Abstract
Background
Correlative evidence indicates that apoptosis is associated with the progression of alcoholic liver disease. If apoptosis contributes to ethanol-induced steatohepatitis and/or fibrosis, then mice deficient in Bid, a key pro-apoptotic Bcl-2 family member, or mice treated with a pan-caspase inhibitor (VX166) should be resistant to ethanol-induced liver injury.
Methods
This hypothesis was tested in mice using a model of chronic, heavy ethanol-induced liver injury, as well as in a model in which moderate ethanol feeding accelerated the appearance of early markers of hepatic fibrosis in response to acute carbon tetrachloride exposure.
Results
Chronic ethanol feeding to mice increased TUNEL- and cytokeratin 18-positive cells in the liver, as well as the expression of receptor interacting protein kinase 3 (RIP3), a marker of necroptosis. In this model, Bid−/− mice or wild-type mice treated with VX166 were protected from ethanol-induced apoptosis, but not ethanol-induced RIP3 expression. Bid-deficiency or inhibition of caspase activity did not protect mice from ethanol-induced increases in plasma alanine and aspartate amino transferase activity, steatosis or mRNA expression of some inflammatory cytokines. Moderate ethanol feeding to mice enhanced the response of mice to acute carbon tetrachloride exposure, resulting in increased expression of α-smooth muscle actin and accumulation of extracellular matrix protein. VX166-treatment attenuated ethanol- mediated acceleration of these early indicators of carbon tetrachloride-induced hepatic fibrosis, decreasing the expression of α-smooth muscle actin and the accumulation of extracellular matrix protein.
Conclusion
Ethanol-induced apoptosis of hepatocytes was mediated by Bid. Apoptosis played a critical role in the accelerating the appearance of early markers of carbon tetrachloride-induced fibrosis by moderate ethanol, but did not contribute to ethanol-induced hepatocyte injury, steatosis or expression of mRNA for some inflammatory cytokines.
Keywords: Alcoholic liver disease, cell death, necroptosis, caspase, Bid
Alcoholic liver disease (ALD) typically progresses through a series of pathologies starting with fatty liver or steatosis. Steatosis often proceeds to steatohepatitis, characterized by inflammation and hepatic cell death (Nagata et al., 2007). Steatohepatitis can further proceed to fibrosis, followed by cirrhosis. Ethanol-induced hepatic inflammation is characterized by infiltration of a variety of pro-inflammatory cells, including macrophages, monocytes, lymphocytes and neutrophils, as well as enhanced production of many pro-inflammatory cytokines (Szabo and Bala, 2010). Macrophage-derived TNFαis central to ethanol-induced hepatic inflammation and injury (Roychowdhury et al., 2009a). Apoptosis is implicated in a variety of pathological conditions in the liver, including ALD (Mundt et al., 2005; Zhou et al., 2001) and non-alcoholic steatohepatitis (NASH) (Galle and Krammer, 1998; Mundt et al., 2005). Induction of apoptosis in hepatocytes closely correlates with the severity of alcoholic and non-alcoholic liver diseases (Benedetti et al., 1988; Guicciardi and Gores, 2005; Witek et al., 2009). Detection of apoptotic hepatocytes in close proximity to inflammatory foci in liver biopsies from patients with ALD suggests that apoptosis could be a critical factor in alcohol-induced inflammation in the liver (Guicciardi and Gores, 2005; Jaeschke, 2002; Ziol et al., 2001).
Apoptosis is a complex, caspase-dependent cell death mechanism, mediated by the extrinsic (death-receptor-mediated) and the intrinsic (mitochondria-mediated) pathways (Elmore, 2007). Interaction of the death receptors (TNFαreceptor or Fas) with their respective ligands initiates the extrinsic pathway, while mitochondrial dysfunction triggers the intrinsic pathway. Bid is a key pro-apoptotic Bcl-2 family member that is crucial for the induction of apoptotic death and serves as a bridge between these two fundamental cell death pathways (Yin, 2000a; Yin, 2000b). Since pathological activation of apoptosis in the liver is implicated in the progression of liver disease in both acute and chronic liver disorders, attenuation of apoptotic events can be considered as a potential therapeutic strategy against liver damage (Witek et al., 2009).
Hepatocytes also undergo a necrosis-like caspase-independent cell death, called programmed necrosis/necroptosis, in conditions such as ischemia (Rosenbaum et al.) or in response to partial hepatectomy (Zorde-Khvalevsky et al., 2009). Necrosis can either be executed in a rapid, uncontrolled manner leading to rupture of cell membranes and leakage of intracellular proteins in the extra-cellular space, known as oncotic necrosis, or it can be orchestrated by a cascade of proteins, termed as programmed necrosis/necroptosis (Vandenabeele et al.2010). Programmed necroptosis is regulated by specific receptor-interacting protein kinases (RIP), including RIP1 and RIP3 (Vandenabeele et al.2010). Upon activation of the necroptotic pathways, damage-associated molecular pattern (DAMP) molecules are released from the dying cells; DAMPs then trigger a variety of pro-inflammatory signals and oxidative stress (Tsung et al., 2007).
Genetic or pharmacological inhibition of apoptosis, using specific caspase-deficient mice or drugs that inhibit caspase activities, is protective in a number of models of liver injury, including the methyl-choline deficient model (MCD) of NASH (Witek et al., 2009), bile duct ligation-induced hepatic fibrosis (Canbay et al., 2004; Nalapareddy et al., 2009), hepatitis C (Masuoka et al., 2009) and liver transplantation (Baskin-Bey et al., 2007). Inhibiting caspases, the central mediators of apoptosis, ameliorates inflammation and fibrosis in the MCD model of NASH, but fails to attenuate liver injury, assessed by increased circulating ALT/AST (Witek et al., 2009). Although correlative evidence indicates that apoptosis is associated with inflammation and hepatic injury during progression of ALD (Malhi et al., 2006), very little is known about the direct role of apoptosis in mediating ethanol-induced liver injury. In a rat model, treatment with a pan-caspase inhibitor ameliorated ethanol-induced sensitization to challenge with LPS (Deaciuc et al., 2001); however, no studies have been carried out to directly link apoptosis to progression of alcohol-induced liver damage.
We hypothesized that chronic ethanol feeding induces apoptosis in the liver in a Bid-dependent manner, which subsequently contributes to liver injury. Making use of Bid-deficient mice and the pan-caspase inhibitor VX166, we investigated whether apoptosis contributes to steatosis, inflammation, hepatocyte injury and fibrosis in response to ethanol using both a model of chronic, heavy ethanol feeding to generate steatohepatitis, as well as moderate ethanol feeding combined with carbon tetrachloride (CCl4) to model the acceleration of fibrosis by ethanol. Chronic, heavy ethanol feeding resulted in Bid-dependent hepatocyte apoptosis. While Bid-deficiency or treatment with the pan-caspase inhibitor VX166 had no effect on ethanol-induced hepatocyte injury, steatosis or expression of mRNA for some inflammatory cytokines, inhibition of caspase activity attenuated the expression of early markers of fibrosis in mouse liver in response to moderate ethanol and CCl4.
Experimental Procedures
Materials
Female C57BL/6J mice (8–10 weeks old) were purchased from Jackson Labs (Bar Harbor, Maine). Lieber-DeCarli high-fat ethanol and control diets were purchased from Dyets (Bethlehem, PA). A colony of Bid−/− mice on a C57BL/6 background (Ding et al., 2004) was established at the Cleveland Clinic from breeders obtained from Dr. Xiao-Ming Yin of the University of Pittsburgh.
Antibodies were from the following sources: 4-hydroxynonenal (Alpha Diagnostics, San Antonio, TX), caspase-generated fragment of cytokeratin-18 (M30) (Roche, Mannheim, Germany), α-smooth muscle actin, clone 1A6 (Sigma-Aldrich, St. Louis, MO), Collagen 1 (Southern Biotech, Birmingham, AL), CYP2E1 (Research Diagnostics, Inc., Flanders, NJ), HSC70 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) and RIP3 (ABGENT, San Diego, CA, cat. no. AP7819b). Alexa fluor-488 conjugated secondary antibodies were purchased from Invitrogen (Carlsbad, CA). TUNEL assay kit Apop Tag @ Plus in Situ apoptosis detection kit was purchased from Chemicon International (Temula, CA, cat. no. S7111). Pan-caspase inhibitor VX166 was a gift from Vertex Pharmaceuticals (Europe) Ltd (Abingdon, UK).
Mouse models
All procedures using animals were approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Female mice were housed in shoe-box cages (2 animals/cage) with microisolator lids. Standard microisolator handling procedures were used throughout the study. Mice were randomized into ethanol-fed and pair-fed groups and then adapted to control liquid diet for 2 days.
Chronic, heavy ethanol-induced liver injury
The ethanol-fed group was then allowed free access to an ethanol containing diet with increasing concentrations of ethanol: 1 and 2% (vol/vol) each for 2 days, then 4 and 5% ethanol each for 7 days, and finally 6% ethanol for 1 more week. The 6% (vol/vol) diet provided ethanol as 32 percent of total calories in the diet (termed “25d, 32%”). Control mice were pair-fed diets which iso-calorically substituted maltose dextrins for ethanol over the entire feeding period. C57BL/6 mice were given one daily dose of VX166 (10mg/Kg) or vehicle by gavage to inhibit caspase activity each afternoon 1 h prior to providing the daily diet. VX166 was dissolved in polyethylene glycol 400 supplemented with vitamin E (vehicle) (Sigma catalog #202398).
Moderate alcohol and carbon tetrachloride model of early markers of fibrosis
The ethanol-fed group was then allowed free access to an ethanol containing diet with increasing concentrations of ethanol: 1 and 2% (vol/vol) each for 2 days; the 2% diet provided 11% of calories as ethanol. After 4 days of ethanol feeding, mice were treated with VX166 (as described above) or vehicle. After 1 h, mice were injected intraperitoneally with a single dose of carbon tetrachloride (CCl4) (Pritchard et al.2010a). CCl4 was prediluted 1:3 in olive oil before administration. Mice received a single dose at 1μL/g body weight of CCl4 administered by intraperitoneal injection using 100 μL Hamilton syringes fitted with 26G 5/8 inch needles. Mice continued on either the control or ethanol diet (11% of calories) and daily gavage of VX166 or vehicle for a further 3 days after CCl4 exposure.
At the end of the feeding protocol mice were anesthetized, blood samples taken into non-heparinized syringes from the posterior vena cava and livers excised. Portions of each liver were then either fixed in formalin or frozen in optimal cutting temperature (OCT) compound (Sakura Finetek U.S.A., Inc., Torrance CA) for histology, frozen in RNAlater (Qiagen, Valencia, CA) or flash frozen in liquid nitrogen and stored at −80 °C until further analysis. Blood was transferred to EDTA-containing tubes for the isolation of plasma. Plasma was then stored at -80°C.
Histopathology
Formalin-fixed tissues were paraffin-embedded, sectioned, coded and stained with hematoxylin and eosin. Steatosis and inflammation were scored by our experienced pathologist (X. Liu) on a scale of 0 to 3 on the basis of inflammatory cells infiltrated. For histological detection of fibrosis, formalin-fixed liver tissues were paraffin-embedded, sectioned, coded and stained with Sirius red.
Biochemical assays
Plasma samples were assayed for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using commercially available enzymatic assay kits (Diagnostic Chemicals, LTD, Oxford, CT) following the manufacturer’s instructions. Total hepatic triglycerides were assayed using the Triglyceride Reagent Kit from Pointe Scientific Inc. (Lincoln Park, Michigan).
Western blot analysis
Frozen liver tissue (0.5–1.0 g) was homogenized in lysis buffer (10 ml/ g tissue) and protein concentration were measured using the BCA assay (Pritchard et al., 2007). Liver lysates were then used for Western blot analysis of Cytochrome P450 2E1 (CYP2E1) and RIP3 expression. HSC 70 was used as the loading control.
Isolation of RNA and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated and reverse transcribed followed by amplification using qRT-PCR. The relative amount of target mRNA was determined using the comparative threshold (Ct) method by normalizing target mRNA Ct values to those of 18S (Mandal et al.)
Measure of apoptosis and immunohistochemistry
Apoptosis was detected using both TUNEL assay and immunostaining for M30, a fragment of cytokeratin-18 resulting from caspase cleavage, as described previously (Cohen et al. 2010)
Formalin-fixed paraffin-embedded liver sections were de-paraffinized and stained for 4- hydroxynonenal (4-HNE) (Roychowdhury et al., 2009b), α-smooth muscle actin (Pritchard and Nagy 2010b) or RIP3 (Zorde-Khvalevsky et al., 2009). Frozen liver sections were used for staining collagen 1. All images presented in the results are representative of at least 3 images per liver and 4 mice per experimental condition.
Statistical analysis
All values presented represent means ± standard error of mean, with n=3–6 experimental points. Data were analyzed by general linear models procedure (SAS, Carey, NC). Data were log transformed if needed to obtain a normal distribution. Follow-up comparisons were made by least square means testing.
Results
Apoptosis in the liver following chronic, heavy ethanol feeding was Bid-dependent
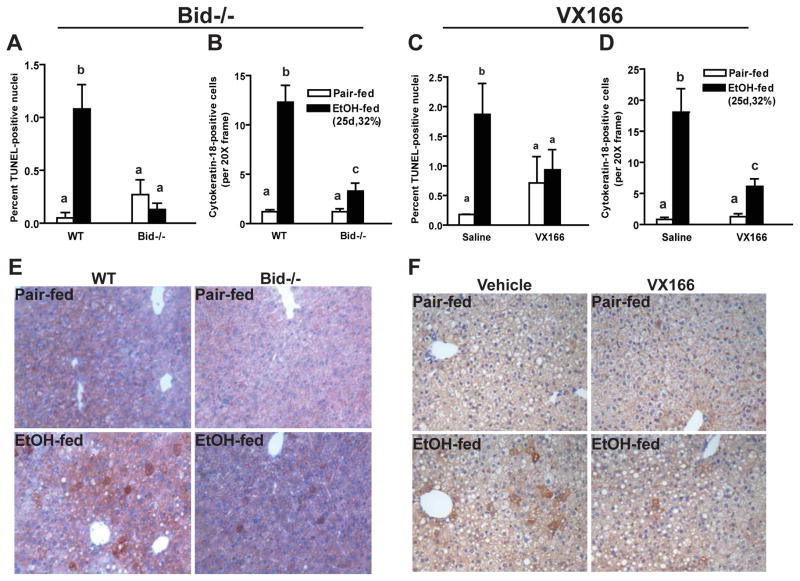
Fragmentation of DNA is one of the hallmarks of apoptosis (Malhi et al., 2006). After chronic, heavy ethanol feeding, the number of TUNEL-positive nuclei, a marker of DNA fragmentation, increased in liver of wild-type mice (Figure 1A/C and Supplemental Figure 1). Accumulation of the hepatocyte-specific, caspase-generated cytokeratin by-product, cytokeratin-18 (CK-18) (Cohen et al.) was also detected in livers of chronic ethanol-fed wild-type mice, but not pair-fed control (Figure 1B/D). Bid-deficient mice were resistant to chronic ethanol-induced increases in apoptosis; both TUNEL and CK-18-positive cells were reduced in Bid-deficient mice compared to wild-type mice after ethanol feeding (Figure 1A, B and E and supplemental Figure 1A). Similarly, wild-type mice treated with the pan-caspase inhibitor VX166 were resistant to chronic ethanol-induced apoptosis in liver (Figure 1C, D and F and Supplemental Figure 1B).
Figure 1.
Ethanol-induced increase of TUNEL and cytokeratin-18 (CK-18)-positive cells are reduced in the livers from Bid-deficient and VX166-treated mice. A/B/E) C57BL/6J wild-type (WT) and Bid−/− mice were allowed free access to increasing concentrations of ethanol diets (25d, 32%) or pair-fed control diets. C/D/F) WT mice were gavaged daily with VX166 (10mg/kg) or vehicle during the ethanol feeding protocol (25d, 32%). Paraffin-embedded liver sections were de-paraffinized followed by TUNEL (A/C) or CK-18 (B,D,E,F) immunostaining. Cells positive for TUNEL were counted and expressed as percent of total nuclei stained with DAPI (Images shown in Supplemental Figure 1). TUNEL and CK-18 images were acquired using 40X and 20X objectives, respectively. CK-18-positive cells were counted and expressed as cells per 20X frame. Values represent means ± SEM, n=4–6. Values with different alphabetical superscripts were significantly different from each other, p< 0.05.
Chronic, heavy ethanol-induced liver injury was not affected by inhibition of apoptosis
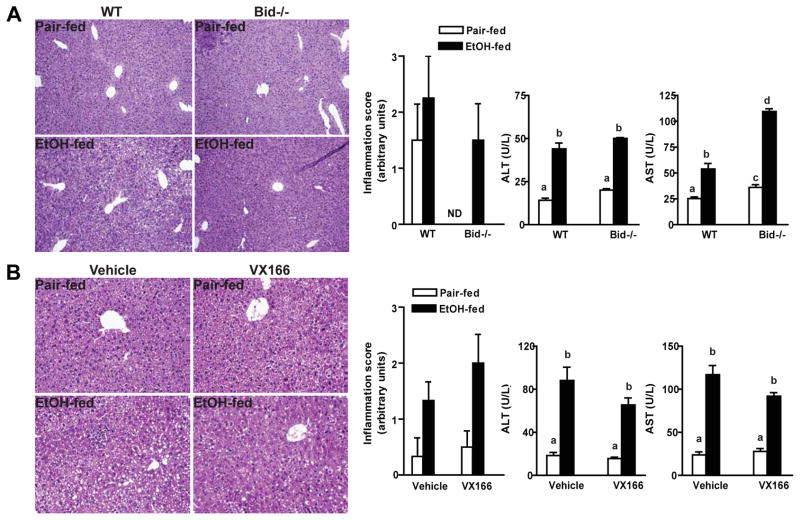
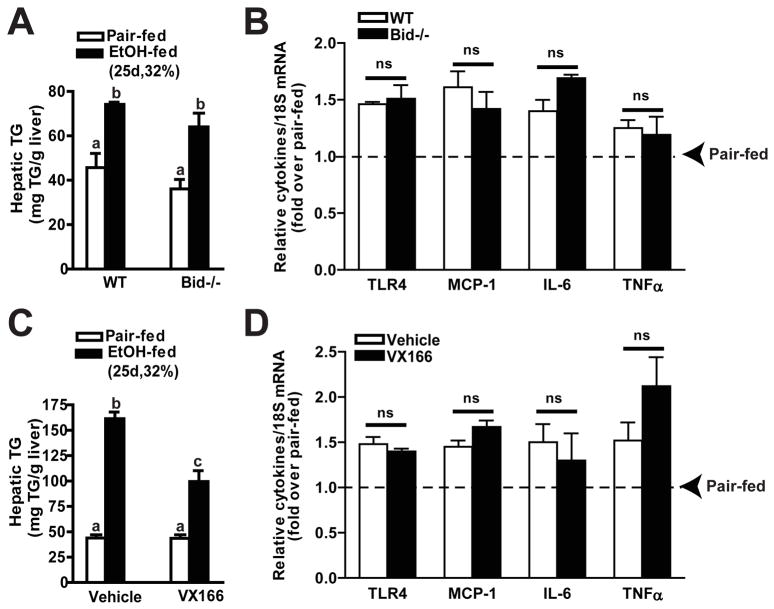
Prolonged, pathological increases in apoptosis in the liver are often associated with the induction of liver damage (Malhi et al., 2006). Chronic ethanol feeding resulted in an increase in circulating liver enzymes (ALT and AST) and an increase in mild to moderate inflammatory activity (Figure 2). Neither Bid deficiency (Figure 2A) nor treatment with VX166 (Figure 2B) prevented chronic ethanol-induced increases in circulating liver enzymes or the appearance of inflammation, based on histopathological scoring. Chronic ethanol feeding also increased the expression of mRNA encoding the pro-inflammatory cytokines and chemokines MCP-1, IL-6, and TNFα, as well as TLR-4 (Figure 3). None of these inflammatory markers were affected by genotype or treatment with the caspase inhibitor (Figure 3). Chronic, heavy ethanol feeding increased hepatic triglycerides (Figure 3). Although this increase was partially reduced in VX166-treated mice (Figure 3B), Bid-deficiency had no effect on triglyceride (Figure 3A). Ethanol-induced accumulation of 4-HNE-protein adducts, a biomarker of oxidative stress, was also sustained in liver from Bid-deficient and VX166-treated mice (Figure S2).
Figure 2.
Ethanol-induced inflammation and liver injury markers in mouse livers. A) C57BL/6J wild-type (WT) and Bid-deficient mice were allowed free access to diets with increasing concentrations of ethanol or pair-fed controls. B) WT mice were gavaged daily with VX166 (10mg/kg) or vehicle during the ethanol feeding protocol (25d, 32%). Paraffin-embedded liver sections were stained with H & E. A,B) Histological scoring for inflammation was carried out by an experienced pathologist using a scale of 0 to 3. ALT and AST activities were measured in plasma. Values represent means ± SEM, n=4–6. Values with different alphabetical superscripts were significantly different from each other, p< 0.05. ND = not detected.
Figure 3.
Bid-deficiency or VX166-treatment did not prevent ethanol-induced increase of hepatic triglyceride or cytokine expression in mice. A,B) C57BL/6J wild-type (WT) and Bid-deficient mice were allowed free access to diets with increasing concentrations of ethanol or pair-fed controls. C,D) WT mice were gavaged daily with VX166 (10mg/kg) or vehicle during the ethanol feeding protocol (25d, 32%). C,D) Hepatic triglyceride concentration was measured by biochemical assay. Values represent means ± SEM, n=4-6.Values with different alphabetical superscripts were significantly different from each other, p< 0.05. B/D) TLR4, MCP-1, IL-6 and TNFαmRNA expression were detected in mouse livers using RT-PCR measurement. Values represent means ± SEM, n=4–6.
Chronic, heavy ethanol feeding elicited markers of non-apoptotic cell death
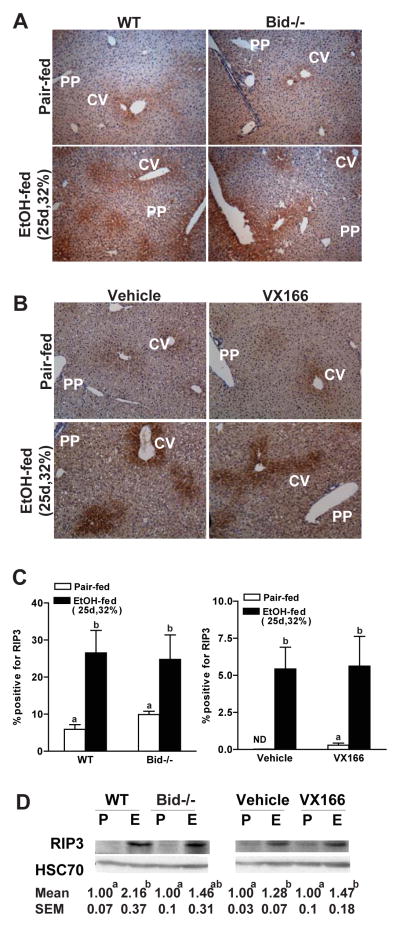
Since deficiency of Bid or VX166-treatment did not prevent ethanol-induced hepatocyte injury, we hypothesized that chronic, heavy ethanol feeding triggers additional cell death mechanisms, such as caspase-independent cell death pathways. RIP3-mediated necroptosis or programmed necrosis is activated in a variety of cell types, particularly when the apoptotic cascade is inhibited (Zhang et al., 2009). Chronic, heavy ethanol feeding induced RIP3 expression; RIP3 expression was observed around the central veins (zone 3) in the liver (Figure 4A/B). Importantly, RIP3 expression was not different between wild type and Bid-deficient mice (Figure 4A/C/D) and not affected by treatment with VX166 (Figure 4B/C/D). Immunoreactive RIP3, assessed by Western blot, was increased after chronic, heavy ethanol feeding in wild-type mice; this increase in expression was not affected by genotype or treatment with VX166 (Figure 4D). These data indicate that induction of RIP3 expression was independent of chronic, heavy ethanol-induced apoptosis.
Figure 4.
Markers of non-apoptotic cell death after chronic, heavy ethanol exposure. A) C57BL/6J wild-type (WT) and Bid-deficient mice were allowed free access to diets with increasing concentrations of ethanol or pair-fed controls. B) WT mice were gavaged daily with VX166 (10mg/kg) or vehicle during the ethanol feeding protocol (25d, 32%). A/B) Paraffin-embedded livers were de-paraffinized followed by immuno-detection for RIP3. All images were acquired using 10X objective. Figures are representative of 3 images per liver and 4 mice per experimental condition. (C) RIP3-positive areas were quantified using Image Pro-Plus software and analyzed. Values with different alphabetical superscripts were significantly different from each other, p< 0.05. ND = not detected. (D) RIP3 protein expression in liver lysates was measured using Western blot. Hsc70 was used as a loading control. Images are representative of 4–6 animals per group. Values represent means ± SEM. Values with different alphabetical superscripts were significantly different from each other, p< 0.05.
Pan caspase inhibitor-treatment reduces stellate cell activation and hepatic fibrosis following combined exposure to moderate ethanol and CCl4
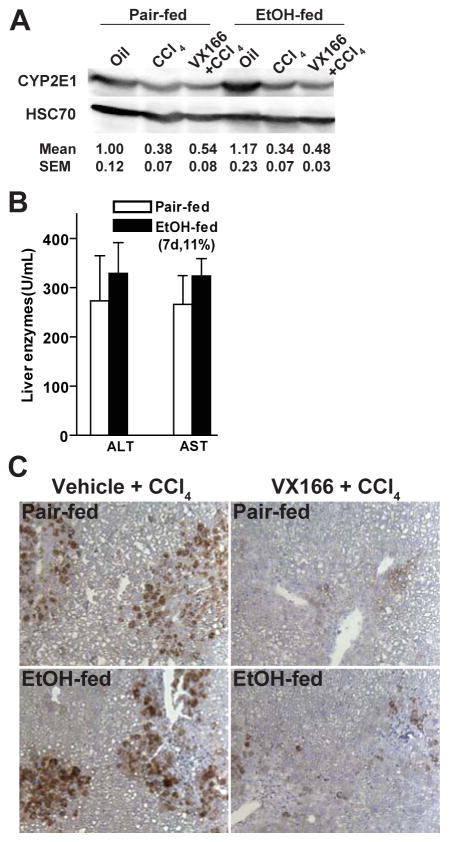
Recently Witek et al. demonstrated that inhibition of caspases, using VX166, reduces hepatic fibrosis in mice fed a MCD diet (Witek et al., 2009). Because the Lieber-DeCarli model of chronic, heavy ethanol feeding does not produce fibrosis in mouse liver, we used an alternative approach to test the contribution of apoptosis to the acceleration of fibrosis by ethanol. Mice were exposed to combined treatment of moderate ethanol and a single dose of CCl4. CCl4 is activated via CYP2E1 and thus chronic, heavy ethanol exposure can result in increased bioactivation of CCl4 (Wong et al., 1998). However, CYP2E1 is not induced in mice exposed to low to moderate doses of ethanol; mice fed diets with 11% of calories as ethanol had baseline levels of CYP2E1 (Figure 5A). Treatment with CCl4 modestly decreased CYP2E1 expression in both pair- and ethanol-fed mice; this decrease was not affected by VX166 treatment (Figure 5A). Consistent with an equivalent bioactivation of CCl4 in pair-and ethanol-fed mice, CCl4- induced increases in ALT/AST were not affected by ethanol feeding (Figure 5B). Gao and colleagues (Jeong et al., 2008) have used an alternative strategy of equalizing ALT/AST in response to ethanol and CCl4 by reducing the dose of CCl4 in ethanol-fed mice in order to normalize CCl4 bioactivation with chronic, heavy ethanol feeding.
Figure 5.
VX166-treatment reduced hepatocyte apoptosis in response to moderate ethanol and CCl4 exposure. C57BL/6J wild-type (WT) mice were allowed free access to diets with ethanol (11%) or pair-fed controls for 2 days. Mice were then given a single injection of CCl4 and maintained on ethanol- or control diets for 3 additional days. Starting on day 3 (1 h prior to the injection of CCl4), mice were treated daily with vehicle or VX166 by gavage. A) CYP2E1 protein expression in liver lysates was measured using Western blot. Images are representative of at least 3 mice per group and values under the image are means and SEM. B) ALT and AST activity was measured detected in plasma. Values represent means ± SEM, n=3-6. C) Paraffin-embedded liver sections were de-paraffinized and stained for CK-18 by immunohistochemistry. Images are representative of 3 images per liver and at least 3 mice per experimental condition.
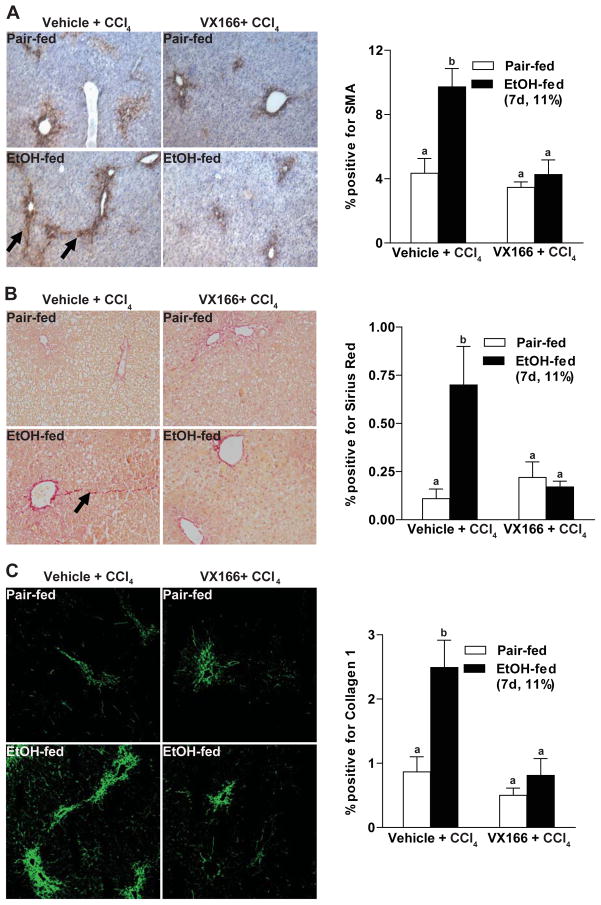
Exposure of mice to a single dose of CCl4 robustly increased CK18 immunostaining (Figure 5C); treatment of mice with VX166 prevented this apoptotic response (Figure 5C). Exposure of mice to a single dose of CCl4 increased expression of αSMA, a marker of hepatic stellate cell activation (Figure 6A). αSMA expression was increased in ethanol-fed mice and exhibited more extensive bridging compared to pair-fed mice (Figure 6A). Treatment with VX166 prior to CCl4 exposure normalized the expression of αSMA in ethanol-fed mice to that of the pair-fed controls (Figure 6A). Sirius red staining paralleled the increased expression of αSMA, with some bridging observed in ethanol-fed mice treated with CCl4 compared to pair-fed mice (Figure 6B). Treatment with VX166 attenuated the impact of CCl4 on increased accumulation of extracellular matrix protein, essentially eliminating the CCl4-induced increase in Sirius Red staining in ethanol-fed mice (Figure 6B). Finally, the accumulation of collagen I protein was detected by immunohistochemistry (Figure 6C); ethanol-fed mice accumulated more collagen I protein in response to CCl4. This response was normalized when mice were pre-treated with VX166 (Figure 6C).
Figure 6.
VX166-treatment reduced hepatic fibrosis after moderate ethanol and CCl4 exposure. C57BL/6J wild-type (WT) mice were allowed free access to diets with ethanol (11%) or pair-fed controls for 2 days. Mice were then given a single injection of CCl4 and maintained on ethanol- or control diets for 3 additional days. Starting on day 3 (1 h prior to the injection of CCl4), mice were treated daily with vehicle or VX166 by gavage. Paraffin-embedded liver sections were de-paraffinized and stained for A) αSMA, B) Sirius red staining and C) Collagen 1 staining. Images are representative of 3 images per liver and at least 3 mice per experimental condition.
Discussion
Chronic ethanol consumption results in hepatic inflammation, steatosis and oxidative stress, leading to hepatocyte injury and the release of the intracellular enzymes, ALT and AST, into the circulation (Roychowdhury et al., 2009a). Although apoptosis and necrosis are associated with ethanol-induced liver damage (Malhi et al., 2006), the contribution of specific modes of cell death mechanisms to liver injury is not known. Here we found that chronic, heavy ethanol exposure results in a Bid-dependent apoptosis in hepatocytes. However, inhibition of apoptosis, either genetically in Bid−/− mice or pharmacologically by treatment with a pan-caspase inhibitor, did not affect several markers of liver injury, including ALT/AST, expression of mRNA for a number of inflammatory cytokines and histological scoring of the liver, in response to chronic, heavy ethanol feeding. Ethanol-induced liver injury, even after pharmacological inhibition of caspases, was associated with increased expression of RIP3 within the liver parenchyma. RIP3 expression is a characteristic feature of non-apoptotic cell death. These data suggest that caspase-independent cell death pathways are important contributors to liver injury induced by chronic, heavy ethanol consumption.
In contrast, apoptosis was a critical contributor to the appearance of early markers of hepatic fibrosis in a novel mouse model of early fibrotic responses which combined moderate ethanol exposure with the hepatotoxin CCl4. Exposure of wild-type mice to moderate concentrations of ethanol in the diet prior to exposure to CCl4 increased the activation of hepatic stellate cells, as well as increased the accumulation of extracellular matrix proteins. This acceleration of in the appearance of early markers of fibrosis in response to moderate ethanol exposure was independent of increased expression of CYP2E1 or enhanced hepatotoxicity. Importantly, these data suggest that therapeutic strategies to inhibit hepatocyte apoptosis may delay or prevent the transition for early stages of ethanol-induced liver injury (i.e. steatosis or modest inflammation) to the onset of fibrosis.
Given the association between hepatic apoptosis and steatohepatitis (Guicciardi and Gores, 2005), we hypothesized that activation of Bid-mediated apoptotic signals would result in amplification of pro-inflammatory cascades and contribute to hepatocyte injury in response to chronic ethanol feeding. However, attenuation of apoptosis by Bid-deficiency or treatment with a pan-caspase inhibitor failed to prevent expression of mRNA for several inflammatory cytokines, increase in circulating ALT/AST levels or hepatic steatosis. These responses in the mouse model of ALD are in part consistent with two recent studies investigating the impact of pan-caspase inhibitors in NAFLD/NASH models. Witek, et al. reported that the pan-caspase inhibitor VX166 fails to prevent increased circulating ALT/AST in the MCD model of NASH (Witek et al., 2009). However, in the MCD model of NASH, VX166 decreased expression of some inflammatory cytokines, as measured by ELISA (Witek et al., 2009). Anstee, et al. investigated the impact of VX166 for the treatment of established steatosis and steatohepatitis (Anstee et al.2010). In this model, VX166 reduced apoptosis as well as histological scores for inflammation, but had little effect on expression of mRNA for TNF-α or MCP-1 when compared to vehicle treated controls (Anstee et al.2010). Our data suggest that inflammatory cytokine expression is predominantly mediated via non-apoptotic pathways in response to ethanol, while apoptotic pathways appear to be more critical in the MCD model of NASH. Possible alternative mechanisms for ethanol-induced inflammation include complement activation and/or TLR4-dependent increases in cytokine expression (Nagy, 2003; Roychowdhury et al., 2009a).
Caspase-independent cell death mechanisms, including necroptosis and autophagy, are implicated in hepatocyte injury in different pathological conditions (Malhi et al., 2006). Moreover, in vitro studies suggest that the fate of individual cells can be shifted between apoptosis and necroptosis, depending on the intracellular environment (Malhi et al., 2006). Programmed necrosis and apoptosis share similar activation pathways (Vandenabeele et al.2010). Upon activation of the death receptor pathway, programmed necrosis results in generation of reactive oxygen species in mitochondria, mitochondrial membrane depolarization and cell death (Zhang et al., 2009). Here we report that chronic ethanol–induced RIP3 expression was sustained in the presence or absence of VX166. Thus, both caspase-dependent and independent pathways of cell death are activated in response to chronic, heavy ethanol exposure. Importantly, inhibition of apoptosis in vivo during ethanol feeding did not exacerbate other types of cell death. Future studies on the mechanisms of non-apoptotic cell death in the context of ethanol exposure are warranted, but beyond the scope of the current investigation.
Apoptosis is also associated with hepatic fibrosis. For example, the pan-caspase inhibitor IDN 6556 prevents bile duct ligation-induced hepatic fibrosis (Canbay et al., 2004) and VX166 attenuates hepatic fibrosis in the MCD model of NASH (Witek et al., 2009). Making use of a model of acceleration of CCl4-induced fibrosis by moderate ethanol exposure, we found that inhibition of caspase activity with VX166 treatment, reduced hepatocyte apoptosis and attenuated the onset of early markers of fibrosis, as indicated by a decrease in the expression of αSMA, a marker of hepatic stellate cell activation, and Sirius red staining, a marker of accumulation of extracellular matrix protein. These data indicate that hepatocyte apoptosis acts as a strong pro-fibrotic signal in the context of moderate ethanol exposure and pan-caspase inhibitors could be considered as potential therapeutic agents to treat ethanol-induced hepatic fibrosis.
In summary, ethanol feeding activated both apoptotic and non-apoptotic cell death pathways. Inhibition of apoptosis had no effect on hepatocyte injury or expression of mRNA for some inflammatory cytokines in response to chronic, heavy ethanol exposure. In contrast, hepatocyte apoptosis was important to the pathophysiological effects of combined moderate ethanol and hepatotoxin exposure. Inhibition of apoptosis in a cell and/or time-specific manner could be useful to identify apoptosis-driven molecular events in specific cell types during progression of steatohepatitis or fibrosis. Our results also suggest that development of agents controlling both apoptosis and non-apoptotic cell death should be further investigated in the development of potential therapeutic strategies against ethanol-induced liver damage.
Supplementary Material
TUNEL images of Bid-deficient and VX166-treated mouse livers. A) C57BL/6J wild-type (WT) and Bid-deficient mice were allowed free access to diets with increasing concentrations of ethanol or pair-fed controls (25d, 32%). B) WT mice were gavaged daily with VX166 (10mg/kg) or vehicle during the ethanol feeding protocol (25d, 32%). Paraffin-embedded liver sections were de-paraffinized followed by staining for TUNEL positive cells. TUNEL images were acquired using a 40X objective. Cells positive for TUNEL were counted and expressed as percent of total nuclei stained with DAPI.
Bid-deficiency or VX166-treatment did not attenuate ethanol-induced accumulation of 4-HNE-protein adducts. A) C57BL/6J wild-type (WT) and Bid-deficient mice were allowed free access to diets with increasing concentrations of ethanol or pair-fed controls (25d, 32%). B) WT mice were gavaged daily with VX166 (10mg/kg) or vehicle during the ethanol feeding protocol (25d, 32%). Paraffin-embedded livers were de-paraffinized followed by immuno-detection of 4-HNE-protein adducts using immunofluorescence. All images were acquired using 40X objective. Figures are representative of 2-3 images per liver and 3 mice per experimental condition. Values with different alphabetical superscripts are significantly different from each other, p< 0.05.
Acknowledgments
Financial support: This work was supported in part by ABMRF/The Foundation for Alcohol Research (SR); NIH grant RO1AA013868, P20 AA17069 and DOD #10248754 (LEN); American Liver Foundation fellowship (DJC); P20 AA17069 (#6017) and R01 DK076852 (AEF). This work was also supported in part by the Case Western Reserve University/Cleveland Clinic CTSA UL1RR024989.
References
- Anstee QM, Concas D, Kudo H, Levene A, Pollard J, Charlton P, Thomas HC, Thursz MR, Goldin RD. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J Hepatol. 2010;53(3):542–50. doi: 10.1016/j.jhep.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, Burgart L, Garrity-Park M, van Vilsteren FG, Oliver LK, Rosen CB, Gores GJ. Clinical Trial of the Pan-Caspase Inhibitor, IDN-6556, in Human Liver Preservation Injury. Am J Transplant. 2007;7(1):218–25. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- Benedetti A, Brunelli E, Risicato R, Cilluffo T, Jezequel AM, Orlandi F. Subcellular changes and apoptosis induced by ethanol in rat liver. J Hepatol. 1988;6(2):137–43. doi: 10.1016/s0168-8278(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308(3):1191–6. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139(2):664–74. doi: 10.1053/j.gastro.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaciuc IV, D'Souza NB, de Villiers WJ, Burikhanov R, Sarphie TG, Hill DB, McClain CJ. Inhibition of caspases in vivo protects the rat liver against alcohol-induced sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res. 2001;25(6):935–43. [PubMed] [Google Scholar]
- Ding WX, Ni HM, DiFrancesca D, Stolz DB, Yin XM. Bid-dependent generation of oxygen radicals promotes death receptor activation-induced apoptosis in murine hepatocytes. Hepatology. 2004;40(2):403–13. doi: 10.1002/hep.20310. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galle PR, Krammer PH. CD95-induced apoptosis in human liver disease. Semin Liver Dis. 1998;18(2):141–51. doi: 10.1055/s-2007-1007150. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54(7):1024–33. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Inflammation in response to hepatocellular apoptosis. Hepatology. 2002;35(4):964–6. doi: 10.1053/jhep.2002.0350964. [DOI] [PubMed] [Google Scholar]
- Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134(1):248–58. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43(2 Suppl 1):S31–44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- Mandal P, Roychowdhury S, Park PH, Pratt BT, Roger T, Nagy LE. Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol. 2010;185(8):4928–37. doi: 10.4049/jimmunol.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka HC, Guicciardi ME, Gores GJ. Caspase inhibitors for the treatment of hepatitis C. Clin Liver Dis. 2009;13(3):467–75. doi: 10.1016/j.cld.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt B, Wirth T, Zender L, Waltemathe M, Trautwein C, Manns MP, Kuhnel F, Kubicka S. Tumour necrosis factor related apoptosis inducing ligand (TRAIL) induces hepatic steatosis in viral hepatitis and after alcohol intake. Gut. 2005;54(11):1590–6. doi: 10.1136/gut.2004.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Suzuki H, Sakaguchi S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J Toxicol Sci. 2007;32(5):453–68. doi: 10.2131/jts.32.453. [DOI] [PubMed] [Google Scholar]
- Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228(8):882–90. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- Nalapareddy P, Schungel S, Hong JY, Manns MP, Jaeschke H, Vogel A. The BH3-only protein bid does not mediate death-receptor-induced liver injury in obstructive cholestasis. Am J Pathol. 2009;175(3):1077–85. doi: 10.2353/ajpath.2009.090304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard MT, Cohen JI, Roychowdhury S, Pratt BT, Nagy LE. Early growth response-1 attenuates liver injury and promotes hepatoprotection after carbon tetrachloride exposure in mice. J Hepatol. 2010a;53(4):655–62. doi: 10.1016/j.jhep.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard MT, Nagy LE. Hepatic fibrosis is enhanced and accompanied by robust oval cell activation after chronic carbon tetrachloride administration to Egr-1-deficient mice. Am J Pathol. 2010b;176(6):2743–52. doi: 10.2353/ajpath.2010.091186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard MT, Roychowdhury S, McMullen MR, Guo L, Arteel GE, Nagy LE. Early growth response-1 contributes to galactosamine/lipopolysaccharide-induced acute liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1124–33. doi: 10.1152/ajpgi.00325.2007. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, Grotta JC, Cuny GD, Yuan J, Savitz SI. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010;88(7):1569–76. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury S, McMullen MR, Pritchard MT, Hise AG, van Rooijen N, Medof ME, Stavitsky AB, Nagy LE. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology. 2009a;49(4):1326–34. doi: 10.1002/hep.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury S, McMullen MR, Pritchard MT, Li W, Salomon RG, Nagy LE. Formation of gamma-ketoaldehyde-protein adducts during ethanol-induced liver injury in mice. Free Radic Biol Med. 2009b;47(11):1526–38. doi: 10.1016/j.freeradbiomed.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16(11):1321–9. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204(12):2913–23. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, Omenetti A, Jung Y, Teaberry V, Choi SS, Guy CD, Pollard J, Charlton P, Diehl AM. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50(5):1421–30. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153(1):109–18. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- Yin XM. Bid, a critical mediator for apoptosis induced by the activation of Fas/TNF-R1 death receptors in hepatocytes. J Mol Med. 2000a;78(4):203–11. doi: 10.1007/s001090000099. [DOI] [PubMed] [Google Scholar]
- Yin XM. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000b;10(3):161–7. doi: 10.1038/sj.cr.7290045. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–6. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sun X, Kang YJ. Ethanol-induced apoptosis in mouse liver: Fas- and cytochrome c-mediated caspase-3 activation pathway. Am J Pathol. 2001;159(1):329–38. doi: 10.1016/S0002-9440(10)61699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziol M, Tepper M, Lohez M, Arcangeli G, Ganne N, Christidis C, Trinchet JC, Beaugrand M, Guillet JG, Guettier C. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J Hepatol. 2001;34(2):254–60. doi: 10.1016/s0168-8278(00)00047-7. [DOI] [PubMed] [Google Scholar]
- Zorde-Khvalevsky E, Abramovitch R, Barash H, Spivak-Pohis I, Rivkin L, Rachmilewitz J, Galun E, Giladi H. Toll-like receptor 3 signaling attenuates liver regeneration. Hepatology. 2009;50(1):198–206. doi: 10.1002/hep.22973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TUNEL images of Bid-deficient and VX166-treated mouse livers. A) C57BL/6J wild-type (WT) and Bid-deficient mice were allowed free access to diets with increasing concentrations of ethanol or pair-fed controls (25d, 32%). B) WT mice were gavaged daily with VX166 (10mg/kg) or vehicle during the ethanol feeding protocol (25d, 32%). Paraffin-embedded liver sections were de-paraffinized followed by staining for TUNEL positive cells. TUNEL images were acquired using a 40X objective. Cells positive for TUNEL were counted and expressed as percent of total nuclei stained with DAPI.
Bid-deficiency or VX166-treatment did not attenuate ethanol-induced accumulation of 4-HNE-protein adducts. A) C57BL/6J wild-type (WT) and Bid-deficient mice were allowed free access to diets with increasing concentrations of ethanol or pair-fed controls (25d, 32%). B) WT mice were gavaged daily with VX166 (10mg/kg) or vehicle during the ethanol feeding protocol (25d, 32%). Paraffin-embedded livers were de-paraffinized followed by immuno-detection of 4-HNE-protein adducts using immunofluorescence. All images were acquired using 40X objective. Figures are representative of 2-3 images per liver and 3 mice per experimental condition. Values with different alphabetical superscripts are significantly different from each other, p< 0.05.