Abstract
We assessed whether melatonin administration would prevent the hyperoxidative status that occurs in lung mitochondria with age. Mitochondria from lungs of male and female senescent prone mice at 5 and 10 months of age were studied. Age-dependent mitochondrial oxidative stress was evaluated by measuring the levels of lipid peroxidation and nitrite, glutathione/glutathione disulfide ratio, and glutathione peroxidase and reductase activities. Mitochondrial respiratory chain and oxidative phosphorylation capability were also measured. Age induces a significant oxidative/nitrosative status in lung mitochondria, which exhibited a significantly reduced activity of the respiratory chain and ATP production. These manifestations of age were more pronounced in males than in females. After 9 months of melatonin administration in the drinking water, the hyperoxidative status and functional deficiency of aged lung mitochondria were totally counteracted, and had increased ATP production. The beneficial effects of melatonin were generally similar in both mice genders. Thus, melatonin administration, as a single therapy, maintained fully functioning lung mitochondria during aging, a finding with important consequences in the pathophysiology of lung aging. In view of these data melatonin, the production of which decreases with age, should be considered a preventive therapy against the hyperoxidative status of the aged lungs, and its use may lead to the avoidance of respiratory complications in the elderly.
Keywords: Lung, Aging, Mitochondria, Respiratory chain, Oxidative phosphorylation, Oxidative stress
Keywords: Life Sciences, Molecular Medicine, Geriatrics/Gerontology, Cell Biology
Introduction
Respiratory system undergoes various structural, physiological, and immunological changes with age, which are directly involved in the frequency and severity of several chronic and acute lung pathologies such as emphysema and pneumonia, respectively (Sharma and Goodwin 2006). Age also increases in the inflammatory response to lung injury due to pollutants (Meyer 2005; Elder et al. 2000). Whereas it is unclear why the elderly are more susceptible to these lung diseases, previous studies have revealed that oxidative stress could be an important component of the mechanism of action of several toxic compounds that reach the lung through inhaled air (Umstead et al. 2009). Moreover, exposure to ozone and/or high oxygen produces a similar susceptibility increase to lung diseases than aging (Baleeiro et al. 2003; Mikerov et al. 2008). These data suggest a relationship between lung disease, age, and oxidative stress.
Lungs are highly vascularised organs and they are directly exposed to atmospheric oxygen. Consequently, lungs themselves are an important source of reactive oxygen (ROS) and nitrogen (RNS) species that play an important role in lung biology and pathology. In particular, ROS and RNS may be produced by the immune cells during chronic or acute inflammation in the lung, which may be used for local defence against pathogens (Umstead et al. 2009). The remainder ROS/RNS may be scavenged by the endogenous antioxidant system preventing the subsequent oxidative/nitrosative damage to the lung. But with age, increased production of ROS and RNS, together with a reduced antioxidant defense activity, may surpass the antioxidative capability of the lungs, leading to lung damage and making lungs more susceptible to other pathologies (Umstead et al. 2009; Kirkwood 2005). ROS/RNS may also be produced in lung mitochondria, constituting a key factor in aging (Pendyala and Natarajan 2010; Shoal et al. 2002). The high concentration of ROS and RNS in the mitochondria as a consequence of aging promotes the subsequent oxidative damage to DNA, proteins and lipids, reducing ATP availability and accelerating cell damage, reducing the repair capacity of lung epithelium and becoming lung tissue more sensitive to diseases (Mora and Rojas 2008).
To clarify the role of aging on the incidence of lung disease, it is mandatory to first know the extent at which ROS and RNS are produced, and whether these radicals affect significantly the lung mitochondrial function. The redox states of glutathione/glutathione disulfide (GSH/GSSG) have been shown to be oxidized with age (Rebrin et al. 2003; Jones 2006). Besides ROS, RNS may also cause lipid peroxidation (LPO), and products of peroxidation are in turn important mediators of oxidative damage (Riahi et al. 2010). In this regard, the senescence-accelerated mouse (SAMP), an established murine model of accelerated aging (Hosokawa 2002; Takeda 1999), is a good model to test this hypothesis since lungs from a SAMP sub-strain shows morphological and functional changes associated to aging (Teramoto et al. 1994). The significant mitochondrial oxidative damage associated to aging in several tissues of SAMP8 mice may explain their hyperoxidative status leading to ATP depletion and death (Hosokawa 2002; Rodríguez et al. 2007a, b). Thus, in this study we analyzed the age-dependent ROS/RNS production by lung mitochondria in male and female SAMP8 mice and their relation with mitochondrial bioenergetics. Because it has been reported that melatonin, an endogenous antioxidant, prevented the age-related free radical generation in SAMP8 mice (Rodríguez et al. 2007a, b), the beneficial effect of chronic melatonin treatment on lung mitochondria in SAMP8 animals was also assessed.
Materials and methods
Animals and treatments
Male and female SAMP8 mice breeding pairs were obtained from the Council for SAM Research (Kyoto, Japan) through Harlan (Barcelona, Spain). The animals were maintained in the university’s facility under a 12:12 h light/dark cycle (lights on at 0700 h) at 22 ± 2°C, and were given regular chow and tap water, under the supervision of veterinarians. All experiments were performed according to the Spanish Government Guide and the European Community Guide for animal care. Animals were used at 5 and 10 months of age. Once newborn mice were separated from their mothers (at the age of 1 month), melatonin or vehicle treatments were initiated. The animals were separated into the following groups (n = 35 animals/group): (a) Veh-5 group, consisting of SAMP8 animals treated with vehicle from 1 to 5 months of age; (b) Veh-10 group, which included SAMP8 mice treated with vehicle from 1 to 10 months of age; and (c) aMT-10 group, which included SAMP8 mice treated with melatonin from 1 to 10 months of age. Melatonin was dissolved in a minimum volume of absolute ethanol and then diluted in the drinking water to yield a dose of 10 mg/kg body weight (b.w.) daily during the months of treatment. The concentration of ethanol in the final drinking water was 0.066%. A fresh melatonin and vehicle solutions were prepared twice a week. Water bottles were covered with aluminum foil to protect from light, and the drinking fluid was changed twice weekly. All mice were killed at the end of their treatment period, i.e. 5 and 10 months, and their lungs were quickly removed and immediately processed for pure mitochondria preparation.
Isolation of lung mitochondria
Animals were killed by cervical dislocation and lung mitochondria were immediately isolated (Escames et al. 2006). All procedures were carried out at 0–4°C. Briefly, the lungs were excised, washed with buffer A (0.32 M sucrose, 1 mM EDTA K+, 10 mM Tris–HCl, pH 7.4, at 4°C), and homogenized (1/10, w/v) in buffer A at 800 rev/min with a Teflon pestle. The homogenate was centrifuged at 1,300 × g for 3 min at 4°C. The pellet was suspended in 5 ml buffer A, and centrifuged again at 1,300 × g for 3 min at 4°C. The supernatants were mixed and centrifuged at 21,200 × g for 10 min at 4°C. Then, the mitochondrial pellets were frozen to −80°C. Mitochondrial protein content was determined in an aliquot of homogenized lung mitochondria without BSA (Lowry et al. 1951).
Lipid peroxidation determination
Mitochondrial fractions were thawed, suspended in ice-cold 20 mM Tris–HCl buffer, pH 7.4, and sonicated to break mitochondria membranes. Aliquots of these samples were either stored at −80°C for total protein determination (Lowry et al. 1951) or used for LPO measurements. For this purpose, a commercial LPO assay kit that estimates both malondialdehide (MDA) and 4-hydroxyalkenals (4HDA) was used (Bioxytech LPO-568 assay kit; OxisResearch, Portland, OR, USA) (Esterbauer and Cheeseman 1990). LPO concentration is expressed in nmol/mg prot.
Nitrite plus nitrate determination
Mitochondrial fractions were thawed, suspended in ice-cold distilled water, and immediately sonicated to break mitochondrial membranes. Aliquots of these samples were either stored at −80°C for total protein determination (Lowry et al. 1951) or used to calculate nitrite levels. Samples were previously treated with nitrate reductase to transform nitrate to nitrite. The total amount of nitrite (nitrite plus nitrate) was measured following the Griess reaction (Green and Ruiz de Luzuriaga 1981) by incubating 100-μl samples with 100 μl Griess reagent [0.1% N-(1-naphthyl) ethylendiamine dihydrochloride; 1% sulfanilamide in 5% phosphoric acid; 1:1] at room temperature for 20 min. The absorbance at 550 nm was measured and nitrite concentrations were calculated by comparison with the absorbance of a standard solution of known sodium nitrite concentration, and expressed as nmol/mg prot.
Measurement of glutathione peroxidase (GPx) and reductase (GRd) activities
Mitochondrial fractions were thawed and suspended in 200 μl of buffer A (potassium phosphate 50 mM and EDTA-K2 1 mM, pH 7.4) and sonicated. To measure GPx activity, 10 μl of each sample were added to 240 μl of a working solution containing buffer A plus 4 mM sodium azide, 4 mM GSH, 0.2 mM NADPH and 0.5 U/ml GRd. After incubation for 4 min at 37°C, the reaction was started by adding 10 μl of cumene hydroperoxide (0.3%) and the GPx activity was determined spectrophotometrically following the oxidation of the NADPH for 3 min at 340 nm (UV-1603 spectrophotometer Shimadzu Deutschland GmBH, Duisburg, Germany) (Griffith 1999). GRd activity was measured in samples (35 μl) added to 465 μl of a working solution containing buffer A plus 2 mM GSSG. After incubation for 4 min at 37°C, the reaction was started by adding 8.5 μl of 9.5 mM NADPH solution, and the GRd activity was spectrophotometrically measured following the oxidation of NADPH for 3 min at 340 nm (Griffith 1999). GPx and GRd activities are expressed as nmol/min/mg prot. In both cases, non-enzymatic NADPH oxidation was subtracted from the overall rates.
Measurement of glutathione and glutathione disulfide
Both GSH and GSSG were measured by a slight modification of an established fluorometric method (Hissin and Hilf 1976). Mitochondrial fractions were deproteinized with ice-cold 10% TCA and centrifuged at 20,000 × g for 15 min. For GSH measurement, 10 μl supernatant was incubated with 10 μl of an ethanol o-ophthalaldehyde solution (1 mg/ml) and 180 μl phosphate buffer (100 mM sodium phosphate, 5 mM EDTA-Na2, pH 8.0) for 15 min at room temperature. Then, the fluorescence of the samples was measured at 340 nm excitation and 420 nm emission in a plate-reader spectrofluorometer (Bio-Tek Instruments, Inc., Winooski, VT, USA). For GSSG measurement, 30-μl aliquots of supernatants were preincubated with 12 μl N-ethylmaleimide solution (5 mg/ml in distilled water) for 40 min at room temperature, and then alkalinized with NaOH 0.1 N. Aliquots of 45 μl were then incubated with 10 ml o-ophthalaldehyde solution and 145 μl NaOH 0.1 N for 15 min at room temperature. The fluorescence was then measured. GSH and GSSG concentrations were calculated with standard curves prepared accordingly. The levels of GSH and GSSG are expressed in nmol/mg prot.
Determination of mitochondrial complex I, II, III, and IV activities
Mitochondrial pellets were thawed, suspended in 350 μl of the incubation medium corresponding to the complex to be measured and immediately sonicated to prepare submitochondrial particles. Mitochondrial protein concentration was measured using BSA as standard (Lowry et al. 1951). To determine the complex I activity, submitochondrial fractions (0.6 mg/ml) were incubated for 3 min in a medium containing 250 mM sucrose, 50 mM potassium phosphate, 1 μM KCN, 50 μM decylubiquinone, 0.8 μM antimycin, pH 7.4. The reaction was initiated by the addition of NADH, and the activity of the complex I (NADH CoQ oxidoreductase, expressed as nmol oxidized NADH/min/mg prot) was measured following the rate of the oxidation of NADH (100 mM) at 340 nm in a UV-1603 spectrophotometer (Shimadzu Deutschland GmBH) (Barrientos 2002). The activity of complex II (succinate: DCIP oxireductase, expressed in nmol reduced DCIP/min/mg prot) was measured in 1 ml medium containing submitochondrial particles (0.03 mg/ml), 100 mM potassium phosphate, 0.5 M succinate, 0.8 μM antimycin, 50 μM rotenone, 2 μM KCN, 50 μM DCIP, pH 7.4. The reaction was initiated by the addition of 50 μM decylubiquinone. The activity of complex II was measured following the rate of reduction of 2, 6-DCIP at 600 nm with 520 nm as reference wavelength (Brusque et al. 2002). The activity of complex III (ubiquinol: cytochrome c reductase, expressed in nmol reduced cytochrome c/min/mg prot) was measured in 1 ml medium containing submitochondrial particles (0.03 mg/ml), 35 mM potassium phosphate, 5 mM MgCl2, 2.5 mg/ml BSA, 1.8 μM KCN, 50 μM rotenone and 2 μM decylubiquinone, pH 7.5. The reaction was started by adding 125 μM cytochrome c and the activity of complex III was measured following the rate of reduction of cytochrome c at 550 nm with 580 nm as the reference wavelength (Brusque et al. 2002). The activity of complex IV (cytochrome c oxidase, expressed as nmol oxidized cytochrome c/min/mg prot) was measured in 1 ml medium containing submitochondrial particles (0.1 mg prot/ml) and 50 mM potassium phosphate, pH 6.8. The reaction was initiated by adding 75 μM cytochrome c previously reduced with sodium borohydride and measuring the absorbance at 550 nm (Brusque et al. 2002).
Measurement of mitochondrial content of adenine nucleotides
Adenine nucleotides were determined by HPLC with a ProPac PA1 column (4 × 250 mm; Dionex, Bannockburn, IL, USA) and a binary gradient of 0.3 M ammonium carbonate and water (Pissarek et al. 1999). Purified mitochondria were rapidly resuspended in ice-cold 0.5 M perchloric acid, mixed during 120 s in vortex (to break the mitochondrial membranes) and centrifuged at 25,000 × g for 15 min at 2°C to precipitate proteins. Pellets were frozen to −80°C to determine protein concentration (Lowry et al. 1951) and the supernatants were mixed with 8 μl 5 M potassium carbonate to neutralize the acid and centrifuged at 12,000 × g for 10 min at 2°C. The resultant supernants were used for HPLC measurements. After stabilizing the column with the mobile phase, 20 μl of each sample were injected onto the HPLC system. The mobile phase consisted in water (phase A) and 0.3 M ammonium carbonate pH 8.9 (phase B), and the following time schedule for the binary gradient (flow rate, 1 ml/min) was used: 5 min, 50% A and 50% B; 5 min 50–100% B and then 100% B during 25 min; 5 min 100–50% B and then another 5 min with 50% B. For calibration, water was used as blank and 3.125, 6.250, 12.5 and 25 μg/ml of each nucleotide (AMP, ADP and ATP) were used for constructing the standard curves. Absorbance of the samples was measured with a UV detector at 254-nm wavelength and the concentration of each nucleotide was calculated based on the peak area. Mitochondrial content of nucleotides is expressed in μg/mg prot.
Statistical analysis
Data are expressed as the mean ± SE of at least six animals analysed in duplicate. An ANOVA followed by Student’s t-test was used to compare the means between groups. A p value lower than 0.05 was considered statistically significant.
Results
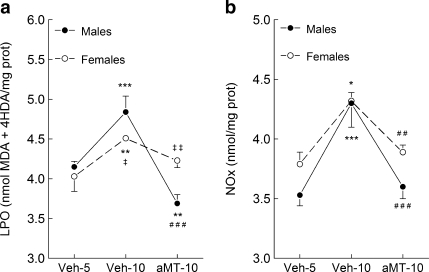
Figure 1a shows the changes in LPO levels in lung mitochondria of SAMP8 mice. Up to 5 months of age (Veh-5 groups) male and female mice had similar LPO levels, increasing them at 10 months of age (Veh-10) mainly in males (p < 0.05 vs. females). The animals treated with melatonin (aMT-10 groups) had an important (p < 0.001) reduction in LPO levels, and the effect of treatment was more pronounced in males than in females (p < 0.01).
Fig. 1.
Effect of age and melatonin treatment on (a) LPO and (b) nitrite levels in lung mitochondria from male and female SAMP8 mice. Animals were given vehicle (0.066% ethanol) or melatonin (10 mg/kg) in the drinking water from 1 month after birth, and sacrificed 4 or 9 months later (at 5 and 10 months of age, respectively). Results are expressed as a mean ± SEM of six experiments measured in duplicate. Veh5 and Veh10, SAMP8 mice treated with vehicle and sacrificed at 5 and 10 months of age; aMT10, SAMP8 mice treated with melatonin and sacrificed at 10 months of age. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with Veh5; ###p < 0.01 compared with Veh10; ‡p < 0.05 and ‡‡p < 0.01 compared with the same group of males
Nitric oxide levels were quantified through the levels of nitrite plus nitrate (NOx, Fig. 1b). Young male and female animals had similar NOx levels in lung mitochondria (Veh-5), increasing significantly at 10 months of age (Veh-10 groups). Melatonin treatment prevented the age-dependent NOx surge, keeping them at the level found at 5 months of age in both genders (p < 0.001 and p < 0.01 for males and females, respectively).
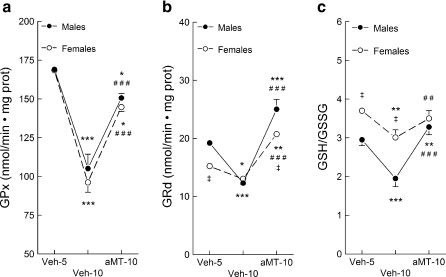
The activity of GPx was similar in male and female animals at 5 months of age (Veh-5), decreasing significantly 5 months later in both genders (Veh-10, Fig. 2a, p < 0.001). Melatonin administration prevented the drop in the age-dependent GPx activity (p < 0.001). GRd activity was higher in males than in females at 5 months of age (Veh-5, Fig. 2b, p < 0.05), decreasing at 10 months of age in both genders (p < 0.001 and p < 0.05 for males and females, respectively). In turn, chronic melatonin administration increased GRd activity in both mice’s gender (p < 0.001) even above the values found at 5 months of age (p < 0.001 and p < 0.05 for males and females, respectively).
Fig. 2.
Effect of age and melatonin treatment on the glutathione peroxidase (GPx) (a) and glutathione reductase (GRd) (b) activities, and on the GSSG/GSH ratio (c) in lung mitochondria from males and females SAMP8 mice. See legend of Fig. 1 for additional information. Results are expressed as a mean ± SEM value of six experiments measured in duplicate. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with Veh5; ##p < 0.01 and ###p < 0.001 compared with Veh10; ‡p < 0.05 and ‡‡p < 0.01 compared with the same group of males
The GSH/GSSG ratio was higher in young females than in males (Fig. 2c, p < 0.05). Age significantly reduced this ratio in males (p < 0.001) and somewhat in females (p < 0.01). The administration of melatonin completely restored the GSH/GSSG ratio in both genders, although the effect was more prominent in males (p < 0.01 and p < 0.001, respectively).
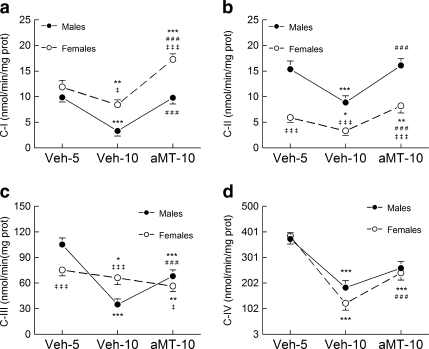
The activity of the respiratory complexes is shown in Fig. 3. Complex I activity (Fig. 3a) decreased with age mainly in males (p < 0.001) than in females (p < 0.01). Melatonin prevented the effect of age on complex I activity in both genders (p < 0.001). Female mice, however, reached higher activity of complex I activity after melatonin treatment than males (p < 0.001). Complex II activity (Fig. 3b) was higher in young males than in females (Veh-5, p < 0.001), whereas the reduction of its activity with age was higher in the former (p < 0.001 vs. p < 0.05, respectively). Melatonin treatment restored the activity of complex II in both mice genders (p < 0.001), although females showed an activity of complex II after melatonin treatment higher than at 5 months of age (p < 0.01). The activity of complex III (Fig. 3c) was higher in young males than in females (p < 0.001), and it was reduced significantly with age (p < 0.001 and p < 0.05 for males and females, respectively). Melatonin treatment partially counteracted the effect of age on complex III activity in males (p < 0.001), whereas its activity remained low in females. Complex IV activity (Fig. 3d) was similar in male and female mice at 5 months of age (Veh-5), decreasing it at the same extent with age (p < 0.001). Melatonin partially counteracted the effects of age, recovering the activity of this complex up to 65% (p < 0.001).
Fig. 3.
Age and melatonin effects on the activity of complex I (a NADH dehydrogenase), complex II (b succinate dehydrogenase), complex III (c cytochrome bc1 complex), and complex IV (d cytochrome c oxidase) of the electron transport chain in lung mitochondria from male and female SAMP8 mice. See legend of Fig. 1 for additional information. Results are expressed as a mean ± SEM value of six experiments measured in duplicate. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with Veh5; ###p < 0.001 compared with Veh10; ‡p < 0.05 and ‡‡‡p < 0.001 compared with the same group of males
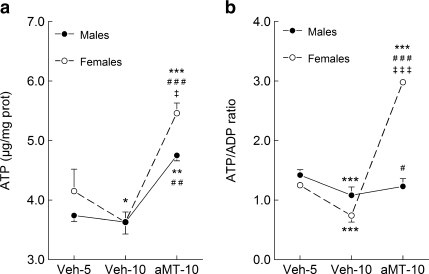
Mitochondrial ATP levels are shown in Fig. 4. Whereas the ATP content in lung mitochondria was similar in both genders at 5 months of age, it decreased 5 months later in females (p < 0.05). Melatonin treatment increased the ATP content over the levels at 5 months of age in males (p < 0.01) and females (p < 0.001). The effect of melatonin on ATP levels was significantly higher in females than in males (p < 0.05). The ATP/ADP ratio decreased significantly (p < 0.001) in both genders with age (Fig. 4b, p < 0.001). The treatment with melatonin increased the ATP/ADP ratio, mainly in females (p < 0.001). The changes observed in ATP/ADP ratio were quite similar to those observed in ATP/AMP ratio and energy charge (data not shown).
Fig. 4.
Effect of age and melatonin treatment on the ATP (a) content and ATP/ADP ratio (b) in lung mitochondria from males and females SAMP8 mice. See legend of Fig. 1 for additional information. Results are expressed as a mean ± SEM value of six experiments measured in duplicate. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with Veh5; #p < 0.05, ##p < 0.01 and ###p < 0.001 compared with Veh10; ‡p < 0.05 and ‡‡‡p < 0.001 compared with the same group of males
Discussion
This study documents the existence of a prominent age-dependent oxidative stress in lung mitochondria of SAMP8 mice. The results suggest that the oxidative stress derived from free-radical generation, combined with a less effective antioxidative system, may underlie the mitochondrial dysfunction in this model of aging. Of interest is the presence of a lower oxidative stress and mitochondrial dysfunction in aged female compared with male mice. Another important finding here reported is the prevention of the age-dependent lung mitochondrial oxidative/nitrosative stress by melatonin, which also increased the ATP availability to the cell. Together, reducing lung mitochondrial dysfunction by melatonin may yield tissues less susceptible to the age-dependent chronic and/or acute lung pathologies such as emphysema and pneumonia. Moreover, the virtual lack of toxicity of melatonin (De Lourdes et al. 2000) supports its chronic administration in these conditions.
The vast majority of the organs produce ROS mainly related to oxidative phosphorylation in mitochondria. Because their physiological function, lungs are highly vascularised and they are directly exposed to a hyperoxidative ambient air, which includes atmospheric oxygen and ozone (Baleeiro et al. 2003; Mikerov et al. 2008; Elder et al. 2000). These conditions increase oxidative damage to the lungs and may explain the higher LPO levels and GPx and GRd activities observed in lung of SAMP8 mice compared with other metabolically active organs such as, heart, diaphragm or brain (Rodríguez et al. 2007a; Carretero et al. 2009). Moreover, cardiolipin oxidation, one of the major sources of 4-hydroxynonenal, a LPO component (Liu et al. 2010) which is involved in apoptosis (Gonzalvez and Gottlieb 2007), contributes to the increased LPO levels and mitochondrial apoptosis during aging. In addition to ROS, lung cells are exposed to RNS, mainly NO● and its metabolite ONOO−, both of them produced by the immune cells through the induction of the inducible NOS (iNOS) (Escames et al. 2003; Nathan and Xie 1994). Whereas at physiological concentration NO● regulates the respiration acting on the complex IV (Brown 2001), high NO● levels reversibly inhibit the activity of complex IV, III and I (Brown and Borutaite 2002). Additionally, the formation of ONOO− transforms two relatively unreactive free radicals, NO● and O2‾• into a much more reactive specie that can irreversibly damage the mitochondrial respiratory complexes (Brown and Boroutaite 2004). Furthermore, the reaction of ONOO− with CO2 accounts for a large fraction of the ONOO− formed in vivo, and the reaction produces reactive intermediates such as nitrites that can affect other biotargets in secondary reactions (Squadrito and Pryor 1998). Among other macromolecules, lipids are attacked not only by ROS, but also by RNS. So, whereas LPO levels express the damage induced by these radicals, NOx levels directly reflect the degree of nitrosative stress. Herein, our results support the increase in NOx and LPO in the aged mitochondria of lungs elsewhere published in other peripheral tissues (Rodríguez et al. 2007a; Carretero et al. 2009; Matsugo et al. 2000).
The increased oxidative/nitrosative stress of lung mitochondria in aged mice reflects a hyperoxidative environment that may negatively affect the activity of the mitochondrial respiratory chain. This hypothesis is further supported by the changes observed in GSH. The GSH redox cycle, the main mitochondrial defense against ROS and RNS, comprises the major reductive force for maintaining intracellular redox balance and regulating cellular defenses (Meister 1988). The GSH/GSSG ratio is indicative of the overall redox state (Jones 2002; Droge 2002), and its decrease in aged SAMP8 mice reflects an oxidizing tendency due to ROS generation, and a reduced biosynthetic activity (Garcia et al. 2010). In support of the GSH–respiratory chain connection, the age-dependent GSH reduction parallels a reduced activity of the respiratory complexes at 10 month of age in both genders of mice. Consequently, these mitochondria have a diminished capacity of ATP production. These aged mice manifested most of the characteristic changes in senile lung, including increments of the airspace size and shape constant obtained from the pressure–volume curve, although without alveolar wall destruction and, thus, absence of emphysema (Teramoto et al. 1994). However, SAM mice develop emphysema when they were exposed to tobacco smoke, supporting the idea that premature aging is not the direct cause of emphysema, but it enhances the susceptibility of the lung to extrinsic insults (Uejima et al. 1990). In this study, the hyperoxidative status and mitochondrial dysfunction in lungs from aged mice could probably account for this increased susceptibility of the elderly to lung diseases.
Melatonin is a potent free radical scavenger which possesses antioxidant and anti-inflammatory properties (Tan et al. 1993, 1998; 2001; 2007; Reiter et al. 2009; Crespo et al. 1999; Escames et al. 2006). When scavenging free radicals, melatonin becomes in a series of metabolites that are also free radical scavengers (Hardeland et al. 2009). Mitochondria are the main targets for these actions of melatonin (Acuña-Castroviejo et al. 2007, 2011; Jou et al. 2010; López et al. 2009; Paradies et al. 2010), and its ability to maintain mitochondrial homeostasis has been reported (Acuña-Castroviejo et al. 2001; 2011; Escames et al. 2007; López et al. 2006). Of note, melatonin—but not other antioxidants, such as vitamins C and E, and N-acetylcystein—was able to maintain mitochondrial GSH homeostasis in extremely oxidative conditions, increasing their capability of ATP production (Martín et al. 2000, 2002). In this context, it is not surprising that the antioxidant and anti-inflammatory potential of melatonin has been elsewhere reported in SAM mice (Rodríguez et al. 2007a, b). Here, the chronic administration of melatonin absolutely prevented the age-dependent dysfunction in lung mitochondria of senescent mice. These changes were accompanied by normalization of the GSH/GSSH ratio and increase in GPx and GRd activities, with the latter probably being responsible for restoring the GSH pool in the mitochondria. Besides the direct scavenger activity, these changes may reflect the well-known genomic effect of indoleamine: inducing GPx and GRd and reducing iNOS expression (Crespo et al. 1999; Escames et al. 2003; Antolin et al. 1996).
Females seem to be more protected against oxidative stress than males, an observation related, at least in part, to estrogens (Candore et al. 2010; Viña and Borrás 2010). At 5 months of age, no differences were found in LPO or NOx levels between male and female mice, but the former had higher LPO levels than females at 10 months of age. At the age of 5 months, GRd activity was lower and GSH/GSSG ratio higher in females than in males, and this difference was maintained at 10 months of age in the case of the GSH/GSSG ratio. Moreover, gender differences among the activity of the complexes II and III at 5 months of age and that of complexes I, II, and III at 10 months of age, were also detected. Thus, it seems that mitochondria from males are more dependent on complex I, whereas female mitochondria are more dependent on complex II. To our knowledge, variable stoichiometries of mitochondrial complexes have been observed in different tissues (Benard et al. 2006), but no data on both genders are as yet available. The substrate of complex I is NADH, which comes from glucose metabolism; the substrate of complex II mainly comes from fatty acid β-oxidation. Since estrogens are involved in the regulation of lipid metabolism (Campbell and Febbraio 2001; Wang et al. 2001), the gender differences in complex I, II and III activities may invoke the regulatory action of sex hormones. The effects of melatonin against age-dependent mitochondrial oxidative/nitrosative stress and GSH/GSSG ratio were similar in male and female mice. Moreover, melatonin restored the activity of the four mitochondrial complexes in all cases except for the complex III in females, increasing the ATP production, especially in females. Although estrogen may contribute to a better antioxidative protection in female mice, the reduction in melatonin production with age, which is observed in SAMP8 mice as well as in humans (Lardone et al. 2006; Waldhauser et al. 1998), may cause, at least in part, the mitochondrial dysfunction reported in this study.
Conclusions
The anti-aging effects of melatonin agree with previous data reported on different animal models of age and disease, suggesting that indoleamine is a universal antioxidant and anti-inflammatory molecule, targeting the mitochondria to improve their function in health and disease (López et al. 2009; Acuña-Castroviejo et al. 2011). Reduction in the apoptosis program and increased life span have been demonstrated in mice after melatonin therapy (Caballero et al. 2009; Rodríguez et al. 2007a, b, 2008).
Our results support the concept that SAMP8 mice exhibit a relatively high level of oxidative stress and respiratory chain impairments in lung mitochondria at the age of 10 months, in comparison with young mice. These results support the mitochondrial oxidative stress hypothesis of aging (Miquel 1998); however, several differences were detected between both genders, suggesting a role for estrogens in the lower mitochondrial impairment among females compared with males. Moreover, differences on mitochondrial complexes activity, ATP levels and ATP/ADP ratio in response to melatonin, were also detected. Because the beneficial effects of melatonin against age-dependent organ dysfunction have been observed, melatonin becomes an optimal candidate for anti-aging studies. Applied to lungs, this observation becomes extremely important. In fact, aging itself is a process involving multiple lung alterations and, among other considerations, changes in respiratory physiology associated with aging must be anticipated to minimize potential complications associated with surgery and anesthesia in the elderly (Sprung et al. 2006). Melatonin may be an excellent therapy against the hyperoxidative status of the aged lungs, preventing clinical complications in the elderly.
Acknowledgements
The authors thank A. Puertas for technical assistance. This study was partially supported by grants from the Instituto de Salud Carlos III (RD06/0013/0008, PI08-1664), and from the Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía (P07-CTS-03135 and CTS-101).
References
- Acuña-Castroviejo D, Martín M, Macías M, Escames G, León J, Khaldy H, Reiter RJ. Melatonin, mitochondria and cellular bioenergetics. J Pineal Res. 2001;30:65–74. doi: 10.1034/j.1600-079X.2001.300201.x. [DOI] [PubMed] [Google Scholar]
- Acuña-Castroviejo D, Escames G, Rodríguez MI, López LC. Melatonin role in the mitochondrial function. Front Biosci. 2007;12:947–963. doi: 10.2741/2116. [DOI] [PubMed] [Google Scholar]
- Acuña-Castroviejo D, López LC, Escames G, López A, García JA, Reiter RJ. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem. 2011;11:221–240. doi: 10.2174/156802611794863517. [DOI] [PubMed] [Google Scholar]
- Antolin I, Rodríguez C, Sainz RM, Mayo JC, Uría H, Kotler ML, Rodríguez-Colunga MJ, Tolivia D, Menéndez-Peláez A. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J. 1996;10:882–890. doi: 10.1096/fasebj.10.8.8666165. [DOI] [PubMed] [Google Scholar]
- Baleeiro CE, Wilcoxen SE, Morris SB, Standiford TJ, Paine R., 3rd Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol. 2003;171:955–963. doi: 10.4049/jimmunol.171.2.955. [DOI] [PubMed] [Google Scholar]
- Barrientos A. In vivo and in organelle assessment of OXPHOS activities. Methods. 2002;26:307–316. doi: 10.1016/S1046-2023(02)00036-1. [DOI] [PubMed] [Google Scholar]
- Benard G, Faustin B, Passerieux E, Galinier A, Rocher C, Bellance N, Delage J-P, Casteilla L, Letellier T, Rossignol R. Physiological diversity of mitochondrial oxidative phosphorylation. Am J Physiol Cell Physiol. 2006;291:C1172–C1182. doi: 10.1152/ajpcell.00195.2006. [DOI] [PubMed] [Google Scholar]
- Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001;1504:46–57. doi: 10.1016/S0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- Brown GC, Boroutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta. 2004;1658:44–49. doi: 10.1016/j.bbabio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic Biol Med. 2002;33:1440–1450. doi: 10.1016/S0891-5849(02)01112-7. [DOI] [PubMed] [Google Scholar]
- Brusque AM, Rosa RB, Schuck PF, Dalcin KB, Ribeiro CAJ, Silva CG, Wannmacher CMD, Dutra-Filho CS, Wyse ATS, Briones P, Wajner M. Inhibition of the mitochondrial respiratory chain complex activities in rat cerebral cortex by methylmalonic acid. Neurochem Int. 2002;40:593–601. doi: 10.1016/S0197-0186(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Caballero B, Vega-Naredo I, Sierra V, Huidobro-Fernández C, Soria-Valles C, Gonzalo-Calvo D, Tolivia D, Pallás M, Camins A, Rodríguez-Colunga MJ, Coto-Montes A. Melatonin alters cell death processes in response to age-related oxidative stress in the brain of senescence-accelerated mice. J Pineal Res. 2009;46:106–114. doi: 10.1111/j.1600-079X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- Campbell SE, Febbraio MA. Effect of ovarian hormones on mitochondrial enzyme activity in the fat oxidation pathway of skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281:E803–E808. doi: 10.1152/ajpendo.2001.281.4.E803. [DOI] [PubMed] [Google Scholar]
- Candore G, Balistreri CR, Colonna-Romano G, Lio D, Listì F, Vasto S, Caruso C. Gender-related immune-inflammatory factors, age-related diseases, and longevity. Rejuvenation Res. 2010;13:292–297. doi: 10.1089/rej.2009.0942. [DOI] [PubMed] [Google Scholar]
- Carretero M, Escames G, López LC, Venegas C, Dayoub JC, García LC, Acuña-Castroviejo D. Long-term melatonin administration protects brain mitochondria from aging. J Pineal Res. 2009;47:192–200. doi: 10.1111/j.1600-079X.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- Crespo E, Macías M, Pozo D, Escames G, Martín M, Vives F, Guerrero JM, Acuña-Castroviejo D. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J. 1999;13:1537–1546. [PubMed] [Google Scholar]
- Lourdes M, Seabra V, Bignotto M, Pinto LR, Tufik S. Randomised double blind clinical trial, controlled with placebo of the toxicology of chronic melatonin treatment. J Pineal Res. 2000;29:193–200. doi: 10.1034/j.1600-0633.2002.290401.x. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Elder AC, Gelein R, Finkelstein JN, Cox C, Oberdorster G. Pulmonary inflammatory response to inhaled ultrafine particles is modified by age, ozone exposure, and bacterial toxin. Inhal Toxicol. 2000;12:227–246. doi: 10.1080/089583700750019585. [DOI] [PubMed] [Google Scholar]
- Escames G, León J, Macías M, Khaldy H, Acuña-Castroviejo D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 2003;17:932–934. doi: 10.1096/fj.02-0692fje. [DOI] [PubMed] [Google Scholar]
- Escames G, López LC, Ortiz F, Ros E, Acuña-Castroviejo D. Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: effects of melatonin treatment. Exp Gerontol. 2006;41:1165–1173. doi: 10.1016/j.exger.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Escames G, López LC, Ortiz F, López A, García JA, Ros E, Acuña-Castroviejo D. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J. 2007;274:2135–2147. doi: 10.1111/j.1742-4658.2007.05755.x. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Cheeseman KH. Determination of aldehidic lipid peroxidation products: malonaldehide and 4-hydroxynonenal. Meth Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-H. [DOI] [PubMed] [Google Scholar]
- Garcia J, Han D, Sancheti H, Yap LP, Kaplowitz N, Cadenas E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J Biol Chem. 2010 doi: 10.1074/jbc.M110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez F, Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- Green LC, Ruiz de Luzuriaga K. Nitrate biosynthesis in man. Proc Natl Acad Sci USA. 1981;78:7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. doi: 10.1016/S0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Tan DX, Reiter RJ. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res. 2009;47:109–126. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- Hissin PJ, Hilf R. A fluorimetric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Hosokawa M. A higher oxidative status accelerates senescence and aggravates age dependent disorders in SAMP strains of mice. Mech Aging Dev. 2002;123:1553–1561. doi: 10.1016/S0047-6374(02)00091-X. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Meth Enzymol. 2002;348:93–112. doi: 10.1016/S0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Jones DP. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuv Res. 2006;9:169–181. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- Jou MJ, Peng TI, Hsu LF, Jou SB, Reiter RJ, Yang CM, Chiao CC, Lin YF, Chen CC. Visualization of melatonin's multiple mitochondrial levels of protection against mitochondrial Ca(2+)-mediated permeability transition and beyond in rat brain astrocytes. J Pineal Res. 2010;48:20–38. doi: 10.1111/j.1600-079X.2009.00721.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Lardone PJ, Alvarez-García O, Carrillo-Vico A, Vega-Naredo I, Caballero B, Guerrero JM, Coto-Montes A. Inverse correlation between endogenous melatonin levels and oxidative damage in some tissues of SAM P8 mice. J Pineal Res. 2006;40:153–157. doi: 10.1111/j.1600-079X.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Liu W, Porter NA, Schneider C, Brash AR, Yin H. Formation of 4-hydroxynonenal from cardiolipin oxidation: intramolecular peroxyl radical addition and decomposition. Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.10.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López LC, Escames G, Tapias V, Utrilla MP, León J, Acuña-Castroviejo D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice. Its relation with mitochondrial dysfunction and prevention by melatonin. Int J Biochem Cell Biol. 2006;38:267–278. doi: 10.1016/j.biocel.2005.09.008. [DOI] [PubMed] [Google Scholar]
- López A, García JA, Escames G, Venegas C, Ortiz F, López LC, Acuña-Castroviejo D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res. 2009;46:188–198. doi: 10.1111/j.1600-079X.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Martín M, Macías M, Escames G, León J, Acuña-Castroviejo D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000;14:1677–1679. doi: 10.1096/fj.99-0865fje. [DOI] [PubMed] [Google Scholar]
- Martín M, Macías M, León J, Escames G, Khaldy H, Acuña-Castroviejo D. Melatonin increases the activity of the complexes I and IV of the electron transport chain and the ATP production in rat brain and liver mitochondria. Int J Biochem Cell Biol. 2002;34:348–357. doi: 10.1016/S1357-2725(01)00138-8. [DOI] [PubMed] [Google Scholar]
- Matsugo S, Kitagawa T, Minami S, Esashi Y, Oomura Y, Tokumaru S, Kojo S, Matsushima K, Sasaki K. Age-dependent changes in lipid peroxide levels in peripheral organs, but not in brain, in senescence-accelerated mice. Neurosci Lett. 2000;278:105–108. doi: 10.1016/S0304-3940(99)00907-6. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- Meyer KC. Aging. Proc Am Thorac Soc. 2005;2:433–439. doi: 10.1513/pats.200508-081JS. [DOI] [PubMed] [Google Scholar]
- Mikerov AN, Gan X, Umstead TM, Miller L, Chinchilli VM, Phelps DS, Floros J. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir Res. 2008;9:24. doi: 10.1186/1465-9921-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel J. An update on the oxygen stress-mitochondrial mutation theory of aging: genetic and evolutionary implications. Exp Gerontol. 1998;33:113–126. doi: 10.1016/S0531-5565(97)00060-0. [DOI] [PubMed] [Google Scholar]
- Mora AL, Rojas M. Aging and lung injury repair: a role for bone marrow derived mesenchymal stem cells. J Cell Biochem. 2008;105:641–647. doi: 10.1002/jcb.21890. [DOI] [PubMed] [Google Scholar]
- Nathan C, Xie QW. Nitric oxide synthases: roles, trolls and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res. 2010;48:297–310. doi: 10.1111/j.1600-079X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- Pendyala S, Natarajan V. Redox regulation of NOx proteins. Respir Physiol Neurobiol. 2010 doi: 10.1016/j.resp.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissarek M, Reinhhardt R, Reichelt C, Manaenko A, Krauss GJ, Illes P. Rapid assay for one-run determination of purine and pyrimidine mucleotide contents in neocortical slices and cell cultures. Brain Res Protoc. 1999;4:314–321. doi: 10.1016/S1385-299X(99)00035-5. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic Biol Med. 2003;35:626–635. doi: 10.1016/S0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Paredes SD, Manchester LC, Tan DX. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol. 2009;44:175–200. doi: 10.1080/10409230903044914. [DOI] [PubMed] [Google Scholar]
- Riahi Y, Cohen G, Shamni O, Sasson S. Signaling and cytotoxic functions of 4-Hydroxyalkenals. Am J Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00508.2010. [DOI] [PubMed] [Google Scholar]
- Rodríguez MI, Escames G, López LC, García JA, Ortiz F, López A, Acuña-Castroviejo D. Melatonin administration prevents cardiac and diaphragmatic mitochondrial oxidative damage in senescence-accelerated mice. J Endocrinol. 2007a;194:637–643. doi: 10.1677/JOE-07-0260. [DOI] [PubMed] [Google Scholar]
- Rodríguez I, Escames G, Lopez LC, Lopez A, Garcia JA, Ortiz F, Sanchez V, Romeu M, Acuña-Castroviejo D. Improved mitocondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exptl Gerontol. 2008;43:479–756. doi: 10.1016/j.exger.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Rodríguez MI, Escames G, López LC, López A, García JA, Ortiz F, Acuña-Castroviejo D. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J Pineal Res. 2007b;42:272–279. doi: 10.1111/j.1600-079X.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1:253–260. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–586. doi: 10.1016/S0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Sprung J, Gajic O, Warner DO. Review article: age related alterations in respiratory function - anesthetic considerations. Can J Anaesth. 2006;53:1244–1257. doi: 10.1007/BF03021586. [DOI] [PubMed] [Google Scholar]
- Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite and carbon dioxide. Free Radic Biol Med. 1998;25:392–403. doi: 10.1016/S0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Takeda T. Senescence-accelerated mouse (SAM): a biogerontological resource in aging research. Neurobiol Aging. 1999;20:105–110. doi: 10.1016/S0197-4580(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J 1:57–60
- Tan DX, Manchester LC, Reiter RJ, Plummer BF, Hardies LJ, Vijayalaxmi WST, Shepherd AM. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: a biomarker of in vivo hydroxyl radical generation. Biochem Biophys Res Commun. 1998;253:614–620. doi: 10.1006/bbrc.1998.9826. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Burkhardt S, Sainz RM, Mayo JC, Kohen R, Shohami E, Huo YS, Hardeland R, Reiter RJ. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001;15:2294–2296. doi: 10.1096/fj.01-0309fje. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivates: a never-ending interacting of melatonin with reactive and oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Fukuchi Y, Uejima Y, Teramoto K, Oka T, Orimo H. A novel model of senile lung: senescence-accelerated mouse (SAM) Am J Respir Crit Care Med. 1994;150:238–244. doi: 10.1164/ajrccm.150.1.8025756. [DOI] [PubMed] [Google Scholar]
- Uejima Y, Fukuchi Y, Nagase T, Matsuse T, Yamaoka M, Tabata R, Orimo H. Influence of inhaled tobacco smoke on the senescence accelerated mouse (SAM) Eur Respir J. 1990;3:1029–1036. [PubMed] [Google Scholar]
- Umstead TM, Freeman WM, Chinchilli VM, Phelps DS. Age-related changes in the expression and oxidation of bronchoalveolar lavage proteins in the rat. Am J Physiol Lung Cell Mol Physiol. 2009;296:L14–L29. doi: 10.1152/ajplung.90366.2008. [DOI] [PubMed] [Google Scholar]
- Viña J, Borrás C. Women live longer than men: understanding molecular mechanisms offers opportunities to intervene by using estrogenic compounds. Antioxid Redox Signal. 2010;13:269–278. doi: 10.1089/ars.2009.2952. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Kovács J, Reiter E. Age-related changes in melatonin levels in humans and its potential consequences for sleep disorders. Exp Gerontol. 1998;33:759–772. doi: 10.1016/S0531-5565(98)00054-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Green PS, Simpkins JW. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J Neurochem. 2001;77:804–811. doi: 10.1046/j.1471-4159.2001.00271.x. [DOI] [PubMed] [Google Scholar]