Abstract
Introduction
Neuropathic pain attenuates opioid facilitation of rewarding electrical stimulation of limbic dopaminergic pathways originating from the ventral tegmental area. Whether neuropathic pain alters opioid effects of other brain-reward systems is unknown.
Methods
Control and spinal nerve ligated (SNL) rats were implanted with electrodes into the paraventricular nucleus of the hypothalamus (PVN) or medial forebrain bundle. Control and SNL rats were trained to lever press for electrical stimulation (ICSS) and modulation by morphine or cocaine was assessed.
Results
Control and SNL rats lever pressed for stimulation of the PVN and medial forebrain bundle. Morphine produced greater reductions in the frequency at which rats emitted 50% of maximal responding for PVN ICSS (maximal effect 24.67 ± 4.60 (mean +/− SEM) and 24.11 ± 5.96 in SNL (n = 6) and control (n = 8) rats, respectively, compared to medial forebrain bundle ICSS (12.38 ± 6.77 (n = 8) and 12.69 ± 1.55 (n = 7)). In contrast, cocaine was less efficacious in potentiating PVN ICSS (maximal effect 11.76 ± 2.86 and 12.38 ± 4.01 in SNL (n = 12) and control (n = 8) rats, respectively) compared to medial forebrain bundle ICSS (30.58 ± 3.40 (n = 9) and 27.55 ± 4.51 (n = 7)).
Conclusions
PVN ICSS is facilitated to a greater extent by morphine than cocaine, and the effects of each drug on this behavior are unaltered following SNL. These effects contrast those observed with direct stimulation of limbic dopamine pathways, suggesting that the PVN may have a greater role in the reinforcing effects of opioids than classical limbic regions, particularly in the presence of chronic pain.
Introduction
Intracranial self-stimulation (ICSS) is an operant paradigm pairing lever presses with electrical stimulation of discrete brain pathways.1 Rats will lever press for stimulation of brain regions within the mesolimbic dopamine system, including the ventral tegmental area (VTA) and medial forebrain bundle (MFB).2 Drugs of abuse such as opioids and cocaine, whose reinforcing effects are mediated through this circuitry, potentiate the reinforcing effects of VTA and MFB ICSS in rats.2 Although neuropathic pain does not alter VTA ICSS, the ability of morphine, heroin, and commonly abused prescription opioids are less effective in facilitating VTA ICSS in spinal nerve ligated (SNL) rats compared to control animals.3,4 It is not known if this effect of SNL is limited to facilitation of ICSS in the VTA.
The hypothalamic paraventricular nucleus (PVN) may play an important role in the reinforcing effects of drugs of abuse through oxytocin release in the limbic system.5 Oxytocin release is stimulated in response to various environmental stimuli and is involved in a host of behavioral effects including maternal and social bonding, anxiety, and pain among others.6 A lesser understood effect of oxytocin is its ability to modulate behavioral effects of drugs of abuse.7 These effects likely arise from release of oxytocin from the PVN into limbic and forebrain regions, where oxytocin may interact with dopamine neurotransmission to modulate aspects of drug addiction.5 For instance, PVN oxytocin fibers project to the VTA,8 which expresses oxytocin receptor messenger RNA,9 and intra-VTA oxytocin stimulates dopamine release in the nucleus accumbens that is reversed by pretreatment with an intra-VTA oxytocin receptor antagonist.8 Intracerebroventricular administration of oxytocin has been shown to inhibit analgesic tolerance to heroin10 and morphine.11 In addition, intravenous self-administration of heroin is reduced following intraaccumbens or intrahippocampal oxytocin administration in rats,12 though it is unclear if this reflects changes in tolerance or in the rewarding properties of heroin. Oxytocin administered intracerebroventricularly has also been shown to inhibit some of the acute behavioral effects of cocaine, including stereotypy.13 It is therefore clear that oxytocin has the potential to modulate effects of drugs of abuse, though it is unclear to what extent the reverse is true.
It is hypothesized that electrical stimulation of the PVN will produce reinforcing effects that may be mediated to some extent through indirect stimulation of mesolimbic dopamine via oxytocin release. It is therefore expected that drugs of abuse, which stimulate mesolimbic dopaminergic neurons, would subsequently enhance the reinforcing effects of PVN ICSS. The current study sought to address this by assessing the effects of morphine and cocaine on the reinforcing effects of PVN ICSS. Given the close proximity of the PVN to the MFB, the modulatory effects of morphine and cocaine on the reinforcing effects of MFB ICSS were also assessed. It is also unclear to what extent nerve injury, which diminishes the potentiating effects of opioids for VTA ICSS,3,4 will alter the ability of morphine and cocaine to facilitate PVN or MFB ICSS. Therefore, studies were performed in both normal and nerve-injured rats.
Materials and Methods
Subjects
Subjects were 39 male, Fisher 344 rats (12 SNL and 11 control rats for PVN experiments; 9 SNL and 7 control rats for MFB experiments) weighing between 275–325 g at the beginning of the experiment (Harlan Laboratories, Raleigh, NC). Rats were group-housed in a temperature and humidity controlled room that was maintained on a reversed light-dark cycle (dark 05:00–17:00); this room was adjacent to the room in which behavioral experiments were performed. Food and water were continuously available except during behavioral experiments. All procedures were conducted according to guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain and were approved by the Animal Care and Use Committee of Wake Forest University School of Medicine, Winston-Salem, North Carolina.
Surgeries
Electrode Implantation
Rats were permanently implanted with electrodes as previously described.3 Briefly, rats were anesthetized with pentobarbital (50 mg/kg, intraperitoneal) and atropine methyl nitrate (10mg/kg, intraperitoneal) and received penicillin G procaine (75,000 U, intramuscular) to prevent infection. Rats were secured in a stereotaxic frame and platinum bipolar stimulating electrodes (Plastics One, Roanoke, VA) were aimed at the left PVN or left MFB (2.5mm and 3.0mm posterior to bregma, 0.3mm and 1.7mm lateral from the midline, and 8.0mm and 8.5mm below the skull, for PVN and MFB, respectively), which were secured by three stainless steel screws embedded in dental acrylic on the skull surface.
Spinal Nerve Ligation
After electrode implantation 21 of the 39 rats were subjected to SNL as previously described.14 Briefly, an incision was made through the skin and muscle of the lower back, and the left transverse process of the fifth lumbar vertebra was removed using bone microrongeurs. The fifth lumbar nerve was exteriorized and ligated with 4.0 silk suture. The sixth lumbar nerve was exteriorized from below the iliac bone at the sciatic notch and similarly ligated. Afterwards, muscle layers were sutured with 4.0 chromic gut, the skin with 4.0 nylon suture, and exterior wounds dressed with antibiotic powder (Polysporin; Pfizer Healthcare, Morris Plains, NJ).
Paw Withdrawal Threshold
Mechanical allodynia was assessed using von Frey filaments (Touch Test Sensory Evaluators; Stoelting, Wood Dale, IL)15 for all animals using Dixon nonparametric statistics.16 Paw Withdrawal Threshold (PWT’s) were assessed 14 days after surgery to verify development of allodynia (PWT<4.0 g). Experimenter-delivered electrical stimulation (30 0.5 s stimulations per minute) of the PVN or MFB was delivered using similar parameters to those during self-stimulation sessions. Specific testing procedures were used to prevent motor abnormalities during testing. Baseline PWT’s were determined prior to stimulation. With the frequency set to 50 Hz, the current was gradually increased (10uA increments) up to that used during self-stimulation sessions (unique to each rat; some rats exhibited motor effects and lower intensities than those used during self-stimulation were used). PWT’s were assessed to various frequencies in ascending order (50, 79, 112, 158 Hz for the PVN; 50, 78, 118, 156 Hz for the MFB). The frequency of stimulation was gradually increased (10 Hz increments until the next scheduled test frequency) after each PWT assessment. Testing lasted between 8–12 min per rat.
Drugs
Morphine sulfate was purchased as a 15 mg/ml sterile solution (Baxter Healthcare; Deerfield, IL). Cocaine hydrochloride was obtained from the Drug Supply Program of the National Institute on Drug Abuse (Rockville, MD), dissolved in 0.9% (wt/vol) saline, and sterilized by filtration through a 0.22 µm nitrocellulose filter. All drugs were diluted using 0.9% (wt/vol) saline, pH 7.4.
Intracranial Self-Stimulation
Apparatus
Operant chambers housed within sound- and light-attenuating enclosures equipped with a houselight and ventilation fan were used (Med Associates Inc., St. Albans, VT). These chambers have a lever 5 cm above a grid bar floor, stimulus lamp 2 cm above the lever, and a tone generator. An ICSS stimulator controlled by computer software (Med Associates Inc.) that controlled all stimulation parameters and data collection was located outside of the enclosure. A 2-channel swivel commutator (Model SLC2C, Plastics One) positioned above the operant chamber connected the electrodes to the ICSS stimulator via 25cm cables (Plastics One).
Behavioral Procedure
Following at least 14 days recovery from surgery, rats were trained to lever press for electrical stimulation of the PVN or MFB as previously described.3 Illumination of the stimulus light indicated stimulus availability and each lever press generated a 0.5-s train of rectangular alternating cathodal and anodal pulses (0.1-ms pulse durations). During stimulation the stimulus light shut off, the houselight turned on, and a tone sounded. Responding during stimulation resulted in no further stimulation and was not recorded.
During training sessions the frequency was held constant (150 Hz) and the intensity adjusted to maintain consistent responding. Following initial training, frequency response curves were generated. For PVN ICSS, 120-min sessions consisted of five 18-min components. Each component consisted of eight 135-s trials. Each trial began with a 5-s timeout followed by a 10-s priming period in which rats received five noncontingent stimulations, and concluded with a 120-s response period. Current intensity remained constant (unique to each animal) and 8 frequencies (158-71 Hz, 0.05 log increments; PVN) corresponding to each trial were made available in descending order. A 30-min timeout between the third and fourth components permitted drug injections. MFB ICSS was similarly performed with the following differences: 90-min sessions consisted of six 10-min components, which consisted of ten 60-s trials. Each trial began with a 5-s timeout, followed by a 5-s priming period, and concluded with a 50-s response period; 10 frequencies (156-45 Hz, 0.06 log increments) corresponded to each trial. At the beginning of the timeout rats received 1 mL/kg intraperitoneal injections of saline (0.9% wt/vol), morphine (0.3 – 6mg/kg), or cocaine (5 – 10 mg/kg). For data analysis, the two components preceding drug injection and the two (PVN) or three (MFB) components following drug injection were averaged and compared using Prism software (sigmoidal-dose response, variable slope; Graph Pad, La Jolla, CA). Test sessions were separated by at least 1 day. Test sessions were performed 1–4 months after initial surgery.
Histology
Rats were sacrificed by carbon dioxide asphyxiation. Brains were rapidly removed, frozen in isopentane (−35°C), and stored at −80°C. Coronal sections (25 µm) around the electrode tract were obtained using a cryostat to confirm electrode placement within the PVN and MFB (fig. 1).
Figure 1. Location of the stimulating electrodes within the paraventricular nucleus of the hypothalamus and medial forebrain bundle for control and spinal nerve-ligated (SNL) rats.
Numbers left of each brain section indicate distance posterior to bregma, according to the atlas of Paxinos and Watson (1998).21
Data Analysis
The EF50 (frequency at which rats emitted 50% of maximal responding) and maximum response rate for PVN or MFB ICSS was calculated using Prism software (sigmoidal-dose response, variable slope; Graph Pad). The effect of drug treatment on PVN and MFB ICSS was analyzed using a two-way ANOVA with drug dose and experimental group (PVN control, PVN SNL, MFB control, MFB SNL) serving as the independent variables and ΔEF50 (EF50 prior to injection – EF50 after injection) or maximal response rates serving as the dependent measures. Post-hoc analyses were made using Tukey’s HSD t-test for multiple pairwise comparisons. A two-tailed p-value of 0.05 or less was considered statistically significant. All statistical analyses were performed using JMP software (version 5.0.1a, SAS Institute Inc., Cary, NC).
Results
Effects of SNL on ICSS of the PVN or MFB
The intensities used for ICSS were not significantly different between SNL and control groups for either the PVN [F(1,22) = 0.2, p = 0.6] or the MFB [F(1,15) = 0.05, p = 0.8]. The maximum response rates also did not differ between control and SNL rats for ICSS in the PVN [F(1,22) = 0.2, p = 0.6] or in the MFB [F(1,15) = 3.7, p = 0.07] (fig. 2). The EF50 values did not differ between SNL and control rats for ICSS in the MFB [F(1,15) = 0.2, p = 0.7], however the EF50 value for ICSS in the PVN was slightly but significantly greater in SNL rats (114.7 ± 1.8) compared to control subjects (109.1 ± 1.8) [F(1,22) = 4.6, p = 0.04].
Figure 2. Baseline responding for electrical stimulation of the paraventricular nucleus of the hypothalamus (PVN) and medial forebrain bundle (MFB) for control and spinal nerve-ligated (SNL) rats.
Frequency-response curves for baseline responding were generated by averaging the second and third components preceding saline administration (prior to any drug treatments). The y-axis indicates the number of self-stimulations (0.5-sec) per 60 seconds for each frequency (x-axis). Data shown are averages across control (n=11, n=7) and SNL (n=12, n=9) rats with PVN and MFB electrodes, respectively. Average current intensities during baseline responding were 246.82 (17.51) uA and 215.71 (26.87) uA for control and 234.58 (19.59) uA and 208.33 (18.86) uA for SNL rats with PVN and MFB electrodes, respectively.
Comparison of ICSS parameters in the PVN and MFB
The intensities used for ICSS did not differ (SNL and control rats combined) between the PVN and MFB [F(1,38) = 2.1, p = 0.16]. The maximum response rate maintained by ICSS in the PVN (23.8 ± 1.1 resp/min) was approximately half that maintained in the MFB (45.1 ± 1.3 resp/min) [F(1,38) = 160, p < 0.0001] (fig. 2). The EF50 was also significantly greater for ICSS in the PVN (112.0 ± 1.6) compared to the MFB (96.0 ± 2.0) [F(1,38) = 39, p < 0.0001].
Effects of morphine on PVN and MGB ICSS in control and SNL rats
There was a significant main effect of morphine dose [F(4,160) = 23.4. p < 0.00001] and group [F(3,160) = 5.6, p = 0.0012] on the effects of EF50 for ICSS in the PVN and MFB in SNL and control rats (fig. 3). Post-hoc analysis found that the effect of morphine differed between the MFB SNL group and the other three groups. Morphine produced significant leftward shifts in the frequency-rate curve for PVN ICSS, decreasing the EF50 at doses of 1, 3, and 6 mg/kg compared to saline in both control and SNL rats (p ≤ 0.05). Morphine also produced a leftward shift in the frequency-rate curve for the MFB control group, decreasing the EF50 at doses of 3 and 6 mg/kg. Morphine did not alter the EF50 at any dose in the MFB SNL group however (p ≤ 0.05).
Figure 3. Effects of morphine on responding for electrical stimulation of the paraventricular nucleus of the hypothalamus (PVN) and medial forebrain bundle (MFB) for control and spinal nerve-ligated (SNL) rats.
(A) Reduction in EF50 (frequency at which rats emitted 50% of maximal responding) was calculated by subtracting the EF50 for the two (PVN) and three (MFB) components following drug injection from the EF50 for the two components preceding drug injection. Data shown are averages across control (n=8, except n=11 for 3mg/kg) and SNL (n=7, except n=8 and n=6 for 3 and 6mg/kg, respectively) rats with PVN electrodes, as well as control (n=7), and SNL (n=8) rats with MFB electrodes. Frequency-response curves before and after 6mg/kg morphine (30-min) are shown for SNL rats with PVN (B, n=6) and MFB (C, n=8) electrodes. * Significantly different from saline treatment P ≤ 0.05.
The effect of morphine on the Rmax was also dependent on the dose [F(4,160) = 8.1, p < 0.0001] and group [F(3,160) = 11.1, p < 0.0001], with 3 mg/kg decreasing the Rmax in both the PVN control and PVN ICSS groups (p ≤ 0.05) and no other significant effects being found.
Effects of cocaine on PVN and MFB ICSS in control and SNL rats
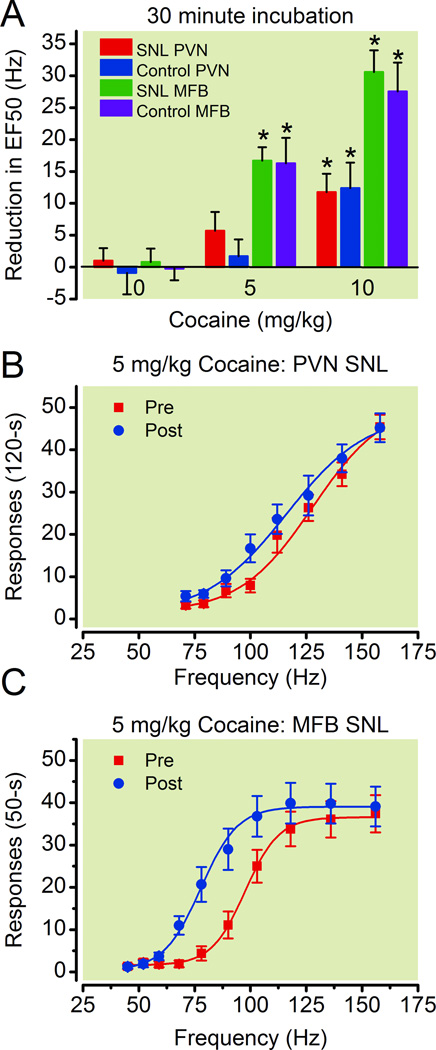
Cocaine produced significant effects on both PVN and MFB ICSS in control and SNL rats. The effect of cocaine on the EF50 values for ICSS was dependent upon both dose [F(2,105) = 46.2, p < 0.0001] and group [F(3,105) = 11.3, p < 0.0001], with a significant dose by group interaction [F(6,105) = 3.5, p = 0.004]. Only the highest dose tested of 10 mg/kg cocaine produced a significant effect on PVN ICSS compared to saline and did so in both the control and SNL groups (p ≤ 0.05). However doses of 5 and 10 mg/kg significantly shifted the frequency-rate curves to the left, thereby reducing the EF50’s for MFB ICSS in both the control and SNL groups compared to saline treatment (p ≤ 0.05) (fig. 4). The effect of cocaine on the EF50’s for ICSS was significantly different between the PVN and MFB groups, but not between the control and SNL groups within each brain region (p ≤ 0.05).
Figure 4. Effects of cocaine on responding for electrical stimulation of the paraventricular nucleus of the hypothalamus (PVN) and medial forebrain bundle (MFB) for control and spinal nerve-ligated (SNL) rats.
(A) Reduction in EF50 (frequency at which rats emitted 50% of maximal responding) was calculated by subtracting the EF50 for the two (PVN) and three (MFB) components following drug injection from the EF50 for the two components preceding drug injection. Data shown are averages across control (n=8 and n=7 for 5 and 10mg/kg, respectively) and SNL (n=9 and n=12 for 5 and 10mg/kg, respectively) rats with PVN electrodes, as well as control (n=7), and SNL (n=8 and n=9 for 5 and 10mg/kg, respectively) rats with MFB electrodes. Frequency-response curves before and after 5mg/kg morphine (30-min) are shown for SNL rats with PVN (B, n=9) and MFB (C, n=8) electrodes. * Significantly different from saline treatment P ≤ 0.05.
Cocaine increased the Rmax values for ICSS at both doses of 5 and 10 mg/kg [F(2,105) = 15.4, p < 0.0001] and there was not a significant effect of ICSS group on this effect [F(3,105) = 1.6, p = 0.2].
Effects of electrical stimulation of the PVN and MFB on PWT in SNL rats
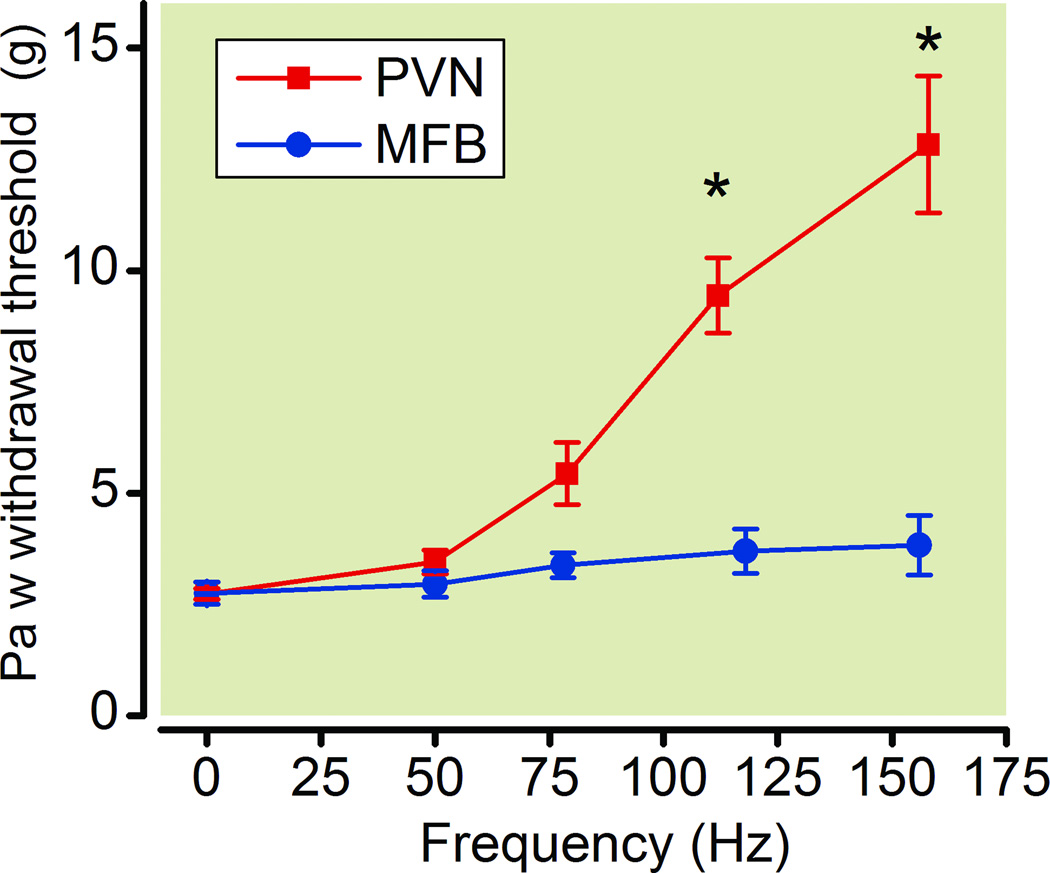
Electrical stimulation of the PVN produced a frequency dependent antiallodynic effect [F(4,39) = 24.9, p < 0.0001], with frequencies of 112 and 158 Hz increasing PWT compared to baseline values (2.7 ± 0.1g) (fig. 5). In contrast to the PVN, electrical stimulation of the MFB failed to produce a significant effect on PWT over a range of 50 – 156 Hz [F(4,39)−1.2, p = 0.3], with PWT not being significantly different from baseline values (2.8 ± 0.3 g) at any frequency (intensity = 189.4 ± 12.1 µA) (fig. 5).
Figure 5. Effects of electrical stimulation of the paraventricular nucleus of the hypothalamus (PVN) and medial forebrain bundle (MFB) on paw withdrawal thresholds (PWT) in spinal nerve-ligated rats (SNL).
Frequency-effect curves for PVN (n=8) and MFB (n=8) stimulation on PWT were determined in SNL rats. * Significantly different from baseline.
Discussion
The current study sought to determine if the reinforcing effects of electrical stimulation of the PVN are modulated by drugs of abuse in a manner previously reported for the VTA, and if the presence of neuropathic pain altered these effects. 3,4 Pharmacology studies revealed that morphine and cocaine potentiated the reinforcing effects of PVN and MFB ICSS, though important differences in drug efficacy for each brain region were observed. Morphine was more efficacious in potentiating the reinforcing effects of PVN compared to MFB ICSS. In contrast, cocaine had decreased potency and efficacy in potentiating PVN compared to MFB ICSS. Studies in SNL rats revealed that nerve injury diminished morphine’s ability to facilitate MFB ICSS, similar to previous results with VTA ICSS,3 but did not diminish morphine’s ability to facilitate PVN ICSS. Cocaine’s effects were unchanged by SNL for either PVN or MFB ICSS, similar to previous findings with VTA ICSS.3 These studies suggest that the reinforcing effects of PVN ICSS may be modulated to some extent through indirect stimulation of mesolimbic dopamine pathways however it is likely that other systems are involved as well, since PVN ICSS is influenced to a greater extent by opioids compared to cocaine while the reverse is true with MFB or VTA ICSS.3
It is hypothesized that PVN stimulation is reinforcing to some extent through indirect stimulation of mesolimbic dopamine neurons of the VTA, a pathway implicated in the reinforcing aspects of rewarding stimuli.17 PVN oxytocin fibers project directly to the VTA and terminate in close proximity to VTA dopamine cell bodies,8 providing a neuroanatomical basis for oxytocin interactions with mesolimbic dopamine circuitry. Therefore, PVN stimulation is expected to induce oxytocin release in the VTA. Exogenous administration of oxytocin in the VTA induces dopamine release in the nucleus accumbens,8 and thus PVN stimulation-induced oxytocin release in this region would similarly be expected to stimulate dopamine neurotransmission. Under these conditions, it would be expected that drugs which stimulate this circuitry would facilitate PVN ICSS, and the fact that morphine and cocaine facilitated PVN ICSS is in agreement for a role of mesolimbic dopamine mediating the reinforcing effects of PVN ICSS.
Opioids and dopamine agonists also potentiate the effects of MFB ICSS.2 Given the close proximity of the PVN to MFB, one possibility is that unintended stimulation of the MFB is responsible for the reinforcing effects of PVN ICSS, and moreover may explain why morphine and cocaine facilitated PVN ICSS. The potency and efficacy of cocaine was diminished in rats responding for PVN compared to MFB ICSS in both control and SNL rats. If PVN ICSS were mediated by unintended stimulation of the MFB, then these results would indicate that weak stimulation of the MFB from a distal electrode diminishes the potency and efficacy of a drug to facilitate ICSS. However, such an interpretation is unlikely since in the same rats morphine produced greater facilitation of PVN compared to MFB ICSS. One of the most striking of the differences observed between PVN and MFB ICSS was the relative inability of cocaine to facilitate PVN ICSS compared to the MFB. For MFB ICSS the highest dose of cocaine assessed produced roughly a 2.5 fold greater facilitation compared to morphine, similar to previous findings using VTA ICSS,3 and consistent with microdialysis data indicating that cocaine stimulates dopamine release in the nucleus accumbens to a greater extent than morphine.18 In contrast, this same dose of cocaine was only half as efficacious as morphine in facilitating PVN ICSS in control and SNL rats. This suggests a greater role for opioid modulation of PVN ICSS compared to MFB ICSS, and further supports the notion that the neuronal mechanisms that support PVN ICSS are fundamentally different than those involved in MFB ICSS.
In the current study all manipulations on ICSS were performed in both control and SNL rats. The only effect of nerve injury on altering drug modulation of PVN and MFB ICSS was to decrease the potency of morphine for potentiation of MFB ICSS. This finding is similar to previous work using VTA ICSS,3 and may relate to impairment of mu opioid receptor function within the VTA.19 The working hypothesis is that opioids are less effective in stimulating the mesolimbic dopaminergic system in the presence of neuropathic pain,19 and therefore are less effective in facilitating either VTA or MFB ICSS in nerve-injured rats. The fact that morphine was equally effective at facilitating PVN ICSS in SNL and control rats is yet another key difference between PVN and MFB ICSS, and indicates that PVN ICSS may not solely be mediated by indirect stimulation of mesolimbic dopaminergic systems, since one would expect morphine to be less efficacious in SNL rats compared to control subjects if this was true. It is possible that morphine facilitates PVN ICSS by stimulating oxytocin release and/or interacting with oxytocin neurotransmission throughout the limbic system, as well as through its own direct actions in stimulating dopamine neurotransmission.20 Such additive effects could also explain why morphine produces greater facilitation of PVN compared to MFB ICSS, and may mask the suppressive effects of SNL on opioid activity within the VTA. Similarly, it is possible that the reduced efficacy of cocaine compared to morphine in potentiating PVN ICSS may be due to cocaine exerting direct effects on limbic dopamine neurotransmission, but having little impact on facilitating oxytocin release and/or interacting at other sites that contribute to the reinforcing effects of PVN ICSS. To this end, increased efficacy of morphine compared to cocaine in facilitating PVN ICSS suggests that opioid interactions with the PVN may play a prominent and relatively unknown role in the reinforcing and abuse related effects of opioids, during both pain and nonpain states.
A major limitation of the ICSS methodology is the lack of selectivity of electrical stimulation, particularly when studying brain regions in close proximity to each other. To combat this, pharmacological studies were performed in groups with electrodes placed in the PVN and MFB, in order to differentiate the modulatory effects of morphine and cocaine. Since morphine and cocaine facilitate PVN and MFB ICSS, albeit to different degrees, it is impossible to rule out the existence of unintended stimulation reaching the opposing brain region. It should be noted, however, that the differential effects of morphine and cocaine in facilitating PVN and MFB ICSS were obtained in both control and SNL rats; this increases confidence in these findings since in all cases critical pharmacological differences were found in two groups of animals for each brain region.
Another limitation of the current work pertains to regional selectivity of the drugs studied. Since morphine and cocaine were given systemically, it is unclear to what degree drug effects are attributable to specific brain regions. This is particularly important when trying to understand the mechanism by which morphine facilitates PVN ICSS, since under the current thinking PVN ICSS could be modulated at the level of the PVN, VTA, or in other limbic and frontal brain regions that contain opioid receptors. Future studies addressing these questions would benefit from the use of site specific intracranial injections of opioid and oxytocin agonists and antagonists.
It was interesting that PVN but not MFB stimulation was effective in reversing mechanical allodynia in SNL rats. This indicates not only that stimulation of limbic dopamine does not alter spinally-mediated hypersensitivity, but that stimulation of the MFB is unlikely causing stimulation of the PVN; it is unclear if the reverse is true, however. It is worth noting that compared to previous findings,7,8 higher frequencies of PVN stimulation were necessary to reverse hypersensitivity following nerve injury. Initial attempts to provide short, constant stimulation followed by assessment of allodynia proved difficult to do in freely moving rats. Therefore, in the current study PVN stimulation was delivered for minutes, and each 0.5-s train of stimulation was followed by a 1.5-s period of no stimulation. This was done because it allowed animals to habituate to the stimulation, reduced motor effects, allowed testing at higher frequencies, and modeled rates of self-stimulation during ICSS sessions. It is quite possible that the repeated use of on and off stimulation may have led to the higher frequency requirements in reversing hypersensitivity. Nonetheless, this protocol was reliable in reversing hypersensitivity during PVN stimulation, and reflects yet another difference between stimulation of the PVN and MFB in the current work.
In conclusion, electrical stimulation of the PVN produces reinforcing effects in control and SNL rats. It is hypothesized that oxytocin release into limbic regions involved in reward (e.g., VTA) likely mediates to some degree the reinforcing effects of PVN ICSS. Morphine was more effective than cocaine in potentiating PVN ICSS, while the reverse is true in the MFB. The reinforcing effects of PVN ICSS are influenced by opioids to a greater extent than cocaine and these effects are unaltered by SNL, suggesting that the PVN may be a brain region intricately involved in the reinforcing effects and abuse liability of opioids under normal as well as chronic pain states.
Summary Statement.
What we already know about this topic
Drugs that produce addiction, facilitate self-stimulation from various brain regions
Facilitation of brain self-stimulation by opioids is attenuated in a chronic neuropathic pain model
What this article tells us that is new
Stimulation of the paraventricular nucleus was enhanced more by morphine than by cocaine and morphine remained enhanced in a chronic neuropathic pain model
Understanding the role of the paraventricular nucleus and the reinforcement of opioids in chronic could improve our understanding of addiction in chronic pain
Acknowledgements
Supported by grants DA-022599 (TJM) and T32-DA-007246 (EEE) from the National Institute on Drug Abuse of the National Institutes of Health, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 2.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 3.Ewan EE, Martin TJ. Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain. Anesthesiology. 2011;114:624–632. doi: 10.1097/ALN.0b013e31820a4edb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewan EE, Martin TJ. Rewarding electrical brain stimulation in rats after peripheral nerve injury: Decreased facilitation by commonly abused prescription opioids. Anesthesiology. 2011;115:1271–1280. doi: 10.1097/ALN.0b013e3182330448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16:e92–e123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, Ueta Y, Zingg HH, Chvatal A, Sykova E, Dayanithi G. REVIEW: Oxytocin: Crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther. 2010;16:e138–e156. doi: 10.1111/j.1755-5949.2010.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarnyai Z. Oxytocin as a potential mediator and modulator of drug addiction. Addict Biol. 16:199–201. doi: 10.1111/j.1369-1600.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- 8.Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri GL, Argiolas A. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci. 2007;26:1026–1035. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- 9.Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139:5015–5033. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs GL, Borthaiser Z, Telegdy G. Oxytocin reduces intravenous heroin self-administration in heroin-tolerant rats. Life Sci. 1985;37:17–26. doi: 10.1016/0024-3205(85)90620-4. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs GL, Sarnyai Z, Izbeki F, Szabo G, Telegdy G, Barth T, Jost K, Brtnik F. Effects of oxytocin-related peptides on acute morphine tolerance: Opposite actions by oxytocin and its receptor antagonists. J Pharmacol Exp Ther. 1987;241:569–574. [PubMed] [Google Scholar]
- 12.Ibragimov R, Kovacs GL, Szabo G, Telegdy G. Microinjection of oxytocin into limbic-mesolimbic brain structures disrupts heroin self-administration behavior: A receptor-mediated event? Life Sci. 1987;41:1265–1271. doi: 10.1016/0024-3205(87)90205-0. [DOI] [PubMed] [Google Scholar]
- 13.Sarnyai Z, Babarczy E, Krivan M, Szabo G, Kovacs GL, Barth T, Telegdy G. Selective attenuation of cocaine-induced stereotyped behaviour by oxytocin: Putative role of basal forebrain target sites. Neuropeptides. 1991;19:51–56. doi: 10.1016/0143-4179(91)90073-r. [DOI] [PubMed] [Google Scholar]
- 14.Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: Relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007;106:312–322. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Nichols ML, Bian D, Ossipov MH, Lai J, Porreca F. Regulation of morphine antiallodynic efficacy by cholecystokinin in a model of neuropathic pain in rats. J Pharmacol Exp Ther. 1995;275:1339–1345. [PubMed] [Google Scholar]
- 16.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 17.Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niikura K, Narita M, Butelman ER, Kreek MJ, Suzuki T. Neuropathic and chronic pain stimuli downregulate central μ-opioid and dopaminergic transmission. Trends Pharmacol Sci. 2010;31:299–305. doi: 10.1016/j.tips.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277:119–127. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Lorenzana G, Espinosa-Lopez L, Carranza M, Aramburo C, Paz-Tres C, Rojas-Piloni G, Condes-Lara M. PVN electrical stimulation prolongs withdrawal latencies and releases oxytocin in cerebrospinal fluid, plasma, and spinal cord tissue in intact and neuropathic rats. Pain. 2008;140:265–273. doi: 10.1016/j.pain.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Miranda-Cardenas Y, Rojas-Piloni G, Martinez-Lorenzana G, Rodriguez-Jimenez J, Lopez-Hidalgo M, Freund-Mercier MJ, Condes-Lara M. Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain. 2006;122:182–189. doi: 10.1016/j.pain.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York, NY: Academic Press; 1998. [Google Scholar]