Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that affects a staggering percentage of the aging population and causes memory loss and cognitive decline. Mitochondrial abnormalities can be observed systemically and in brains of patients suffering from AD, and may account for part of the disease phenotype. In this review, we summarize some of the key findings that indicate mitochondrial dysfunction is present in AD-affected subjects, including cytochrome oxidase deficiency, endophenotype data, and altered mitochondrial morphology. Special attention is given to recently described perturbations in mitochondrial autophagy, fission-fusion dynamics, and biogenesis. We also briefly discuss how mitochondrial dysfunction may influence amyloidosis in Alzheimer’s disease, why mitochondria are a valid therapeutic target, and strategies for addressing AD-specific mitochondrial dysfunction.
Keywords: Alzheimer’s disease, autophagy, bioenergetics, biogenesis, fission, mitochondria, mitochondrial DNA, mitochondrial dysfunction, mitochondrial medicine
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized clinically by cognitive decline and memory loss. It is estimated that approximately 5.4 million Americans suffer from AD (Thies and Bleiler, 2011). One in eight individuals over 65 have AD, and 40–50% of those over 85 years of age are affected (Evans et al., 1989).
Mutations in the amyloid precursor protein (APP) gene and in two genes that encode proteins that participate in, among other things, APP processing associate with rare, autosomal dominant, pre-senile AD cases (Goate et al., 1991; Levy-Lahad et al., 1995; Sherrington et al., 1995; Wolfe et al., 1999). The vast majority of AD, however, occurs in individuals over the age of 65, and both prevalence and incidence increase with advancing age. This suggests that age-related physiologic changes play a critical role in the vast majority of those affected with AD (Corrada et al., 2008; Corrada et al., 2010).
Although much remains to be learned about both AD etiology and pathogenesis, it is clear that mitochondrial function in the brains of AD subjects differs from those of non-AD subjects. Equally interesting, and perhaps pertinent to the issue of AD etiology, is the fact that mitochondrial function in the non-brain tissues of AD subjects also differs from that of control subjects. This review will discuss mitochondrial dysfunction and its potential role in AD, and will emphasize data that show bioenergetic homeostasis is perturbed in AD.
Is sporadic Alzheimer’s disease a primary or secondary amyloidosis?
In 1906, Alois Alzheimer described a 55-year old patient with severe dementia. Post-mortem evaluation revealed extensive amyloid plaques in this patient’s cerebral cortex (Alzheimer, 1907; Verhey, 2009). In the 1970s, Katzman and colleagues extended the term “Alzheimer’s disease” to include senile dementia, a condition not previously thought to critically overlap with the pre-senile dementia disorder described by Alzheimer. This was accomplished by using the presence of amyloid plaques and neurofibrillary tangles to argue the existence of a common etiology, and infer that all tangle and plaque dementias were a single disease (Katzman, 1976). The identification of deterministic mutations within the APP gene, from which beta amyloid (Aβ) derives (Goate et al., 1991), subsequently gave rise to the amyloid cascade hypothesis (Hardy and Allsop, 1991; Hardy and Higgins, 1992). This hypothesis has profoundly influenced AD conceptual thinking. Most investigators in the AD field currently assume that pre-senile, autosomal dominant cases of AD with APP mutations are instigated through abnormal amyloid processing, although the possibility that disease arises as a consequence of perturbed APP function has not been fully excluded (Galvan et al., 2006).
Problems arise when using the amyloid cascade hypothesis to explain the pathophysiology of senile, sporadic forms of AD. For example, unlike in autosomal dominant forms, no apparent mutations exist in APP or processing proteins. Even genetic polymorphisms in APP seem to have little or no effect on sporadic AD risk (Guyant-Marechal et al., 2007). Amyloid levels in the brain can exist in the absence of clinical symptoms, and elevated Aβ levels preclude biochemical and clinical signs of neurodegeneration by years. Also, it is well recognized that elevated brain parenchyma Aβ does not necessarily associate with clinical dementia (Berlau et al., 2009; Sperling et al., 2011). If amyloidosis were sufficient to cause disease, it seems unlikely that individuals with elevated Aβ could remain asymptomatic for so long.
A key point to keep in mind, though, is that even if all AD cases include brain amyloidosis, the question of whether particular forms of AD represent primary or secondary amyloidoses must still be considered. The extremely rare APP mutation cases have the highest likelihood of being primary amyloidoses. In the absence of deterministic mutations, other physiologic events may be required to induce amyloidosis. If this presumption is correct then these cases are secondary amyloidoses. Recently, it has become abundantly clear that APP metabolism is an exquisitely regulated process, and bioenergetic status or states indeed regulate APP metabolism (Gabuzda et al., 1994; Gasparini et al., 1997; Khan et al., 2000; Webster et al., 1998).
Mitochondria in AD
Mitochondrial structural and functional perturbations in AD have been recognized for some time. For example, in 1985 the morphology of mitochondria in degenerating dendrites from brains of AD patients was noted to be abnormal, and postulated to perhaps even precede dendritic degeneration (Saraiva et al., 1985). One year later, Peterson et al. described altered calcium homeostasis in AD patient fibroblasts, a finding potentially consistent with the presence of a systemic bioenergetic defect (Peterson and Goldman, 1986). The activities of several mitochondria-localized enzymes, including α-ketoglutarate dehydrogenase complex and pyruvate dehydrogenase complex, are reduced in AD, and this observation encouraged some to propose AD is a disease of perturbed brain energy metabolism (Blass et al., 2002; Gibson et al., 1998; Gibson et al., 1988; Sorbi et al., 1983).
Cytochrome oxidase activity is systemically reduced in AD
Cytochrome oxidase (COX) is the final enzyme in the mitochondrial respiratory electron transport chain (ETC). It receives electrons from cytochrome c. Mammalian COX exists as a dimer with 13 protein subunits per monomer. Ten are encoded by nuclear and three by mitochondrial DNA (mtDNA) genes. In addition to its protein moiety, COX contains two redox active hemes and two redox active copper centers.
In 1990, Parker et al. reported that AD patients have a non-brain limited reduction in COX activity, as the COX defect in this study was observed in AD subject platelet mitochondria (Parker et al., 1990). This observation was subsequently confirmed in brains of AD patients (Kish et al., 1992). Since then, other studies and investigators have documented that COX activity is systemically altered in AD subjects (Bosetti et al., 2002; Cardoso et al., 2004a; Curti et al., 1997; Mutisya et al., 1994; Parker et al., 1994; Parker and Parks, 1995; Swerdlow, 2011b; Wong-Riley et al., 1997). COX reduction has also been reported to occur at the mild cognitive impairment (MCI) stage of AD (Valla et al., 2006).
Animal studies suggest COX dysfunction may represent an early manifestation of Aβ toxicity. In AD mouse models, Aβ co-localizes with mitochondria, and Aβ also appears to co-localize with mitochondria in human AD brains (Caspersen et al., 2005; Crouch et al., 2005; Hansson Petersen et al., 2008; Lustbader et al., 2004; Manczak et al., 2006). APP transgenic mice display early evidence of mitochondrial perturbation, as manifested by an increased expression of COX genes at only two months of age (Reddy et al., 2004).
The role of COX dysfunction in AD, whether genetically transmitted, somatically acquired, or induced through toxic insults such as Aβ has been debated (Farber et al., 2000; Parker et al., 1990; Swerdlow, 2011b; Swerdlow and Kish, 2002). In addition to Aβ, another candidate toxic insult derives from studies of metal and ion toxicity. It is known that Al3+, Fe3+ and Ca2+ are elevated in AD brains (Good et al., 1992; Huang et al., 2004; Ito et al., 1994). Alleyne and colleagues (Alleyne et al., 2011), using a rabbit model (Klatzo et al., 1965), evaluated the impact of elevated metal ions on brain COX catalytic activity (Vmax) and substrate binding ability (Km). They reported that 10 days after intra-cerebral injection of 1.4% solutions of AlCl3, FeCl3, CaCl2, or MgCl2, the isolated brain mitochondria showed significantly decreased COX Vmax activities. The increased metal ion content that developed within the mitochondria enhanced the ionic strength of the inter-membrane space, which slowed the interaction between COX and its substrate, cytochrome c. One immediate consequence of this included excess oxygen radical production, which damaged the enzyme itself, damaged surrounding structures, and decreased ATP synthesis.
AD mitochondrial dysfunction is perpetuated in cybrid cell lines
Experimental data exist that suggest COX impairment is a downstream consequence, and not an initiating cause, of AD pathology. In the study by Fukui et al., it was found that the brains of mice expressing mutant APP and mutant presenilin 1 in a neuron-specific COX-deficient background exhibited significantly fewer amyloid plaques, a reduction in Aβ42 levels, and reduced oxidative changes as compared to COX-competent transgenic mice (Fukui et al., 2007). The authors of this study concluded that COX impairment may not be responsible for Aβ accumulation in the AD brain.
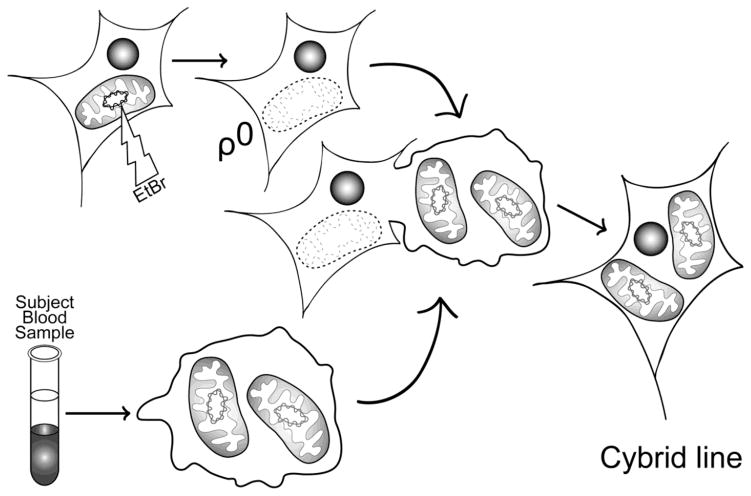
Other data though, argue that the systemic AD COX defect can drive a number of disease-typical pathologic phenomena. Much of these data derive from studies of cytoplasmic hybrid (cybrid) cell lines; cybrid studies further suggest mtDNA is also at least partly responsible for reduced COX activity in AD patients (Swerdlow et al., 1997). When platelet mtDNA from AD patients is expressed within neuronal cell lines grown in culture, the resulting cybrid cell lines manifest a reduction in COX activity (Figure 1). This specific biochemical defect persists over time in the AD cybrid lines (Swerdlow, 2007; Swerdlow, 2011b; Swerdlow et al., 1997). It has further been observed that cybrid lines containing AD subject mitochondria/mtDNA overproduce oxygen radicals, accumulate Aβ, and have decreased ATP levels compared to control cybrid lines containing mtDNA from unaffected individuals (Cardoso et al., 2004b; Khan et al., 2000; Swerdlow, 2007). Since three of the 13 COX subunits are encoded by mtDNA, this phenomenon suggests mtDNA differs between AD patients and control subjects, and supports the view that mtDNA contributes to the AD-associated COX activity reduction. Taken together, these results support hypotheses which place mitochondrial dysfunction at the center of AD pathogenesis (Swerdlow et al., 2010; Swerdlow and Khan, 2004; Swerdlow and Khan, 2009; Swerdlow et al., 1997).
Figure 1.
Generation of cybrid cell lines. Tumor or immortalized cell lines are grown in the presence of ethidium bromide, which effectively eliminates functional mtDNA to result in a ρ0 cell line. ρ0 cells are then fused with a patient’s platelets, which contain mitochondria but not nuclei. This creates cytoplasmic hybrid (cybrid) cells that can be isolated and expanded. The expanded cybrid cell cultures are biochemically analyzed. Differences in function between cell lines mostly likely arise through differences in their mtDNA.
Mitochondrial cascade hypothesis
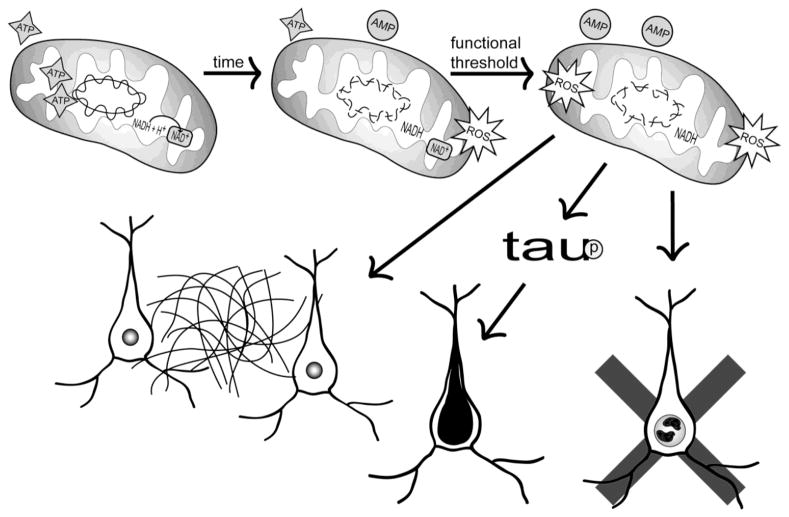
The apparent importance of mitochondria in AD and the inability of Aβ protein accumulation to fully account for the epidemiology and pathology of sporadic AD, as well as systemic biochemical perturbations in AD subjects, led Swerdlow and Khan to propose the mitochondrial cascade hypothesis (Swerdlow and Khan, 2004). Briefly, this hypothesis proposes that inherited mutations in mtDNA determine the basal functional ability of mitochondria and their ability to respond to and recover from stress signaling that is mediated by molecules such as reactive oxygen species (ROS). At the point at which a critical threshold of mitochondrial dysfunction is reached, the histopathology of AD develops, and includes neuronal apoptosis, β-amyloid deposition, and neurofibrillary tangles (Figure 2).
Figure 2.
Mitochondrial cascade hypothesis. Bi-parental inheritance of genes required for mitochondrial function and maintenance, as well as the specific maternal inheritance of mitochondrial genes, determines intrinsic mitochondrial function and durability. Over time, mitochondrial injuries accumulate and these injuries perturb mitochondrial function. While these perturbations are initially compensated for, compensatory changes eventually prove inadequate and a critical point is reached where the neuron comes to favor anaerobic over aerobic bioenergetics. Activation of various cell stress pathways, combined with a shift from an aerobic to an anaerobic bioenergetic profile, gives rise to AD-typical histology changes including Aβ accumulation in the brain parenchyma, tau phosphorylation and neurofibrillary tangle formation in neurons, and synaptic degeneration,
Maternal transmission of AD
Although gene mutations have been identified in the early onset familial forms of AD, and some risk factor genes have been uncovered in the far more common late-onset forms (Corder et al., 1993), the genetic factors that influence sporadic AD risk are overall less clear-cut. Other such factors likely exist, though, since for cognitively normal individuals of advanced age having a first-degree family history of late-onset AD constitutes a robust risk factor (Farrer et al., 1997; Silverman et al., 1994). First-degree relatives of affected probands have a four to tenfold higher risk of developing AD than the general population, with some variability depending on which family members are affected (Cupples et al., 2004; Green et al., 2002; Silverman et al., 2005).
The possibility that maternally inherited mtDNA may influence disease risk and pathology has been considered. While several studies conclude there is no evidence of a unique maternally-inherited AD risk factor or even that there is predominant paternal transmission (Ehrenkrantz et al., 1999; Payami and Hoffbuhr, 1993), other epidemiological studies find maternal inheritance strongly influences AD risk (Duara et al., 1993; Edland et al., 1996). Among AD patients with one affected parent, the ratio of mothers to fathers affected is 3:1. For cases in which affected proband relations include one affected parent and at least one sibling, the mother to father ratio increases to 9:1 (Edland et al., 1996).
Brain imaging techniques also provide evidence of a unique maternal genetic contribution. Positron emission tomography (PET) imaging, when using 2-[18F] fluoro-2-deoxy-D-glucose (FDG) as the ligand, can be used to determine the cerebral metabolic rate of glucose (CMRglc). It has been demonstrated that in AD patients, CMRglc is reduced in several neuroanatomic areas including the parietotemporal, posterior cingulate, and to a lesser extent frontal cortex and medial temporal lobe regions (Mosconi, 2005). These reductions occur years before AD symptom onset (Jack et al., 2010). One FDG-PET study reported that cognitively normal subjects (aged from 46–80) with AD mothers but not AD fathers had AD-like patterns of CMRglc reduction even after accounting for other possible AD risk factors including age, gender, education, apolipoprotein E (APOE) genotype, and subjective memory complaints (Mosconi et al., 2007).
In a related study, COX activity was determined in platelet mitochondria from 36 cognitively normal individuals with or without a family history of late-onset AD. It was found that the subjects with an AD-affected mother had 30% lower COX activity when compared to subjects with a paternal history or no family history. No differences were found between the latter two groups (Mosconi et al., 2011). This result remained significant even after controlling for age, gender, education, and APOE genotype.
Other studies report evidence of a maternally-inherited AD endophenotype. These studies include demonstrations of increased brain amyloid deposition in the cognitively intact children of AD mothers, increased isoprostanes and AD-like Aβ42 changes in cerebrospinal fluid from cognitively intact children of AD mothers, increased degrees and rates of brain atrophy in cognitively intact children of AD mothers, and a potential relative softening of memory test performance in cognitively intact children of AD mothers (Berti et al., 2011; Debette et al., 2009; Honea et al., 2011; Honea et al., 2010; Mosconi et al., 2010a; Mosconi et al., 2009; Mosconi et al., 2010b).
AD endophenotype and some epidemiologic studies, therefore, infer that although maternal and paternal inheritance influences AD risk, maternal inheritance has a bigger impact. These studies, though, cannot directly prove that mtDNA represents the responsible genetic factor, although reduced COX activity in the children of AD mothers, as well as AD cybrid data, indicate mtDNA should be strongly suspect. The rules of mitochondrial genetics, which deviate from those of Mendelian genetics, also make mtDNA a particularly attractive candidate. Indeed, a hypothesis of mtDNA-mediated sporadic disease inheritance was previously proposed by Parker (Parker, 1990). This hypothesis stresses that, due to factors such as maternal inheritance, heteroplasmy, threshold, and mitotic segregation, mtDNA inheritance may not only give rise to matrilineal patterns of disease transmission, but also to apparent sporadic disease transmission.
Heteroplasmy refers to the fact that not all mtDNA molecules within a cell are equivalent. Different distributions of a heteroplasmic mutation may allow the sparing versus impairment of different brain regions, and also mtDNA variation between oocytes. Thus, as individual mitochondria segregate during embryonic development, major regional differences in mtDNA may result (Parker et al., 1990; Swerdlow and Khan, 2004).
Mitochondrial function affects Aβ processing
Accumulating data indicate mitochondrial bioenergetics, bioenergetic homeostasis, and probably brain metabolism in general affect APP processing (Brody et al., 2008; Gabuzda et al., 1994; Gasparini et al., 1997; Kang et al., 2009; Khan et al., 2000; Webster et al., 1998). To better understand this phenomenon, it is worth considering how mitochondrial function impacts certain cell signaling pathways.
NAD+/NADH ratio and the cell redox status
Mitochondria oxidize reduced NADH to NAD+. This activity is integrated into other cell bioenergetics-related pathways, such as glycolysis, which requires the presence of NAD+ to proceed. The primary role of complex I (NADH:ubiquinone oxidoreductase) in the ETC is to accept high energy electrons from NADH, which is produced during glycolysis, the tricarboxylic acid cycle, or other specific redox reactions. A malate-aspartate shuttle allows the transfer of NADH reducing equivalents from the cytosol and into the mitochondrial matrix. When mitochondria are inhibited experimentally or functioning suboptimally, the ratio of NAD+/NADH within the cell can substantially decrease (Braidy et al., 2011; Schuchmann et al., 2001; Stefanatos and Sanz, 2011). Conversely, cells with relatively healthy mitochondria may have a high NAD+/NADH ratio. NAD+ is utilized by and activates other enzymes, including the sirtuin (silent mating type information regulation two homolog) family of proteins. Sirtuins are increasingly believed to play roles in neuroprotection and longevity, and sirtuins may represent AD therapeutic targets (Guarente, 2011; Lombard et al., 2011; Rahman and Islam, 2011; Zhang et al., 2011a; Zhang et al., 2011b).
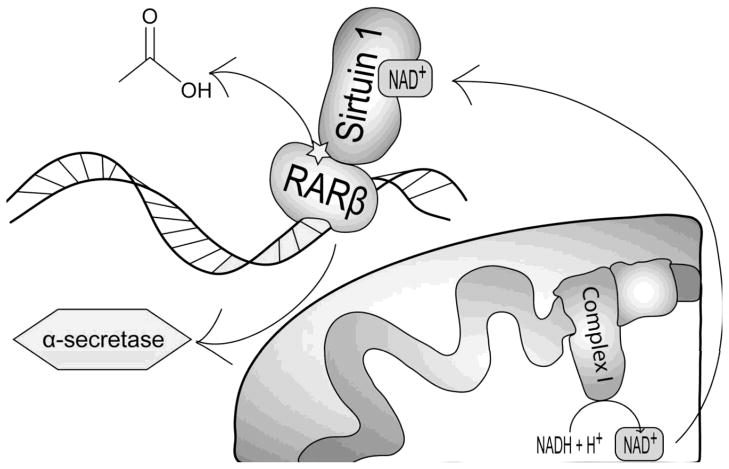
Recently, sirtuin 1 protein, which is encoded by the gene SIRT1, has been shown to play a potential role in APP processing. In 2010, Donmez et al. showed that sirtuin 1 upregulates expression of ADAM10, which encodes an α-secretase (Donmez et al., 2010). Specifically, the authors showed that in a transgenic mouse model of AD, co-transgenic overexpression of SIRT1 resulted in a significant reduction of amyloid plaques and Aβ42 levels. Brain-specific deletion of SIRT1 induced significant mortality at age 3–5 months in mice transgenic for mutant human APP and presenilin transgenes. It was further demonstrated that SIRT1 overexpression resulted in significantly higher α-secretase protein levels, which coincided with increased levels of α-secretase cleavage products. From a mechanistic perspective, sirtuin 1 seemed to interact with and deacetylate the retinoic acid receptor β (RARβ) to increase ADAM10 transcription, as the ADAM10 promoter contains a retinoic acid receptor element (Figure 3).
Figure 3.
Role of NAD+/NADH in APP processing. In the mitochondria, NADH is oxidized to NAD+, which in turn increases cytosolic NAD+. Cytosolic NAD+ activates sirtuin 1, which leads to de-acetylation of retinoic acid receptor β. De-acetylation of retinoic acid receptor β allows it to interact with the α-secretase promoter and increase α-secretase expression. α-secretase-mediated APP cleavage prevents processing of APP to Aβ.
By shifting the cell NAD+/NADH redox balance towards a more reduced state, mitochondrial dysfunction could reduce the amount of NAD+ that is available to sirtuin 1. This in turn could reduce α-secretase levels and divert APP processing towards its β-secretase derived Aβ product.
Oxidative stress
Oxidative stress is believed to represent an early manifestation of AD pathology (Nunomura et al., 2001). Numerous studies indicate that AD brains have oxidized RNA, nuclear and mtDNA, lipids, and proteins (Gabbita et al., 1998; Mecocci et al., 1994; Nunomura et al., 1999; Sayre et al., 1997; Smith et al., 1991). It has long been held that mitochondria are the primary source of intracellular ROS, and that elevated ROS may correlate with mitochondrial dysfunction (Shigenaga et al., 1994). Depending on cell type and intracellular localization, ROS can have a vast array of functions, some beneficial and necessary, others detrimental and pathological (Finkel, 2011). Considerable data perhaps implicate a mechanistically relevant role for ROS in AD. In particular, evidence suggests elevated ROS upregulate Aβ production (Tamagno et al., 2002).
Studies in COS, PC12, and neuroglioma cell lines indicate that perturbation of mitochondrial function with sodium azide, oligomycin, or carbonyl cyanide m-chlorophenylhydrazone shifts APP processing away from its α-secretase cleavage, thus decreasing soluble APP derivatives and likely promoting the amyloidogenic beta secretase-mediated cleavage of APP (Gabuzda et al., 1994; Gasparini et al., 1997; Webster et al., 1998). In one study, glutathione supplementation restored soluble APP processing, suggesting oxidative stress was to some degree responsible for diverting APP processing away from the α-secretase cut (Gasparini et al., 1997).
The identification of signaling molecules sensitive to ROS has helped to further implicate a role for ROS in driving amyloidosis (Figure 4). For example, a 1998 report by Saitoh et al. reported the redox homeostatic protein thioredoxin binds to and inactivates the mitogen activator protein kinase kinase kinase (MAPKKK) ASK1 (apoptosis signal-regulating kinase) (Saitoh et al., 1998). ROS can directly oxidize thioredoxin, and when this occurs thioredoxin dissociates from ASK1 (Finkel, 2011; Gotoh and Cooper, 1998; Saitoh et al., 1998; Shen and Liu, 2006). Free ASK1 can undergo phosphorylation, and subsequently activate its downstream targets. One of these targets includes c-Jun N-terminal kinase (JNK) (Filomeni et al., 2003; Filomeni et al., 2005).
Figure 4.
Role of ROS in APP processing. (A) Excess ROS are produced by dysfunctional mitochondria. (B) ROS oxidizes thioredoxin, releasing it from ASK1. (C) ASK1 causes JNK activation. (D) Alternatively, ROS oxidizes GST, releasing JNK. (E) Activated JNK deacetylates histones and demethylates APP, BACE, and presenilin gene promoters, which leads to increased Aβ production.
In addition to its upregulation by ASK1, ROS may activate JNK through other mechanisms (Figure 4). Adler et al. showed that glutathione S-transferase (GST) associates with and inhibits JNK (Adler et al., 1999a; Adler et al., 1999b). This may partly explain why, in the absence of oxidative stress, JNK activity tends to remain low. In the presence of oxidative stress, however, GST is oxidized. Oxidized GST no longer binds JNK, thus preventing GST-mediated JNK inhibition.
Recently, Guo et al. showed that anisomycin-induced JNK activation significantly increased Aβ production in SH-SY5Y neuroblastoma cells (Guo et al., 2011). These investigators further showed that activation of JNK caused upregulation of APP, β-site APP cleaving enzyme 1 (BACE1), and the presenilin 1 gene through demethylation of their respective promoters and histone deacetylation. These effects would collectively be expected to increase Aβ production. Studies such as these provide additional insight into how a primary mitochondrial defect might drive amyloidosis.
Mitochondrial biomass and homeostasis in AD
Because of their high energy demands, neurons are especially dependent on mitochondrial dynamics, and tightly control their mitochondrial mass (Santos et al., 2010). When neurons accumulate dysfunctional mitochondria or experience increased metabolic or bioenergetic demands, they must pursue one or a combination of several strategies to ensure sustained function. One response includes autophagy, an important mechanism through which abnormal mitochondrial are decommissioned and eliminated. Fusion and fission events help traffic abnormal mitochondria toward autophagic processing, and help maintain normal mitochondrial numbers throughout the neuron. Biogenesis produces new mitochondria and increases mitochondrial mass. Evidence suggests that cells tightly regulate mitochondrial fission, fusion, autophagy, and biogenesis in order to maintain a healthy mitochondrial population (Twig et al., 2008b).
Mitochondrial autophagy in AD
Autophagy helps cells eliminate unnecessary cytoplasmic contents via phagosome formation and lysosomal degradation (Yang and Klionsky, 2011; Youle and Narendra, 2011). In hepatic cells, inhibition of mitochondria by the antiretroviral drug efavirenz significantly upregulates mitochondrial autophagy, and ultimately appears to have a protective effect (Apostolova et al., 2011a; Apostolova et al., 2011b). Findings like these support the view that autophagic elimination of dysfunctional mitochondria under some conditions could prove physiologically advantageous. This may have important implications for AD, since AD is characterized by the presence of dysfunctional mitochondria.
In 2001, Hirai et al. reported that when mtDNA contained within phagocytized mitochondria are specifically accounted for, AD subject hippocampal neurons can actually contain elevated levels of mtDNA (Hirai et al., 2001). This finding suggests that mitochondrial autophagy rates are increased in AD. It has also been shown that in AD brains other mitochondrial components such as COX and lipoic acid, a cofactor utilized by pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, concentrate within autophagosomes (Hirai et al., 2001; Moreira et al., 2007b). Ultrastructural analysis of dystrophic neurites in AD brains indicates that most organelles present are autophagosomes, further supporting the view that autophagy is increased in AD neurites (Nixon et al., 2005). In healthy neurons, by contrast, autophagosome accumulation is rarely observed (Boland et al., 2008; Nixon and Yang, 2011).
Some studies find that autophagic fluxes are impaired in AD (Cataldo et al., 1995; Nixon and Yang, 2011), which could potentially account for increased numbers of autophagosomes in AD neurons. Whether increased mitochondrial autophagy in AD is due to a break-down in autophagy pathways or increased flux through functioning autophagy pathways remains to be seen. The need to dispose of elevated numbers of dysfunctional mitochondria could conceivably lead to either or both possibilities.
Several pathways that monitor cell energy status link mitochondria with autophagy and permit mitochondrial function to regulate autophagy activity. Nutrient deprivation and decreased bioenergetic capacity of cells have been shown to inhibit cytosolic mammalian target of rapamycin (mTOR) signaling (Noda and Ohsumi, 1998; Scott et al., 2004; Tee et al., 2005). Inhibition of mTOR allows the initiation of autophagosome formation (Crespo et al., 2005), thus promoting autophagy. Decreased mitochondrial function can also increase cell AMP/ATP ratios. Elevating AMP relative to ATP activates AMP kinase (AMPK), which modifies a wide range of cellular functions in order to maintain energy homeostasis (Carling et al., 1994; Hardie and Hawley, 2001; Mihaylova and Shaw, 2011). Interestingly, AMPK can inhibit the mTOR signaling pathway, and thus upregulate autophagy (Meley et al., 2006), suggesting that high cellular AMP levels due to mitochondrial dysfunction may activate autophagic clearance of mitochondria. Indeed, one study found that in AD patients, lymphocyte mTOR levels directly correlate with cognitive decline (Paccalin et al., 2006). This study is consistent with the view that systemic mitochondrial deficits occur in AD, and further suggests a potential role for perturbed mTOR signaling-autophagy relationships in AD.
Mitochondrial fission and fusion
Mitochondrial fission and fusion are necessary for proper mitochondrial function (Santos et al., 2010). For example, inhibition of fusion by genetic knockout of mitofusin 2 (Mfn2) causes severe mitochondrial dysfunction that drives neuronal degeneration (Chen et al., 2007).
Impaired fission also interferes with the cell’s ability to target dysfunctional mitochondria for autophagic removal. It is believed that fission divides a dysfunctional mitochondrion into two unequal daughter mitochondria, one with seemingly normal mitochondrial markers and the other with dysfunctional markers. The dysfunctional daughter unit is then packaged for autophagy, while the healthy unit is free to undergo fusion (Twig et al., 2008a).
In AD, fission seems to be particularly perturbed (Manczak et al., 2011; Wang et al., 2008a; Wang et al., 2009; Wang et al., 2008b). This phenomenon is not brain limited, but appears to also apply to fibroblasts from sporadic AD subjects (Wang et al., 2008a). Wang et al. reported that in 19% of fibroblasts from AD patients, mitochondria were excessively localized to the perinuclear region and were dramatically elongated. Additionally, it was found that dynamin-related protein 1 (Drp1), a protein that plays a major role in mitochondrial fission, was significantly decreased and that experimental over-expression of Drp1 in these cells rescued the abnormal mitochondrial morphology. In this study, the exaggerated mitochondrial perinuclear distribution further emphasizes that fission-fusion dynamics influence mitochondrial transport, since abnormally sized mitochondria may distribute throughout the cell differently than normal mitochondria.
In possible contrast to the Wang et al. study, Manczak et al. more recently reported Drp1 mRNA and protein were increased in AD subject autopsy brains (Manczak et al., 2011). However, this group also described a significant increase in Fis1, a mitochondrial fission protein, and a decrease in the Mfn1 and Mfn2 fusion proteins. These findings further support the case that mitochondrial fission and fusion are disrupted in AD.
Mitochondrial biogenesis
Mitochondrial biogenesis is the process by which cells generate new mitochondria and, if necessary, increase mitochondrial mass. This process involves coordinated expression of proteins encoded by nuclear and mitochondrial DNA. To accomplish this, peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α) coordinates mitochondrial biogenesis in at least some tissues such as muscle, heart, liver, and pancreas via co-activation of various transcription factors (Finck et al., 2006; Finck and Kelly, 2006; Lehman et al., 2000; Lin et al., 2002; Onyango et al., 2010; Rhee et al., 2003).
PGC1α is regulated by several metabolism-responsive elements. Sirtuin 1, which is activated by NAD+, de-acetylates PGC1α. This is an activating de-acetylation that helps to increase mitochondrial biogenesis (Lagouge et al., 2006; Nemoto et al., 2005). AMP kinase (AMPK), which is activated by elevated AMP/ATP ratios, can phosphorylate and thus directly activate PGC1α (Jager et al., 2007; Reznick and Shulman, 2006). For example, AMPK activation of PGC1α in rat visual cortical neurons increases mitochondrial mass (Yu and Yang, 2010). AMPK can also modulate the activities of other proteins, such as mTOR and forkhead box containing protein O (FOXO), to further affect PGC1α activity (Cunningham et al., 2007; Daitoku et al., 2003; Greer et al., 2009; Nakae et al., 2008a; Nakae et al., 2008b).
It was recently shown that PGC1α mRNA and protein levels are reduced in AD subject brains (Qin et al., 2009; Sheng et al., in press; Swerdlow, 2011a). Even if PGC1α changes represent a consequence as opposed to cause of AD pathology, PGC1α remains an attractive target for therapeutic intervention. Whether mitochondrial mass changes in AD constitute an upstream or downstream physiologic event, it is reasonable to postulate that increasing mitochondrial mass may alleviate bioenergetics-related stress in the AD brain.
Mitochondrial transport
Evidence suggests that mitochondria can be rapidly transported to areas of high bioenergetic demand as required by the neuron (Hollenbeck and Saxton, 2005; MacAskill et al., 2010). This mitochondrial transport appears to be important for the development, stability, and function of synapses and dendritic spines (Li et al., 2004; Mattson et al., 2008), and impaired mitochondrial trafficking has been implicated in a number of neurodegenerative disorders such as Huntington’s disease (Reddy and Shirendeb, 2011), Parkinson’s disease (Sterky et al., 2011), and amyotrophic lateral sclerosis (De Vos et al., 2007). Altered mitochondrial transport may also play a role in AD (Wang et al., 2008a). One study found that mitochondrial transport in AD patient brains was decreased compared to control brains (Dai et al., 2002). Trimmer and Borland showed that the transport of fluorescently-labeled mitochondria in cybrids generated from AD patients is significantly reduced compared to control cybrids, suggesting that mitochondrial transport may be impaired in AD (Trimmer and Borland, 2005). Mitochondrial transport may also be altered in mouse models of AD (Calkins et al., 2011; Massaad et al., 2010; Pigino et al., 2003) and in cell cultures treated with Aβ (Calkins and Reddy, 2011). These studies together argue that impaired mitochondrial transport impacts the pathogenesis of AD.
Mitochondria as an AD therapeutic target
Data discussed thus far suggest that abnormal mitochondrial dynamics and function play an important role in AD neurodysfunction and neurodegeneration. Certainly, if anti-amyloid treatments are found to inadequately benefit AD patients (Herrmann et al., 2011), alternative targets such as mitochondrial function will prove increasingly important.
Antioxidants
To date, a variety of compounds with free radical scavenging properties have been evaluated in AD subjects or AD models. Lipoic acid, a coenzyme of mitochondrial pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, is an antioxidant that readily penetrates the blood-brain barrier. It can chelate redox-active transition metals, reduce inflammation, and increase levels of acetylcholine, a neurotransmitter which is severely reduced in some AD structures (Bielarczyk et al., 2006). It can also recycle other antioxidants such as vitamin C, vitamin E, and glutathione (Su et al., 2010). Lipoic acid treatment has been shown to improve cognition and AD-related pathology in Tg2756 mice that express a mutant human APP transgene (Quinn et al., 2007). A cell-culture based study has also demonstrated that lipoic acid with or without N-acetyl cysteine decreases oxidative stress and apoptotic markers in AD subject fibroblasts (Moreira et al., 2007a). A clinical study of 43 AD patients reported that over the course of approximately one year, taking 600 mg per day of lipoic acid appeared to slow disease progression as measured by neuropsychological tests (Hager et al., 2007). This study, though, was not placebo-controlled, double-blinded, or randomized and therefore more conclusive studies are needed.
Coenzyme Q10 (CoQ10), which participates in ETC electron transfer, also functions as an endogenous antioxidant. In the ETC, CoQ10 accepts electrons from complex I and II. Animal studies using aged APP/presenilin 1 transgenic mice report oral CoQ10 attenuates oxidative stress, tau-related pathology, intracellular Aβ levels, and amyloid plaque deposition (Yang et al., 2010; Yang et al., 2008). Idebenone, a water-soluble, synthetic variant of CoQ10, has also been tested in AD patients. Two randomized, double-blind, placebo-controlled studies with over 300 mild-to-moderate AD patients reported that idebenone (90 mg three times daily) for either one year (Gutzmann and Hadler, 1998; Weyer et al., 1997) benefited cognitive outcome measures. A third idebenone trial tested three different idebenone doses (120, 240, or 360 mg) in 536 AD subject. Although each individual dose failed to demonstrate clinical benefits, when all three idebenone groups were combined, cognitive decline overall appeared to slow in the idebenone-treated subjects (Thal et al., 2003).
When it comes to designing potential antioxidant approaches, cell compartmentalization issues require consideration. Antioxidants that only access the cytosol may have a limited impact on mitochondrial ROS (Su et al., 2010). To address this concern, a mitochondrial-targeted antioxidant, MitoQ, has been produced by conjugation of the lipophilic triphenylphosphonium cation to coenzyme Q (Murphy and Smith, 2000). This molecule’s cationic properties allow it to concentrate within negatively charged mitochondrial matrices.
Studies have shown that in N2a neuroblastoma cells and primary neurons from Tg2576 mice, MitoQ treatment mitigates the effects of Aβ exposure, which include altered peroxiredoxin expression, altered mitochondrial structural gene expression, mitochondrial oxidative stress, reduced COX activity, and reduced ATP levels (Manczak et al., 2010). Another study reported that MitoQ prevents Aβ-induced long-term potentiation impairments in hippocampal slices from transgenic mice (Ma et al., 2011). These results suggest mitochondrial antioxidant targeting may confer more robust physiologic effects than non-targeted antioxidants. This is important because antioxidant trials in AD have to date yielded disappointing results.
PGC1α
As discussed above, PGC1α is a key regulator of mitochondrial biogenesis and respiration. In the first reported study of AD brain PGC1α levels, Qin et al. analyzed postmortem brain samples and found that relative to control brains PGC1α mRNA expression and protein levels were decreased (Qin et al., 2009). PGC1α expression was negatively associated with both AD-type neuritic plaque pathology and total Aβ levels, suggesting that either Aβ interferes with PGC1α, or that reduced amyloidosis is a downstream effect of sustained PGC1α expression. This study went on to show that viral-mediated PGC1α expression in primary Tg2576 cortico-hippocampal neurons reversed glucose-induced Aβ peptide production.
Overexpression of PGC1α also protects neurons from degeneration induced by oxidative stress or mutant huntingtin, and increases neurite mitochondrial density (Cui et al., 2006; St-Pierre et al., 2006; Wareski et al., 2009). Overall, data suggest reversing the AD PGC1α deficit, or otherwise enhancing PGC1α activity or expression, constitutes a reasonable AD therapeutic target.
Exercise
Exercise has been shown to decrease ROS production rates in different organs, including the brain (Molteni et al., 2004; Ogonovszky et al., 2005; Radak et al., 2001). Given that there are abnormal levels of oxidative stress in AD brains, exercise may help to mitigate this phenomenon. In one pertinent study that used 13-month old NSE/APPsw transgenic mice, a 16-week treadmill running intervention (13.2m/min, 60min/day, 5 days/week) improved spatial learning and memory, significantly decreased Aβ42 protein levels, and increased brain superoxide dismutase- 1 and catalase expression (Cho et al., 2010). These data are consistent with data from studies of TgCRND8 and 3xTg AD mice that found voluntary wheel running reduced plaque formation (Adlard et al., 2005; Garcia-Mesa et al., 2011).
Since physical exercise most likely indirectly affects the brain, exercise’s brain effects are presumably mediated by reducing some factor that is normally within the brain, or else by adding a factor. Regarding the latter possibility, one candidate that has not previously been considered is lactate. Lactate is generated through sustained or repetitive muscle contraction. Several human studies using nuclear magnetic resonance spectroscopy techniques have shown blood lactate can constitute an important neuron energy fuel that is ultimately consumed and oxidized within mitochondria (Boumezbeur et al., 2010; Gallagher et al., 2009; Quistorff et al., 2008; van Hall et al., 2009; Wyss et al., 2011). Lactate generated via astrocyte glycolysis increasingly appears to serve as a key neuron energy fuel that is essential for long-term memory formation (Suzuki et al., 2011). Glucose and lactate are not functionally interchangeable, which suggests lactate may play a unique role in brain bioenergetic metabolism. Further studies are needed to explore whether exercise-produced lactate can specifically supplement a neuron’s astrocyte-derived lactate supply.
Although cell and animal based studies suggest exercise training impacts brain mitochondria and Aβ accumulation at least in mice (Adlard et al., 2005; Boveris and Navarro, 2008), to date no studies have tested whether lactate contributes in any way to these effects. If lactate does play a role, it would have important implications for a commonly asked question: how much and what forms of exercise are likely to have the most robust impact on the brain?
Conclusions
It increasingly appears that mitochondria play an important role in AD pathogenesis. In AD subjects, mitochondrial dysfunction is not brain-localized, but rather appears to be systemic. By influencing cell bioenergetic states and fluxes, as well as the cell redox environment, mitochondria are in a position to influence brain amyloidosis. In this capacity it is conceivable that in the AD brain, mitochondrial dysfunction actually lies upstream of brain amyloidosis. Moreover, various aspects of mitochondrial function, maintenance, and homeostasis are perturbed in AD, and many of these disrupted physiologies could potentially serve as AD therapeutic targets.
Highlights.
Alzheimer’s disease patients have systemic mitochondrial dysfunction
Mitochondrial dysfunction in Alzheimer’s disease may drive other pathologies
Mitochondrial autophagy, fission, fusion, and biogenesis are altered in Alzheimer’s
Mitochondria represent a reasonable Alzheimer’s disease therapeutic target
Acknowledgments
JES is supported by the University of Kansas School of Medicine Physician Scientist Training Program. RHS is supported by the University of Kansas Alzheimer’s Center (NIA P30 AG035982) and the Frank and Evangeline Thompson Alzheimer’s Therapeutic Development Fund.
Abbreviations
- Aβ
beta amyloid
- AD
Alzheimer’s disease
- ADAM10
a disintegrin and metalloproteinase domain-containing protein 10
- AMP
adenosine monophosphate
- AMPK
AMP kinase
- APOE
apolipoprotein E
- APP
amyloid precursor protein
- ASK1
apoptosis signal-regulating kinase
- BACE
β-site APP cleaving enzyme 1
- CMRglc
cerebral metabolic rate of glucose
- COX
cytochrome oxidase
- Drp1
dynamin-related protein 1
- EtBr
ethidium bromide
- FDG
2-[18F] fluoro-2-deoxy-D-glucose
- Fis1
fission 1
- FOXO
forkhead box containing protein O
- GST
glutathione-S-transferase
- JNK
c-Jun N-terminal kinase
- Mfn
mitofusin
- mtDNA
mitochondrial DNA
- mTOR
mammalian target of rapamycin
- NAD
nicotinamide adenine dinucleotide
- PET
positron emission transmission
- PGC1α
peroxisome proliferator-activated receptor-γ-coactivator 1α
- ROS
reactive oxygen species
- SIRT
silent mating type information regulation two homolog
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, et al. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–21. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler V, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999a;18:1321–34. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler V, et al. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999b;18:6104–11. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- Alleyne T, et al. Unraveling the role of metal ions and low catalytic activity of cytochrome C oxidase in Alzheimer’s disease. J Mol Neurosci. 2011;43:284–9. doi: 10.1007/s12031-010-9436-8. [DOI] [PubMed] [Google Scholar]
- Apostolova N, et al. Autophagy as a rescue mechanism in Efavirenz-induced mitochondrial dysfunction: A lesson from hepatic cells. Autophagy. 2011a;7:1402–4. doi: 10.4161/auto.7.11.17653. [DOI] [PubMed] [Google Scholar]
- Apostolova N, et al. Compromising mitochondrial function with the antiretroviral drug efavirenz induces cell survival-promoting autophagy. Hepatology. 2011b doi: 10.1002/hep.24459. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, et al. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72:829–34. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti V, et al. Structural brain changes in normal individuals with a maternal history of Alzheimer’s. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.01.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielarczyk H, et al. RS-alpha-lipoic acid protects cholinergic cells against sodium nitroprusside and amyloid-beta neurotoxicity through restoration of acetyl-CoA level. J Neurochem. 2006;98:1242–51. doi: 10.1111/j.1471-4159.2006.03966.x. [DOI] [PubMed] [Google Scholar]
- Blass JP, et al. The role of the metabolic lesion in Alzheimer’s disease. J Alzheimers Dis. 2002;4:225–32. doi: 10.3233/jad-2002-4312. [DOI] [PubMed] [Google Scholar]
- Boland B, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28:6926–37. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti F, et al. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol Aging. 2002;23:371–6. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Boumezbeur F, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30:13983–91. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med. 2008;44:224–9. doi: 10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Braidy N, et al. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brody DL, et al. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–4. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins MJ, et al. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20:4515–29. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins MJ, Reddy PH. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer’s disease neurons. Biochim Biophys Acta. 2011;1812:507–13. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso SM, et al. Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiol Aging. 2004a;25:105–10. doi: 10.1016/s0197-4580(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, et al. Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Abeta toxicity. J Neurochem. 2004b;89:1417–26. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- Carling D, et al. Mammalian AMP-activated protein kinase is homologous to yeast and plant protein kinases involved in the regulation of carbon metabolism. J Biol Chem. 1994;269:11442–8. [PubMed] [Google Scholar]
- Caspersen C, et al. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–1. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, et al. Gene expression and cellular content of cathepsin D in Alzheimer’s disease brain: evidence for early up-regulation of the endosomal-lysosomal system. Neuron. 1995;14:671–80. doi: 10.1016/0896-6273(95)90324-0. [DOI] [PubMed] [Google Scholar]
- Chen H, et al. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–62. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Cho JY, et al. The combination of exercise training and alpha-lipoic acid treatment has therapeutic effects on the pathogenic phenotypes of Alzheimer’s disease in NSE/APPsw-transgenic mice. Int J Mol Med. 2010;25:337–46. doi: 10.3892/ijmm_00000350. [DOI] [PubMed] [Google Scholar]
- Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corrada MM, et al. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–43. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- Corrada MM, et al. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010;67:114–21. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JL, et al. Inhibition of target of rapamycin signaling by rapamycin in the unicellular green alga Chlamydomonas reinhardtii. Plant Physiol. 2005;139:1736–49. doi: 10.1104/pp.105.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch PJ, et al. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1–42. J Neurosci. 2005;25:672–9. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, et al. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Cunningham JT, et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–40. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- Cupples LA, et al. Estimating risk curves for first-degree relatives of patients with Alzheimer’s disease: the REVEAL study. Genet Med. 2004;6:192–6. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- Curti D, et al. Oxidative metabolism in cultured fibroblasts derived from sporadic Alzheimer’s disease (AD) patients. Neurosci Lett. 1997;236:13–6. doi: 10.1016/s0304-3940(97)00741-6. [DOI] [PubMed] [Google Scholar]
- Dai J, et al. Impaired axonal transport of cortical neurons in Alzheimer’s disease is associated with neuropathological changes. Brain Res. 2002;948:138–44. doi: 10.1016/s0006-8993(02)03152-9. [DOI] [PubMed] [Google Scholar]
- Daitoku H, et al. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes. 2003;52:642–9. doi: 10.2337/diabetes.52.3.642. [DOI] [PubMed] [Google Scholar]
- De Vos KJ, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16:2720–8. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, et al. Association of parental dementia with cognitive and brain MRI measures in middle-aged adults. Neurology. 2009;73:2071–8. doi: 10.1212/WNL.0b013e3181c67833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G, et al. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–32. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Duara R, et al. A comparison of familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1377–84. doi: 10.1212/wnl.43.7.1377. [DOI] [PubMed] [Google Scholar]
- Edland SD, et al. Increased risk of dementia in mothers of Alzheimer’s disease cases: evidence for maternal inheritance. Neurology. 1996;47:254–6. doi: 10.1212/wnl.47.1.254. [DOI] [PubMed] [Google Scholar]
- Ehrenkrantz D, et al. Genetic epidemiological study of maternal and paternal transmission of Alzheimer’s disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 1999;88:378–82. doi: 10.1002/(sici)1096-8628(19990820)88:4<378::aid-ajmg15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Evans DA, et al. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–6. [PubMed] [Google Scholar]
- Farber SA, et al. Acceleration of phosphatidylcholine synthesis and breakdown by inhibitors of mitochondrial function in neuronal cells: a model of the membrane defect of Alzheimer’s disease. FASEB J. 2000;14:2198–206. doi: 10.1096/fj.99-0853. [DOI] [PubMed] [Google Scholar]
- Farrer LA, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- Filomeni G, et al. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003;63:5940–9. [PubMed] [Google Scholar]
- Filomeni G, et al. Disulfide relays and phosphorylative cascades: partners in redox-mediated signaling pathways. Cell Death Differ. 2005;12:1555–63. doi: 10.1038/sj.cdd.4401754. [DOI] [PubMed] [Google Scholar]
- Finck BN, et al. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–22. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H, et al. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:14163–8. doi: 10.1073/pnas.0705738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbita SP, et al. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J Neurochem. 1998;71:2034–40. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- Gabuzda D, et al. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J Biol Chem. 1994;269:13623–8. [PubMed] [Google Scholar]
- Gallagher CN, et al. The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain. 2009;132:2839–49. doi: 10.1093/brain/awp202. [DOI] [PubMed] [Google Scholar]
- Galvan V, et al. Reversal of Alzheimer’s-like pathology and behavior in human APP transgenic mice by mutation of Asp664. Proc Natl Acad Sci U S A. 2006;103:7130–5. doi: 10.1073/pnas.0509695103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mesa Y, et al. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J Alzheimers Dis. 2011;24:421–54. doi: 10.3233/JAD-2011-101635. [DOI] [PubMed] [Google Scholar]
- Gasparini L, et al. Effect of energy shortage and oxidative stress on amyloid precursor protein metabolism in COS cells. Neurosci Lett. 1997;231:113–7. doi: 10.1016/s0304-3940(97)00536-3. [DOI] [PubMed] [Google Scholar]
- Gibson GE, et al. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105:855–70. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- Gibson GE, et al. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol. 1988;45:836–40. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- Goate A, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–6. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Good PF, et al. Selective accumulation of aluminum and iron in the neurofibrillary tangles of Alzheimer’s disease: a laser microprobe (LAMMA) study. Ann Neurol. 1992;31:286–92. doi: 10.1002/ana.410310310. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Cooper JA. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem. 1998;273:17477–82. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- Green RC, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–36. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- Greer EL, et al. AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Ann N Y Acad Sci. 2009;1170:688–92. doi: 10.1111/j.1749-6632.2009.04019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235–44. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- Guo X, et al. Epigenetic mechanisms of amyloid-beta production in anisomycin-treated SH-SY5Y cells. Neuroscience. 2011;194:272–81. doi: 10.1016/j.neuroscience.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Gutzmann H, Hadler D. Sustained efficacy and safety of idebenone in the treatment of Alzheimer’s disease: update on a 2-year double-blind multicentre study. J Neural Transm Suppl. 1998;54:301–10. doi: 10.1007/978-3-7091-7508-8_30. [DOI] [PubMed] [Google Scholar]
- Guyant-Marechal L, et al. Variations in the APP gene promoter region and risk of Alzheimer disease. Neurology. 2007;68:684–7. doi: 10.1212/01.wnl.0000255938.33739.46. [DOI] [PubMed] [Google Scholar]
- Hager K, et al. Alpha-lipoic acid as a new treatment option for Alzheimer’s disease--a 48 months follow-up analysis. J Neural Transm Suppl. 2007:189–93. doi: 10.1007/978-3-211-73574-9_24. [DOI] [PubMed] [Google Scholar]
- Hansson Petersen CA, et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U S A. 2008;105:13145–50. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–9. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–8. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Herrmann N, et al. Current and emerging drug treatment options for Alzheimer’s disease: a systematic review. Drugs. 2011;71:2031–65. doi: 10.2165/11595870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hirai K, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–23. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–9. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, et al. Progressive regional atrophy in normaladults with a maternal history of Alzheimer disease. Neurology. 2011;76:822–829. doi: 10.1212/WNL.0b013e31820e7b74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, et al. Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology. 2010;74:113–20. doi: 10.1212/WNL.0b013e3181c918cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, et al. Redox-active metals, oxidative stress, and Alzheimer’s disease pathology. Ann N Y Acad Sci. 2004;1012:153–63. doi: 10.1196/annals.1306.012. [DOI] [PubMed] [Google Scholar]
- Ito E, et al. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:534–8. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R. Editorial: The prevalence and malignancy of Alzheimer disease. A major killer. Arch Neurol. 1976;33:217–8. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- Khan SM, et al. Alzheimer’s disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000;48:148–55. [PubMed] [Google Scholar]
- Kish SJ, et al. Brain cytochrome oxidase in Alzheimer’s disease. J Neurochem. 1992;59:776–9. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- Klatzo I, et al. Experimental Production of Neurofibrillary Degeneration. I. Light Microscopic Observations. J Neuropathol Exp Neurol. 1965;24:187–99. doi: 10.1097/00005072-196504000-00002. [DOI] [PubMed] [Google Scholar]
- Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lehman JJ, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–56. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Lahad E, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–7. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Li Z, et al. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lin J, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Lombard DB, et al. Mitochondrial sirtuins in the regulation of mitochondrial activity and metabolic adaptation. Handb Exp Pharmacol. 2011:163–88. doi: 10.1007/978-3-642-21631-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader JW, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–52. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Ma T, et al. Amyloid beta-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J Neurosci. 2011;31:5589–95. doi: 10.1523/JNEUROSCI.6566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, et al. Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur J Neurosci. 2010;32:231–40. doi: 10.1111/j.1460-9568.2010.07345.x. [DOI] [PubMed] [Google Scholar]
- Manczak M, et al. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–49. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Manczak M, et al. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011;20:2495–509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, et al. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010;20(Suppl 2):S609–31. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaad CA, et al. Mitochondrial superoxide contributes to blood flow and axonal transport deficits in the Tg2576 mouse model of Alzheimer’s disease. PLoS One. 2010;5:e10561. doi: 10.1371/journal.pone.0010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, et al. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–66. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, et al. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol. 1994;36:747–51. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- Meley D, et al. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281:34870–9. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, et al. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–40. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Moreira PI, et al. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis. 2007a;12:195–206. doi: 10.3233/jad-2007-12210. [DOI] [PubMed] [Google Scholar]
- Moreira PI, et al. Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol. 2007b;66:525–32. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. European Journal of Nuclear Medicine and Molecular Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- Mosconi L, et al. Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104:19067–72. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, et al. Reduced Mitochondria Cytochrome Oxidase Activity in Adult Children of Mothers with Alzheimer’s Disease. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-110866. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, et al. Oxidative stress and amyloid-beta pathology in normal individuals with a maternal history of Alzheimer’s. Biol Psychiatry. 2010a;68:913–21. doi: 10.1016/j.biopsych.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72:513–20. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, et al. Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer’s. Proc Natl Acad Sci U S A. 2010b;107:5949–54. doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev. 2000;41:235–50. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- Mutisya EM, et al. Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. J Neurochem. 1994;63:2179–84. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- Nakae J, et al. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008a;57:563–76. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- Nakae J, et al. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008b;582:54–67. doi: 10.1016/j.febslet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Nemoto S, et al. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–60. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Nixon RA, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–22. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Yang DS. Autophagy failure in Alzheimer’s disease-locating the primary defect. Neurobiol Dis. 2011;43:38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–6. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- Nunomura A, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–67. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Nunomura A, et al. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 1999;19:1959–64. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogonovszky H, et al. The effects of moderate-, strenuous- and over-training on oxidative stress markers, DNA repair, and memory, in rat brain. Neurochem Int. 2005;46:635–40. doi: 10.1016/j.neuint.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Onyango IG, et al. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta. 2010;1802:228–34. doi: 10.1016/j.bbadis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Paccalin M, et al. Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:320–6. doi: 10.1159/000095562. [DOI] [PubMed] [Google Scholar]
- Parker WD. In: Pascuzzi RM, editor. Sporadic neurologic disease and the electron transport chain: a hypothesis; Proceedings of the 1989 Scientfic Meeting of the American Society for Neurological Investigation: New Developments in Neuromuscular Disease Indiana University Printing Services; Bloomington, Indiana. 1990. [Google Scholar]
- Parker WD, Jr, et al. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40:1302–3. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, et al. Electron transport chain defects in Alzheimer’s disease brain. Neurology. 1994;44:1090–6. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Parks JK. Cytochrome c oxidase in Alzheimer’s disease brain: purification and characterization. Neurology. 1995;45:482–6. doi: 10.1212/wnl.45.3.482. [DOI] [PubMed] [Google Scholar]
- Payami H, Hoffbuhr K. Lack of evidence for maternal effect in familial Alzheimer’s disease. Genetic epidemiology. 1993;10:461–464. doi: 10.1002/gepi.1370100622. [DOI] [PubMed] [Google Scholar]
- Peterson C, Goldman JE. Alterations in calcium content and biochemical processes in cultured skin fibroblasts from aged and Alzheimer donors. Proc Natl Acad Sci U S A. 1986;83:2758–62. doi: 10.1073/pnas.83.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, et al. Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci. 2003;23:4499–508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, et al. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66:352–61. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JF, et al. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28:213–25. doi: 10.1016/j.neurobiolaging.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Quistorff B, et al. Lactate fuels the human brain during exercise. FASEB J. 2008;22:3443–9. doi: 10.1096/fj.08-106104. [DOI] [PubMed] [Google Scholar]
- Radak Z, et al. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Rahman S, Islam R. Mammalian Sirt1: insights on its biological functions. Cell Commun Signal. 2011;9:9–11. doi: 10.1186/1478-811X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, et al. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum Mol Genet. 2004;13:1225–40. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Shirendeb UP. Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington’s disease. Biochim Biophys Acta. 2011;1822:101–110. doi: 10.1016/j.bbadis.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–9. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J, et al. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci U S A. 2003;100:4012–7. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RX, et al. A synergistic dysfunction of mitochondrial fission/fusion dynamics and mitophagy in Alzheimer’s disease. J Alzheimers Dis. 2010;20(Suppl 2):S401–12. doi: 10.3233/JAD-2010-100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva AA, et al. Mitochondrial abnormalities in cortical dendrites from patients with Alzheimer’s disease. J Submicrosc Cytol. 1985;17:459–64. [PubMed] [Google Scholar]
- Sayre LM, et al. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68:2092–7. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, et al. Monitoring NAD(P)H autofluorescence to assess mitochondrial metabolic functions in rat hippocampal-entorhinal cortex slices. Brain Res Brain Res Protoc. 2001;7:267–76. doi: 10.1016/s1385-299x(01)00080-0. [DOI] [PubMed] [Google Scholar]
- Scott RC, et al. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–78. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–39. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Sheng B, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem. doi: 10.1111/j.1471-4159.2011.07581.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–60. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, et al. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–8. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, et al. Variability of familial risk of Alzheimer disease across the late life span. Arch Gen Psychiatry. 2005;62:565–73. doi: 10.1001/archpsyc.62.5.565. [DOI] [PubMed] [Google Scholar]
- Silverman JM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part VI. Family history assessment: a multicenter study of first-degree relatives of Alzheimer’s disease probands and nondemented spouse controls. Neurology. 1994;44:1253–9. doi: 10.1212/wnl.44.7.1253. [DOI] [PubMed] [Google Scholar]
- Smith CD, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–3. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbi S, et al. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol. 1983;13:72–8. doi: 10.1002/ana.410130116. [DOI] [PubMed] [Google Scholar]
- Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Stefanatos R, Sanz A. Mitochondrial complex I: a central regulator of the aging process. Cell Cycle. 2011;10:1528–32. doi: 10.4161/cc.10.10.15496. [DOI] [PubMed] [Google Scholar]
- Sterky FH, et al. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci U S A. 2011;108:12937–42. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, et al. Abnormal mitochondrial dynamics--a novel therapeutic target for Alzheimer’s disease? Mol Neurobiol. 2010;41:87–96. doi: 10.1007/s12035-009-8095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–23. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007;85:3416–28. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Beta-apptists and Tauists, it’s Time for a Sermon from the Book of Biogenesis. J Neurochem. 2011a doi: 10.1111/j.1471-4159.2011.07561.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria and cell bioenergetics: Increasingly recognized components and a possible etiologic cause of Alzheimer’s disease. Antioxid Redox Signal. 2011b doi: 10.1089/ars.2011.4149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, et al. The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20(Suppl 2):S265–79. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218:308–15. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Kish SJ. Mitochondria in Alzheimer’s disease. Int Rev Neurobiol. 2002;53:341–85. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, et al. Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 1997;49:918–25. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- Tamagno E, et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–88. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- Tee AR, et al. Analysis of mTOR signaling by the small G-proteins, Rheb and RhebL1. FEBS Lett. 2005;579:4763–8. doi: 10.1016/j.febslet.2005.07.054. [DOI] [PubMed] [Google Scholar]
- Thal LJ, et al. Idebenone treatment fails to slow cognitive decline in Alzheimer’s disease. Neurology. 2003;61:1498–502. doi: 10.1212/01.wnl.0000096376.03678.c1. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7:208–44. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Trimmer PA, Borland MK. Differentiated Alzheimer’s disease transmitochondrial cybrid cell lines exhibit reduced organelle movement. Antioxid Redox Signal. 2005;7:1101–9. doi: 10.1089/ars.2005.7.1101. [DOI] [PubMed] [Google Scholar]
- Twig G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008a;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, et al. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008b;1777:1092–7. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla J, et al. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion. 2006;6:323–30. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–9. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008a;173:470–82. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008b;105:19318–23. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareski P, et al. PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem. 2009;284:21379–85. doi: 10.1074/jbc.M109.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MT, et al. The effects of perturbed energy metabolism on the processing of amyloid precursor protein in PC12 cells. J Neural Transm. 1998;105:839–53. doi: 10.1007/s007020050098. [DOI] [PubMed] [Google Scholar]
- Weyer G, et al. A controlled study of 2 doses of idebenone in the treatment of Alzheimer’s disease. Neuropsychobiology. 1997;36:73–82. doi: 10.1159/000119366. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–7. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M, et al. Cytochrome oxidase in Alzheimer’s disease: biochemical, histochemical, and immunohistochemical analyses of the visual and other systems. Vision Res. 1997;37:3593–608. doi: 10.1016/S0042-6989(96)00210-6. [DOI] [PubMed] [Google Scholar]
- Wyss MT, et al. In vivo evidence for lactate as a neuronal energy source. J Neurosci. 2011;31:7477–85. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, et al. Coenzyme Q10 reduces beta-amyloid plaque in an APP/PS1 transgenic mouse model of Alzheimer’s disease. J Mol Neurosci. 2010;41:110–3. doi: 10.1007/s12031-009-9297-1. [DOI] [PubMed] [Google Scholar]
- Yang X, et al. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci. 2008;34:165–71. doi: 10.1007/s12031-007-9033-7. [DOI] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2011;12:814–22. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Yang SJ. AMP-activated protein kinase mediates activity-dependent regulation of peroxisome proliferator-activated receptor gamma coactivator-1alpha and nuclear respiratory factor 1 expression in rat visual cortical neurons. Neuroscience. 2010;169:23–38. doi: 10.1016/j.neuroscience.2010.04.063. [DOI] [PubMed] [Google Scholar]
- Zhang F, et al. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011a;95:373–95. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, et al. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011b;95:373–395. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]