Abstract
Dendrites from a single neuron may be highly branched but typically do not overlap. This self-avoidance behavior has been shown to depend on cell-specific membrane proteins that trigger mutual repulsion. Here we report the surprising discovery that a diffusible cue, the axon guidance protein UNC-6/Netrin, is required for self-avoidance of sister dendrites from the PVD nociceptive neuron in C. elegans. We used time lapse imaging to show that dendrites fail to withdraw upon mutual contact in the absence of UNC-6/Netrin signaling. We propose a model in which the UNC-40/DCC receptor captures UNC-6/Netrin at the tips of growing dendrites for interaction with UNC-5 on the apposing branch to induce mutual repulsion. UNC-40/DCC also responds to dendritic contact through an additional pathway that is independent of UNC-6/Netrin. Our findings offer a new model for how an evolutionarily conserved morphogenic cue and its cognate receptors can pattern a fundamental feature of dendritic architecture.
Sensory neurons form highly branched networks of dendritic processes. Despite the complexity of these structures, dendrites arising from a given neuron rarely overlap. This phenomenon of self-avoidance is widely observed and is presumptively employed to maximize coverage of the receptive field1-3. Studies in Drosophila have revealed that the cell surface proteins Dscam, turtle and Flamingo can mediate self-avoidance and thus suggest that physical contact between sister dendrites is sufficient to trigger mutual repulsion4-8. Differential expression of the large number of available Dscam isoforms offers an elegant solution to the problem of distinguishing self vs non-self by providing unique combinations of markers for specific neuron types. A much smaller array of distinct Dscam isoforms is produced in mammals, however, and thus is unlikely to account for the majority of self-avoidance decisions in vertebrate neural development9. Overall, the molecular roles of other determinants of dendritic architecture are also poorly understood3. In contrast, the outgrowth of axons have been linked to a wide array of guidance cues and receptors. For example, the extracellular protein, UNC-6/Netrin, is secreted from specific donor cells to generate a graded signal that directs axon growth10-12. UNC-6/Netrin can also function as a short-range cue on either the membrane of the secreting cell or after capture by distal guidepost cells to direct local axon trajectory13-17. The axon guidance function of UNC-6/Netrin is evolutionarily conserved and depends on interaction with specific receptor proteins including UNC-40/DCC and UNC-5 18,19. Here we exploit the morphological simplicity of the PVD nociceptive neuron20-23 in the model organism C. elegans and its accessibility to live cell imaging to detect a new function for UNC-6/Netrin in dendritic self-avoidance. We also show that this mechanism depends on physical contact between sister dendrites. Our finding provides the first example of a diffusible cue in this role and therefore expands the repertoire of potential self-avoidance components to include other established extracellular signaling molecules and the pathways that they control.
PVD neurons exhibit dendritic self-avoidance
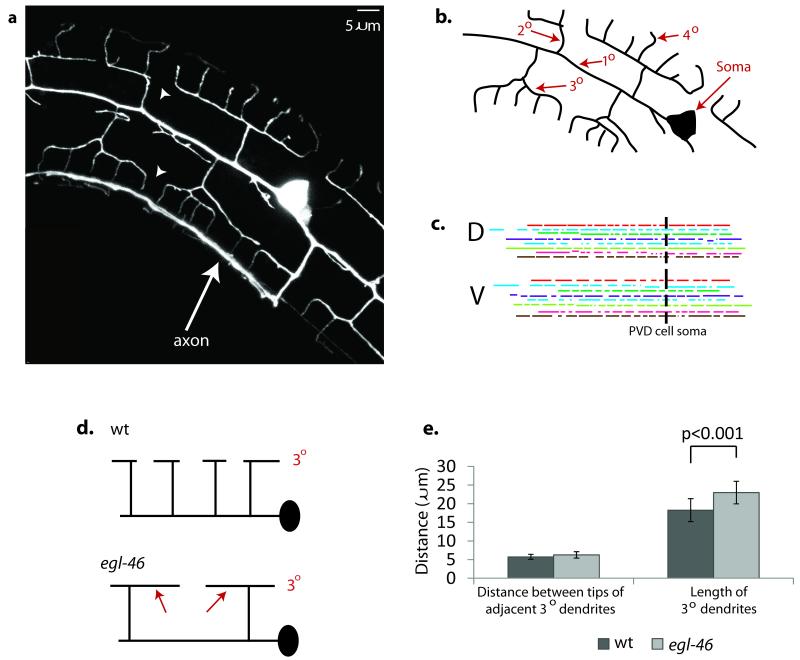
PVD neurons display a highly branched network of sensory processes in which a collection of dendritic trees or “menorahs” is rooted in a common 10 dendrite (Fig.1a, b)21,24-26. This well-ordered and non-overlapping array of PVD dendrites is generated by a combination of defined branching events and an error correction mechanism in which sister dendrites are repelled by mutual contact24,25. The patterning role of self-avoidance is strikingly evident in the outgrowth of 3o dendrites. In each menorah, paired 3o dendrites project along a sublateral nerve cord in either an anterior or posterior direction (Fig.1a,b). We used time-lapse imaging to establish that growth continues until the tip of one 3o dendrite contacts another 30 branch pointing in the opposite direction24. Touch evokes rapid withdrawal that results in an eventual gap between 3o dendrites from adjacent menorahs. This mechanism readily accounts for the observation that the inter-tip gap distance is constant between flanking 30 dendrites but that adult branch length and termination points are highly variable for PVD neurons in different animals (Fig.1c). As an additional test of a self-avoidance model, we used a mutant of the egl-46/Zn finger/Nerfin transcription factor to reduce the overall number of PVD menorahs24. This genetic background effectively widens the spacing between the branch initiation points of adjacent 30 dendrites (Fig.1d). Thus, this approach uses a genetic strategy to answer the question: If repulsion specifies the regular layout of PVD dendrites, what is the consequence of branch ablation? Our results show that 30 branches are significantly longer in the egl-46 mutant but that the inter-tip gap is maintained (Fig.1e)24. Thus, these observations rule out models in which branch length is determined by a fixed yardstick or defined by external landmarks but favor the idea that the non-overlapping PVD dendritic architecture is achieved through a contact-dependent mechanism of self-recognition.
Figure 1. UNC-6/Netrin signaling is required for contact-dependent self-avoidance.
(a) Fluorescent image of PVD neuron labeled with GFP to show the non-overlapping pattern of PVD dendrites. Arrowheads denote gaps between 30 dendrites of adjacent menorahs. The single PVD axon (arrow) marks the location of the ventral nerve cord. (b) Tracing of PVD branches to show numbering scheme. (c) Tracings of 3o dendrites from dorsal (D) and ventral (V) regions of ten individual PVD neurons are denoted with matching colors. Note that 3o dendrites do not terminate at specific anatomical regions or exhibit a single length as would be expected if outgrowth were governed by external landmarks or limited by an intrinsic mechanism of length determination. (d) egl-46 mutants show fewer 2o branches and longer 3o dendrites (arrows). (e) PVD neurons have fewer 2o branches in egl-46 (n1076) mutants (32.3 + 5.3) than wild type (wt) (38.9 + 5.4). Despite the consequent reduction in the overall number of 3o branches in egl-46 mutants, the normal distance between the tips of adjacent 3o dendrites is maintained by extending the average outgrowth length of 3o dendrites. Error bars represent s.d.
UNC-6 signaling is required for dendritic self-avoidance
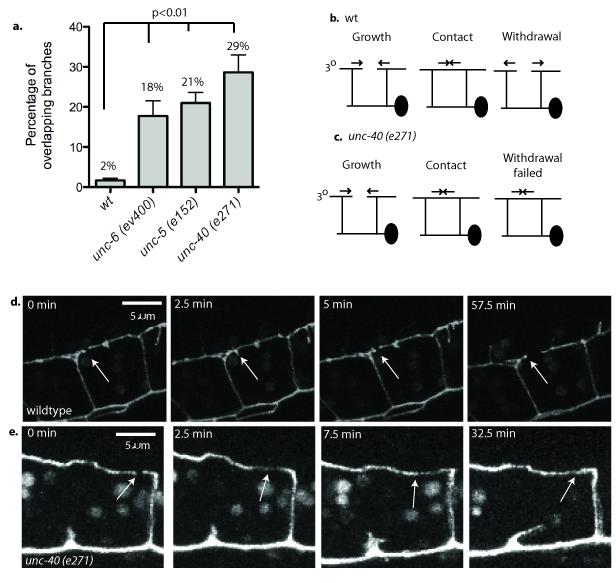
We generated a PVD expression profile24 and used genetic analysis of known axon guidance molecules suggested by this list to test for potential roles in dendritic morphogenesis (supplemental table 1). We noted that genetic ablation of the UNC-6 receptors UNC-40/DCC and UNC-5 altered several aspects of PVD morphology (supplemental figure 124,26) including the aberrant occurrence of overlaps between flanking menorahs in the adult (Fig.2a). We used time-lapse imaging to establish that this mutant phenotype arises from a self-avoidance defect. In the wild type, 3o dendrites from adjacent menorahs grow toward each other but quickly retract with over > 50% regressing within 3 minutes of contact and < 13% still touching at the 10 min mark; ultimately, less than 1% of wild-type 3o dendrites overlap with each other (Fig.2b, d, supplemental movie 1, supplemental figure 2). In contrast, in unc-40(e271) mutants, a majority (76%) of adjacent 3o dendrites failed to withdraw within 10 min of contact and almost one third (29%) never regressed (Fig.2a,c,e) (supplemental movie 2, supplemental figure 2). Similar defects were captured in time-lapse movies of unc-5(e152) (supplemental movie 3, supplemental figure 2). Motivated by these results, we examined the mutant unc-6(ev400) and detected 3o self-avoidance defects resembling those of unc-40/DCC and unc-5 mutants (Fig.2a). If these genes function in a common pathway, double mutants between unc-6 and each of its receptors should fail to enhance the self-avoidance defect of either single mutant. This prediction is confirmed for the self-avoidance defect arising from the combination of unc-5 and unc-6 which is comparable to that of either unc-5 or unc-6 alone (supplemental figure 3). However, the unc-40 mutation enhances both the Unc-5 and Unc-6 single mutant self-avoidance phenotypes. These results are consistent with model in which unc-40 exercises a role in self-avoidance that is independent of unc-6 signaling through unc-5. In addition, because neither unc-5 nor unc-6 enhance the Unc-40 self-avoidance defect (supplemental figure 3), we conclude that unc-40 also functions in the unc-6- and unc-5-dependent pathway. Here we describe experiments designed to establish the mechanism whereby UNC-6/Netrin and its receptors, UNC-40/DCC and UNC-5, mediate dendritic self-avoidance.
Figure 2. UNC-6/Netrin signaling is required for contact-dependent self-avoidance.
(a) 3o branches from adjacent menorahs overlap in unc-6 (ev400), unc-5(e152) and unc-40 (e271) mutants more frequently than in wild type (wt). p<0.01. Error bars represent s.d. (b) Schematic showing 3o outgrowth, contact and retraction in wild type (wt). (c) 3o branches fail to withdraw after contact in an unc-40 mutant. (d) Images captured from a time-lapse movie showing contact then rapid withdrawal (< 2.5 min) (arrow) of 3o branches in wild type.(e) Successive images showing that 3o branches fail to withdraw within 30 min of mutual contact in unc-40(e270) (arrow).
Self-avoidance requires UNC-6 but not a graded UNC-6 signal
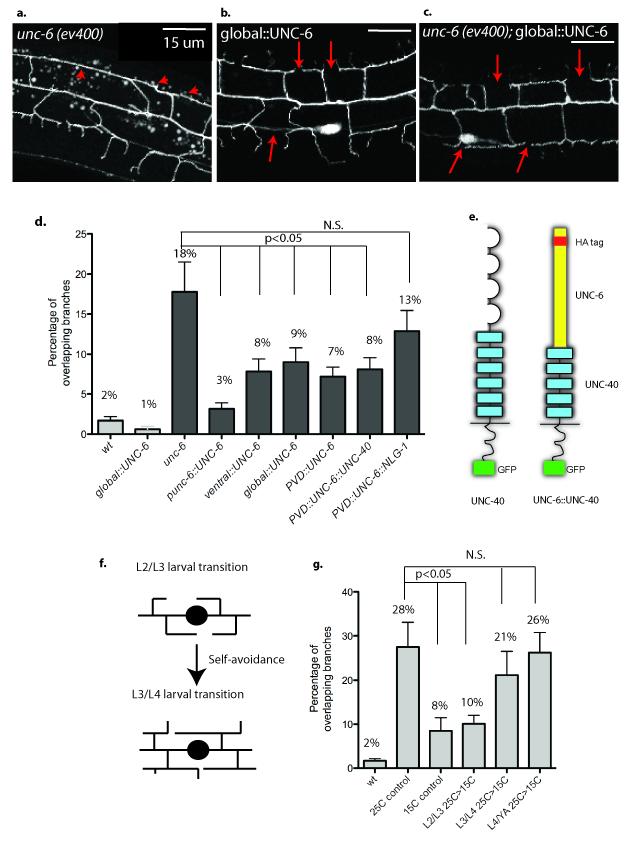
UNC-6 is secreted from ventral cells to direct axonal outgrowth and cell migration along the D/V body axis10,27. When this ventral source of UNC-6 is removed in unc-6(ev400) mutants, 18% of PVD 3o branches overlap per animal (Fig.3a,d). This defect is complemented by UNC-6 expression with the native unc-6 promoter (Fig.3d). Transgenic expression of UNC-6 in a ventral neuron (AVA) with the rig-3 promoter28 also improves the self-avoidance response (8% overlapping branches, p=0.02 vs unc-6) and thus indicates that UNC-6 expression from ventrally located cells is sufficient to mediate PVD 3o branch self-avoidance (Fig.3d).
Figure 3. UNC-6/Netrin functions as a permissive cue to prevent dendritic branch overlap.
(a, d) unc-6(ev400) shows overlapping 3o PVD branches (arrowheads) (b, d) Expression of UNC-6 with a heat shock promoter (global∷UNC-6) restores self-avoidance (arrows) to unc-6(ev400) but (c, d) does not induce PVD dendritic outgrowth defects in a wild-type background. (d) UNC-6 expression with the native unc-6 promoter (unc-6∷UNC-6), a ventral neuron-specific promoter (ventral∷UNC-6) or the PVD promoter (PVD∷UNC-6) rescues unc-6(ev400) self-avoidance defects (see Methods). Expression of UNC-6 fused to the extracellular domain of UNC-40 (PVD∷UNC-6∷UNC-40) restores self-avoidance but UNC-6 fused to the membrane protein neuroligin (PVD∷UNC-6∷NLG-1) does not rescue 3o branch self-avoidance defects in unc-6(ev400). Genetic backgrounds are wild type (wt) (light grey boxes) or unc-6(ev400) (dark gray boxes). (e) Schematic of UNC-40 protein (Ig domain = loops, Fibronectin domains = rectangles, intracellular domain and GFP tag = dark rectangle) and UNC-6∷UNC-40 chimera (UNC-6 contains HA tag). (f) PVD 3o branch self-avoidance occurs during the L3 larval stage. (g) The temperature sensitive allele unc-6(rh46) was shifted from restrictive (25C) to permissive temperature (15C) at successively later stages (L2/L3, L3/L4, L4/adult transitions) during larval development. Temperature shift from 25C to 15C at the L2/L3 transition rescues the 3o branch self-avoidance defect (p<0.01) but downshifts at later developmental stages do not restore self-avoidance. Continuous growth at 25C (25C control) results in a higher fraction of overlapping branches than continuous growth at 15C (15C control). PVD 3o branch overlap was scored in the adult. Error bars represent s.d.
Although extracellular UNC-6 protein is presumptively distributed in a ventral to dorsal gradient, we did not observe a significant difference in the extent of self-avoidance errors in ventral vs. dorsal 3o branches in unc-6 (ev400) (supplemental figure 4). We next used a heat shock promoter (hsp16.2) to drive expression of UNC-6 in all cells29 and thereby directly determine if a ventral to dorsal gradient of UNC-6 is required for self-avoidance. Although global expression of UNC-6 is known to disrupt axon guidance along the D/V axis29, ubiquitous UNC-6 expression in a wild-type background during multiple larval stages did not perturb PVD 3o branch self-avoidance (Fig.3b,d).
We reasoned that UNC-6/Netrin might function as a permissive cue in this case such that a specific source or gradient of UNC-6/Netrin is not necessary provided sufficient ligand is available. This idea is substantiated by our finding that global expression of UNC-6/Netrin in unc-6(ev400) with the heat shock promoter before the L3 larval stage rescues 3o branch self-avoidance (9% overlapping branches, p=0.04 vs unc-6) (Fig.3c,d). In addition, we showed that expression of UNC-6/Netrin in PVD with the F49H12.4 promoter30 also rescued unc-6 (ev400) self-avoidance defects (7% overlapping branches, p=0.01 vs unc-6) (Fig.3d). Based on these results, we conclude that PVD dendritic self-avoidance is independent of the UNC-6/Netrin gradient and therefore that UNC-6/Netrin does not provide a directional signal to repel dendritic outgrowth. We considered an alternative model in which the mere availability of UNC-6 is sufficient to trigger repulsion and next asked the question of when this function is required.
UNC-6 is required during the period when dendrites self-avoid
Time-lapse imaging established that PVD 3o branch self-avoidance occurs during the L3 larval stage24. If UNC-6 is directly involved in self-avoidance then UNC-6 function should be required during this period. We used a conditional unc-6 allele (rh46) to test this idea in temperature shift experiments that regulate temporal UNC-6 activity31.
unc-6(rh46) mutants grown at the restrictive temperature (25C) display a self-avoidance defect (28% of overlapping 3o branches, Fig.3g) comparable to that of the unc-6(ev400) null allele (p=0.14 ev400 vs rh46) which therefore suggests that the rh46 point mutation results in a dysfunctional UNC-6 protein at restrictive temperature31. The self-avoidance defect is weaker but still significant at 15C (Fig.3g, 8% of overlapping 3o branches, p =0.006 vs 25C control, p=0.047 vs N2) indicating that the rh46 mutant UNC-6 protein is only partially active at permissive temperature. We shifted unc-6(rh46) animals from restrictive temperature (25C) to permissive temperature (15C) at succeeding developmental stages (Fig.3f,g). unc-6(rh46) larvae downshifted at the L2/L3 transition and then maintained at permissive temperature until the adult, showed a self-avoidance defect comparable to that of control animals grown continuously at 15C (Fig.3g, 10% overlapping branches, p = 0.007 vs 25C control). In contrast, downshifts to permissive temperature (i.e., restoration of UNC-6 activity) after the L2/L3 transition resulted in a self-avoidance defect as severe as unc-6(rh46) animals grown continuously at the restrictive temperature (Fig.3g, p = 0.42 for L3/L4, p = 0.86 for L4/YA vs 25C control). These results indicate that self-avoidance does not depend on UNC-6 function during embryonic and early larval development but that UNC-6 is required after the beginning of the L3 stage. Similar temperature upshift experiments confirmed that loss of UNC-6 function during the L3 larval period enhances the rh46 self-avoidance defect but later shifts to restrictive temperature (i.e., L3/L4 or L4/YA) after 30 branch outgrowth is complete do not result in a severe branch overlap phenotype (supplemental figure 5). Thus, our results are consistent with a model in which UNC-6 function is required for self-avoidance during a brief developmental window in the L3 larval stage in which 30 dendrites are actively engaged in outgrowth and contact-dependent repulsion. This finding is important because it argues against the possibility that UNC-6/Netrin signaling fulfills an earlier, indirect role in which it primes PVD dendrites for self-avoidance by regulating expression32, for example, of an alternative set of interacting components.
UNC-40/DCC and UNC-5 function cell-autonomously in PVD
Genetic ablation of UNC-5 and UNC-40 resulted in significant overlap of 3o dendrites (Fig.2a). Because UNC-5 and UNC-40 have been previously shown to function as receptors for UNC-6/Netrin and because UNC-5 and UNC-40 transcripts are enriched in our PVD microarray data set24, we reasoned that UNC-5 and UNC-40 are likely to act in PVD to prevent overlap of 3o dendrites. This model predicts that expression of UNC-5 and UNC-40/DCC in PVD should be sufficient to restore self-avoidance to the corresponding unc-5 or unc-40 mutants.
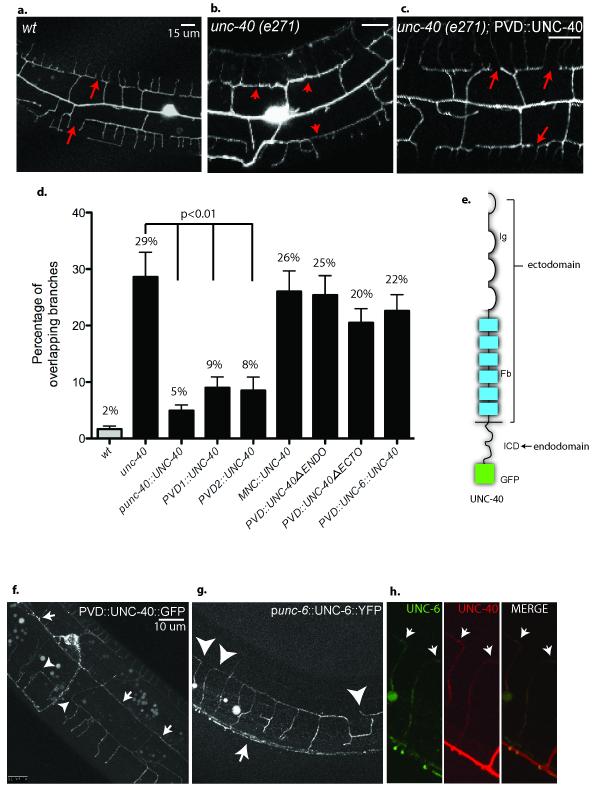
Expression of UNC-40 with its endogenous promoter in unc-40(e271) reduced the frequency of overlapping branches from 29% to 5% (p = 3E−5 vs unc-40) (Fig.4d). UNC-40 expression with the PVD promoters F49H12.430 or ser2prom333 also showed significant rescue (8% overlapping 3o branches, p=5E−7 vs unc-40) (Fig.4c,d). Thus, these results are indicative of the cell-autonomous function of UNC-40 in PVD. We have previously noted that a significant fraction of PVD 20 branches fasciculate with motor neuron commissures that also project from the ventral to dorsal side of the animal24. To determine if PVD dendritic self-avoidance is indirectly compromised by commissural axon guidance defects in unc-40(e271)18, we restored UNC-40 expression to ventral cord motor neurons with the unc-25 promoter34. Motor neuron expression of UNC-40 largely rescued commissural axon outgrowth to the dorsal cord, as expected18 (supplemental fig 6) but did not restore PVD self-avoidance (Fig.4d).
Figure 4. UNC-40/DCC functions in PVD to mediate self-avoidance and captures exogenous UNC-6/Netrin at the PVD cell surface.
(a) PVD 3o dendrites do not overlap in wild-type (wt) adults (arrows). (b, c) Expression of UNC-40 (PVD∷UNC-40) in unc-40 (e271) rescues (arrows) the Unc-40 self-avoidance defect (arrowheads). (d) Quantification confirms that expression of UNC-40/DCC with the native unc-40 promoter (unc-40∷UNC-40) and with two different PVD promoters (PVD1∷UNC-40, PVD2∷UNC-40) restores PVD dendritic self-avoidance whereas expression with a motor neuron-specific promoter (MNC∷UNC-40) does not. PVD expression of UNC-40/DCC lacking either the extracellular UNC-6 binding domain (PVD∷UNC-40deltaECTO) or intracellular signaling domain (PVD∷UNC-40deltaENDO) fails to rescue self-avoidance. Genetic backgrounds are wild type (wt) (grey box) or unc-40(e271) (black boxes). Error bars represent s.d. (e) Schematic of UNC-40 protein (Ig domain = loops, Fibronectin domains = rectangles, intracellular domain and GFP tag = dark rectangle). (f) PVD expression of GFP labeled UNC-40 (PVD∷UNC-40∷GFP) results in GFP puncta in PVD processes (arrows) and at tips of growing dendrites (arrowheads). (g) YFP-labeled UNC-6 expressed from its native promoter in ventral cells (unc-6∷UNC-6∷YFP) decorates PVD neurons (arrowheads) expressing UNC-40∷mCherry. Arrow denotes UNC-6∷YFP labeling of ventral nerve cord. (h) UNC-6∷YFP (UNC-6, green) labeling of PVD expressing UNC-40∷mCherry (UNC-40, red). Merged image showing co-localization of UNC-6∷YFP and UNC-40∷mCherry puncta (arrows).
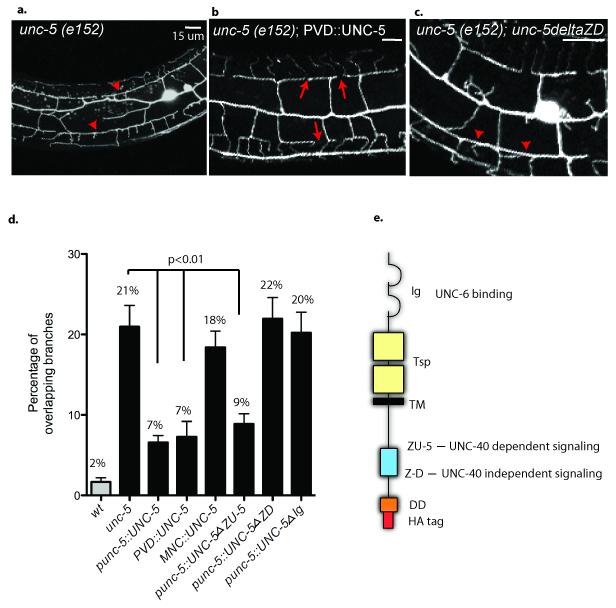
In similar experiments, expression of UNC-5 under its endogenous promoter resulted in a significantly reduced fraction of overlapping branches in the unc-5(e152) mutant (Fig.5d) (7%, p = 8.56E-5 vs unc-5). A cell-autonomous role for UNC-5 in PVD is consistent with our finding that UNC-5 expression with the F49H12.4 promoter also rescues the Unc-5 self-avoidance defect (Fig.5b,d, p = 1.8E−4 vs unc-5). Restoration of UNC-5 expression in motor neurons did not complement unc-5(e152) PVD self-avoidance errors (Fig.5d) but does repair the uncoordinated phenotype that arises from misguided motor axon outgrowth (data not shown)35,36. The results of these cell-specific rescue experiments show that UNC-5 function is required in the PVD dendrites to prevent overlap of 3o branches.
Figure 5. UNC-5 is required in PVD and utilizes UNC-40-independent signaling to mediate self-avoidance.
(a, d) unc-5(e152) shows PVD self-avoidance defects (arrowheads) (b) Expression of UNC-5 with the PVD promoter (PVD∷UNC-5) prevents 3o branch overlap in unc-5 (e152) but (c) UNC-5 lacking the Z-D domain does not rescue. (d) Expression of UNC-5 with the native unc-5 promoter (unc-5∷UNC-5) or with the PVD promoter (PVD∷UNC-5) restores PVD self-avoidance but expression of the UNC-5 with the motor neuron promoter (MNC∷UNC-5) does not. Expression of UNC-5 proteins lacking either an UNC-40-independent cytoplasmic signaling domain (unc-5∷UNC-5deltaZD) or UNC-6-binding domain (unc-5∷UNC-5deltaIg) fails to rescue self-avoidance in unc-5 (e152) mutants whereas expression of an UNC-5 protein lacking an UNC-40-dependent signaling domain (unc-5∷UNC-5deltaZU-5) prevents 3o branch overlap. Genetic backgrounds are wild type (wt) (grey box) or unc-5(e151) (black boxes). Error bars represent s.d. (e) Schematic of UNC-5 protein showing functional regions (Ig domain = loops, Thrombospondin domain (Tsp) = square, TM = transmembrane domain, and ZU-5, Z-D, Death domain (DD) intracellular domains and C-terminal HA tag).
UNC-40/DCC localizes UNC-6/Netrin to PVD dendrites
Consistent with the hypothesis that UNC-40/DCC function is required in PVD dendrites, PVD expression of a functional GFP-tagged UNC-40 protein (UNC-40∷GFP)29 resulted in distinct GFP puncta in PVD processes (Fig.4f). Moreover, UNC-40∷GFP puncta can be readily seen at the tips of 3o dendrites where contact-dependent self-avoidance occurs (Fig.4f,h).
We considered the possibility that UNC-6 functions as a contact-dependent repellent and tested this idea with an experiment designed to detect UNC-6 at the surface of PVD dendrites. We used the endogenous UNC-6 promoter to drive expression of UNC-6∷YFP37. Although this transgenic line rescues Unc-6 axon guidance defects and therefore must secrete a functional UNC-6∷YFP protein, UNC-6∷YFP is too diffuse to detect in a wild-type animal outside of the ventral cells in which it is expressed (supplemental figure 7)37. To enhance the sensitivity of this assay, we expressed mCherry-labeled-UNC-40/DCC in PVD. In this background, UNC-6∷YFP is strikingly evident as YFP puncta that overlap with mCherry∷UNC-40 (Fig.4g,h). In contrast, expression of UNC-5 in PVD rescued the Unc-5 self-avoidance defect (Fig.5d) did not result in detectable localization of UNC-6∷YFP on PVD (data not shown). These results are consistent with a model in which UNC-40/DCC, but not the UNC-5 receptor, captures UNC-6 from the extracellular space at the surface of PVD dendrites. This idea is supported by our finding that PVD expression of a truncated UNC-40 protein lacking the UNC-6-binding extracellular domain (PVD∷UNC-40deltaECTO) fails to restore 3o branch self-avoidance in unc-40 (e271) (20% overlapping dendrites, p=0.23 vs unc-40) whereas PVD expression of intact UNC-40/DCC protein is sufficient (Fig.4d). To rule out the possibility of a dominant negative effect, we determined that the PVD∷UNC-40deltaECTO protein does not disrupt self-avoidance in a wild-type background (data not shown).
UNC-6 bound to UNC-40 functions as a short-range cue
Our results show that UNC-6/Netrin secreted from a ventral source can be captured by UNC-40/DCC at the surface of PVD dendrites. Because PVD sister dendrite repulsion depends on direct contact, we wondered if UNC-6 bound to UNC-40 at the tips of touching 30 dendrites could trigger this response. In this model, UNC-40/DCC might adopt a role in which it positions UNC-6/Netrin at this critical location to activate withdrawal of an apposing dendrite. This idea mirrors the observation that Drosophila UNC-40/Fra/DCC can sequester exogenous NetrinB at the surface of guidepost cells to steer local axon outgrowth in a contact-dependent mechanism13. Our model predicts that UNC-6/Netrin protein tethered to the UNC-40/DCC receptor can function as a short-range cue. To test this idea, we used a chimeric protein in which UNC-6 is fused to the extracellular region of UNC-40 and expressed it in PVD. This membrane bound form of UNC-6/Netrin (PVD∷UNC-6∷UNC-40) rescued the dendritic self-avoidance defects of unc-6 (ev400) (8% overlapping 3o branches, p=0.02 vs unc-6) (Fig.3d). Expression in AVA interneurons in the ventral nerve cord with the rig-3 promoter28 (ventral∷UNC-6∷UNC-40), however, fails to restore self-avoidance to unc-6(ev400) and therefore confirms that the UNC-6∷UNC-40 fusion protein is not released from the cell surface (supplemental figure 8). Thus, our results are consistent with a model in which UNC-40/DCC localizes exogenous UNC-6/Netrin to the surface of PVD dendrites where it functions as a short-range cue to trigger self-avoidance. This configuration may be specifically required because PVD expression of UNC-6 fused to the N-terminus of a different transmembrane protein, NLG-1/Neuroligin (PVD∷UNC-6∷NLG-1),17 did not rescue self-avoidance in unc-6(ev400) (Fig.3d). The next problem to consider was how apposing PVD dendrites might detect this local UNC-40-bound, UNC-6/Netrin ligand. Because of the well-established role of UNC-5 in mediating repulsive responses to UNC-6/Netrin14,19,35, and our finding that UNC-5 expression in PVD is necessary for self-avoidance, we imagined that UNC-5 could provide this function
Self-avoidance is mediated by UNC-5 signaling
A mutation in the UNC-5 extracellular Ig domain that disrupts UNC-6/Netrin binding fails to rescue self-avoidance when expressed in unc-5(e152) (Fig.5d, 20% overlapping branches, p=0.83 vs unc-5). This finding is consistent with genetic results (supplemental figure 3) showing that unc-5 and unc-6 function in a common pathway to mediate self-avoidance and with the proposal that UNC-6 binding to UNC-5 is necessary for this interaction. Genetic analysis in C. elegans has shown that UNC-5 can mediate UNC-6-mediated repulsion either in concert with UNC-40 or independently36. These UNC-5 functions depend on specific conserved cytoplasmic domains; the Z-D sequence is necessary for UNC-40-independent signaling whereas the ZU-5 region is required for UNC-40-dependent activity36. To distinguish between these models, we tested mutant versions of the UNC-5 protein that lack either the ZU-D region (UNC-40-independent signaling) or the ZU-5 domain (UNC-40-dependent signaling). Previous work has shown that UNC-5 localization is not disrupted by these mutations36. Transgenic expression of the UNC-5 protein lacking the Z-D domain (UNC-5deltaZD) did not restore self-avoidance to an unc-5 (e152) mutant (Fig.5c,d 22% overlapping branches, p=0.79 vs unc-5). In contrast, deletion of the ZU-5 region (UNC-5deltaZU-5) that is required for UNC-40-dependent signaling significantly improved the frequency of self-avoidance in comparison to the unc-5 (e152) mutant alone (Fig.5d, 9% overlapping branches, p=3E−4). These results are consistent with a model in which UNC-5-mediated repulsion does not depend on interactions in cis with the UNC-40 protein but that UNC-40 function is required for localizing UNC-6/Netrin for binding in trans to UNC-5 at the apposing tip of the adjacent 30 dendrite.
UNC-40/DCC signaling is required for self-avoidance
Although the UNC-6∷UNC-40 fusion protein rescues the unc-6 mutant (Fig.3d) and therefore likely functions as a membrane-bound cue to trigger dendrite repulsion, UNC-6:UNC-40 does not restore self-avoidance to unc-40(e271) (Fig.4d). One explanation for this result is that unc-40 signaling is not active in the UNC-6∷UNC-40 fusion protein and that this UNC-40 function is necessary for self-avoidance. We tested this idea with a modified UNC-40 protein that lacks the intracellular domain (ICD) that mediates UNC-40/DCC downstream signaling38,39. Interestingly, PVD expression of this truncated UNC-40/DCC protein (PVD∷UNC-40deltaENDO) in unc-40(e271) fails to rescue dendrite repulsion (Fig.4d). Thus, our results indicate that UNC-40 provides the dual roles of capturing UNC-6 at the PVD cell surface for interaction with UNC-5 as well as activating a downstream pathway to mediate self-avoidance.
Discussion
Dendrites from a single neuron may be highly branched but rarely touch one another2,9. The absence of overlap arises from a mechanism in which sister dendrites are mutually repelled by transient encounters during outgrowth. The necessity of physical contact for self-avoidance is indicative of interaction between surface markers that trigger repulsion2. This model is substantiated by the recent discovery that membrane proteins can mediate self-avoidance in Drosophila sensory neurons5-8. Here we describe a novel mechanism in the nematode, C. elegans, in which this self-recognition function is provided by a diffusible cue (supplemental figure 9).
Our results show that UNC-6/Netrin is secreted from ventral cells to modulate self-avoidance of PVD sensory neuron dendrites in distal, lateral locations. We propose that UNC-6/Netrin is sequestered at the surface of PVD dendritic branches by the canonical receptor UNC-40/DCC where it is positioned to trigger a repulsive response upon contact with UNC-5 on the apposing dendrite. PVD self-avoidance also depends on UNC-40/DCC function in a separate pathway that does not require unc-5 and unc-6 (supplemental fig 9).
In some respects, our model parallels an earlier finding in Drosophila in which UNC-40/Frazzled/DCC functions in guidepost cells to capture Netrin as a local guidance cue for nearby axons13,40,41. In this setting, however, the Netrin receptor in the responding cells is unknown and this signaling event occurs between separate cells. In the model that we have proposed, UNC-5 mediates a negative response to UNC-6 between spatially distinct membrane regions of the same cell. Netrin has also been shown to function as a short-range signal for axonal and dendritic guidance in other contexts and for defining the placement of synapses between specific neurons14-17. The phenomenon of self-avoidance that we have detected includes additional features that point to a complex mechanism. In addition to the proposed role for UNC-40/DCC of sequestering UNC-6/Netrin for interaction with UNC-5, our genetic evidence (supplemental fig 3) indicates that UNC-40/DCC also functions in a parallel self-avoidance pathway that does not involve unc-5 and unc-6. UNC-6-independent signaling by UNC-40/DCC has been previously observed18,32,42 and is suggestive of additional UNC-40/DCC activating ligands. Previous work has shown that UNC-5 and UNC-40 can signal independently of each other to mediate repulsion to UNC-6/Netrin14,18,43,44 but our findings include the additional observation that this activity requires physical contact between apposing dendrites.
In addition to expanding the repertoire of self-avoidance proteins, our discovery that UNC-40/DCC and UNC-5 are involved suggests that other established UNC-6/Netrin signaling proteins could also be utilized to trigger repulsion. For example, we note that the UNC-6/Netrin pathway components UNC-34/Ena, CED-10/Rac and MIG-10/Lamellipodin45-47 are highly expressed in PVD and therefore available for this role24. The significance of this possibility is underscored by the fact that little is known of the downstream mechanisms that reorganize the dendritic cytoskeleton to effect mutual repulsion48. For example, the intracellular proteins tricornered (trc) and furry (fry) are required for dendritic self-avoidance in a subset of Drosophila sensory neuron but mechanisms that activate these components are poorly defined7,49. In addition, the cytoplasmic domain of Dscam is necessary for self-avoidance but no downstream effectors have been identified6.
We have established that UNC-6/Netrin is required for self-avoidance of PVD dendrites. However, our results also point to additional mechanisms for regulating iso-neuronal repulsion. We note that most (~ 65%) of PVD 30 dendrites undergo normal self-avoidance in strong loss-of-function alleles of UNC-6/Netrin pathway genes (Fig.2). This result parallels the observation that mutants in self-avoidance genes in Drosophila (e.g., Dscam, Turtle) and C. elegans (e.g., eff-1) are also incompletely penetrant6,8,25.
Although our results reveal a new role for UNC-6/Netrin signaling in dendritic self-avoidance, the model that we have proposed involving a single cue and its receptors is unlikely to provide a general solution to the problem that individual neurons face of distinguishing self from non-self. The cell surface markers Dscam and protocadherins which can be expressed in many different alternative forms, are proposed to fulfill this role by providing unique combinations of labels for marking single neuron types in complex neural environments9. However, our discovery of a mechanism whereby an exogenous cue can be utilized to pattern dendritic self-avoidance suggests that other extracellular signals and their receptors could be similarly employed. This possibility significantly expands the potential utility of this self-avoidance strategy.
Nature Methods
Nematode Strains and Genetics
The wild-type C. elegans Bristol strain N2 was used for all experiments and cultured as previously described 50.
Mutants used in this study
unc-6 (ev400); unc-6 (rh46); unc-5 (e152); unc-40 (e271); unc-129 (ev554); slt-1 (eh15); sax-3 (ky123); vab-2 (ju1); ptp-3 (ok244); madd-2 (ok2266); nid-1 (cg119). Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). All studies in this work used C. elegans hermaphrodites.
Additional strains
NC1686 [wdIs51 (F49H12.4∷GFP + unc-119)]24; NC1687 [wdIs52 (F49H12.4∷GFP + unc-119)]24; CX6488 [kyIs299, hsp16.2∷unc-6∷HA + ord-1∷RFP]29, YC149 [unc-6 (ev400); ghIs9 (unc-6∷venus + odr-1∷RFP)]37; CZ1200 [juIs76 (unc-25∷GFP)]; NW1454 [unc-5 (e53); dpy-20 (e1282); evIs105 (pU5∷HA delta ZO-1)]; NW1151 [unc-5 (e53); evIs906 (pU5∷HA Ig # 1m + dpy-20)]; NW1180 [unc-5 (e53); evIs91 (pU5∷HA delta BamH1 + dpy-20)]; NW1137 [unc-5 (e53); evIs886 (pU5∷HA + dpy-20)]36
Transgenic strains generated by microinjection
NC2099 [pha-1 (e2123ts); wdEx682 (MVC119 (rig-3∷unc-6) + pBx (pha-1) + dat-1∷mcherry)]; NC2099 [pha-1 (e2123ts); wdEx682 (MVC119 (rig-3∷unc-6) + pBx (pha-1) + dat-1∷mcherry); NC2182 [pha-1 (e2123ts); wdEx692 (pCJS28, F49H12.4∷unc-6∷HA + pBx, pha-1 + dat-1∷mcherry)]; NC1893 [pha-1 (e2132ts); wdEx640 (F49H12.4∷unc-40∷mcherry + pBx (pha-1) + odr-1∷mcherry)]; NC2059 [pha-1 (e2123ts); wdEx662 (pCJS52 (ser2prom3∷unc-40∷mcherry) + pBx (pha- 1) + dat-1∷mcherry)]; NC2098 [pha-1 (e2123ts); wdEx681 (pCJS68 (unc-25∷unc-40∷mRFP) + pBx (pha-1) + dat-1::mcherry)]; TV1788 [unc-40 (e271); wyIs45; wyEx650 (unc-40 minigene w/ mcherry injected at 20 ng/ul has co-selector marker GFP in coelomocytes)]; NC2301 [pha-1 (e2123ts); wdEx746 (pCJS93, F49H12.4∷unc-40∷GFP + pBx, pha-1 + pCJS85, dat-1∷mcherry)]; N2315 [pha-1 (e2123ts); wdEx748 (pCJS98, F49H12.4::unc-40deltaECTO∷mcherry + dat-1∷mcherry + pBx, pha-1)]; NC2044 [pha-1 (e2123ts); wdEx660 (pCJS65 (unc-5∷unc-5∷CFP) + dat-1∷mcherry + pBx (pha-1))]; NC2247 [pha-1 (e2123); lon-2 (e678); wdEx716 (pCJS72, unc-25∷unc-5∷mRFP + dat-1∷mcherry + pBx)];
Molecular Biology
UNC-40, UNC-6 and UNC-5 expression plasmids were constructed using conventional cloning and gateway recombinase technology as previously described 10,29,35,51. Detailed descriptions of plasmids constructions are available on request.
Confocal Microscopy
Nematodes were immobilized with 15 mM levamisole on a 2% agarose pad in M9 buffer 24. Images were obtained in a Leica TCS SP5 confocal microscope. Z-stacks were collected with either 40X (1 um/step), 63X (0.75 um/step) or 100x (0.75 um/step) objectives; single plane projections were generated with Leica Application Suite Advanced Fluorescence software. Brightness and contrast were enhanced using Adobe Photoshop CS5.
Time-Lapse Imaging
Nematodes were imaged as previously described24. For each time point, the 40X, 63X or 100X objective was used to collect a Z-stack (0.75 um/step) spanning the focal depth of the PVD neuron and its dendritic branches. Dendritic branch outgrowth at each time point was evaluated from a Z-projection. Larval stages were identified from morphological features: L2 (postdeirid); L3, L4, and young adult (vulval development) 52. At least three independent movies verified each example of dynamic dendritic growth described in this report.
Measuring 3o dendrites length
Images from L4 larval animals were measured using the vector tool in ImageJ.
Scoring self-avoidance defects
Each genotype was visualized in a PVD∷GFP reporter line (wdIs51 or wdIs52). At least 20 animals (> 600 of 3o gaps) were visualized for each genotype. Confocal images were collected in a z-stack to span the depth of PVD with a step size of 1 um. PVD morphology was scored from Z-stack projections. A self-avoidance defect is identified as any two adjacent 3o branches that lack an intervening gap between them. Adjacent 3o branches are defined as physically linked to 2o branches that project from flanking locations on the PVD 1o branch. The number of self-avoidance errors (i.e., absence of intervening space between 3o branches of adjacent menorahs) was divided by the total number of potential 3o branch gaps per animal (i.e., number of 20 branches – 2]) to provide the fraction of overlapping 3o branches per animal. The average fraction of overlapping 3o branches for each genotype was calculated for histograms summarizing these results.
Statistical analysis
An unpaired Student’s t-test was used to calculate statistical significance.
Heat Shock Experiments
Animals were heat shocked at the appropriate age as previously described 29. All animals were imaged at the late L4 larval stage after PVD development is complete.
Temporal requirement for UNC-6/Netrin
unc-6 (rh46) worms were maintained at the appropriate temperature 31 and treated with hypochlorite to release embryos for overnight incubation in M9 buffer. Synchronized L1s were placed on a bacterial lawn to initiate larval development and then shifted to either the permissive (15C) or restrictive (25) temperature at specific larval intervals (L2/L3 larval transition, L3/L4 larval transition, end of L4 larval stage) for growth until the late young adult stage for imaging. Animals grown at either the permissive or restrictive temperature throughout development were used as controls.
Supplementary Material
Acknowledgements
We thank Cori Bargmann for unc-86∷UNC-40∷GFP and hsp16.2∷UNC-6∷HA and the CX6488 strain; William Wadsworth for the pIM97 unc-6 expression construct and unc-6 (rh46); Kang Shen for constructs used to make pCJS01, F49H12.4∷gateway∷mcherry; Peter Roy for the unc-40 (e271) sequence; Yoshio Goshima for ghIs9; Joe Culotti for the unc-5 rescue construct and for the modified UNC-5 protein strains used for structure/function analysis, members of the D. Miller lab, R. Blakely lab and the D. Colón-Ramos lab for technical advice and for comments on the manuscript. Some of the strains used in this work were provided by the C. elegans Genetics Center which is supported by NIH NCRR. This work was supported by NIH R01 NS26115 (DMM), NIH R21 NS06882 (DMM), NIH F31 NS071801 (CJS), NIH R00 NS057931 (DCR), Klingenstein Foundation and the Alfred P. Sloan Foundation fellowship (DCR), and NIH MBRS SC3 GM089595 (MKV).
Footnotes
Author Contributions
C.J.S. and D.M.M. designed the experiments. C.J.S. performed experiments with advice from D.M.M. J.D.W. helped with the phenotypic analysis of UNC-6/Netrin signaling mutants. D.C.R. and M.K.V. provided reagents to test the cell-specific requirement of UNC-40 and UNC-6 and helpful advice. C.J.S and D.M.M. wrote the paper with input from coauthors.
References
- 1.Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grueber WB, Sagasti A. Self-avoidance and tiling: Mechanisms of dendrite and axon spacing. Cold Spring Harb Perspect Biol. 2010;2:a001750. doi: 10.1101/cshperspect.a001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corty MM, Matthews BJ, Grueber WB. Molecules and mechanisms of dendrite development in Drosophila. Development. 2009;136:1049–1061. doi: 10.1242/dev.014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hattori D, Millard S, Wojtowicz W, Zipursky S. Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol. 2008;24:597–620. doi: 10.1146/annurev.cellbio.24.110707.175250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes M, et al. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews B, et al. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Matsubara D, Horiuchi SY, Shimono K, Usui T, Uemura T. The sevenpass transmembrane cadherin Flamingo controls dendritic self-avoidance via its binding to a LIM domain protein, Espinas, in Drosophila sensory neurons. Genes Dev. 2011;25:1982–1996. doi: 10.1101/gad.16531611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long H, Ou Y, Rao Y, van Meyel DJ. Dendrite branching and selfavoidance are controlled by Turtle, a conserved IgSF protein in Drosophila. Development. 2009;136:3475–3484. doi: 10.1242/dev.040220. [DOI] [PubMed] [Google Scholar]
- 9.Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Wadsworth W, Bhatt H, Hedgecock E. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- 11.Serafini T, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell K, et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 13.Hiramoto M, Hiromi Y, Giniger E, Hotta Y. The Drosophila Netrin receptor Frazzled guides axons by controlling Netrin distribution. Nature. 2000;406:886–889. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- 14.Keleman K, Dickson B. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron. 2001;32:605–617. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 15.Colon-Ramos D, Margeta M, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teichmann HM, Shen K. UNC-6 and UNC-40 promote dendritic growth through PAR-4 in Caenorhabditis elegans neurons. Nature Neuroscience. 2010:1–9. doi: 10.1038/nn.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J, et al. A conserved juxtacrine signal regulates synaptic partner recognition in Caenorhabditis elegans. Neural Dev. 2011;6:28. doi: 10.1186/1749-8104-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan S, et al. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- 19.Leonardo E, et al. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 1997;386:833–838. doi: 10.1038/386833a0. [DOI] [PubMed] [Google Scholar]
- 20.Smith CJ, et al. Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Dev Biol. 2010;345:18–33. doi: 10.1016/j.ydbio.2010.05.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albeg A, et al. C. elegans multi-dendritic sensory neurons: Morphology and function. Mol Cell Neurosci. 2010 doi: 10.1016/j.mcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oren-Suissa M, Hall DH, Treinin M, Shemer G, Podbilewicz B. The Fusogen EFF-1 Controls Sculpting of Mechanosensory Dendrites. Science. 2010 doi: 10.1126/science.1189095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall DH, Treinin M. How does morphology relate to function in sensory arbors? Trends Neurosci. 2011;34:443–451. doi: 10.1016/j.tins.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith CJ, et al. Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Developmental Biology. 2010;345:18–33. doi: 10.1016/j.ydbio.2010.05.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oren-Suissa M, Hall DH, Treinin M, Shemer G, Podbilewicz B. The Fusogen EFF-1 Controls Sculpting of Mechanosensory Dendrites. Science. 2010;328:1285–1288. doi: 10.1126/science.1189095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguirre-Chen C, Bülow HE, Kaprielian ZC. elegans bicd-1, homolog of the Drosophila dynein accessory factor Bicaudal D, regulates the branching of PVD sensory neuron dendrites. Development. 2011;138:507–518. doi: 10.1242/dev.060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishii N, Wadsworth W, Stern B, Culotti J, Hedgecock E. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9:873–881. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz V, Pan J, Voltmer-Irsch S, Hutter H. IgCAMs redundantly control axon outgrowth in Caenorhabditis elegans. Neural Dev. 2009;4:13. doi: 10.1186/1749-8104-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler C, Fetter R, Bargmann C. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson J, et al. Complementary RNA amplification methods enhance microarray identification of transcripts expressed in the C. elegans nervous system. BMC Genomics. 2008;9:84. doi: 10.1186/1471-2164-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Li H, Wadsworth W. The Roles of Multiple UNC-40 (DCC) Receptormediated Signals in Determining Neuronal Asymmetry Induced by the UNC-6 (netrin) Ligand. Genetics. 2009 doi: 10.1534/genetics.109.108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Garbe D, Bashaw G. A frazzled/DCC-dependent transcriptional switch regulates midline axon guidance. Science. 2009;324:944–947. doi: 10.1126/science.1171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsalik EL, Hobert O. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol. 2003;56:178–197. doi: 10.1002/neu.10245. [DOI] [PubMed] [Google Scholar]
- 34.Eastman C, Horvitz HR, Jin Y. Coordinated transcriptional regulation of the unc-25 glutamic acid decarboxylase and the unc-47 GABA vesicular transporter by the Caenorhabditis elegans UNC-30 homeodomain protein. J Neurosci. 1999;19:6225–6234. doi: 10.1523/JNEUROSCI.19-15-06225.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung-Hagesteijn C, et al. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell. 1992;71:289–299. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- 36.Killeen M, et al. UNC-5 function requires phosphorylation of cytoplasmic tyrosine 482, but its UNC-40-independent functions also require a region between the ZU-5 and death domains. Dev Biol. 2002;251:348–366. doi: 10.1006/dbio.2002.0825. [DOI] [PubMed] [Google Scholar]
- 37.Asakura T, Ogura K, Goshima Y. UNC-6 expression by the vulval precursor cells of Caenorhabditis elegans is required for the complex axon guidance of the HSN neurons. Dev Biol. 2007;304:800–810. doi: 10.1016/j.ydbio.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 38.Bashaw GJ, Goodman CS. Chimeric axon guidance receptors: the cytoplasmic domains of slit and netrin receptors specify attraction versus repulsion. Cell. 1999;97:917–926. doi: 10.1016/s0092-8674(00)80803-x. [DOI] [PubMed] [Google Scholar]
- 39.Gitai Z, Yu T, Lundquist E, Tessier-Lavigne M, Bargmann C. The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron. 2003;37:53–65. doi: 10.1016/s0896-6273(02)01149-2. [DOI] [PubMed] [Google Scholar]
- 40.Hiramoto M, Hiromi Y. ROBO directs axon crossing of segmental boundaries by suppressing responsiveness to relocalized Netrin. Nature Neuroscience. 2006;9:58–66. doi: 10.1038/nn1612. [DOI] [PubMed] [Google Scholar]
- 41.Kuzina I, Song JK, Giniger E. How Notch establishes longitudinal axon connections between successive segments of the Drosophila CNS. Development. 2011;138:1839–1849. doi: 10.1242/dev.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexander M, et al. An UNC-40 pathway directs postsynaptic membrane extension in Caenorhabditis elegans. Development. 2009;136:911–922. doi: 10.1242/dev.030759. [DOI] [PubMed] [Google Scholar]
- 43.MacNeil L, Hardy W, Pawson T, Wrana J, Culotti J. UNC-129 regulates the balance between UNC-40 dependent and independent UNC-5 signaling pathways. Nat Neurosci. 2009;12:150–155. doi: 10.1038/nn.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merz DC, Zheng H, Killeen MT, Krizus A, Culotti JG. Multiple signaling mechanisms of the UNC-6/netrin receptors UNC-5 and UNC-40/DCC in vivo. Genetics. 2001;158:1071–1080. doi: 10.1093/genetics/158.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang C, et al. MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr Biol. 2006;16:854–862. doi: 10.1016/j.cub.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 46.Quinn C, Pfeil D, Wadsworth W. CED-10/Rac1 mediates axon guidance by regulating the asymmetric distribution of MIG-10/lamellipodin. Curr Biol. 2008;18:808–813. doi: 10.1016/j.cub.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleming T, et al. The role of C. elegans Ena/VASP homolog UNC-34 in neuronal polarity and motility. Developmental Biology. 2010;344:94–106. doi: 10.1016/j.ydbio.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grueber WB, Sagasti A. Self-avoidance and tiling: Mechanisms of dendrite and axon spacing. Cold Spring Harb Perspect Biol. 2010;2:a001750. doi: 10.1101/cshperspect.a001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emoto K, Parrish J, Jan L, Jan Y-N. The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature. 2006;443:210–213. doi: 10.1038/nature05090. [DOI] [PubMed] [Google Scholar]
- 50.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poon V, Klassen M, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 2008;455:669–673. doi: 10.1038/nature07291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sulston J, Horvitz H. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.