 subjects with the same atherosclerosis risk factors, 68 male and 52 female, 70 young–middle-aged subjects (mean age ± SD 49.5 ± 10.5 years) and 50 old-aged subjects (76.2 ± 7.7 years). Patients with DVT showed a significant decrease of FMD compared to patients without DVT (6.8 ± 5.5% vs. 10.9 ± 3.5%, p < 0.001). Moreover, old-aged patients showed a significant decrease of FMD compared to the young–middle-aged subjects (7.4 ± 4.1% vs. 9.8 ± 5.3%, p = 0.005). In the whole study population, DVT was strongly associated with FMD (risk factors adjusted β = −4.14, p < 0.001). A significant interaction between age and the presence of DVT on predicting FMD was found (p = 0.003) suggesting a differential behavior of DVT as predictor of FMD. In young–middle-aged group, multivariate model confirmed that DVT was the most significant predictor of continuous FMD (β = −6.06, p < 0.001). On the contrary, DVT was no more a predictor of FMD in the old age group (β = −0.73, p=0.556). Furthermore, old-aged patients without DVT showed a statistically significant decrease of FMD compared to the young–middle-aged subjects without DVT (8.2±2.1% vs. 12.6±2.7%, p<0.001) and old-aged patients with DVT showed a not statistically significant decrease of the FMD compared to the young–middle-aged patients with DVT (6.7±5.3% vs. 6.8±5.7%, p = 0.932). In conclusion, young–middle-aged patients with spontaneous DVT show an impaired FMD, whereas this impairment in old-aged subjects is evident independently from the presence or absence of DVT. Aging per se may be associated with physiologic abnormalities in the systemic arteries and with endothelial dysfunction.
Keywords: Endothelial dysfunction, Venous thrombosis, Atherosclerosis, Flow-mediated dilation, Aging
Keywords: Life Sciences, Molecular Medicine, Geriatrics/Gerontology, Cell Biology
Introduction
The possible link between venous thrombosis and atherosclerosis is not yet clear and the role of the respective risk factors and the biological mechanisms underlying this association remain to be elucidated. This is of clinical interest, since the possible interactions between cardiovascular disease and venous thrombosis may have important clinical implications with respect to the risk assessment, the modification of the risk factors, and the medical treatment (Jerjes-Sanchez, 2005; Prandoni et al. 2003). Endothelial dysfunction has been reported to be the initial step in atherosclerosis and represents overall functional changes characterized by vasospasm, coagulation abnormalities, and increased vascular proliferation. Measurement of the flow-mediated dilation (FMD) of the brachial artery with high-resolution ultrasound scanning represents a valuable method to evaluate non-invasively peripheral endothelial dysfunction (Agouni et al. 2008). Disturbance of vascular dilatation due to reduced nitric oxide bio-availability in the vascular wall leads to predominance of vasoconstriction, which is usually associated with increased platelet adhesivity and increased endothelial permeability for lipoproteins and white blood cells leading to connective tissue proliferation. For many years, venous thrombosis and atherosclerotic disease have been considered two different disease entities with important differences in their pathways to thrombosis, but they may be associated, with a high proportion of patients with venous thrombosis demonstrating endothelial dysfunction, preclinical atherosclerotic changes, and higher risk of subsequent development of atherosclerotic disease (Prandoni et al. 2006; Prandoni 2007; Sorensen et al. 2007; Tsai et al. 2002). In particular, a recent paper has evidenced that in patients with primary arterial or venous disease, the arterial and venous vessel walls deteriorate simultaneously, as the prevalence of preclinical indicators of atherosclerosis is higher in patients with spontaneous venous thrombosis than in healthy subjects, demonstrating a relationship between asymptomatic atherosclerotic lesions and idiopathic venous thrombosis of the lower limbs (Jezovnik et al. 2010a). On the other hand, flow-mediated and glyceryl-trinitrate-mediated dilation of the brachial artery have resulted impaired in patients with idiopathic venous thrombosis, indicating that these patients may have functional disturbances of the venous wall, which could be involved in thrombus formation, especially in subjects without known risk factors of venous thrombosis (Jezovnik et al. 2010b).
The aim of this study was to investigate whether DVT was a significant predictor for FMD, assessed in a peripheral artery by high-resolution ultrasound scanning, considering the overall study population and young–middle-aged and old-aged patients separately. DVT exposed and not exposed groups were matched for cardiovascular risk profile.
Materials and methods
Patients
We enrolled consecutive patients hospitalized in the Department of Internal Medicine with and without DVT assessed through the compression ultrasonography (CUS) of lower limb veins. The study was approved by the local Scientific and Ethical Committee, the subjects gave written informed consent and the investigation conformed to the principles outlined in the Declaration of Helsinki. The subjects were chosen to obtain two matched groups homogeneous with respect to the common risk factors for atherosclerosis (age, male gender, obesity, current cigarette smoking, dyslipidemia, diabetes mellitus, and arterial hypertension), with respect to cardiovascular diseases (stable and unstable angina, myocardial infarction, and cerebrovascular accident such as nonhemorrhagic stroke and peripheral vascular disease) and with respect to medication history (use of statins, antihypertensive agents, nitrates, and platelet anti-aggregating agents) and biochemical parameters (total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol; triglycerides, plasma glucose, and erythrocyte sedimentation rate (ESR)). Exclusion criteria included pregnancy, cancer, concomitant infection, leg trauma or bone fracture, cast therapy, surgical procedures in the last 90 days, condition of being chronically ill, bedridden or nonambulatory, and treatment with prothrombotic agents (steroids, estrogens). Patients with DVT were selected in the acute phase (within 2 weeks from onset) of disease and heparin was administered to the patients with venous thrombosis after the ultrasonographic evaluations in the form of low-molecular-weight heparin (enoxaparin, at a dosage of 1 mg/kg twice a day).
Measurements and biochemical parameters
In all enrolled subjects we measured weight, standing height, and level of overweight was measured by body mass index. We asked questions on number of cigarettes smoked per day and duration of smoking; responses to these questions provided the foundation for the definitions of smoking status and pack-years of smoking. Blood pressure was measured using the same protocol. Three measurements were taken with a random-zero sphygmomanometer, and the mean of the last two of three measurements was used. A venous blood sample was drawn after an overnight fast for the determination of lipid parameters (total, LDL, and HDL cholesterol and triglycerides), plasma glucose, and erythrocyte sedimentation rate. Lipids, plasma glucose, and ESR were measured by standard methods. The following cut-off values were considered: systolic arterial pressure >140 mmHg and diastolic arterial pressure >90 mmHg for definition of arterial hypertension, a total cholesterol level >200 mg/dl, a low-density lipoprotein cholesterol level >100 mg/dl, a high-density lipoprotein cholesterol level <40 mg/dl for male subjects or <50 mg/dl for female subjects and a triglyceride level ≥150 mg/dl for definition of dyslipidemia, a fasting glycemia ≥125 mg/dl for definition of diabetes mellitus, ESR ≥20 mm/h for definition of inflammation in the body. The screening for mutations and conditions associated with thrombophilia (antithrombin III, protein C or protein S deficiency, factor V Leiden, prothrombin G20210A mutation, hyperhomocysteinemia, and lupus-like anticoagulants) was performed in all the subjects and the subjects with alterations of these factors were excluded from the study.
Echo-Doppler study
The ultrasonographic evaluations have been effectuated for diagnosis and measurements at day 1.
The presence of DVT was assessed by CUS that combines real-time imaging of the deep veins with venous compression. Lower extremity venous imaging is performed with the patient supine, the head elevated (10–20°), the leg positioned with the knee slightly elevated and the hips slightly rotated externally. The ultrasonographic evaluations of the endothelial function and the flow-mediated endothelial-dependent vasodilatation have been effectuated through flow-mediated dilatation measurement in the brachial artery of the non-dominant arm. The validity of the method has been confirmed in previous studies (Corretti et al. 2002; Drexler et al. 2002; Roman et al. 2006; Bots et al. 2005; Peretz et al. 2007). The diameter of the brachial artery was measured by an expert sonographer on ultrasound images obtained in B-mode with a high-resolution 5–10-MHz multi-frequency linear probe connected to an ESAOTE Technos-MPX (Esaote, Genova, Italy). All the patients were fasting and had not been smoking, drinking alcohol and coffee, or taking anti-oxidative agents for 12 h at least. Subjects were examined in the morning (between 9 and 11 a.m.) and after 10 min of rest in a supine position in a quiet, temperature-controlled room (21°C to 23°C) the brachial artery was explored at the antecubital fossa level on a longitudinal plane, optimizing the depth and the acoustic window pre-sets and keeping them in the same position during the study, by using a mechanical probe-holder arm. After the basal measurement of the diameter and of the speed of the brachial artery flow, a sphygmomanometer cuff has been inflated around the forearm at a pressure of 50 mmHg higher than the basal systolic pressure and deflated after 5 min. The measurement of the vessel diameter and the speed of the flow have been effectuated before and after the deflation every 30 s for 3 min, registered in telediastole, coinciding with R wave of the ECG, which was continuously monitored. The variations of the diameter during the reactive hyperemia have been expressed as percentage increase of the diameter compared to the basal value. The measurement has been registered at the media–adventitia interface of anterior (near) and posterior (far) vessel walls. For each scanning the diameters have been measured in four heart cycles and the average has been calculated by dividing the difference between the maximum diameter and the basal one by the maximum diameter. The diameter of the brachial artery was measured by the same expert sonographer (MG), who was blinded to clinical data. The intraobserver variability for the repeated measurements of resting arterial diameter were 0.023 ± 0.004 and 0.051 ± 0.005 mm, respectively. When this study was performed on two separate days in 28 patients, the within-patient difference for the measurement of the percent increase in the arterial diameter during reactive hyperemia was 1.4 ± 0.3%.
Statistical analysis
Patients’ baseline characteristics were reported as mean ± standard deviation (SD) or frequencies and percentages for continuous and categorical variables, respectively. Baseline comparisons between groups were made using Chi-square test for categorical variables and Mann–Whitney U test for continuous variables.
Univariate and multivariate linear regression models (adjusting for the established risk factors) were used to investigate possible association between continuous FMD and presence of DVT. Results were reported as regression coefficients (β) along with their standard errors (SE) and p values.
Interaction between age and DVT in predicting continuous FMD was evaluated including in the multivariate model the age by DVT multiplicative term. Young–middle-aged and old-aged patients subgroups analyses were performed in the same fashion.
Possible interactions among risk factors in predicting FMD were also investigated using RECursive Partitioning and AMalgamation (RECPAM) method (Ciampi et al. 1995; De Berardis et al. 2007), separately for patients with age <65 years and patient with age ≥65 years. This tree-based method investigates the interactions among clinical covariates. At each partitioning step, the method chooses the covariate and its best binary split to maximize the difference in the outcome of interest. The algorithm stops when user-defined conditions (stopping rules) are met. To obtain more robust and stable split, a permutation approach was adopted to choose the best splitting variable.
A p value <0.05 was considered for statistical significance. All analyses were performed using SAS Release 9.1 (SAS Institute, Cary, NC, USA). For the RECPAM analysis, we used a SAS macro routine written by F. Pellegrini.
Results
The final study population consisted of 120 subjects with the same atherosclerosis risk factors, 68 men (56.7%) and 52 women (43.3%). The mean age was 60.6 ± 16.2 years (range 17 to 96 years). Baseline clinical characteristics of patients divided according to the presence of DVT were reported on Table 1. There were 70 young–middle-aged subjects (mean age, 49.5 ± 10.5 years; range, 17 to 64 years) and 50 old-aged subjects (mean age, 76.2 ± 7.7 years; range, 65 to 96 years). The patients were then divided in four subgroups: 36 young–middle-aged subjects without spontaneous DVT (with a mean age ± SD of 51.2 ± 9.5 years, range 23 to 64 years, 21 men and 15 women), 34 young–middle-aged patients with spontaneous DVT (with a mean age ± SD of 47.7 ± 11.4 years, range 18 to 63 years, 19 men and 15 women), 24 old-aged subjects without spontaneous DVT (with a mean age ± SD of 76.0 ± 7.9 years, range 65 to 92 years, 13 men and 11 women), and 26 old-aged patients with spontaneous DVT (with a mean age ± SD of 75.9 ± 6.8 years, range 66 to 90 years, 15 men and 11 women).
Table 1.
Patients’ baseline characteristics reported as mean ± standard deviation (SD) in subjects with and without deep venous thrombosis (DVT)
| Variable | Not exposed (without DVT) | Exposed (with DVT) | p value |
|---|---|---|---|
| Number of subjects n (%) | 60 (50) | 60 (50) | – |
| Age (years) | 61.2 ± 15.1 | 60.1 ± 17.4 | 0.729 |
| Gender male n (%) | 34 (56.67) | 34 (56.67) | 1.000 |
| Obesity n (%) | 17 (28.33) | 23 (38.33) | 0.245 |
| Smoke n (%) | 19 (31.67) | 13 (21.67) | 0.216 |
| Anti-aggregating drugs n (%) | 17 (28.33) | 18 (30.00) | 0.841 |
| Statins n (%) | 17 (28.33) | 12 (20.00) | 0.286 |
| Dyslipidemia n (%) | 16 (26.67) | 20 (33.33) | 0.426 |
| Diabetes mellitus n (%) | 5 (8.33) | 7 (11.67) | 0.543 |
| Arterial hypertension n (%) | 17 (28.33) | 26 (43.33) | 0.087 |
| Cardiovascular disease n (%) | 4 (6.67) | 3 (5.00) | 0.697 |
| Continuous FMD (%) | 10.7 ± 3.5 | 6.6 ± 5.5 | <0.001 |
No differences in risk factors were detected between DVT exposed and not exposed groups.
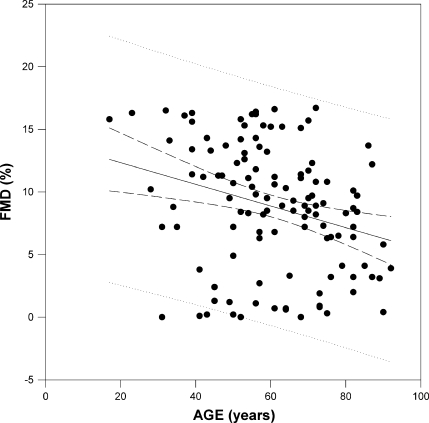
Patients with DVT showed a significant decrease of FMD compared to patients without DVT (6.8 ± 5.5 vs. 10.9 ± 3.5, p < 0.001), old-aged patients showed a significant decrease of FMD compared to the young–middle-aged subjects (7.4 ± 4.1 vs. 9.8 ± 5.3, p = 0.005) and a statistically significant negative correlation was found between age and FMD (r = −0.28, p < 0.01; Fig. 1). Then we tested the interaction between age and the presence of DVT on predicting continuous FMD. As a significant interaction was found (p = 0.003), subgroup analyses for young–middle-aged and old-aged groups were performed, separately.
Fig. 1.
x–y plot showing a regression line with 95% confidence limits between flow-mediated dilation (FMD) and age in the study population (n = 120). Linear regression evidences a statistically significant negative trend between FMD and age (r = −0.28, p < 0.01)
As shown in Table 2, unadjusted and adjusted regression coefficients were estimated in all patients from univariate and multivariate linear regression models, respectively, the latter adjusted for the following risk factors: age, gender, obesity, smoke, dyslipidemia, diabetes mellitus, arterial hypertension, cardiovascular diseases, use of platelet anti-aggregating drugs, use of statins, and presence of DVT. Univariate DVT regression coefficient (−4.10, p < 0.001) did not substantially change after adjustment for the established risk factors (−4.14, p < 0.001), confirming that DVT was the most significant predictor of endothelium-dependent vasodilatation.
Table 2.
Univariate and multivariate linear regression analyses for continuous flow-mediated dilation
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | Beta (SE) | p value | Beta (SE) | p value |
| Deep venous thrombosis | −4.10 (0.83) | <0.001 | −4.14 (0.80) | <0.001 |
| Age | −0.05 (0.03) | 0.089 | −0.01 (0.03) | 0.589 |
| Gender (male) | −1.66 (0.91) | 0.070 | −1.37 (0.77) | 0.079 |
| Obesity | −2.46 (0.94) | 0.010 | −2.04 (0.85) | 0.018 |
| Smoke | −0.06 (1.03) | 0.952 | −0.25 (0.86) | 0.774 |
| Dyslipidemia | −2.84 (0.96) | 0.004 | −0.34 (1.36) | 0.805 |
| Cardiovascular disease | −1.02 (1.95) | 0.602 | −2.43 (1.66) | 0.145 |
| Arterial hypertension | −1.89 (0.94) | 0.046 | −0.23 (1.02) | 0.818 |
| Diabetes mellitus | −1.25 (1.52) | 0.413 | 0.10 (1.35) | 0.943 |
| Platelet anti-aggregating drugs | −2.35 (0.98) | 0.018 | −1.66 (0.89) | 0.066 |
| Statins | −1.93 (1.05) | 0.069 | −1.30 (1.43) | 0.366 |
In the same way, univariate obesity regression coefficient (−2.46, p = 0.010) did not substantially change after adjustment for the established risk factors (−2.04, p = 0.018).
In young–middle-aged group, univariate and multivariate models confirmed that DVT was the most significant predictor of continuous FMD: unadjusted and adjusted regression coefficients were: −6.20 (p < 0.001) and −6.06 (p < 0.001), respectively (Table 3). On the contrary, DVT was no more a predictor of FMD in old-aged group (β = −0.73, p = 0.556; Table 4).
Table 3.
Univariate and multivariate linear regression analyses for continuous flow-mediated dilation in patients with age <65 years
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | Beta (SE) | p value | Beta (SE) | p value |
| Deep venous thrombosis | −6.20 (1.03) | <0.001 | −6.06 (1.08) | <0.001 |
| Age | 0.06 (0.06) | 0.287 | 0.04 (0.05) | 0.419 |
| Gender (male) | −1.70 (1.25) | 0.180 | −1.84 (1.04) | 0.082 |
| Obesity | −3.09 (1.26) | 0.017 | −1.27 (1.13) | 0.265 |
| Smoke | −1.43 (1.36) | 0.299 | −0.05 (1.05) | 0.962 |
| Dyslipidemia | −3.12 (1.45) | 0.035 | −1.15 (1.83) | 0.532 |
| Cardiovascular disease | −1.67 (3.77) | 0.659 | −1.27 (2.91) | 0.662 |
| Arterial hypertension | −1.60 (1.66) | 0.338 | −0.12 (1.60) | 0.941 |
| Diabetes mellitus | 1.68 (3.10) | 0.590 | 4.44 (2.50) | 0.081 |
| Platelet anti-aggregating drugs | −2.81 (1.69) | 0.102 | −1.19 (1.30) | 0.367 |
| Statins | −1.57 (1.72) | 0.363 | −1.92 (1.97) | 0.336 |
Table 4.
Univariate and multivariate linear regression analyses for continuous flow-mediated dilation in patients with age ≥65 years
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | Beta (SE) | p value | Beta (SE) | p value |
| Deep venous thrombosis | −1.20 (1.15) | 0.301 | −0.73 (1.23) | 0.556 |
| Age | −0.04 (0.05) | 0.552 | −0.01 (0.07) | 0.836 |
| Gender (male) | −1.67 (1.15) | 0.152 | −1.54 (1.19) | 0.205 |
| Obesity | −1.91 (1.24) | 0.130 | −2.24 (1.25) | 0.080 |
| Smoke | 1.62 (1.38) | 0.246 | 1.55 (1.50) | 0.310 |
| Dyslipidemia | −1.75 (1.16) | 0.138 | −0.24 (1.97) | 0.905 |
| Cardiovascular disease | 0.34 (1.94) | 0.861 | −1.05 (1.94) | 0.591 |
| Arterial hypertension | −0.42 (1.20) | 0.727 | 0.292 (1.27) | 0.818 |
| Diabetes mellitus | −1.18 (1.50) | 0.436 | −0.89 (1.50) | 0.555 |
| Platelet anti-aggregating drugs | −0.76 (1.16) | 0.513 | −1.01 (1.18) | 0.398 |
| Statins | −1.19 (1.20) | 0.326 | −0.93 (2.00) | 0.644 |
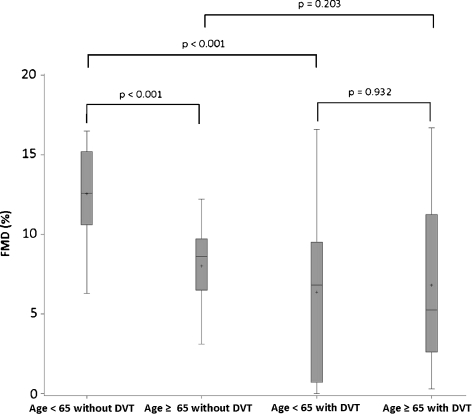
Furthermore, young–middle-aged patients with DVT showed a statistically significant decrease of the FMD compared to the subjects without DVT (6.8 ± 5.7% vs. 12.6 ± 2.7%, p < 0.001). Old-aged patients with DVT showed a not statistically significant decrease of the FMD compared to the subjects without DVT (6.7 ± 5.30% vs. 8.2 ± 2.1%, p = 0.203). Old-aged patients without DVT showed a statistically significant decrease of the FMD compared to the young–middle-aged subjects without DVT (8.2 ± 2.1% vs. 12.6 ± 2.7%, p < 0.001). Old-aged patients with DVT showed no statistically significant difference of the FMD compared to the young–middle-aged patients with DVT (6.7 ± 5.3% vs. 6.8 ± 5.7%, p = 0.932; Fig. 2). Such results suggested that the presence of DVT was strongly associated with decreasing of FMD values, while age plays a role on FMD only in patients without DVT.
Fig. 2.
FMD of the brachial artery in patients with and without deep venous thrombosis (DVT). Young–middle-aged patients with DVT show a statistically significant decrease of the FMD compared to the subjects without DVT (6.8 ± 5.7% vs. 12.6 ± 2.7%, p < 0.001). Old-aged patients with DVT show a not statistically significant decrease of the FMD compared to the subjects without DVT (6.7 ± 5.30% vs. 8.2 ± 2.1%, p = 0.203). Old-aged patients without DVT show a statistically significant decrease of the FMD compared to the young–middle-aged subjects without DVT (8.2 ± 2.1% vs. 12.6 ± 2.7%, p < 0.001). Old-aged patients with DVT show no statistically significant difference of the FMD compared to the young–middle-aged patients with DVT (6.7 ± 5.3% vs. 6.8 ± 5.7%, p = 0.932). Box plots with means, medians, quartiles, minimum, and maximum values
Moreover, in order to investigate possible interactions among age, gender, obesity, smoke, dyslipidemia, diabetes mellitus, arterial hypertension, cardiovascular diseases, use of platelet anti-aggregating drugs, use of statins, and presence of DVT on FMD, RECPAM analyses were performed, separately for patients with age <65 years and patient with age ≥65 years. No statistical significant interactions were found.
Discussion
The pathophysiology of venous thrombosis involves blood stasis, hypercoagulability, and endothelial damage. The pathways to arterial and venous thrombosis have been considered completely different for many years, but recent epidemiological studies have evidenced an association between venous thrombosis and indicators of atherosclerosis and/or arterial thromboembolic events (Van der Hagen et al. 2006; Reich et al. 2006; Ageno et al. 2008; Heit 2008; Isma et al. 2009; Holst et al. 2010). A common denominator might be represented by endothelial dysfunction. Endothelial dysfunction has been conclusively shown to be a primal event in the progression of atherothrombosis and to have a predictive value for future ischemic cardiovascular events (Gresele et al. 2010; Migliacci et al. 2007). FMD renders the endothelium-dependent relaxation of a conduit artery following the post-ischemic increase of blood flow. Brachial artery reactivity, frequently used for non-invasive ultrasonographic assessment of FMD, indicates endothelium-dependent response to increased wall shear stress and when impaired is a marker for endothelial dysfunction and increased cardiovascular risk, correlating with impaired endothelium-dependent relaxation in the coronary arteries (Takase et al. 1998; Matsuo et al. 2004; Thanyasiri et al. 2005). In agreement with previous results (Yavuz et al. 2008), the measurement of endothelial-dependent vasodilatation in our old-aged population has evidenced that FMD declines with increasing age (Figs. 1 and 2) and we could confirm that advanced age is a predictor of impaired endothelial function in human subjects.
As evidenced in our study, young–middle-aged subjects show impaired FMD when affected by DVT, whereas in the elderly there is no further decrease of endothelial-dependent vasodilatation in the presence of DVT.
Univariate and multivariate linear regression models, the latter adjusted for the common atherosclerosis risk factors (age, gender, obesity, smoke, dyslipidemia, diabetes mellitus, and arterial hypertension), cardiovascular diseases, use of platelet anti-aggregating drugs, use of statins, and presence of DVT indicate that DVT was the most significant predictor of endothelium-dependent vasodilatation in the whole study population and in the young–middle-aged subjects (Tables 2 and 3), but not in the elderly (Table 4). The results of this study suggest that FMD is comparable in old-aged patients with and without DVT and that DVT does not predict impairment of FMD and endothelial dysfunction in the elderly.
This evidence confirms that aging in itself is associated with impaired FMD and consequently with an increased risk of cardiovascular events, in which endothelial dysfunction is an early marker. Previous studies have evidenced a decrease of FMD in elderly subjects with obstructive sleep apnea syndrome (Chung et al. 2009), arterial hypertension (Kimura et al. 1999), and microalbuminuria with or without diabetes mellitus (Stehouwer et al. 2004).
A borderline level of significance was reached for the correlation between male gender and FMD in the whole study population and in the group of young–middle-aged subjects (p = 0.08), but not in the old age group. This evidence is in agreement with the results of a pioneer study evaluating FMD in the elderly, where aging resulted associated with progressive endothelial dysfunction in normal humans and the decrease of endothelial-dependent vasodilation occurred earlier in men, whereas women showed a steep decline at around the time of the menopause (Celermajer et al. 1994). This gender difference in the pattern of age-related decline in endothelial function supports the concept of a hormonal influence on vascular disease and risk. Male gender remains a cardiovascular risk factor, even after controlling for other influences, such as cigarette smoking and hypertension and life expectancy is greater for women than for men, in large part because of their lower incidence of cardiovascular death in middle age. The protective effect of estrogens on the arterial wall decreases with advancing age and this phenomenon, considering the advanced mean age of the overall study population (60.6 ± 16.2 years) and of the two subgroups (49.5 ± 10.5 and 76.2 ± 7.7 years, respectively), might explain why the interaction between gender and impaired endothelial function found in our study did not reach statistical significance.
We have to consider some limitations to the present study. We included a small number of patients and we excluded from the study patients who did not meet stringent criteria of inclusion. Therefore, the results obtained in this study may not apply to a more general population of patients. Further studies are needed to evaluate the utility of FMD as a screening test for cardiovascular risk in elderly patients with idiopathic DVT and to evaluate the association between FMD and future cardiac events in elderly patients with DVT.
In conclusion, we have examined young–middle-aged and old-aged subjects with and without unprovoked DVT and we have evaluated the arterial endothelial function by FMD measurement in the brachial artery. Data obtained show that old-aged subjects have altered endothelial function and that aging per se, irrespective of exposure to known risk factors, may be associated with physiologic abnormalities in the systemic arteries. Endothelial dysfunction, an early indicator of pathology valuable to guide risk factors modification and therapeutic intervention, is present in the elderly and is not aggravated by other concomitant diseases as in the case of spontaneous deep phlebothrombosis.
Contributor Information
Gianluigi Mazzoccoli, Email: g.mazzoccoli@tin.it.
Andrea Fontana, Email: a.fontana@operapadrepio.it.
Massimo Grilli, Email: massimogrilli@hotmail.com.
Mariangela Pia Dagostino, Email: dagostinomariangela@libero.it.
Massimiliano Copetti, Email: m.copetti@operapadrepio.it.
Fabio Pellegrini, Email: f.pellegrini@operapadrepio.it.
Gianluigi Vendemiale, Email: g.vendemiale@operapadrepio.it.
References
- Ageno W, Becattini C, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol. 2008;173(4):1210–1219. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bots ML, Westerink J, Rabelink TJ, Koning EJ. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. Eur Heart J. 2005;26(4):363–368. doi: 10.1093/eurheartj/ehi017. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Ahn HJ. Endothelial dysfunction and inflammatory reactions of elderly and middle-aged men with obstructive sleep apnea sindrome. Sleep Breath. 2009;13:11–17. doi: 10.1007/s11325-008-0210-x. [DOI] [PubMed] [Google Scholar]
- Ciampi A, Negassa A, Lou Z. Tree-structured prediction for censored survival data and the Cox model. J Clin Epidemiol. 1995;48:675–689. doi: 10.1016/0895-4356(94)00164-L. [DOI] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Herman MG, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. A report of the International Brachial Artery Reactivity Task Force. JACC. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Berardis G, Pellegrini F, Franciosi M, et al. Clinical and psychological predictors of incidence of self-reported erectile dysfunction in patients with type 2 diabetes. J Urol. 2007;177:252–257. doi: 10.1016/j.juro.2006.08.102. [DOI] [PubMed] [Google Scholar]
- Drexler H, Herman MG, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. A report of the International Brachial Artery Reactivity Task Force. JACC. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Gresele P, Momi S, Migliacci R. Endothelium, venous thromboembolism and ischaemic cardiovascular events. Thromb Haemost. 2010;103(1):56–61. doi: 10.1160/TH09-08-0562. [DOI] [PubMed] [Google Scholar]
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28(3):370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism results from the Copenhagen City Heart Study. Circulation. 2010;121:1896–1903. doi: 10.1161/CIRCULATIONAHA.109.921460. [DOI] [PubMed] [Google Scholar]
- Isma N, Svensson PJ, Gottsäter A, Lindblad B. Prospective analysis of risk factors and distribution of venous thromboembolism in the population-based Malmö Thrombophilia Study (MATS) Thromb Res. 2009;124(6):663–666. doi: 10.1016/j.thromres.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Jerjes-Sanchez C. Venous and arterial thrombosis: a continuous spectrum of the same disease? Eur Heart J. 2005;26(1):3–4. doi: 10.1093/eurheartj/ehi041. [DOI] [PubMed] [Google Scholar]
- Jezovnik MK, Poredos P, Lusa L. Idiopathic venous thrombosis is associated with preclinical atherosclerosis. J Atheroscler Thromb. 2010;17:304–311. doi: 10.5551/jat.3079. [DOI] [PubMed] [Google Scholar]
- Jezovnik MK, Poredos P, Stalc M. Impairment of the vasodilatation capability of the brachial artery in patients with idiopathic venous thrombosis. J Atheroscler Thromb. 2010;17:1190–1198. doi: 10.5551/jat.4960. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Matsumoto M, Deng YB, Iwai K, Munehira J, Hattori H, Hoshino T, Yamada K, Kawanishi K, Tsuchiya H. Impaired endothelial function in hypertensive elderly patients evaluated by high resolution ultrasonography. Can J Cardiol. 1999;15:563–568. [PubMed] [Google Scholar]
- Migliacci R, Becattini C, Pesavento R, Davi G, Vedovati MC, Guglielmini G, Falcinelli E, Ciabattoni G, Dalla Valle F, Prandoni P, Agnelli G, Gresele P. Endothelial dysfunction in patients with spontaneous venous thromboembolism. Haematologica. 2007;92(6):812–818. doi: 10.3324/haematol.10872. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Matsumoto T, Takashima H, Ohira N, Yamane T, Yasuda Y, Tarutani Y, Horie M. The relationship between flow-mediated brachial artery vasodilation and coronary vasomotor responses to bradykinin: comparison with those to acetylcholine. J Cardiovasc Pharmacol. 2004;44(2):164–170. doi: 10.1097/00005344-200408000-00004. [DOI] [PubMed] [Google Scholar]
- Peretz A, Leotta DF, Sullivan JH, Trenga CA, Sands FN, Aulet MR, Paun M, Gill EA, Kaufman JD. Flow mediated dilation of the brachial artery: an investigation of methods requiring further standardization. BMC Cardiovasc Disord. 2007;7:11–19. doi: 10.1186/1471-2261-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prandoni P. Venous thromboembolism and atherosclerosis: is there a link? J Thromb Haemost. 2007;5(1):270–275. doi: 10.1111/j.1538-7836.2007.02467.x. [DOI] [PubMed] [Google Scholar]
- Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, Lensing A, Prins MH, Girolami A. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;349(4):1435–1441. doi: 10.1056/NEJMoa022157. [DOI] [PubMed] [Google Scholar]
- Prandoni P, Ghirarduzzi A, Prins MH, Pengo V, Davidson BL, Soresen H, Pesavento R, Iotti M, Casiglia E, Iliceto S, Pagnan A, Lensing AW. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost. 2006;4(9):1891–1896. doi: 10.1111/j.1538-7836.2006.02058.x. [DOI] [PubMed] [Google Scholar]
- Reich LM, Folsom AR, Key NS, Boland LL, Heckbert SR, Rosamond WD, Cushman MJ. Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemost. 2006;4(9):1886–1890. doi: 10.1111/j.1538-7836.2006.02121.x. [DOI] [PubMed] [Google Scholar]
- Roman MJ, Naqvi TZ, Gardin JM, Gerhard-Herman M, Jaff M, Mohler E. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr. 2006;19(8):943–954. doi: 10.1016/j.echo.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Sørensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20- year cohort study. Lancet. 2007;370(9601):1773–1779. doi: 10.1016/S0140-6736(07)61745-0. [DOI] [PubMed] [Google Scholar]
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl. 2004;92:S42–S44. doi: 10.1111/j.1523-1755.2004.09211.x. [DOI] [PubMed] [Google Scholar]
- Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82(12):1535–1539. doi: 10.1016/S0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- Thanyasiri P, Celermajer DS, Adams MR. Endothelial dysfunction occurs in peripheral circulation patients with acute and stable coronary artery disease. Am J Physiol Heart Circ Physiol. 2005;289(2):H513–H517. doi: 10.1152/ajpheart.01086.2004. [DOI] [PubMed] [Google Scholar]
- Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162(10):1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- Hagen PB, Folsom AR, Jenny NS, Heckbert SR, O’Meara ES, Reich LM, Rosendaal FR, Cyshman M. Subclinical atherosclerosis and the risk of future venous thrombosis in the cardiovascular health study. J Thromb Haemost. 2006;4(9):1903–1908. doi: 10.1111/j.1538-7836.2006.02096.x. [DOI] [PubMed] [Google Scholar]
- Yavuz BB, Yavuz B, Sener DD, Cankurtaran M, Halil M, Ulger Z, Nazli N, Kabakci G, Aytemir K, Tokgozoglu L, Oto A, Ariogul S. Advanced Age Is Associated with Endothelial Dysfunction in Healthy Elderly Subjects. Gerontology. 2008;54:153–156. doi: 10.1159/000129064. [DOI] [PubMed] [Google Scholar]