Abstract
Like humans, rhesus monkeys show cognitive decline and this review considers what structural age-related changes underlie this decline. Some structural measures do not alter significantly with age. These include brain weight, overall cortical thickness; numbers of cortical neurons; and numbers of astrocytes and microglial cells. Other structural measures change with age, but the change does not correlate with cognitive decline. These changes include nerve fiber loss from some fiber tracts, degeneration and regeneration of myelin sheaths, and increase in the frequency of oligodendrocytes. Among the structural measures that increase in frequency with age and also correlate with cognitive decline are the increased frequency of degenerating myelin sheaths and a loss of nerve fibers from some fiber tracts; and the loss of synapses and dendritic spines from upper layers of prefrontal cortex. Consequently, the existing data suggest that cognitive decline correlates with changes in myelinated nerve fibers and with disconnections between and within cortical areas, as reflected by the age-related loss of synapses and of dendritic spines from some cortical areas.
Keywords: Macaca mulatta, normal aging, cerebral cortex, synapses, myelinated nerve fibers, neuroglia
Introduction
This article reviews what has been published and what is currently known about the effects of age on the structure of the cerebral cortex and its associated white matter tracts in the rhesus monkey (Macaca mulatta). The review examines the relationship of these structural changes to the age-related decline in cognitive function. An advantage of studying the rhesus monkey is that these primates have complex behavioral patterns that approach those of humans (e.g., Moss et al., 1999). In particular they have an ability to perform complex short-term memory tasks, so that their cognitive status can be analyzed using behavioral tasks that are similar to ones used to study the effects of age in humans. This allows investigators to try to answer the important question of what structural changes in the brain underlie age-related cognitive decline. This can be done because the cognitive status of a monkey can be accurately assessed before it’s brain is preserved to determine what structural alterations occur with increasing age, the nature of these alterations, and whether or not their frequency correlates with cognitive decline. The fact that the brains can be optimally fixed is especially important for ultrastructural studies of the effects of aging, because as will become evident, a number of the structural alterations that occur with age can only be accurately assessed by ultrastructural evaluations. Such evaluations cannot be made on human brains due to the structural degenerative changes that occur between the time of death and when the tissue can be fixed. Also, since the rhesus monkey does not develop Alzheimer’s disease (Peters et al., 1996), studies of the rhesus monkey brain are valuable for unraveling the likely effects of normal aging on cognitive function in the human brain, unconfounded by changes that are related to Alzheimer’s disease pathology. In humans mild to severe Alzheimer’s disease occurs in over 40% of individuals 85 years and older (Hebert et al., 2003) and its preclinical stage, which occurs with typical Alzheimer’s pathology (Price et al., 2009), may last as long as 15 years before the symptoms of cognitive dementia become evident (Kawas et al., 2003; Elias et al., 2000; Amieva et al., 2005).
In terms of age equivalency between humans and rhesus monkeys, few humans live as long as 100 years and the maximum life span of the rhesus monkey is about 35 years (Tigges et al., 1988), so that one rhesus monkey year is equivalent to about three human years. This equivalency is supported by the fact that rhesus monkeys become sexually mature at about 5 years of age, while humans become sexually mature at 13 to 15 years of age.
In the earlier studies of the effects of age on the brain, behavioral assessments were not carried out, but most monkeys in recent studies have been behaviorally tested using one or more cognitive tasks. The behavioral tasks most commonly used to ascertain the cognitive status of rhesus monkeys are the Delayed Non-Matching to Sample (DNMS), Delayed Response (DR), and the spatial and object versions of the Delayed Recognition Span Task (DRST). Detailed descriptions of these tasks and how they are administered can be found in Presty et al. (1987), Moss et al. (1988), Rapp and Amaral (1989), Arnsten and Goldman-Rakic (1990), Moss et al. (1999), Killiany et al. (2000), Dumitriu et al. (2010), and Hara et al. (2010 and 2011a). The DNMS task is a benchmark visual recognition task of memory and employs a paradigm in which a monkey has to learn to correctly identify a novel object from a previously presented familiar object. A different pair of objects is used for each trial. In the basic acquisition task there is generally a 10 second intra-trial delay before the new object is presented. To make the memory task more demanding the delay between presentation of the sample and the representation of the sample with the novel object may be increased from 10 seconds to 2, or even 10 minutes. The Delayed Response (DR) task is considered a spatial working memory task in which the subject must remember, over varying delays, which of the two identical stimuli positioned to the left or to the right of the subject is baited. The DRST is a test of working memory and memory loading, in which a subject must identify a novel stimulus from an increasing number of familiar stimuli, using spatial or non-spatial clues. In studies by our own group at Boston University, performance on this task by aged monkeys has been reported separately (Moss et al., 1997; Killiany et al., 2000), or as part of a combined “functional score”, with performance obtained by individual monkeys on the basic DNMS acquisition, and on the DNMS 2-minute delay. The combined data are then transformed to scores normalized to the performance of a population of fifty-three adult monkeys, as described by Herndon et al. (1997), producing a composite score of global cognitive ability, the Cognitive Performance Index (CPI). The inverse of the CPI is the CII, or the Cognitive Impairment Index, which is calculated individually for each monkey and is a measure of cognitive impairment. Essentially the higher the CII, the more is a monkey cognitively impaired.
Hara et al. (2011b) have recently presented a review of the effects of age on prefrontal area 46 and dentate gyrus, and this review includes some useful information about not only the effects of age on the morphology of neurons, but also on molecular markers and mechanisms of aging.
A survey of the literature on the effects of age on the rhesus monkey cerebral hemispheres shows that the effects of age on the cerebral cortex and its associated fibers tracts fall into three categories. These effects are shown in Tables 1 – 3: Table 1 lists the structures that do not alter significantly with age; Table 2 lists structural alterations that increase in frequency with age, but for which the increased frequency does not correlate with, or has not been correlated with, cognitive decline; and Table 3 lists the structural alterations that increase in frequency with age, and the frequency of which also correlates with the cognitive impairment displayed by the monkeys. These latter alterations are likely to be the most significant ones in terms of what structural alterations underlie cognitive decline. In Tables 2 and 3 the percentages given in parentheses after the type of structural change show (1) the frequency with which that particular type of change or structure occurs in monkeys 25 years and older, or (2) in the case of nerve fibers and synapses, the percentage loss of nerve fibers and synapses when young (5 years of age and less) and old monkeys (25 years of age and older) are compared.
TABLE 1.
MORPHOLOGICAL MEASURES THAT DO NOT CHANGE SIGNIFICANTLY WITH AGE
| OVERALL | |
| Total brain weight | Herndon et al., 1998 |
| Overall cortical thickness | Koo et al., 2010 |
| Total volumes of prefrontal cortex, hippocampus, and calcarine cortex. | Shamy et al., 2011 |
| CORTICAL AREAS | |
| Area 17:Total thickness, surface area and volume | Peters et al., 1997 |
| Area 46:Thickness and volume | O’Donnell et al., 1999 |
| CROSS SECTIONAL AREAS OF FIBER TRACTS | |
| Splenium of corpus callosum | Peters and Sethares, unpublished. |
| Genu of corpus callosum | Bowley et al., 2010 |
| Fornix | Peters et al., 2010 |
| Optic nerve | Sandell and Peters, 2001 |
| Cingulate bundle | Bowley et al., 2010 |
| NUMBERS OF NEURONS | |
| Area 4: total number of neurons | Tigges et al., 1990 |
| Area 4: number of Betz cells | Tigges et al., 1990 |
| Hippocampus | West et al., 1993 |
| Hippocampus | Keuker et al., 2003 |
| Entorhinal cortex: Layer II | Gazzaley et al., 1997 |
| Entorhinal cortex : Layers II, III and V/VI | Merrill et al., 2000 |
| Visual cortex | Vincent et al., 1989 |
| Visual cortex | Peters et al., 1997 |
| Numbers of Meynert cells | Peters and Sethares, 1993 |
| Number of large layer IVB and Meynert cells | Hof et al., 2000 |
| Area 46 | Peters et al., 1994 |
| Area 46 | Smith et al., 2004 |
| Area 46; neuronal density | Cruz et al., 2009 |
| Areas 17 and 46:layer 1 | Peters and Sethares, 2002a |
| SYNAPSES | |
| Axosomatic synapses on Betz cells | Tigges et al., 1992 |
| Dentate gyrus supragranular layer: | Tigges et al., 1996; Hara et al., 2011a |
| Dentate gyrus, outer molecular layer | Tigges et al., 1995. |
| Dentate gyrus, inner and outer molecular layer | Hara et al. 2010 |
| Axospinous synapse frequency and lengths of postsynaptic densities. | Hara et al. 2010 |
| FREQUENCY OF ALTERED AXONS | |
| Area 17 | Peters et al., 2000 |
| Splenium of corpus callosum | Peters and Sethares, unpublished. |
| MYELINATED NERVE FIBERS | |
| Area 17:No. of myelinated nerve fibers | Nielsen and Peters, 2000 |
| Area 17:Axonal diameter | Peters et al., 2001 |
| Area 17:Lengths of nodes and paranodes | Peters and Sethares, 2003 |
| Area 46:Lengths of nodes and paranodes | Peters and Sethares, 2003 |
| FREQUENCY OF NEUROGLIAL CELLS | |
| Area 17: astrocytes and microglia | Peters et al., 2008 |
| Area 46: astrocytes and microglia | Peters and Sethares, unpublished. |
| Layer I of areas 46 and 17: All neuroglia | Peters and Sethares, 2002a |
| Anterior commissure: All neuroglia | Sandell and Peters, 2003 |
TABLE 3.
STRUCTURAL CHANGES THAT CORRELATE WITH AGE AND COGNITIVE DECLINE
| Correlation with age | Behavioral Correlation(CII) | Source | |
|---|---|---|---|
| CORTICAL VOLUMES | |||
| Dorsal prefrontal cortex reduction | p=0.045 | DNMS p=0.003 | Shamy et al., 2011 |
| Anterior cingulate cortex reduction | p=0.008 | DNMS p=0.026 | Shamy et al., 2011 |
| CORTICAL THICKNESS | |||
| Thinning of prefrontal and sup. temporal cortices | p= 0.003 | DR task. p<0.0081 | Alexander et al., 2008 |
| LAYER 1 | |||
| Area 46;decreased thickness | p<0.01 | DNMS 2″ p<0.252 | Peters et al., 1998b |
| MICROCOLUMN STRENGTH | |||
| Area 46:neuron displacement; reduction in “strength” on minicolumns. | p<0.05 | p<0.01 |
Cruz et al., 2004 Cruz et al., 2009 |
| NERVE CELL LOSS | |||
| Area 8A of frontal cortex (32%) | overall p<0.0001 | DR p<0.01 | Smith et al., 2004 |
| DECREASED FREQUENCY OF SYNAPSES | |||
| Layer 1:Area 46 (30 – 60%) | p<0.01 | p<0.01 | Peters et al., 1998b |
| Layer 2/3: A 46. asymm. synapses (30%) | p<0.01–0.001 | p=0.008 | Peters et al., 2008a; Dumitriu et al., 2010 |
| Layer 2/3: A 46; symm. synapses (30%) | p=0.016 | p=0.07 | Peters et al., 2008a |
| Layer 3:A 46 axospinous synapses (32%) | p<0.01 | DNMS p=0.023 | Dumitriu et a.l, 2010 |
| Layer 2/3:A 46; Increased No. of vesicles in axosomatic terminals | p<0.005 | p=0.022 | Soghomonian et al., 2010 |
| AXONAL BOUTONS | |||
| Dentate gyrus, outer molecular layer | DNMS acquisition | ||
| Increased proportion of nonsynapsing boutons | p<0.01 | p=0.026 | Hara et al., 2011a |
| Decreased proportion of multisynaptic boutons | p<0.044 | p<0.05 | Hara et al., 2011a |
| DENDRITIC SPINES | |||
| A 46;layer3: Loss of thin spines (33%) (No loss of mushroom spines) | p=0.02 | DNMS p=0.043 | Dumitriu et al., 2010 |
| NERVE FIBER LOSS | |||
| Splenium of corpus callosum (30%) | p<0.01 | DRST p>0.052 | Peters and Sethares, unpublished |
| Fornix:EM count (26%) | p<0.0001 | p=0.015 | Peters et al., 2010 |
| Anterior commissure (45%) | p<0.01 | p<0.01 | Sandell and Peters, 2003 |
| FREQUENCY OF ALTERED SHEATHS | |||
| Area17 (5.4%) | p<0.001 | p<0.001 | Peters et al., 2000 |
| Area 46 (6%) | p<0.001 | p=0.001 | Peters and Sethares, 2002b |
| Splenium of corpus callosum (6%) | p>0.001 | p=0.06 | Peters and Sethares, 2002b & unpublishe |
| Fornix (3.5%) | p<0.001 | p=0.06 | Peters et al., 2010 |
| Cingulate bundle (7%) | p<0.0001 | p=0.001 | Bowley et al., 2010 |
| FREQUENCY OF PARANODES | |||
| Area 46 (12%) | p<0.001 | p<0.01 | Peters and Sethares, 2003 |
| FREQUENCY OF ALTERED AXONS | |||
| Cingulate bundle (0.6%) | p<0.0001 | DNMS 2″ p<0.005 | Bowley et al., 2010 |
| Genu of corpus callosum (0.6%) | p<0.0001 | Bowley et al., 2010 | |
| Fornix (0.3%) | p<0.01 | p=0.01 | Peters et al., 2010 |
| FREQUENCY OF NEUROGLIAL CELLS | |||
| Area 17: increase in oligos.(50%) | p=0.012–0.001 | p=0.1 | Peters et al., 2008b |
No correlation with DNMS task results
No correlations with other behavioral tasks used to determine CII
No correlations with DRST task results
TABLE 2.
STRUCTURES THAT CHANGE WITH AGE: BUT THE CHANGES HAVE NOT BEEN SHOWN TO CORRELATE WITH COGNITIVE DECLINE
| Correlation with age | Correlation with Behavior | Source | |
|---|---|---|---|
| FOREBRAIN | |||
| Gray matter volume reduction (2.08%) | p=0.037 | No correlation | Wisco et al., 2008 |
| White matter volume reduction (11.53%) | p= 0.0003 | No correlation | Wisco et al., 2008 |
| CEREBRAL CORTEX OVERALL | |||
| Thickening of sup. temp. and cingulate cortices | p=0.01–0.05 | Not done | Koo et al., 2010 |
| Thinning of somatosensory and motor cortices | p<0.01 | Not done | Koo et al., 2010 |
| SENILE PLAQUES | |||
| Increased senile plaque burden | p=0.05–0.09 | No correlation |
Struble et al., 1985; Sloane et al., 1997 Heilbroner and Kemper, 1990 |
| LAYER I | |||
| Area 17: decrease in thickness | p<0.01 | No correlation | Peters et al., 2001 |
| Area 17: decreased density of synapses | p<0.01 | No correlation | Peters et al., 2001 |
| DENDRITIC AND SPINE CHANGES | |||
| Decrease in spine density on layer 3 long projecting neurons in Sup. Temp Gyrus (25%) | p<0.01 | Not done | Duan et al., 2003; Kabaso et al., 2009 |
| Decreased spine density on layer 3 locally projecting neurons in Area 46 (18%) | p<0.01 | Not done | Kabaso et al., 2009 |
| SYNAPSES | |||
| Area 46, Layer 5:Loss of asymmetric synapses (20%) | p=0.02 | No correlation | Peters et al., 2008a |
| Area 46, Layer 5:Loss of symmetric synapses(10%) | p=0.31 | No correlation | Peters et al., 2008a |
| Area 46, Layer 2/3 increased size of axosomatic terminals | p=0.02 | No correlation | Soghomonian et al., 2010 |
| Area 46, Layer 2/3: increased size of axodendritic terminals | p<0.0001 | No correlation | Soghomonian et al., 2010 |
| Dentate gyrus; outer molecular layer; loss of axodendritic synapses | no p value | not done | Tigges et al., 1995 |
| NERVE FIBER LOSS | |||
| Genu of corpus callosum (20%) | p<0.05 | No correlation | Bowley et al., 2010 |
| Cingulate bundle (20%) | p<0.005 | No correlation | Bowley et al., 2010 |
| Optic nerve (45%) | p<0.0001 | Not done | Sandell and Peters, 2001 |
| FREQUENCY OF ALTERED AXONS | |||
| Anterior commissure (0.4%) | p<0.01 | No correlation | Sandell and Peters, 2003 |
| Splenium of corpus callosum (0.1%) | No correlation | No correlation | Peters and Sethares. Unpublished |
| FREQUENCY OF ALTERED SHEATHS | |||
| Anterior commissure (5.4%) | p<0.001 | No correlation | Sandell and Peters, 2003 |
| Genu of corpus callosum (7.9%) | p=0.005–0.0001 | No correlation | Bowley et al., 2010 |
| FREQUENCY OF PARANODES | |||
| Area 17 (13%) | p<0.001 | No correlation | Peters and Sethares, 2003 |
| Splenium of corpus callosum (8%) | p<0.0001 | No correlation | Peters and Sethares. Unpublished |
| Genu of corpus callosum (9%) | p<0.0001 | No correlation | Bowley et al., 2010 |
| Anterior commissure (7.3%) | p<0.01 | No correlation | Sandell and Peters, 2003 |
| Fornix (7.2%) | p<0.0002 | No correlation | Peters et al., 2010 |
| Cingulate bundle (7%) | p<0.05 | No correlation | Bowley et al., 2010 |
| INCREASE IN THICKNESS OF MYELIN SHEATHS | |||
| Area 17 | p=0.001 | No correlation | Peters et al., 2001 |
| FREQUENCY OF NEUROGLIAL CELLS | |||
| Area 46: increase in oligodendrocytes (45%) | p=0.05–0.003 | No correlation | Peters and Sethares. Unpublished |
| Fornix: increase in oligodendrocytes (20%) | p=0.003 p<0.01 |
No correlation Not done |
Peters et al., 2010 Sandell and Peters, 2002 |
| CAPILLARIES | |||
| Thickening of basal lamina | p=0.01 | No correlation | Peters and Sethares. Unpublished |
| Splitting of basal lamina | p=0.004 | No correlation | Peters and Sethares. Unpublished |
Table 4 presents a summary of the increased frequency of occurrence of myelin sheath alterations and of the increased frequency of paranodes in various structures and their correlations with age and cognitive decline. In this Table the figures in parentheses record how many more times profiles of altered sheaths and of paranodes are encountered in old monkeys as compared to young ones.
TABLE 4.
MYELIN PATHOLOGY
| AGE RELATED CORRELATIONS | CORRELATIONS WITH COGNITIVE DECLINE | ||||
|---|---|---|---|---|---|
| Increased Frequency of Altered Sheaths | Increased Frequency of Paranodes | Frequency of Altered Sheaths | Frequency of Paranodes | Source | |
| Area 46 | p <0.001(x4) | p <0.001(x1.9) | p <0.001 | p <0.01 | Peters and Sethares, 2002b, |
| Area 17 | p <0.001(x6) | p <0.001(x1.5) | p <0.001 | N.C. | Peters et al., 2010 |
| Genu C.C. | p <0.001(x10) | p <0.0001(x1.5) | N.C. | N.C. | Bowley et al., 2010 |
| Splenium C.C. | p <0.001(x20) | p <0.0001(x1.6) | p=0.06 | N.C. | Peters and Sethares, 2002b a |
| Ant. Comm. | p <001(x13) | p <0.01(x1.2) | N.C. | N.C. | Sandell and Peters, 2003 |
| Cingulate | p <0.0001(x10) | p <0.05(x1.3) | p<0.001 | N.C. | Bowley et al., 2010 |
| Fornix | p <0.001(x3) | p <0.0002(x1.4) | p=0.06 | N.C. | Peters et al., 2010 |
| Optic Nerve | not done | not done | |||
The numbers in parentheses give the increase in frequency when young and old monkeys are compared
NC : no correlation
Morphological Measures That Do Not Change Significantly With Age
Morphological measures that do not change significantly with increasing age are listed in Table 1. One of these measures is brain weight. Herndon et al. (1998) obtained data from necropsies of 399 rhesus monkeys of ages covering the entire life span of this species and while the brains of the male monkeys weigh 12% more than those of female monkeys, the data show that the weight of the brain is stable throughout the adult life span of the rhesus monkey. The sizes of most parts of the cerebral cortex also appear to be unaltered with age. O’Donnell et al. (1999) have shown that the volume of area 46 of the prefrontal cortex does not change with age and Peters et al. (1997) have shown the same to be true for primary visual cortex. However, in a more recent MRI study Shamy et al. (2011) report that while the total volume of prefrontal cortex does not alter with age, the volumes of dorsal prefrontal cortex, area 46, and of anterior cingulate cortex are reduced. The volumes of other prefrontal areas, hippocampus and calcarine cortex are not altered.
MRI scanning, based on a surface modeling technique, shows that the mean overall thickness of the cortex does not change with age (Koo et al., 2010), even though, as indicated in Table 2, specific cortical areas such as area 7 and motor areas 4 & 5 have been reported to become somewhat thinner, while the cortices in the superior temporal lobes and cingulate cortex become slightly thicker (Koo et al., 2010). The thickness of the cortex in other areas, such as the primary visual cortex (Peters et al., 1997) and area 46, are reported to remain unchanged with age (O’Donnell et al., 1999), but which cortical areas are affected by age cannot yet be said to be completely resolved.
As for white matter, although the MRI studies of Wisco et al. (2008) have shown the overall volume of the white matter in the cerebral hemispheres of the rhesus monkey to decrease by as much as 11.5% with age (Table 2), measurements of the cross-sectional areas of several structures such as the genu (Bowley et al., 2010) and splenium (Peters and Sethares, unpublished) of the corpus callosum, the fornix (Peters et al., 2010), the cingulate bundle (Bowley et al., 2010), and the optic nerve (Sandell and Peters, 2001) are not altered with age, even though these bundles lose nerve fibers (see Tables 2 and 3). However, it seems that there is not an extensive loss of myelinated nerve fibers from the cerebral cortex with age, since Neilsen and Peters (2000) found no significant loss of fibers from the vertical bundles of myelinated nerve fibers that extend though the lower layers of the primary visual cortex.
Early investigators (e.g., Brody, 1955, 1970) came to the conclusion that there is a progressive loss of cortical neurons with age. However, in recent years it has become evident that the early accounts describing such losses in normal brains are incorrect and that the erroneous conclusions were due to problems with differential shrinking of young and old brains during fixation and problems with counting techniques (e.g., Morrison and Hof, 1997; Peters et al., 1998; Hof and Morrison, 2004). Overall, there is no significant loss of cortical neurons during normal aging in rhesus monkeys. This has been shown in studies of primary motor cortex (Tigges et al., 1990), hippocampus (West et al., 1993; Keuker et al., 2003), entorhinal cortex (Gazzaley et al., 1997; Merrill et al., 2000), primary visual cortex (Vincent et al., 1989; Peters and Sethares, 1993; Peters et al., 1997; Hof et al., 2000), and prefrontal area 46 (Peters et al., 1994; Smith et al., 2004, and Cruz et al., 2009). The one exception may be area 8A of prefrontal cortex (Table 3). As a result of their stereological analyses of prefrontal cortex Smith et al. (2004) concluded that there is a focal loss of 32% of Nissl stained neurons from area 8A. Area 8A is an area of cortex associated with working memory, and Smith et al. (2004) found that the age-related loss of neurons from area 8A correlated significantly with the working memory performance (Delayed Response) displayed by the monkeys (see Table 3). Smith et al. (2004) also report there is a 50% reduction in the immunologically stained cholinergic nerve fibers arising from the nucleus basalis and projecting to this cortical area. Interestingly, in agreement with other studies (e.g., Peters et al., 1994; Cruz et al., 2009), Smith et al. (2004) found no loss of neurons from the adjacent area 46.
Tigges et al. (1992) found that there is no loss of axosomatic synapses on Betz cells in motor cortex, but as far as we are aware, this is the only study that has examined the effects of age on the frequency of axosomatic synapses in any area of rhesus monkey cortex. Tigges et al. (1995 and 1996) also examined the dentate gyrus and determined that there is no overall changes in the numbers and sizes of synapses in either the outer molecular layer or the supragranular layer, and Hara et al. (2010 and 2011a) obtained a similar result for the outer and inner molecular layers. However, although Tigges et al. (1995) found no overall loss of synapses from the supragranular layer, they did detect a loss of axodendritic (shaft) synapses with age from the outer molecular layer of the dentate gyrus (see Tables 2 and 3).
It also seems that in some structures there is no overall change in the numbers of neuroglial cells with age. This is true for the anterior commissure (Sandell and Peters, 2003) and for layers 1 of the cortex in areas 17 and 46 (Peters and Sethares, 2002a). On the other hand, while there is no change in the frequency of astrocytes or microglia with age throughout the depth of area 17 (Peters et al., 2008b) and area 46 (Peters and Sethares, unpublished), there is a significant 45% age-related increase in the numbers of oligodendrocytes in area 46 (Table 2), a 20% increase in the fornix (Sandell and Peters, 2002), and a 50% increase in area 17 (Table 3).
With the exception of the unsubstantiated reported loss of neurons from area 8A (Smith et al., 2004), the above are the main entities that have been reported not to alter during normal aging in the rhesus monkey cerebral hemispheres. Moreover, as a basis for what will be considered later on the effects of age on nerve fibers and their myelin sheaths, it is pertinent to record that there is no indication that the diameters of axons alter significantly with age (Peters et al., 2001), or that there is a change in either the lengths of nodes of Ranvier or of paranodes of myelinated nerve fibers (Peters and Sethares, 2003).
Structures That Change With Age, But The Change Does Not Correlate, Or Has Not Been Correlated, With Cognitive Decline
Structures that change with age, but the frequency of occurrence of which does not correlate, or has not been correlated with, cognitive decline, are listed in Table 2. In most cases the age-related structural changes have been examined to determine if there is a correlation between their frequencies and cognitive decline. In the few cases, in which a correlation with behavior has not been determined, this is indicated by the comment “not done”. These cases will be described individually as each particular age change is considered.
In rhesus monkeys senile plaques increase in number with age, but their frequency does not correlate with cognitive status. Struble et al. (1985) found several middle-aged (14 to 25 years) monkeys to have senile plaques and suggested that senile plaques may play a role in the cognitive decline that begins in middle age. However, in middle–aged monkeys senile plaques are generally sparse. And indeed, only a few old monkeys have large numbers of senile plaques. The plaques that are present have a predilection for the frontal and somatosensory cortices (Struble et al., 1985; Heilbroner and Kemper, 1990; Sloane et al., 1997), and although the number of senile plaques increases with age, there is no correlation between plaque density and the cognitive status of the monkeys (Sloane et al., 1997). In this context it is of interest that while the predominant form of amyloid is Aβ40 in both healthy monkeys and humans, in Alzheimer’s disease in humans the prevalence of Aβ42 increases. However, the proportion of this form of amyloid is low in monkeys (Gearing et al., 1996; Kanemaru et al., 1996). This suggests there is a difference in the processing of the APP molecule in monkeys and humans, so that the monkey does not accumulate amyloid in the same manner as the human. It is also pertinent to point out that monkeys do not develop neurofibrillary tangles, which are related to neuronal loss in humans.
Even though there is no significant loss of cortical neurons in monkeys with age (see Table 1), MRI studies have shown that there is an overall decrease in forebrain volume of 5%, due to a 2.08% loss of gray matter and an 11.53% loss of white matter volume in the cerebral hemispheres (Wisco et al., 2008). These losses occur even though overall brain weight does not change with age (Herndon et al., 1998), but the losses in the volumes of forebrain white and gray matters did not correlate with cognitive decline (Wisco et al., 2008). In another MRI study Alexander et al. (2008) reported that with age there is a small loss of gray matter from several cortical areas, including prefrontal cortex and portions of the temporal cortex and the right visual cortex, and that these losses do correlate with the decline in working memory (Delayed Response) (see Table 3). It should also be pointed out that Shamy et al. (2011) have reported an age-related reduction in the volumes of the dorsal prefrontal and anterior cingulate cortices, and that these reductions also correlate with behavioral changes (see Table 3).
The situation is made more confusing by the data of Koo et al. (2010). They concluded that while the overall cortical thickness does not change significantly with age (Table 1), there are changes in the thicknesses of some cortical areas. For example, they find that the superior temporal and cingulate cortices become thicker with age, and the somatosensory and motor cortices become thinner. Whether these changes correlate with cognitive decline was not assessed. In summary, Wisco et al. (2008) found no correlation between any behavioral measures and age changes in white and grey matter (Table 2), while Alexander et al. (2008) found correlations between age-related thinning of the prefrontal and temporal cortices and cognitive decline (Table 3), and Shamy et al. (2011) found correlations between the reduction in volume of area 46 and of anterior cingulate cortex and cognitive decline (see Table 3).
As pointed out in the previous section, despite the loss of white matter from the cerebral hemispheres the cross sectional areas of white matter tracts do not alter significantly with age (see Table 1). Nevertheless, there is a loss of nerve fibers from white matter tracts, as evidenced in the anterior commissure (Sandell and Peters, 2003), the splenium and genu of the corpus callosum and the cingulate bundle (Bowley et al., 2010), and the fornix (Peters et al., 2010), and electron micrographs show 0.1–0.6% of the axons in myelinated nerve fibers to be degenerating at any give time.
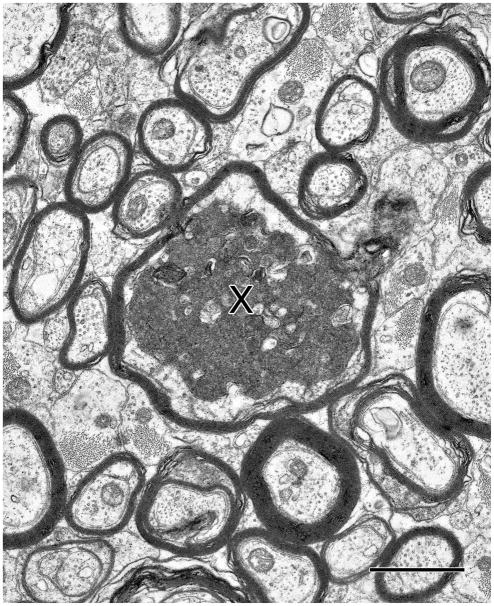
In electron micrographs, profiles of degenerating axons are electron dense (Fig. 1). Ultimately such axons degenerate completely. In the case of myelinated nerve fibers this results in the production of empty myelin sheaths (Fig. 2:1), which also eventually degenerate. This degeneration results in an extensive loss of myelinated nerve fibers from a number of white matter tracts. Thus, with age the genu of the corpus callosum and cingulate bundle (Bowley et al., 2010) lose some 20% of myelinated nerve fibers. And although the losses increase significantly with age, in these particular nerve fiber bundles the losses do not correlate with cognitive decline. This lack of correlation is puzzling, since the genu of the corpus callosum forms interhemispheric forebrain connections and the cingulate bundle links the medial prefrontal, cingulate and parahippocampal cortices that are important in working memory, recognition memory and higher cognitive functions. However, as shown in Table 3, there are other nerve fiber bundles that lose a greater number of myelinated nerve fibers. The splenium of the corpus callosum (Peters and Sethares, unpublished) and the fornix (Peters et al., 2010) lose about 30% of their nerve fibers, while the loss from the anterior commissure (Sandell and Peters, 2003) is as high as 45% (Table 3). In these latter three cases the fiber losses do correlate significantly with cognitive decline. Consequently it would seem that it is the extent of fiber loss, which is important in determining whether or not cognition is affected.
Figure 1.
A myelinated nerve fiber containing a degenerating axon (X) with dark cytoplasm. Anterior commissure of a 32 year old rhesus monkey. Scale bar = 1μm.
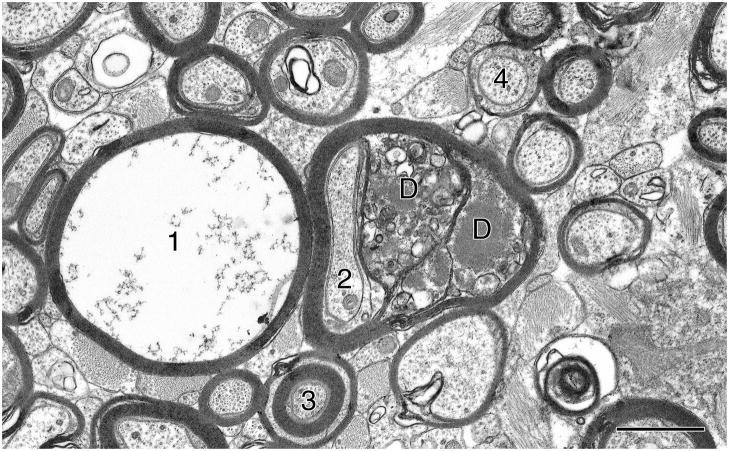
Figure 2.
Nerve fibers in the fornix of a 29 year old rhesus monkey. One myelinated nerve fiber (1) has lost its axon, leaving an empty myelin sheath. In an adjacent nerve fiber the sheath is degenerating, so that the lamellae of the myelin have split to enclose dense cytoplasm (D), pushing the axon (2) to one side. Also present in the field is a myelinated nerve fiber with a thick sheath composed of two sets of compact lamellae (3). Note the nerve fiber sectioned at the level of the paranode (4), which can be identified by the close apposition between the axolemma and the membrane on the inside of the sheath. Scale bar = 1μm.
Despite this extensive loss of myelinated nerve fibers from white matter tracts, as pointed out above, there is no significant loss of cortical neurons with age. It has been suggested that the reason why neurons are not lost is that although some cortical neurons lose their long projecting axons, they do not die because they are maintained by their extensive local plexuses, which extend locally within the cortex (Peters and Rosene, 2003).
Another fiber tract that shows an extensive loss of nerve fibers is the optic nerve. The optic nerve can lose as many as 45% of its total nerve fibers with age (Sandell and Peters, 2001), but the situation is different from the other myelinated nerve fibers tracts discussed above in that the nerve fibers of the optic nerve arise from ganglion cells in the eye. Whether the loss of nerve fibers from the optic nerve correlates with cognitive decline has not been determined.
In addition to the degeneration of axons there are age-related alterations in myelin sheaths. These alterations have been described in detail in a number of reviews (e.g., Peters, 2002b, 2007, 2009), and so they will only be summarized here. Some of the myelin sheath alterations that occur with aging are brought about by the degeneration of the ensheathed axon, which in turn leads to the degeneration of its myelin sheath, resulting in nerve fiber loss. But in other cases myelin sheaths degenerate even though the ensheathed axon is intact. In these situations the most common alteration is splitting of the myelin sheath lamellae at the major dense line to form pockets in which varying amounts of dense cytoplasm accumulate (see Fig. 2; D). Such altered sheaths are referred to as dense sheaths. Since the dense cytoplasm is within the major dense line, it must be derived from the oligodendrocyte forming the sheath, because the major dense line is formed by the apposition of the cytoplasmic faces of successive turns of the oligodendrocyte plasma membrane. Less commonly the intraperiod line of the sheath splits and this can result in the formation of fluid-filled balloons. Most myelin balloons are quite small, but some can become as large as 10 microns in diameter, so that in light microscopic preparations they appear to be holes in the neuropil. Since these balloons are formed by splitting of the intraperiod line, their interstices are effectively in continuity with the extracellular space of the neuropil. In the anterior commissure (Sandell and Peters, 2003) and the genu of the corpus callosum (Bowley et al., 2010) the frequency of occurrence of profiles of nerve fibers with altered or degenerating sheaths does not correlate with cognitive decline (Table 2), but as will be described later, for other nerve fiber tracts and for some cortical areas there are strong correlations between the frequency of altered myelin sheaths and cognitive decline.
There are two other structural alterations displayed by myelin sheaths in aging monkeys that are not degenerative. One is the continued production of myelin, leading to an overall thickening of myelin sheaths by an increase in the numbers of lamellae (see Fig. 2; 3). This type of change has been quantified in primary visual cortex, in which it has been shown that the average number of lamellae in the myelin sheaths of young monkeys is 5.6, while in old monkeys the number increases to an average of 7.0 lamellae (Peters et al., 2001). But as recorded in Table 2, this sheath thickening does not correlate with cognitive decline. Another change, which is also believed to reflect the continued formation of myelin with age, is the formation of sheaths with redundant myelin. Such sheaths are much too large for the enclosed axons, so that the sheaths do not fit snuggly around the axons. At present little is known about these redundant sheaths, although it has been suggested they may be sheaths that are being remodeled (e.g., Cullen and Webster, 1979).
Paranodes are regions at the ends of internodal lengths of myelin where the spiraled myelin lamellae gradually terminate as a length, or segment, of myelin approaches a node of Ranvier. At the paranode the major dense line opens up to accommodate a spiral tunnel of cytoplasm, which in longitudinal sections appears as a series of pockets on each side of the axon. Where the plasma membrane on the inner faces of these pockets abuts against the axolemma, the two membranes become closely apposed to form a junctional complex, which is quite distinctive, making profiles of paranodes easy to recognize in thin sections examined by electron microscopy (see Fig. 2; 4). When the frequencies of profiles of nodes of Ranvier, paranodes and internodes are determined, it is found that with age the frequency of profiles of paranodes increases, so that in old monkeys as many as 7–13% of profiles of myelinated nerve fibers are through paranodes, as compared to 4–5% of profiles in most young monkeys (see Table 2). One reason for this increase could be that the paranodes become longer. But although paranodes do become slightly longer with age as myelin sheaths become thicker, this lengthening of the paranodes is not sufficient to account for the increase in paranodal profiles (Peters and Sethares, 2003). An age-related increase in the frequency of paranodal profiles has been found in all the cortical areas and white matter tracts that have so far been examined and commonly the increase in frequency is between 7–9% (Table 2). The exception is area 46 in which 13% of myelinated nerve fiber profiles are of paranodes, as compared to 8% in young monkeys (Table 3).
Such an increase in the frequency of paranodal profiles would occur if remyelination were taking place, such that the original internodal lengths of myelin degenerate and are replaced by a series of shorter internodes. The accepted landmarks for remyelination are the presence of both short internodes and myelin sheaths that are inappropriately thin for the size of the ensheathed axons (e.g., see Ludwin, 1995; Bruck et al., 2003), and indeed both thin sheaths and internodes as short as 3 – 5μm have been encountered in the cortices of old monkeys (Peters and Sethares, 2003).
It has been suggested that the increase in the numbers of internodal lengths of myelin, with its concomitant increase in the numbers of nodes of Ranvier, leads to a decrease in the conduction velocity along myelinated nerve fibers. This suggestion is supported by the earlier report of a reduction in conduction velocity along nerve fibers in the spinal cords of aging cats (Morales et al., 1987). It has been confirmed more directly by the observations of Lasiene et al. (2008), who have shown that as the rubrospinal tract of the mouse recovers from crush lesions, short internodes are generated and conduction velocity is slowed. Presumably something similar is happening in the brain of the aging monkeys as a consequence of the remyelination. However it is only in area 46 of prefrontal cortex (see Table 3), that there is a correlation between the age-related increase in the frequency of paranodes and cognitive decline (p<0.01). In area 17 and in the five nerve fiber tracts that have been examined (Tables 2 and 4), there is no correlation between the frequency of paranodes and CII or any of the individual behavioral tasks, even though in old monkeys area 17 shows a similar frequency of paranodal profiles (13%) to area 46. It can only be suggested that the difference between area 46 and the other structures is because area 46 is intimately involved in cognition.
Correlated with the increased frequency of paranodes is the age-related 20 to 50% increase in the numbers of oligodendrocytes that has been recorded in areas 17 (Peters et al., 2008b) and 46 (Peters and Sethares, unpublished) and in the fornix (Peters et al., 2010). It is presumed that the increased numbers of oligodendrocytes are necessary to generate the increased numbers of internodes that are formed as a consequence of remyelination. But only in area 17 is there a suggestion the increase in the numbers of oligodendrocytes may correlate (p<0.1) with cognitive decline (Table 3).
As shown in Table 2, Duan et al. (2003) and Kabaso et al. (2009) have demonstrated that with age there are decreases in the numbers of spines on the dendrites of both long and locally projecting pyramidal cells. While these authors did not determine whether these spine losses correlate with cognitive decline, it is likely that they do correlate, since more recently, Dumitriu et al. (2010) have described a significant age-related loss of thin spines from pyramidal cells in area 46 and the loss does correlate with cognitive decline. This, along with the synapse changes listed in Table 2, will be discussed in more detail in the next section of this review.
Another alteration that occurs with age is an increase in the thickness of the basal lamina of cerebral capillaries, a thickening that is often considered to affect the blood-brain barrier properties of blood vessels and the transfer of materials from the capillaries to the brain parenchyma (e.g., see Farkas and Luiten, 2001). However, in a recent study, the effects of age on capillaries have been examined in area 46 of the prefrontal cortex of monkeys whose cognitive status had been assessed. It was found that while the thickness of the outer basal lamina, that is the lamina abutting the astrocytic end-feet, increased significantly by about 20% and shows frequent splitting, neither of these two age-related changes correlated with cognitive decline. There was no change in the thickness of the basal lamina between endothelial cells and pericytes, and no change in the frequency of occurrence of pericytes with age. (Peters and Sethares, to be published).
Structural Changes That Correlate With Both Age And Cognitive Decline
In evaluating what underlies cognitive decline in the aging rhesus monkey these structural changes are the most important ones, because they are the ones that correlate with cognitive decline. Hopefully, by extension, they are also the ones underlying cognitive decline in normally aging humans. These changes are listed in Table 3, which also gives the behavioral tasks used to assess cognitive decline. Where no specific behavioral task is noted in Table 3, the correlation is based on the cognitive impairment index (CII) used by the Boston University group. As pointed out earlier, this index is derived from the composite score obtained by individual monkeys on the basic DNMS acquisition task, the DNMS task with a 2-minute delay, and the DRST task (Herndon et al., 1997). In some other cases the correlation between cognitive status and specific structural alterations pertains to only one behavioral task, sometimes with no correlation with other behavioral tasks. This is indicated in Table 3 by the superscripts.
As pointed out above, a reduction in volume and thinning of some cortical areas, such as dorsal prefrontal cortex, anterior cingulate cortex and superior temporal cortex correlates to cognitive decline (Alexander et al., 2008; Shamy et al. 2011), but what underlies these reductions is not yet clear.
In the forebrain loss of nerve fibers from white matter tracts correlates with age. But for only three fiber tracts, namely the splenium of the corpus callosum (Peters and Sethares, unpublished), the fornix (Peters et al., 2010) and the anterior commissure (Sandell and Peters, 2003), have significant correlations been shown between nerve fiber losses and cognitive decline. As pointed out above, whether or not there is a correlation between nerve fiber loss and cognitive decline appears to depend upon the extent of the loss. Thus, in fiber tracts for which there is no correlation with cognitive decline the nerve fiber loss is only 20% (see Table 2), compared to the 26–45% loss from forebrain tracts for which the fiber loss does correlate with cognitive decline (see Table 3). Presumably the more extensive nerve fiber loss leads to a greater disconnection and greater degradation in information processing between the structures connected by the fiber bundles, and this may be a basis for the effect on cognition.
Unlike the anterior commissure and the genu of the corpus callosum (see Table 2), the frequency of altered myelin sheaths in other forebrain cortical tracts that have been examined, namely the splenium of the corpus callosum (Peters and Sethares, unpublished), the fornix (Peters et al., 2010) and the cingulate bundle (Bowley et al., 2010), as well as two cortical areas, areas 46 (Peters and Sethares, 2002b) and 17 (Peters et al., 2000), show strong correlations with cognitive decline. The underlying basis for this correlation is not yet clear, but it might be that the degenerative sheath alterations slow down conduction velocity along the nerve fibers in the tracts, so that the timing in neuronal circuits is affected. However, this can only be ascertained if nerve fibers with degenerating sheaths are isolated and their conduction velocities determined, which has not yet been done in monkeys.
As pointed out in the previous section, it is surprising that only in area 46 is there a correlation between paranodal frequency and cognitive decline (also see Table 4), since increasing the number of internodal lengths along a nerve fiber slows down conduction velocity (Lasiene et al., 2008). However, there are strong correlations between the frequencies of paranodal profiles and the frequencies of profiles of altered myelin sheaths (p< 0.05 – 0.001) for all of the areas of cortex and fiber tracts that have been examined. This would be expected if degeneration of myelin sheaths leads to remyelination.
The loss of synapses from the neuropil of prefrontal cortex with age was first reported by Uemura (1980), and in area 46 there is a strong correlation between the age-related loss of synapses from the neuropil of the upper layers of this cortical area and cognitive decline (Table 3). The loss of asymmetric, or excitatory synapses amounts to about 30% (Peters et al., 2008a; Dumitriu et al., 2010), and there is a similar percentage loss of symmetric, or inhibitory synapses from the neuropil (Peters et al., 2008a). Both sets of authors have shown that these synaptic losses correlate with increasing cognitive decline. In contrast to the upper cortical layers, the overall loss of synapses from layer 5 is only about 20%. This loss is almost entirely due to asymmetric synapses, and while the loss correlates with increasing age, it does not correlate with cognitive decline (Table 2). These differential losses of synapses from the upper and lower layers of the cortex are made even more interesting because Luebke and her colleagues (Luebke and Rosene, 2003; Luebke et al., 2004; Chang et al., 2005; Chang and Luebke, 2006; Luebke and Chang, 2007) have shown that in in vitro slices from area 46 the basic electrophysiological properties of layer 5 neurons are largely unaltered with age, while the properties of layer 3 pyramidal neurons are changed significantly and are associated with decreased cognitive performance. For example, with age the frequency of action potential firing of layer 3 neurons is dramatically increased, while the firing rates of layer 5 pyramidal cells are largely unaffected (also see Luebke et al., 2010).
The loss of synapses from the cerebral cortex with age is accompanied by a loss of dendritic spines, which are the main recipients of synapses on pyramidal neurons (Fig. 3). Cupp and Uemura (1980) first demonstrated the spine loss in their study of the effects of age on Golgi impregnated neurons in the superior frontal gyrus (area 9) of the rhesus monkey. More recently Duan et al. (2003) carried out a similar study by examining dye-filled layer 3 pyramidal cells that project from the superior temporal gyrus to prefrontal area 46, and they found that with age some 25% of spines are lost from the apical and basal dendritic trees. Later, Kabaso et al. (2009) provided more information by not only re-examining these projection neurons, but also examining the locally projecting neurons within area 46. They found that with age, spine densities, dendritic diameters, dendritic lengths and branching complexity of the long projecting pyramidal neurons are all reduced, but only spine densities are reduced in the locally projecting pyramidal neurons. As pointed out, in these two studies no correlations were made with cognitive statuses of the monkeys (Table 2). But recently Dumitriu et al. (2010) have more closely examined the effects of age on layer 3 pyramidal neurons in area 46 of behaviorally tested rhesus monkeys. They injected the neurons with Lucifer yellow and found a 33% loss of spines from the pyramidal cells. Using electron microscopy they also found a 32% decrease in the density of axospinous synapses in the neuropil of layer 3, which is in agreement with the results of previous studies (Peters et al., 2008a).
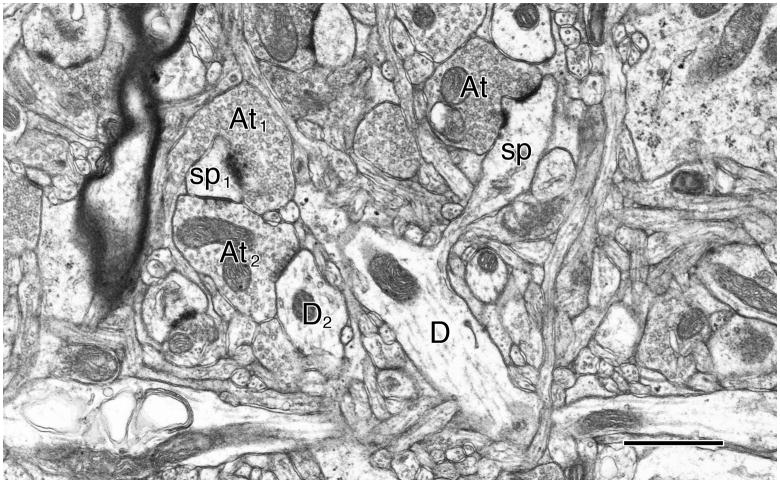
Figure 3.
Neuropil in the upper layer of area 46 in the prefrontal cortex of an 18 year old rhesus monkey. In the middle of the field is a dendrite (D) that gives rise to a spine (sp) forming an asymmetric perforated synapse with an axon terminal (At). Nearby is another dendritic spine (sp1) forming an asymmetric synapse with an axon terminal (At1) that contains spherical synaptic vesicles. This spine is also forming a symmetric, inhibitory, synapse with a second axon terminal (At2) containing smaller, pleomorphic synaptic vesicles. This same axon terminal (At2) also synapses with a dendrite (D2). Scale bar = 1μm.
Dumitriu et al. (2010) then turned their attention to the dendritic spines and used the sizes of the spine heads to separate the spines into two groups: thin spines with heads smaller than 0.6μm in diameter and a maximum length of at least twice the head diameter, and mushroom spines with heads greater than 0.6μm in diameter. Based upon this classification Dumitriu et al. (2010) find that in young monkeys 75% of spines are thin, while in old monkeys the percentage is reduced to 61%. Spines that did not meet these criteria were left unclassified. Dumitriu et al. (2010) then calculated the mean spine density per unit length of dendrites and found that the age-related overall loss of spines is due to a selective loss of thin spines, since the density of mushroom and other spines does not change with age. When their data were correlated with performance of the monkeys on the DNMS acquisition task, it became apparent that that loss of synapses from layer 3 showed a significant correlation with cognitive decline (p= 0.02), as did the density of thin spines (p=0.04), although there was no correlation with DR acquisition. The density of mushroom spines remained stable and was unrelated to cognitive performance. A small age-related increase in the sizes of the heads of thin spines also correlated with cognitive decline as measured by the DNMS task, while sizes of mushroom spines had no correlation with behavior. This led the authors to conclude, as had been proposed by some earlier investigators (see Kasai et al., 2003) that mushroom spines are stable and unchanged during aging, and that they may be involved in long-term memory, while thin spines are plastic and linked to learning. These results have been presented in some detail because this study by Dumitriu et al. (2010) was the first one to test the relationship between thin spines and learning.
However, during aging in area 46 the greatest loss of synapses is from layer 1, which becomes thinner with age and loses 30 – 60% of its synapses (Peters et al., 1998b). The loss of synapses is largely due to the fact that the postsynaptic targets of many synapses in layer 1, namely the apical dendritic tufts of pyramidal cells, are reduced in extent. Although the thinning of layer 1 is only weakly correlated with the DNMS task with a 2-minute delay (p<0.25), the loss of synapses correlates more strongly with overall cognitive decline (p<0.01). This is interesting since as described in more detail in the review by Luebke et al. (2010), there is a massive input of thalamic afferents from the M (matrix) neurons in the thalamic nuclei to layer 1 (see Jones, 2007; Rubio-Garrido et al., 2009). A reduction in the input from the M neurons to layer 1 would interfere with the feedback interactions between area 46 and thalamic nuclei, a feedback that appears to be crucial for associative learning and attention. However, it needs to be stated that even though Peters et al. (1998b) reported a thinning of layer 1 in area 46, O’Donnell et al. (1999) found no change in the volume of layer 1 in area 46 with age (Table 1). Why there is a disparity between the conclusions from these two studies is not apparent. It may be due to the fact that while Peters et al. (1998b) measured the thickness of layer 1 in semithick plastic sections, O’Donnell et al. (1999) used Nissl stained sections and applied stereology to arrive at their conclusion that the volume of layer 1 does not alter with age.
The focus of most of the studies on synaptic loss with age has been on dendrites and their spines in area 46 (Fig. 3). The only other structure in which synapses have been examined in detail is the molecular layer of the dentate gyrus, (see Table 1) in which the frequency of axospinous, asymmetric synapses appears to be stable with age and the lengths of postsynaptic densities do not alter (Tigges, et al., 1995, 1996; Hara et al., 2010, 2011a). Interestingly though, although the frequency of synapses appears not to alter with age, in the outer molecular layer of the dentate gyrus there is an increase in the proportion of axonal boutons that are not forming synapses and a decrease in the proportion of the axonal boutons that are forming multiple synapses. Both of these age changes correlate with DNMS acquisition (Hara et al., 2011a). The only other structure in which the effects of age on synapses has been examined is the motor cortex, in which Tigges et al. (1992) examined the axosomatic synapses. Tigges et al. (1992) concluded that there is no loss of axosomatic synapses from Betz cells in motor cortex as they age (Table 1). Whether there is a similar retention of axosomatic synapses in other parts of the cerebral cortex is not known. However, in an analysis of the effects of age on inhibitory terminals forming axodendritic and axosomatic symmetric synapses in layer 2/3 of area 46, Soghomonian et al. (2010) found that in young monkeys axosomatic terminals are significantly larger than axodendritic ones and that both types of terminals become larger with age. Further, the mitochondria in axosomatic terminals become larger and these terminals also show an increase in numbers of synaptic vesicles. Interestingly, when these changes are analyzed, the increase in the numbers of vesicles in the axosomatic terminals is linked to cognitive impairment. This may result from the enhanced axosomatic inhibitory input to aging pyramidal neurons brought about by increased terminal size and vesicle numbers. It would be of interest to determine if similar age-related changes occur in inhibitory axon terminals forming axosomatic synapses in other areas of the cortex.
Another factor that changes with age and correlates with cognitive decline is the integrity of minicolumns of neurons in area 46 in the ventral bank of the principal sulcus in prefrontal cortex (Cruz et al., 2004). From digitized neuronal density maps of the upper layers of the cortex it is evident that the neurons in area 46 are arranged into vertically oriented minicolumns, each of which is about 22μm wide. As in other cortices, it is likely that this arrangement is brought about because the apical dendrites of pyramidal cells in area 46 are clustered together to form bundles, the center–to-center spacing of which is probably reflected by the digitized image map (see Peters, 2010). Cruz et al. (2004) report that while there is no change with age in total neuronal density in area 46, the width of its minicolumns, or their periodicity, there is a reduction in the “strength” of the minicolumns. This is indicated by the cell bodies of the neurons being randomly displaced by as much as 3μm in old monkeys. Moreover this reduction in minicolumnn “strength’ correlates with cognitive decline as measured by the CII. While the reason for the correlation is not yet understood, Cruz et al. (2004) suggest that the change in minicolumn organization may reflect alterations in connectivity. Recently, this study has been extended to compare the alterations in ventral area 46 with those in the dorsal portion of area 46, and Cruz et al. (2009) determined that while there are age-related reductions in the strength of minicolumns in both parts of area 46, only the alterations in ventral area 46 correlate with cognitive decline. The authors suggest that the reason for the difference in the two parts of area 46 may be because the battery of behavioral tasks used to assess cognitive impairment are insensitive to the age-related structural changes in the dorsal part of area 46.
A Summary with Conclusions
To date few cortical areas in the rhesus monkey have been examined to ascertain the effects of normal aging. The focus has been on primary visual cortex and on area 46 of prefrontal cortex, with a few observations on motor cortex, entorhinal cortex and hippocampus. The remainder of the cortex has been neglected. On the other hand, there are a number of studies on the fiber tracts of the cerebral hemispheres.
With age the overall size of the rhesus monkey brain does not appear to alter significantly, and the overall thickness of the cortex is reported to be either stable or to lose about 2% of its volume, even though there are some local alterations in overall thickness. In general the only layer of the cerebral cortex that appears to lose a significant amount of its thickness is layer 1. Furthermore, relatively few senile plaques occur in the aging rhesus monkey cortex and although their frequency increases with age, the increase does not correlate with cognitive decline.
One consistent feature of the aging cortex is the absence of significant neuronal loss from the neocortex and the hippocampal complex. The possible exception is a reported focal loss of neurons from area 8A of prefrontal cortex, even though the adjacent area 46 appears to remain intact. But although there may not be significant changes in cell numbers with age in area 46, Cruz et al. (2004; 2009) report that with age there is a reduction in the “strength” of minicolumns of neurons in area 46, indicating that neurons become displaced relative to one another. Not only are neuronal numbers not significantly reduced with age, but also there is little change in the overall numbers of astrocytes and microglial cells. However, there are increases in the numbers of oligodendrocytes, which relates to the remyelination of some nerve fibers and the consequent increase in the numbers of internodes along them.
Detailed studies of the effects of aging on synaptic organization of the cerebral cortex have only been carried out in prefrontal area 46 and dentate gyrus. In area 46 various studies have demonstrated a loss of some 30% of the asymmetric and symmetric synapses from layer 2/3, and these losses correlate with cognitive decline. But the greatest loss of synapses is from layer 1, where the apical tufts of the pyramidal neurons are located and in area 46 this loss also correlates with cognitive decline. These losses of synapses are paralleled by losses of dendritic spines, mainly thin spines, since the frequency of the more complex mushroom spines remains stable with age. Also in layer 2/3 the axon terminals forming axosomatic and axodendritic inhibitory synapses become larger with age and there is an increase in the numbers of synaptic vesicles and in the sizes of mitochondria in terminals forming axosomatic synapses. The age changes in layer 5 are different. In layer 5 there is a significant loss of asymmetric synapses, but not of symmetric ones, and these losses do not correlate with cognitive decline. No parallel studies of dendritic spine changes appear to have been carried out in layer 5. At present the sources of the terminals that are lost with age are not known, but most of them probably arise intrinsically, since some 90% of axon terminals forming excitatory synapses and almost all of the inhibitory synapses arise intracortically (see Peters, 2002a).
An important question that remains unanswered is whether the loss of synapses from area 46 is unique to that area, or whether a similar loss of synapses occurs throughout the cortex. It has been shown that with age there is also a loss of dendritic spines, and presumably of synapses, from layer 3 cortical neurons in the superior temporal gyrus and in area 46, but it is possible that synapse and spine loss are not a general phenomena, since it has been reported that the number of synapses in the neuropil of the dentate gyrus remains stable with age (Table 1; Tigges et al., 1995 and 1996; Hara et al., 2010 and 2011a), as do the numbers of axosomatic synapses on Betz cells in motor cortex (Tigges et al., 1992).
In contrast to the limited amount of information about synapses, there are detailed data on the effects of age on myelinated nerve fibers in two cytoarchitectonic areas (area 17 and 46) and in five subcortical forebrain fiber systems related to the cerebral cortex. There is also some data on the optic nerve. There appears to be no significant loss of myelinated nerve fibers from the cortical gray matter with age. In contrast, as they age all of the subcortical white matter systems lose between 20 to 45% of nerve fibers, but none of them show a change in cross sectional area (Table 1), even though in an MRI study Wisco et al. (2008) have reported a significant 11.5% overall reduction in the volume of white matter. In parallel with the nerve fiber loss it is not surprising that each of the subcortical white matter systems shows some myelinated nerve fibers with axons that are degenerating. But the percentage of axons that show signs of degeneration at any one time is small, and for only three of the five fiber systems examined are there correlations between the frequency of degenerating axons and cognitive decline.
Areas 17 and 46 and all of the subcortical fiber tracts show profiles of myelin sheaths that are degenerating and such profiles are 3 to 20 times more common in old as compared to young monkeys (Table 4). This degeneration is accompanied by an increase in the frequency of paranodes, which are 1.2 to 1.9 times more common in old than in young monkeys (Table 4). This is an indication that remyelination is taking place in the older monkeys. An important question is whether these structural alterations are related to cognitive decline. Interestingly, for fiber tracts such as the cingulate bundle and the genu, which lose only 20% of their nerve fibers, the loss does not correlate with cognitive decline. There is only a correlation when the nerve fiber losses reach 26 – 45%, as they do in the splenium, the fornix and the anterior commissure (Table 3). This suggests that there may be a cognitive reserve built into the brain, such that there is a level of loss and disconnection between structures that can be tolerated, but beyond which cognition becomes affected.
The age-related losses of nerve fibers appear to reflect a pathological process in white matter, rather than in the cortex itself, since there is no significant loss of cortical neurons with age. Thus it is corticocortical axon branches of cortical neurons that degenerate, leaving the neuronal cell bodies and their intracortical plexuses more or less intact. That is not to suggest that the intracortical axonal plexuses are unaffected, since they do lose some axon terminals, as shown by the loss of cortical synapses with age from area 46.
As shown in Table 4, the frequency of altered myelin sheaths in cortices and fiber tracts in old monkeys increases significantly as monkeys get older. While the frequency of altered sheaths correlates with cognitive decline in the two cortical areas examined and in two of the subcortical fiber bundles, the morphological data on altered sheath frequency in the genu of corpus callosum and in the anterior commissure does not correlate with cognitive decline (Table 4). For paranode frequency the results are more clear-cut: the only significant correlation between increased paranode frequency and cognitive decline is in area 46. When young and old monkeys are compared, area 46 shows a profound, 90% (1.9x), increase in the frequency of paranodal profiles (Table 4). The age-related increased frequencies in paranodal profiles in area 17 and in the subcortical fiber tracts are less pronounced. The numbers of paranodes are not increased as dramatically with aging and do not correlate with cognitive decline (Table 4). This is an unexpected result, since it would be expected that the reported slowing of conduction velocity, due to the interposition of shorter internodal lengths along some nerve fibers, would affect the timing in neuronal circuits and hence affect cognition.
What is becoming clear is that cognitive decline during normal aging in the rhesus monkey is not due to a single factor, and that both degenerative and reparative changes occur. In the former category are the degeneration of some axons, which leads to a loss of myelinated nerve fibers, and the degeneration of myelin sheaths around axons that remain intact. Another degenerative change is the loss of synapses from the cortex and its accompanying loss of thin dendritic spines, losses that appear to primarily affect the supragranular layers of the cortex. The most obvious reparative change is the remyelination that leads to the formation of shorter internodes, and the accompanying increase in the numbers of paranodes and oligodendrocytes.
Although the catalog of normal age changes is growing, only cortical areas 46 and 17 and dentate gyrus have been examined to any extent. And as yet, almost nothing is known about what brings about the age related changes in nerve fibers, axons, and cerebrocortical neurons, and the relationships of the changes to each other. This needs to be explored if we are to achieve the ultimate goal of generating interventions that might retard some of the age changes and slow down normal cognitive decline.
Acknowledgments
We wish to thank Claire Folger for her support in helping to carry out our studies of the effects of aging on the monkey brain. We also wish to thank Dr Mark Moss for correcting our errors in citations of the behavioral data referred to in this article; and Dr Jennie Luebke for her very careful reading of this review and for her suggestions about how some of the statements in the text could be more clearly written.
Grant support: NIH/NIA P01 AG00001
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Chen K, Aschenbrenner M, Merkley TL, Santerre-Lemmon LE, Shamy JL, Skaggs WE, Buonocore MH, Rapp PR, Barnes CA. Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. J Neurosci. 2008;28:2710–2718. doi: 10.1523/JNEUROSCI.1852-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva H, Jacqmin-Gadda H, Orgogozo JM, Le Carret N, Helmer C, Letenneur L, Barberger-Gateau P, Colette Fabrigoule C, Dartigues JF. The 9-year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain. 2005;128:1093–1101. doi: 10.1093/brain/awh451. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic P. Analysis of alpha-2 adrenergic agonist effects on the delayed nonmatch-to-sample performance of aged rhesus monkeys. Neurobiol Aging. 1990;11:583–590. doi: 10.1016/0197-4580(90)90021-q. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Cabral H, Rosene DL, Peters A. Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey. J Comp Neurol. 2010;518:1096–9861. doi: 10.1002/cne.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody HD. Organization of the cerebral cortex. III A study of aging in the human cerebral cortex. J Comp Neurol. 1955;1023:511–556. doi: 10.1002/cne.901020206. [DOI] [PubMed] [Google Scholar]
- Brody H. Structural changes in the aging nervous system. Interdisc Top Gerontol. 1970;7:9–21. [Google Scholar]
- Brück W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206:181–5. doi: 10.1016/s0022-510x(02)00191-0. [DOI] [PubMed] [Google Scholar]
- Chang Y, Luebke JI. Age related increase in the slow outward calcium-activated potassium current in layer 3 and layer 5 pyramidal cells in area 46 of the rhesus monkey. J Neurophysiol. 2006;98:2622–2632. doi: 10.1152/jn.00585.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex. 2005;15:409–418. doi: 10.1093/cercor/bhh144. [DOI] [PubMed] [Google Scholar]
- Cruz L, Roe DL, Urbanc B, Cabral H, Stanley HE, Rosene DL. Age-related reduction in microcolumnar structure in area 46 of the rhesus monkey correlates with behavioral decline. Proc Natl Acad Sci USA. 2004;101:15846–158451. doi: 10.1073/pnas.0407002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L, Roe DL, Urbanc B, Inglis A, Stanley HE, Rosene DL. Age-related reduction in microcolumnar structure correlates with cognitive decline in ventral but not dorsal area 46 of the rhesus monkey. Neuroscience. 2009;158:1509–1520. doi: 10.1016/j.neuroscience.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen MJ, Webster HD. Remodelling of optic nerve myelin sheaths and axons during metamorphosis in Xenopus laevis. J Comp Neurol. 1979;184:353–62. doi: 10.1002/cne.901840209. [DOI] [PubMed] [Google Scholar]
- Cupp CJ, Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: quantitative analysis of dendritic branching patterns. Exp Neurol. 1980;69:143–163. doi: 10.1016/0014-4886(80)90150-8. [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex. 2003;13:950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, White RF, D’Agostino RB. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Thakker MM, Hof PR, Morrison JH. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol Aging. 1997;18:549–53. doi: 10.1016/s0197-4580(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra SS. A beta40 is a major form of beta-amyloid in nonhuman primates. Neurobiol Aging. 1996;17:903–908. doi: 10.1016/s0197-4580(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WGM, Roberts MT, Morrison JH, Rapp PR. Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.09.014. Advanced online publication. Retrieved October 26, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen GM, Punsoni M, Rapp PR, Morrison JH. Synaptic characteristics of dentate gyrus axonal boutons and their relationship with aging, menopause, and memory in female rhesus monkeys. J Neurosci. 2011a;31:7737–7744. doi: 10.1523/JNEUROSCI.0822-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Rapp PR, Morrison JH. Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age. 2011b doi: 10.1007/s11357-011-9278-5. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scher PA, Bienias JL, Bennett DS, Evans DA. Alzheimer disease in the US population. Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Heilbroner PL, Kemper TL. The cytoarchitectonic distribution of senile plaques in three aged monkeys. Acta Neuropathol. 1990;81:60–65. doi: 10.1007/BF00662638. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Tigges J, Klumpp SA, Anderson DC. Brain weight does not decrease with age in adult rhesus monkeys. Neurobiol Aging. 1998;19:267–272. doi: 10.1016/s0197-4580(98)00054-2. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hof PR, Nimchinsky EA, Young WG, Morrison JH. Numbers of Meynert and layer IVB cells in area V1: a stereologic analysis in young and aged macaque monkeys. J Comp Neurol. 2000;420:113–126. doi: 10.1002/(sici)1096-9861(20000424)420:1<113::aid-cne8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. Cambridge University Press; New York: 2007. [Google Scholar]
- Kabaso D, Coskren PJ, Henry BI, Hof PR, Wearne SL. The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging. Cereb Cortex. 2009;19:2248–2268. doi: 10.1093/cercor/bhn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaru K, Iwatsubo T, Ihara Y. Comparable amyloid beta-protein (A beta) 42(43) and A beta 40 deposition in the aged monkey brain. Neurosci Lett. 1996;214:196–198. doi: 10.1016/0304-3940(96)12893-7. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Nogichi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends in Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kawas CH, Corrada MM, Brookmeyer R, Morrison A, Resnick SM, Zondermann AB, Arenberg D. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24:157–165. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Moss MB, Rosene DL, Herndon J. Recognition memory function in early senescent rhesus monkeys. Psychbiology. 2000;28:45–56. [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–9. [PubMed] [Google Scholar]
- Koo BB, Schettler SP, Murray DE, Lee JM, Killiany RJ, Rosene DL, Kim DS, Ronen I. Age-related effects on cortical thickness patterns of the Rhesus monkey brain. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.07.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiene J, Shupe L, Perlmutter S, Horner P. No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. J Neurosci. 2008;28:3887–3896. doi: 10.1523/JNEUROSCI.4756-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin SK. Pathology of the myelin sheath. In: Waxman SG, Kocsis JD, Stys PK, editors. The axon: structure, function and pathophysiology. Oxford University Press; New York: 1995. pp. 412–437. [Google Scholar]
- Luebke J, Barbas H, Peters A. Effects of normal aging on prefrontal area 46 in the rhesus monkey. Brain Res Rev. 2010;62:212–232. doi: 10.1016/j.brainresrev.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Chang YM. Effects of aging on the electrophysiological properties of layer 5 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2007;150:556–562. doi: 10.1016/j.neuroscience.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125:277–288. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Rosene DL. Aging alters dendritic morphology, input resistance, and inhibitory signaling in dentate granule cells of the rhesus monkey. J Comp Neurol. 2003;460:573–584. doi: 10.1002/cne.10668. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Roberts JA, Tuszynski MH. Conservation of neuron number and size in entorhinal cortex layers II, III, and V/VI of aged primates. J Comp Neurol. 2000;422:396–401. doi: 10.1002/1096-9861(20000703)422:3<396::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Morales FR, Boxer PA, Fung SJ, Chase MH. Basic electrophysiological properties of spinal cord motoneurons during old age in the cat. J Neurophysiol. 1987;58:180–94. doi: 10.1152/jn.1987.58.1.180. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Moss MB, Killiany RJ, Lai ZC, Rosene DL, Herndon J. Recognition memory span in rhesus monkeys of advanced age. Neurobiol Aging. 1997;18:13–19. doi: 10.1016/s0197-4580(96)00211-4. [DOI] [PubMed] [Google Scholar]
- Moss MB, Killiany RJ, Herndon JG. Age-related cognitive decline in rhesus monkey. Neurodegenerative and age-related changes in structure and function of the cerebral cortex. In: Peters A, Morrison JH, editors. Cerebral Cortex. Vol. 14. Kluwer Academic/Plenum Publishers; New York: 1999. pp. 21–48. [Google Scholar]
- Moss MB, Rosene DL, Peters A. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging. 1988;9:495–502. doi: 10.1016/s0197-4580(88)80103-9. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Peters A. The effects of aging on the frequency of nerve fibers in rhesus monkey striate cortex. Neurobiol Aging. 2000;21:621–628. doi: 10.1016/s0197-4580(00)00169-x. [DOI] [PubMed] [Google Scholar]
- O’Donnell KA, Rapp PR, Hof PR. Preservation of prefrontal cortical volume in behaviorally characterized aged macaque monkeys. Exp Neurol. 1999;160:300–310. doi: 10.1006/exnr.1999.7192. [DOI] [PubMed] [Google Scholar]
- Peters A. Examining neocortical circuits. Some background and facts. J Neurocytol. 2002a;31:183–193. doi: 10.1023/a:1024157522651. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002b;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on nerve fibers and neuroglia in the central nervous system. In: Riddle DR, editor. Brain Aging: Models, Methods and Mechanisms. CRC Press; Baton Rouge: 2007. pp. 97–125. [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Front Neuroanat. 2009;3:11. doi: 10.3389/neuro.05.011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The morphology of minicolumns. In: Blatt GJ, editor. The Neurochemical Basis of Autism. Springer; New York: 2010. pp. 45–68. [Google Scholar]
- Peters A, Leahu D, Moss MB, McNally KJ. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cereb Cortex. 1994;4:621–35. doi: 10.1093/cercor/4.6.621. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Peters A, Moss MB, Sethares C. Effects of aging on myelinated nerve fibers in monkey primary visual cortex. J Comp Neurol. 2000;419:364–376. doi: 10.1002/(sici)1096-9861(20000410)419:3<364::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Peters A, Moss MB, Sethares C. The effects of aging on layer 1 of primary visual cortex in the rhesus monkey. Cerebral Cortex. 2001;11:93–103. doi: 10.1093/cercor/11.2.93. [DOI] [PubMed] [Google Scholar]
- Peters A, Nigro NJ, McNally KJ. A further evaluation of the effect of age on striate cortex of the rhesus monkey. Neurobiol Aging. 1997;18:29–36. doi: 10.1016/s0197-4580(96)00208-4. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL. In aging, is it gray or white? J Comp Neurol. 2003;462:139–143. doi: 10.1002/cne.10715. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. Neuropathol Exp Neurol. 1996;55:861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the Meynert cells in rhesus monkey primary visual cortex. Anat Rec. 1993;236:721–729. doi: 10.1002/ar.1092360416. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. The effects of age on the cells in layer 1 of primate cerebral cortex. Cerebr Cortex. 2002a;12:27–36. doi: 10.1093/cercor/12.1.27. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002b;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Is there remyelination during aging of the primate central nervous system? J Comp Neurol. 2003;460:238–254. doi: 10.1002/cne.10639. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Killiany RJ. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J Comp Neurol. 2001;435:241–8. doi: 10.1002/cne.1205. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008a;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998b;8:671–684. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. How the primate fornix is affected by age. J Comp Neurol. 2010;518:3962–3980. doi: 10.1002/cne.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Verderosa A, Sethares C. The neuroglial population in the primary visual cortex of the aging rhesus monkey. Glia. 2008b;56:1151–61. doi: 10.1002/glia.20686. [DOI] [PubMed] [Google Scholar]
- Presty SK, Bachevalier J, Walker LC, Struble RG, Price DI, Mishkin M, Cork LC. Age differences in recognition memory of the rhesus monkey (Macaca mulatta) Neurobiol Aging. 1987:435–440. doi: 10.1016/0197-4580(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith DG, Schmitt FA, Markesbery WR, Kay J, Kurlan R, Hulettte C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondementing aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp R, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Garrido P, Pérez-de-Manzo F, Porrero C, Galazo MJ, Clascá F. Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb Cortex. 2009;19:2380–2395. doi: 10.1093/cercor/bhn259. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Effects of age on nerve fibers in the rhesus monkey optic nerve. J Comp Neurol. 2001;429:541–553. doi: 10.1002/1096-9861(20010122)429:4<541::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Effects of age on the glial cells in the rhesus monkey optic nerve. J Comp Neurol. 2002;445:13–28. doi: 10.1002/cne.10162. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Disrupted myelin and axon loss in the anterior commissure of the aged rhesus monkey. J Comp Neurol. 2003;466:14–30. doi: 10.1002/cne.10859. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Habeck C, Hof PR, Amaral DG, Fong SG, Buonocore MH, Stern Y, Barnes CA, Rapp PR. Volumetric correlates of spatiotemporal working and recognition memory impairment in aged rhesus monkeys. Cereb Cortex. 2011;21:1559–1573. doi: 10.1093/cercor/bhq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane JA, Pietropaolo MF, Rosene DL, Moss MB, Peters A, Kemper T, Abraham CR. Lack of correlation between plaque burden and cognition in the aged monkey. Acta Neuropathol. 1997;94:471–478. doi: 10.1007/s004010050735. [DOI] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24:4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian JJ, Sethares C, Peters A. Effects of age on axon terminals forming axosomatic and axodendritic inhibitory synapses in prefrontal cortex. Neuroscience. 2010;168:74–81. doi: 10.1016/j.neuroscience.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struble RG, Price DL, Jr, Cork LC, Price DL. Senile plaques in cortex of aged normal monkeys. Brain Res. 1985;361:267–275. doi: 10.1016/0006-8993(85)91298-3. [DOI] [PubMed] [Google Scholar]
- Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. Am J Primatol. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- Tigges J, Herndon JG, Peters A. Neuronal population of area 4 during the life span of the rhesus monkey. Neurobiol Aging. 1990;11:201–208. doi: 10.1016/0197-4580(90)90546-c. [DOI] [PubMed] [Google Scholar]
- Tigges J, Herndon JG, Peters A. Axon terminals on Betz cell somata of area 4 in rhesus monkey throughout adulthood. Anat Rec. 1992;232:305–315. doi: 10.1002/ar.1092320216. [DOI] [PubMed] [Google Scholar]
- Tigges J, Herndon JG, Rosene DL. Mild age-related changes in the dentate gyrus of adult rhesus monkeys. Acta Anat (Basel) 1995;153:39–48. doi: 10.1159/000147713. [DOI] [PubMed] [Google Scholar]
- Tigges J, Herndon JG, Rosene DL. Preservation into old age of synaptic number and size in the supragranular layer of the dentate gyrus in rhesus monkeys. Acta Anat (Basel) 1996;157:63–72. doi: 10.1159/000147867. [DOI] [PubMed] [Google Scholar]
- Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: synaptic density. Exp Neurol. 1980;69:164–172. doi: 10.1016/0014-4886(80)90151-x. [DOI] [PubMed] [Google Scholar]
- Vincent SL, Peters A, Tigges J. Effects of aging on the neurons within area 17 of rhesus monkey cerebral cortex. Anat Rec. 1989;223:329–341. doi: 10.1002/ar.1092230312. [DOI] [PubMed] [Google Scholar]
- West MJ, Amaral DG, Rapp PR. Preserved hippocampal cell number in aged rhesus monkeys with recognition memory deficits. Soc Neurosci. 1993;19:599. (Abstr.) [Google Scholar]
- Wisco JJ, Killiany RJ, Guttmann CRG, Warfield SK, Moss MB, Rosene DL. An MRI study of age-related volume changes in the rhesus monkey. Neurobiol Aging. 2008;29:1563–1575. doi: 10.1016/j.neurobiolaging.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]