Summary
Sulfs are secreted sulfatases that catalyse removal of sulfate from Heparan Sulfate Proteoglycans (HSPGs) in the extracellular space. These enzymes are well known to regulate a number of crucial signalling pathways during development. In this study, we report that DSulfatase-1 (DSulf1), the unique Drosophila Sulf protein, is a regulator of Hedgehog (Hh) signalling during wing development. DSulf1 activity is required in both Hh source and Hh receiving cells for proper positioning of Hh target gene expression boundaries. As assessed by loss- and gain-of-function experiments in specific compartments, DSulf1 displays dual functions with respect to Hh signalling, acting as a positive regulator in Hh producing cells and a negative regulator in Hh receiving cells. In either domain, DSulf1 modulates Hh distribution by locally lowering the concentration of the morphogen at the apical pole of wing disc cells. Thus, we propose that DSulf1, by its desulfation catalytic activity, lowers Hh/HSPG interaction in both Hh source and target fields, thereby enhancing Hh release from its source of production and reducing Hh signalling activity in responding cells. Finally, we show that Dsulf1 pattern of expression is temporally regulated and depends on EGFR signalling, a Hh-dependent secondary signal in this tissue. Our data reveal a novel Hh regulatory feedback loop, involving DSulf1, which contributes to maintain and stabilize expression domains of Hh target genes during wing disc development.
Introduction
Multicellular organisms develop through the specification of particular tissue types in well-defined spatial positions. Such patterning is largely mediated by secreted morphogens, such as FGFs, Wnts, Bone Morphogenetic Proteins (BMPs) and Hedgehog (Hh), which are produced locally and diffuse into adjacent tissues specifying distinct cellular fates in a dose dependant manner (Tabata and Takei, 2004). A remarkable feature of morphogen activities is the precision and robustness of the resulting cell fate patterns. Understanding how morphogen distribution is regulated and how their graded activities are established and maintained remains a major challenge. Extracellular matrix proteins belonging to the Heparan Sulfate Proteoglycans (HSPGs) family are known to play a major role in both stabilisation and transport of secreted molecules (Hacker et al., 2005; Yan and Lin, 2009). Based on mathematical modelling, it has also been proposed that HSPGs might be part of mechanisms that enhance robustness of morphogen patterning activities (Irons et al., 2010). HSPGs are formed by a core protein to which Heparan Sulfate (HS) glycosaminoglycan chains, composed of disaccharide units, are covalently attached. To date, most HSPGs studies have demonstrated the importance of HS chains in regulating their function (Bornemann et al., 2004; The et al., 1999). After polymerization, HS chains undergo modifications such as sulfate addition to the 2-O position of iduronic acid and N, 3-O and 6-O positions of the glucosamine HS units (Esko and Selleck, 2002; Ori et al., 2008). The importance of HS sulfation state for interactions with specific ligands was confirmed by genetic studies showing that mutations in genes encoding for either N-deacetylase/N-sulfotransferase or HS sulfotransferases that catalyze these critical sulfations, cause defects in various signalling pathways both in Drosophila and mice (Gorsi and Stringer, 2007; Ori et al., 2008). In this context, the characterization of Sulf proteins has attracted particular attention. Indeed, these secreted 6-O-endosulfatases, called Sulf proteins, are unique in their ability to catalyze removal of 6-O-sulfate within the HS chains in the extracellular space or in the Golgi, thus modulating the activity of different signalling pathways (Ai et al., 2003; Dhoot et al., 2001; Kleinschmit et al., 2010; Morimoto-Tomita et al., 2002). Sulfs have been involved in the regulation of Wnts, FGFs, BMPs and Shh morphogen activities in vertebrates (Danesin et al., 2006; Freeman et al., 2008; Lamanna et al., 2007; Otsuki et al., 2010). So far, Sulf enzyme activity has been reported to regulate positively or negatively the availability of ligands for binding to their receptors by modulating interactions of ligands or their antagonists with HS chains (Ai et al., 2003; Dhoot et al., 2001; Rosen and Lemjabbar-Alaoui; Viviano et al., 2004; Wang et al., 2004). Moreover, due to their ability to concomitantly modulate several signalling pathways within a given tissue, Sulfs have been proposed to serve as integrators of morphogen activities (Freeman et al., 2008; Otsuki et al., 2010). In Drosophila, wing development is a paradigm for studying integration of signalling pathways during development. The adult wing blade originating from the wing pouch of the wing imaginal disc is characterised by the stereotyped alternation of vein (named L1 to L5) and intervein tissues. The positioning and elaboration of ectodermal veins in the wing pouch of the disc rely on at least four different signalling pathways: Hh, Decapentaplegic (Dpp), Epidermal Growth Factor Receptor (EGFR) and Wingless (Wg) (Blair, 2007). Hh signalling is required to pattern the imaginal disc epithelium along the antero-posterior (AP) axis (Crozatier et al., 2004). Subsequent activation of Hh-dependant secondary signals such as Dpp and EGFR are further responsible for positioning the four provein domains (L2 to L5) corresponding to the prospective adult longitudinal veins (Blair, 2007). Hh is produced by cells of the posterior (P) compartment and diffuses in the anterior (A) compartment where it activates target genes in a dose dependant manner: engrailed (en), patched (ptc), collier (col/knot) and decapentaplegic (dpp), recognized as high-, mid- and low-level target genes, respectively (Crozatier et al., 2004; Strigini and Cohen, 1997). Col specifies the presumptive L3-L4 intervein domain in a cell autonomous way but also contributes to induce L3 and L4 provein cells in adjacent domains by modulating EGFR signalling (Crozatier et al., 2002; Vervoort et al., 1999). Likewise, Dpp, recognized as a long distance signalling molecule, is involved in positioning L2 and L5 provein domains (Blair, 2007). In the wing disc, the dorsal-ventral (DV) patterning depends on Wg signalling (Neumann and Cohen, 1997). Interestingly, the 6-O-endosulfatase DSulfatase-1 (DSulf1) has recently been reported to regulate Wg signalling in this process (Kleinschmit et al., 2010; You et al., 2011). Moreover, Kleinschmit and collaborators proposed that DSulf1 also contributes to regulate Dpp signalling in this tissue (Kleinschmit et al., 2010).
Here, we show that DSulf1 is a novel modulator of Hh signalling required for correct antero-posterior (AP) patterning of the wing. By analysing Dsulf1 null mutants, we first evidenced a mild Hh gain-of-function wing phenotype. Unexpectedly, depleting DSulf1 in either Hh producing or receiving cells of the posterior (P) and anterior (A) compartments, respectively, led to more severe and opposite Hh phenotypes. Indeed, DSulf1 behaves as a positive regulator of Hh in its source but down-regulates Hh signalling activity in its responding field. We provided evidence that DSulf1 regulates Hh distribution by locally lowering its concentration threshold at the apical pole of Dsulf1-expressing cells in both compartments, indicating that DSulf1 promotes the release of the morphogen from the cell surface. Our functional data further involved the glypicans, Dally and Dally-like (Dlp), as potential substrates of DSulf1 activity in the wing disc. Together, our findings lead to the proposal that DSulf1 by reducing Hh/HSPG interaction prevents local Hh retention in producing cells, then promoting Hh release that results in a higher Hh activity in the receiving field. Concomitantly, it controls the morphogen activity in Hh receiving cells, again by reducing its concentration at their apical pole that results in lowering Hh signalling. Finally, we found that Dsulf1 expression is controlled by the EGFR signalling, itself positioned by Hh in the central region of the wing imaginal disc. Therefore, DSulf1 is part of a novel Hh regulatory feedback loop that contributes to define accurate Hh target gene expression domains during wing development. Our results highlight the importance of modulating 6-O-sulfation state of HSPGs to fine-tune Hh patterning activity and bring novel experimental support to the emerging morphogen concept viewing positional specification as a dynamic process driven by feedback adaptation mechanisms.
Materials and Methods
Mutant and transgenic Drosophila strains
The following strains were used: wild-type (OregonR); lacZ expressing enhancer trap allele of dpp (dpp-lacZP10638) (Blackman et al., 1991); dallygem; dallyMH32; dlp1; dlp2; dlpMH20; ci-Gal4 (a gift from G. Struhl); UAS-EGFRDN (Freeman, 1996); UAS-EGFRCA (Queenan et al., 1997); UAS-rho (de Celis et al., 1997); UAS-Ciact also named UAS-CiPKA (Méthot and Bassler, 2000); UAS-GFP:Dally (Eugster et al., 2007); UAS-GFP:Dlp (Han et al., 2004b). We used either UAS-sulf1 (Kamimura et al., 2006) or new UAS-sulf1 transgenic lines inserted on ATTP platforms on 2nd or 3rd chromosome (Sulf1 open-reading-frame amplified by genomic PCR from UAS-sulf1 flies and inserted into the pUASattIns vector). sulf1ΔP1 line was generated by local hopping using the strain carrying P-element inserted in the Dsulf1 locus (PSulf1GT-000656) and selected for imprecise excision (Kleinschmit et al., 2010). UAS-HhNp:HRP line was constructed similarly to UAS-HhNp:GFP line with addition of the HRP tag before the auto-proteolytic cleavage site of Hh, allowing further additions of palmitic acid and cholesterol moieties (Torroja et al., 2004).
Clonal Analysis
Mutant clones were induced by FLP-mediated mitotic recombination (Xu and Rubin, 1993). sulf1ΔP1 was recombined onto FRT82B chromosome and crossed to w,hsflp; FRT82B, UbGFP, RpS3 / TM6B, Tb1, RpS3 being a homozygous cell lethal Minute mutation. The larvae were heat shocked at first instar and dissected at late L3 stage. To localize clones of cells lacking Dsulf1 expression or over-expressing DSulf1 in adult wings, we visualized GFP positive cells in wings dissected from very young adults (less than 1 hour after emerging from pupa). EGFRDN clonal cells were induced by the GAL4/UAS system (Brand and Perrimon, 1993; Ito et al., 1997) in y,w,hsFLP1; Act>y+>Gal4,UAS-GFP strain.
In situ hybridization (ISH)
Dsulf1 expression was monitored using digoxigenin-labeled (DIG, ROCHE) antisense RNA probe, synthesized from SD04414 cDNA. Experiments combining ISH and immunohistochemistry (IHC) were performed as previously described (Kozopas et al., 1998), except that a Proteinase K treatment followed by a post-fixation step were added. Probes were detected using anti-DIG antibody conjugated to alkaline phosphatase (1:1000, ROCHE) and revealed using Fast Red (ROCHE).
Immunohistochemistry and image capture
Wing discs were fixed either in 4% PAF in PBS or in an Absolute Ethanol/1% Acetic Acid solution (EtOH/AA) (Tuckett and Morriss-Kay, 1988). Antibodies were used at the following dilutions: mouse anti-Col, 1:50 (Dubois et al., 2007); rabbit anti-β-gal, 1:1000 (Cappell); mouse anti-Wingless 4D4, 1:200 (Hybridoma Bank); rabbit and mouse anti-GFP, 1:500 (Torrey, ROCHE); mouse anti-Invected 4D9, allowing detection of Engrailed, 1:50 (Hybridoma Bank); rabbit anti-Hh, 1:200 (Taylor et al., 1993); mouse anti-Dally, 1:200 (Abcam); mouse anti-Dallylike I3Q8, 1:50 (Hybridoma Bank) and mouse anti-Ptc Apa1, 1:100 (Hybridoma Bank). Secondary antibodies conjugated to Alexa Fluor fluorescent dyes (Molecular Probes) were used. Detection of HhHRP was done on living tissues by incubation in tyramide alexa fluor 488, 1:100 (Molecular Probes) for 20 minutes and further fixed in 4% PAF in PBS. Discs were mounted in polyvinyl alcohol 4-88 (Fluka). Fluorescence imaging was obtained from a Leica Sp5 confocal microscope. Captured images were assembled using Adobe Photoshop.
Quantifications
In figures 1 to 4, expression of Hh target genes in wing discs were quantified by counting cell rows of positive cells in the A compartment. Cell countings were performed every 20 μm from dorsal to ventral borders of the wing pouch and results were expressed as mean number of cells ± standard deviation. The number (n) of wing discs analysed is indicated in each bar graph. The background measured in cells of the posterior compartment or in wt cells of the A compartment for experiments done by generating small mutant clones, served as internal control. For En staining, the background was measured in the most anterior part of the wing disc. Average pixel intensity was quantified using the Image J software and plotted against distance from the AP boundary. Adult phenotypes were quantified by measuring the surface of L3-L4 spacing of wings and/or by counting the number of trichomes between L3 and L4 veins, at the level of the posterior crossvein. Results were expressed as mean number of trichomes ± standard deviation. Significance was analyzed using the Student's t-test. Image capture for Hh immunostaining was done apically and quantification of Hh distribution was performed using Image J and Excel software. Fluorescence intensity was measured in a 75 μm wide rectangle centred at the level of the AP boundary and positioned between the margin and the dorsal border of the wing pouch (n=15). Average pixel intensity was plotted against distance from the AP boundary.
Results
Dsulf1 loss-of-function mutant impairs Hedgehog dependant antero-posterior patterning of the wing
At least two genes, sulf1 and sulf2, encoding extracellular 6-O-endosulfatases have been identified in vertebrate genomes. By contrast, only one locus named Dsulf1 was found in the annotated Drosophila genome encoding a protein that contains the four characteristic Sulf protein domains (FBgn0040271; Fig. 1A). Protein sequence alignment indicated that the Drosophila enzyme is not preferentially related to Sulf1 or Sulf2 (Fig. 1A).
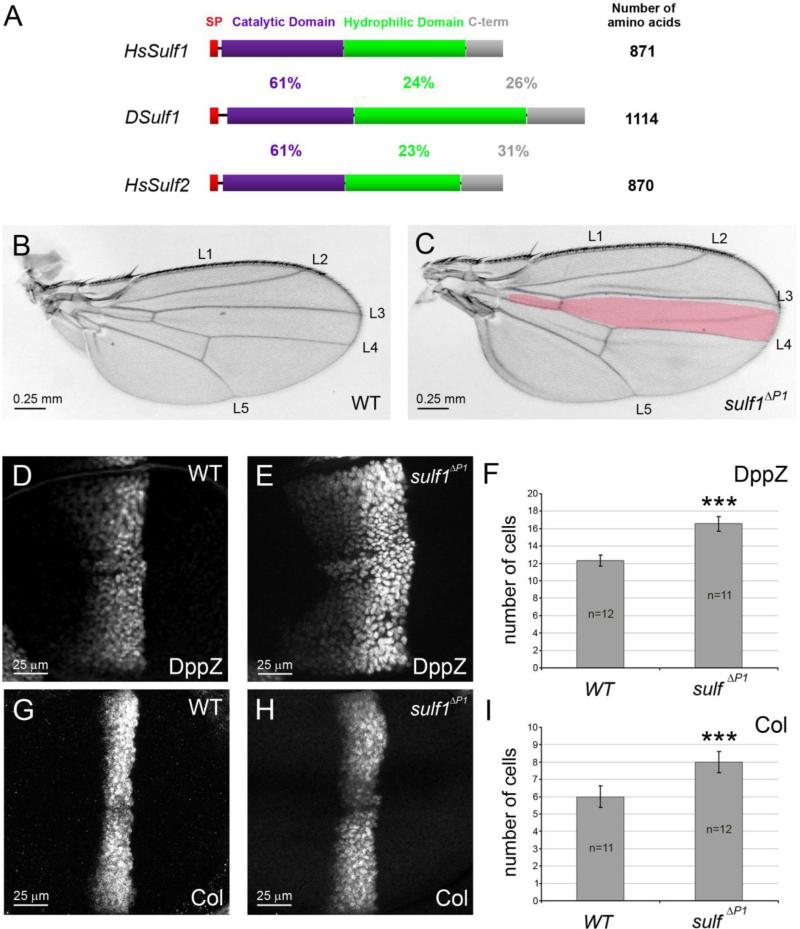
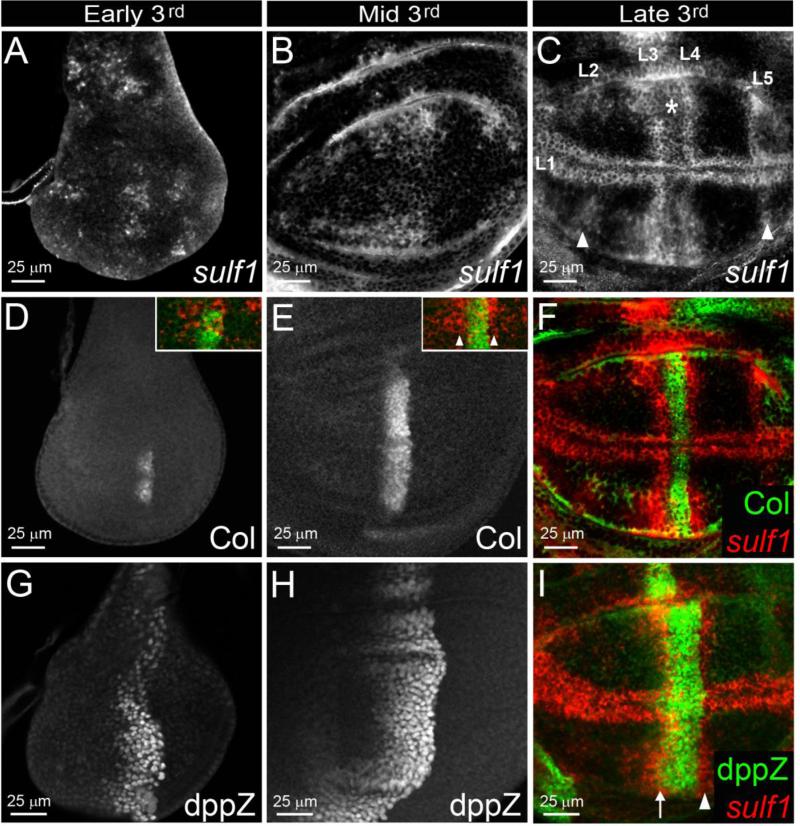
Figure 1. Dsulf1 loss-of-function allele impairs AP patterning of the wing by modulating Hh signalling.
(A) Schematic representation and alignment of Drosophila and Human Sulf proteins named DSulf1 and HsSulf1/HsSulf2, respectively. The size of each protein is indicated on the right. The region corresponding to the signal peptide (SP) is in red, the catalytic domain in purple, the hydrophilic domain in green and the C-terminal domain (C-term) in grey. Percentages of identity between DSulf1 and HsSulf1 or HsSulf2 are indicated for each conserved structural domain. (B, C) Here, as in all subsequent panels, adult wings are oriented with anterior to the top and distal to the right and the wild-type L3-L4 intervein domain, represented in red, is superimposed to the mutant wing. In adult Dsulf1ΔP1 mutants (C), the global size of the wing is enhanced and the L3-L4 intervein domain is enlarged (35±2 trichomes, C) compared to wild type (30±1, B). (D-H) Detection of Hh target gene expression in wt (D, G) and Dsulf1ΔP1 (E, H) 3rd instar wing discs oriented, in this figure and all the following ones, with anterior to the left, posterior to the right, dorsal up and ventral down. Magnification is indicated by scale bar in each figure. Note the enlargement of dpp-lacZ (dppZ, E) and Collier (Col, H) domains of expression in Dsulf1ΔP1 compared to wt discs (D, G). (F, I) Number of cell rows expressing dppZ (F) and Col (I) in wt and Dsulf1ΔP1 wing discs. Error bars represent the standard deviation (***=P<0.0005 using a t-test).
To assess whether Dsulf1 is involved in Hh antero-posterior (AP) patterning activity during wing development, we used the Dsulf1 null mutant allele, called sulf1ΔP1 (Kleinschmit et al., 2010). This mutant showed no lethality at embryonic or larval stages and homozygous sulf1ΔP1 adults were viable and fertile. As previously reported, we observed that sulf1ΔP1 mutant adult wings displayed supernumerary chemosensory and mechanosensory bristles on the anterior wing margin related to an increased Wg signalling (Kleinschmit et al., 2010). However, these mutants also showed fully penetrant wing patterning defects along the AP axis. Indeed, sulf1ΔP1 wings were larger and presented apposition defects of the two wing surfaces. Furthermore, we noticed a mild broadening of the spacing between L3 and L4 veins compared to wt (Fig. 1B and C). This was confirmed by counting the number of trichomes between each vein (35±2 in sulf1ΔP1 compared to 30±1 in wt). In addition, while loss of Dsulf1 function did not lead to defect in the formation of veins, surnumerary and mispositionned sensory organs, called campaniform sensilla, were observed on the L3 vein. Altogether these defects, even mild, were characteristic of a Hh gain-of-function phenotype (Blair, 2007; Crozatier et al., 2003; Johnson et al., 1995). To precisely assess Hh activity, we examined the expression of Hh target genes, dpp, col and en in late 3rd instar wt and sulf1ΔP1 wing discs. We observed that both dppZ and Col expression domains extended anteriorly in sulf1ΔP1 wing discs compared to wild type (wt) (Fig. 1D-I). Cell counting indicated that, while dpp was expressed over 12 cells in wt discs, its expression domain reached 16 cells in sulf1ΔP1 mutants (Fig. 1D,E and F). Likewise, Col was expressed in 6 cells in wt discs and extended to 8 cells in sulf1ΔP1 mutants (Fig. 1G,H and I). Thus, a 33% enlargement of dppZ and Col expression domains was apparent in sulf1ΔP1 wing discs. However, En expression, which depends on high Hh activity, was not modified in sulf1ΔP1 mutants (data not shown). Together, these results indicated a role of DSulf1 in negatively regulating the range of Hh mid- and low-level target genes in the wing disc.
DSulf1 removal from either anterior or posterior compartment induces opposite Hh phenotypes
In order to assess how DSulf1 controls Hh signalling, by regulating its production, diffusion and/or reception, we removed DSulf1 function in either A or P compartment. For this, we used the FLP/FRT system to randomly generate clones of sulf1ΔP1 mutant cells (Xu and Rubin, 1993). In these clones, Dsulf1 expression was not detectable, confirming the invalidation of this gene in this context (data not shown).
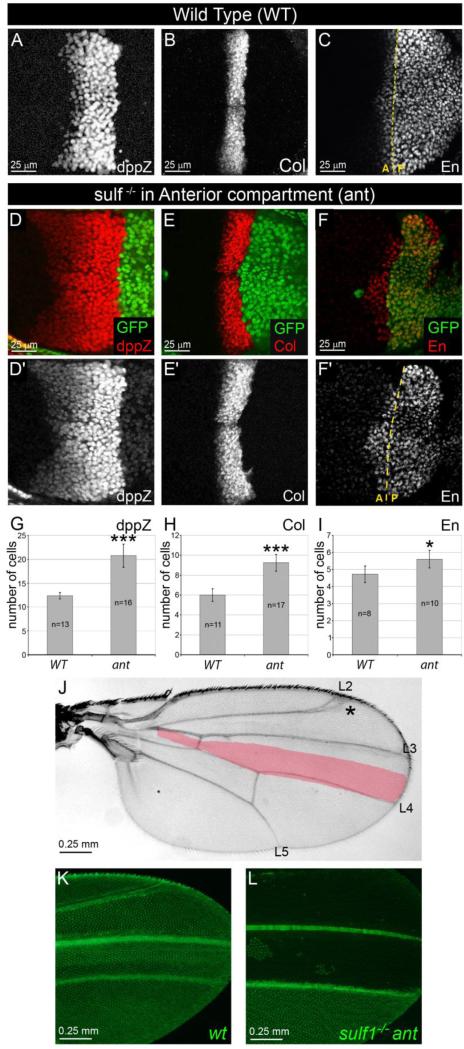
To our surprise, sulf1ΔP1 mutant clones, when encompassing the entire A compartment, caused a strong Hh gain-of-function phenotype which was much more severe than that observed in sulf1ΔP1 mutant wings. Indeed, the dppZ domain reached an average of 20 cells instead of 12 in wt and 16 in sulf1ΔP1 mutant (compare Fig. 2D,G to 2A and Fig. 1E-F). Likewise, the Col expression domain extended to 9 cells instead of 6 in wt and 8 in sulf1ΔP1 mutant (Fig. 2E,H compared to 2B and Fig.1G-I). Additionnally, we observed that the En expression domain, not modified in sulf1ΔP1 discs, reached an average number of 6 cells in sulf1ΔP1 anterior clones (Fig. 2F-I) compared to 5 in wt (Fig. 2C). These observations were further confirmed by analysing adult wings carrying mutant clones visualised by the absence of GFP that was still observable in flies just emerging from the pupal case (Fig. 2K and L). Anterior Dsulf1 mutant clones led to a strong enlargement of the L3-L4 spacing (40±3, Fig. 2J and 2L) compared to both wt (30±1, Fig. 2K) and sulf1ΔP1 homozygous mutant (35±2, Fig. 1C) adult wings. To define whether DSulf1 controls Hh signalling in an autonomous manner, we selected wing discs in which clonal Dsulf1 mutant cells did not encompass the entire A compartment. Analysis of Col expression in this context clearly showed that while the Col-expressing domain was not modified in wt cells, its expression extended anteriorly in adjacent Dsulf1 mutant cells (Fig. S1A,A’ and S1B). We next examined Ptc expression, a Hh transcriptional target also involved in controlling the spreading of the morphogen in its responsive field (Chen and Struhl, 1996), and observed both an up-regulation of Ptc and an enlargement of its domain of expression in Dsulf1 mutant cells compared to adjacent wt cells in the A compartment (Fig. S2A,A’ and S2B,B’). Similarly, but to a lesser extent, Ptc was up-regulated in sulf1ΔP1 mutant compared to wt discs (Fig. S2C,D and S2E). Together, these results showed that DSulf1 acts as a negative regulator of Hh signalling in the A compartment of the wing disc.
Figure 2. Removal of DSulf1 activity in Hh receiving compartment results in Hh gain-of-function phenotype.
(A-F’) Expression patterns of dppZ (A, D and D’), Col (B, E and E’) and En (C, F and F’) in wt (A-C) and in Dsulf1ΔP1 mitotic clones restricted to the A compartment (D-F’) as visualised by the absence of GFP staining (green). Note the anterior extension of dpp (D, D’), Col (E, E’) and En (F, F’) expression domains compared to wt (A, B and C, respectively). (G-I) Number of cell rows expressing dppZ (G), Col (H) and En (I) in wt and in wing discs containing anterior (ant) Dsulf1ΔP1 clones. Error bars represent the standard deviation (*=P<0.05; ***=P<0.0005 using a t-test). (J) Adult wing carrying anterior mutant clones showing a broadening of the L3-L4 spacing (40±3 trichomes) compared to wt (30±1 trichomes, red in J). Note additional defects affecting the positioning of the L2 vein (asterisk). (K-L) Visualization of GFP in wings obtained from young emerging adults. Note the broadening of the L3-L4 spacing in wing containing Dsulf1ΔP1 mutant cells in the A compartment (L) compared to wt (K).
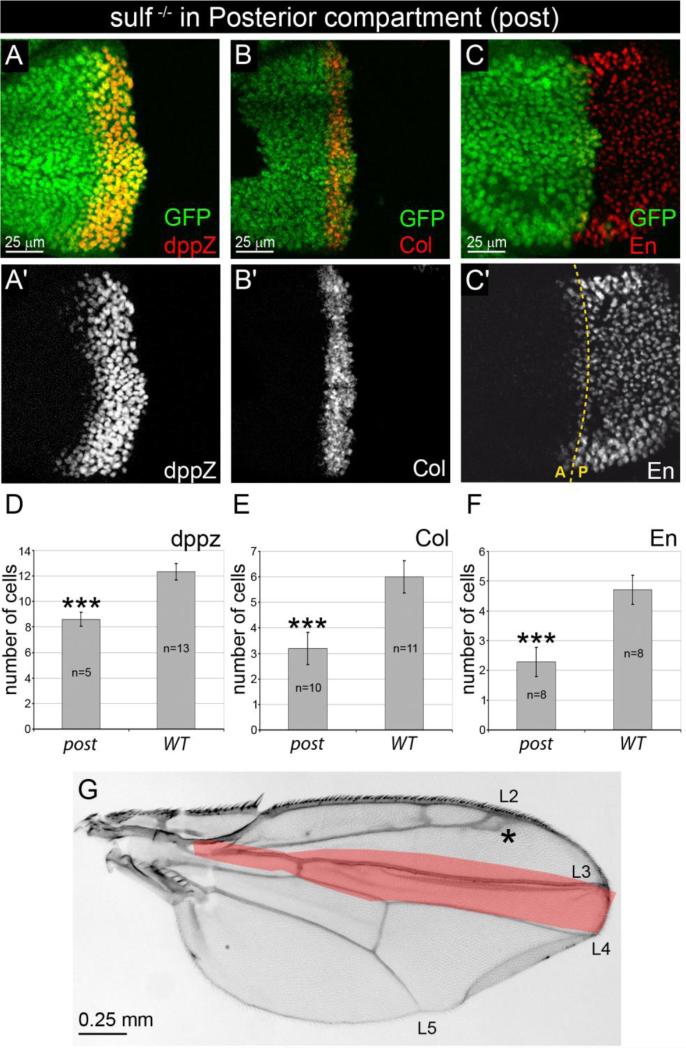
Intriguingly, in these clonal experiments, the Hh gain-of-function phenotype was more pronounced than in the sulf1ΔP1 homozygous mutant. One possible explanation was that DSulf1 may also play a role in the Hh producing compartment by positively regulating Hh signalling in the receiving cells. To test this hypothesis, we analysed wing discs in which Dsulf1 clones arose exclusively in the P compartment. In agreement with our hypothesis, we observed a strong narrowing of domains expressing Hh target genes: dppZ expression domain spanned over 8 cells instead of 12 in wt (Fig. 3A,A’ and D) and 16 in sulf1ΔP1 mutants (Fig. 1E and F). Similarly, Col expression was 3 cells wide in sulf1ΔP1 posterior clones compared to 6 in wt (Fig. 3B,B’ and E) and 8 in sulf1ΔP1 mutant (Fig. 1H and I) and En expression in A compartment was restricted to an average of 2 cells in discs carrying sulf1ΔP1 posterior clones compared to 5 in wt (Fig. 3C and F). Accordingly, phenotypic analysis of adult wings carrying posterior clones showed a characteristic decrease of L3-L4 spacing (21±2, Fig. 3G), consistent with a Hh loss-of-function phenotype. Thus, removing DSulf1 in Hh producing cells led to a clear Hh loss-of-function phenotype.
Figure 3. Removal of DSulf1 activity in Hh producing posterior compartment results in Hh loss-of-function phenotype.
(A-C’) Expression patterns of dppZ (A, A’), Col (B, B’) and En (C, C’) in Dsulf1ΔP1 mitotic clone restricted to the P compartment, detected by absence of GFP staining (green). (D-F) Number of cell rows expressing dppZ (D), Col (E) and En (F) in wing discs containing posterior (post) Dsulf1ΔP1 clones compared to wt. Note the narrowing of dppZ, Col and anterior En expression domains compared to wt. Error bars represent the standard deviation (***=P<0.0005 using a t-test). (G) Adult wing carrying posterior mutant clones showing a narrowing of the L3-L4 spacing (21±2 trichomes), compared to wt (30±1 trichomes, red in G). Note that positioning of the L2 vein is also affected (asterisk).
Taken together, our data, showing that preventing DSulf1 activity in anterior or posterior compartment led to activation or repression of Hh target gene expression, respectively, provided evidence that DSulf1 acts as a negative regulator of Hh signalling in Hh receiving cells and a positive regulator in Hh producing cells.
DSulf1 over-expression impairs Hh signalling in the wing disc
Based on the observation that Hh signalling is up-regulated in sulf1ΔP1 mutant discs, we asked whether DSulf1 gain-of-function could lead to an opposite phenotype. For this purpose, we expressed DSulf1 in the entire dorsal (D) compartment using the apterous-Gal4 driver and analysed Col expression. In such experiments, we observed a strong narrowing of Col-expressing domain in the D compartment of the wing disc (an average of 2 cells compared to 6 in wt), while its pattern of expression was not modified in the ventral compartment (Fig. 4A and B), indicating that DSulf1 over-expression both in the A and P compartments of the wing disc led to a Hh loss-of-function phenotype, opposite to the one observed in sulf1ΔP1 mutant discs. Analysis of adult wings confirmed these results by showing a reduction of the L3-L4 spacing (Fig. 4C). Interestingly, in the wing disc, down-regulation of Hh signalling was restricted to the compartment where DSulf1 was over-expressed, supporting a local activity of this enzyme (Fig. 4A and B).
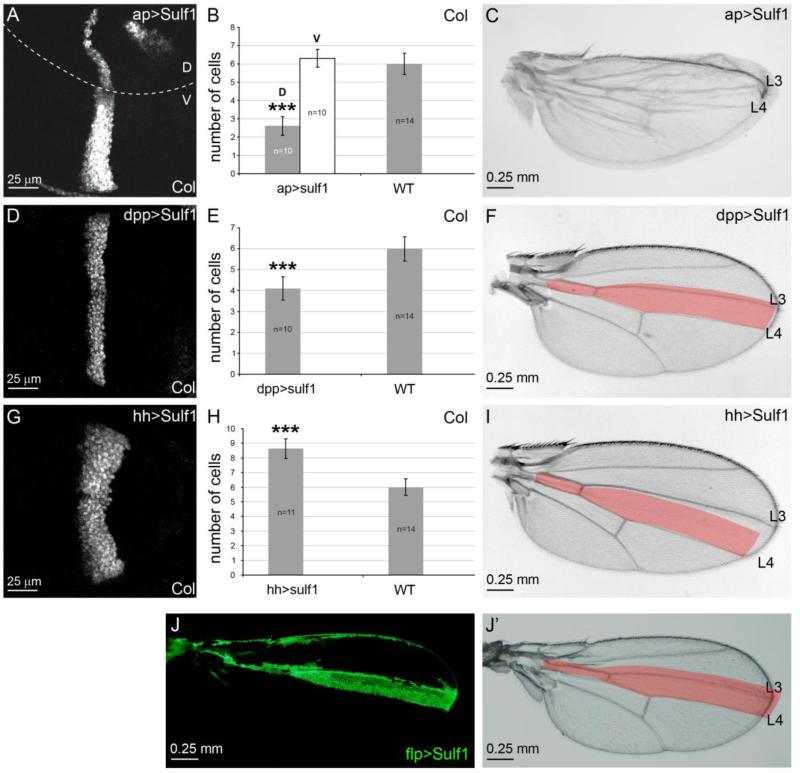
Figure 4. Over-expression of Dsulf1 in D, A or P compartment results in distinct Hh phenotypes.
(A, D and G) Col immunostaining in wing discs over-expressing Dsulf1 in the D compartment (ap>Sulf1, A), in the A compartment (dpp>Sulf1, D) or in the P compartment (hh>Sulf1, G). (B, E and H) Quantification of cell rows number in discs over-expressing Dsulf1 under the control of the three distinct drivers compared to wt. In B, Col-expressing cells have been quantified separately in D and V compartments of the same wing disc. Note that over-expression of Dsulf1 in dorsal cells leads to a reduction of the Col-expressing domain within the D compartment while this domain is not modified in the V compartment, compared to wt. Error bars represent the standard deviation (***=P<0.0005 using a t-test). (C, F and I) Adult wings obtained after Dsulf1 over-expression in the D compartment (ap>Sulf1, C), A compartment (dpp>Sulf1, F) or P compartment (hh>Sulf1, I). Note in C defects in apposition of the two wing surfaces due to impairment of Hh signalling restricted to the D compartment (see A and B). Dsulf1 over-expression in A cells results in mild reduction of the Col expression domain in wing disc (D,E) and of the L3-L4 spacing in adult wing (27±1 trichomes compared to 30±1 in wt, F). Dsulf1 over-expression in P cells leads to an enlargement of the Col-expressing domain in wing disc (G,H) and a broadening of adult L3-L4 spacing in adult wing (39±2 trichomes compared to 30±1 in wt, I). (J, J’) Adult wings carrying flp-out clonal cells over-expressing Dsulf1 in the A compartment (green cells in J) showing a narrowing of the L3-L4 spacing compared to wt (J’, red).
We further over-expressed DSulf1 either in the A or P compartment to define whether opposite effects on Hh signalling could be observed as in mutant clones. Dsulf1 was over-expressed in the Hh target field using two Gal4 drivers: dpp-Gal4 (Fig. 4D-F) and col-Gal4 (data not shown). This resulted in a narrowing of Col expression domain in the wing disc (Fig. 4D,E), and a reduction of the L3-L4 spacing (27±1) in the adult wing compared to wt (Fig. 4F), supporting the Hh negative regulatory activity of DSulf1 in the receiving field. The drivers used to express DSulf1 in the A compartment being Hh transcriptional targets and hence themselves possibly subjected to regulation by DSulf1, we turned to clonal approach and generated clones of cells over-expressing DSulf1 at random locations throughout the wing disc. Examination of adult wings over-expressing DSulf1 in A cells again showed a clear narrowing the L3-L4 spacing, confirming our data (Fig. 4J and J’). We next over-expressed DSulf1 only in the P compartment using the hedgehog-Gal4 (hh>Sulf1) driver. This led to a strong enlargement of Col expression domain in the A compartment of the wing disc (Fig. 4G,H) and a broadening of the L3-L4 spacing in adult wings (39±2) compared to wt (Fig. 4I), confirming that DSulf1 in the P compartment is a positive regulator of Hh signalling.
Together, our data provided further support to our conclusion that DSulf1 has distinguishable roles in Hh receiving and producing cells, acting as a negative and positive regulator of Hh signalling, respectively.
Dsulf1 is dynamically expressed during wing imaginal disc development
Due to its opposite functions on Hh activity in A and P compartments, we turned to a detailed analysis of Dsulf1 expression pattern during wing disc development. Dsulf1 mRNA was first detected from early 3rd instar larval stage, in the central region of the wing pouch and in several spots located in the notum region of the disc (Fig. 5A). In the wing pouch, Dsulf1 expression was further patterned during mid and late 3rd instar stages with higher levels of expression in stripes parallel to the AP boundary and along the margin, reminiscent of the provein domains (Fig. 5B and C). This indicated that Dsulf1 expression, initiated at early 3rd instar stage, was progressively patterned during development of the disc. To precisely position Dsulf1 expression domains, relative to the A and P compartments, we performed double stainings with Col and dppZ. In early 3rd instar stage, both Col and dppZ were detected in the wing disc, their expression covering 4 and 8 cell rows, respectively (Fig. 5D and G). Initial Dsulf1 expression in the centre of the wing pouch overlapped the Col-expressing domain (inset Fig. 5D), showing that Dsulf1 expression was initiated in the Hh responding field of the wing pouch. At mid 3rd instar stage, dppZ- and Col-expressing domains were enlarged, reaching 6 (Col) and 12 (dppZ) cell rows, respectively (Fig. 5E and H). From this stage, Dsulf1 expression was detected on both sides of the Col expression domain (inset Fig. 5E), i.e. in both Hh producing and receiving fields. Thus, Dsulf1 expression was initially restricted to the A compartment and subsequently extended to the P compartment.
Figure 5. Dynamic expression of Dsulf1 in Hh producing and receiving cells during wing imaginal disc development.
(A-C) Temporal Dsulf1 expression in wing discs of early (A), mid (B) and late (C) wt 3rd instar larvae. (A) Dsulf1 expression is detected at early 3rd instar stage in groups of cells located in the central region as well as in anterior and posterior domains of the wing pouch. (B) At mid 3rd instar stage, Dsulf1 expression domain in the central region of the wing pouch appears much broader. (C) At late 3rd instar stage, a regionalisation of the Dsulf1-expressing domain was observed, defining stripes along the AP and DV axes reminiscent to provein domains (L1 to L5). Note a lower level of staining in the presumptive L3-L4 intervein region (asterisk). White arrowheads in C indicate Dsulf1 expression detected in the L2 and L5 provein domains. (D-H) Temporal expression of Col (D-F) and dppZ (G-I) in early (D, G), mid (E, H), and late (F, I) 3rd instar larval wing discs. Note that Col and dpp expression domains extend from early (D,G) to mid (E,H) 3rd instar larval stages. (F and insets in D and E) Double detections of Dsulf1 (red) and Col (green). In the wing pouch, Dsulf1 expression starts in the A compartment as assessed by comparison with Col expression (D, inset). From mid 3rd instar larval stage, Dsulf1 expression is detected on each side of the Col-expressing domain, indicating that Dsulf1 is expressed both in A and P compartments (E, arrowheads in inset and F). (I) Comparison of Dsulf1 expression to dppZ at late 3rd instar larval stage shows that the anterior stripe of high Dsulf1 expression in the A compartment overlaps the dpp expression domain (arrow) and that the posterior Dsulf1-expressing domain abuts the AP boundary (arrowhead).
At late 3rd instar stage, dppZ and Col expression domains did not change (Fig. 5E, F, H and I), indicating that their anterior boundaries have been stabilized. High Dsulf1 expression was observed adjacent to the Col-expressing domain (Fig. 5F and I), in an anterior stripe overlapping the anterior-most dppZ-expressing cells and a posterior stripe abutting the AP boundary. This indicated that, Dsulf1 was highly expressed in L3 and L4 provein domains. However, low level of Dsulf1 expression was still detected in the Col-expressing domain (asterisk in Fig. 5C). These results evidenced that, at late 3rd instar stage, when Hh target gene expression domains were fixed, Dsulf1 was strongly expressed in L3 and L4 provein domains, located in the Hh receiving and producing fields, respectively. In addition to L3 and L4 domains, Dsulf1 was expressed in L2 and L5 proveins, as assessed by comparison with the expression of dpp targets Spalt major (Salm) and optomotor blind (omb) and along the DV boundary, adjacent to wg expressing cells (Fig. 5C and data not shown).
Altogether, these results showed that Dsulf1 expression is dynamically regulated throughout wing disc development, Dsulf1 initial expression being restricted to the Hh receiving field. Based on our functional studies, this suggests that at early stages of wing development, DSulf1 negatively controls Hh signalling. Its expression further turns on in Hh producing cells adjacent to the AP border (L4) and is concomitantly strengthened in a restricted domain of the Hh target field, abutting the Col expression domain (L3). This suggests that the opposite positive and negative regulatory activities of DSulf1 are effective only from mid 3rd instar stage on, when dpp and col-expressing domains are established, strongly supporting a role of DSulf1 in stabilizing the anterior boundaries of Hh target genes.
DSulf1 regulates Hh distribution at the apical pole of wing disc cells
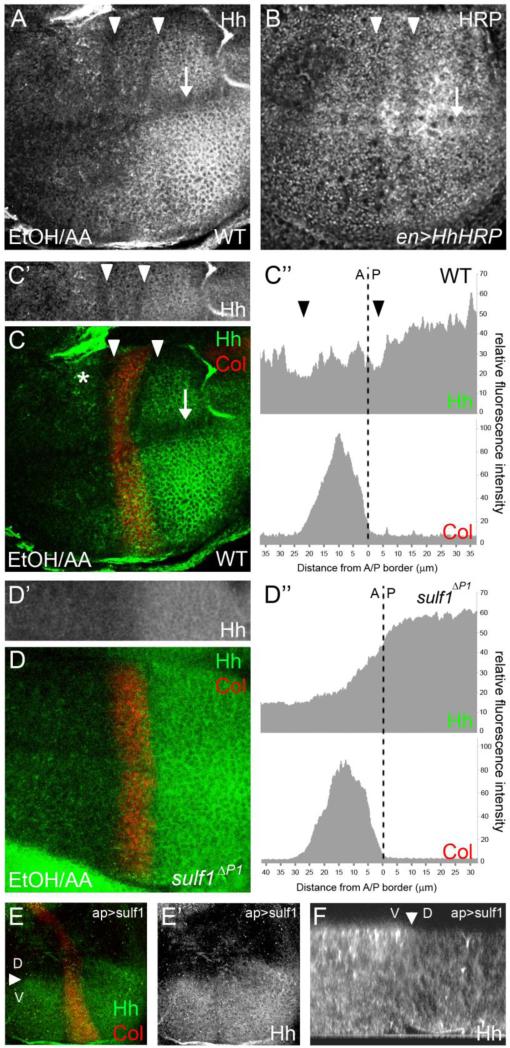
In two recent reports, DSulf1 has been shown to regulate Wg protein distribution during wing development (Kleinschmit et al., 2010; You et al., 2011). We therefore asked whether DSulf1 also regulates Hh activity by controlling its distribution. To test this, we analysed Hh localisation in wt and sulf1ΔP1 mutants. As we did not succeed in detecting Hh at distance from its source in wing imaginal discs using classical paraformaldehyde (PAF) fixation protocols, we turned to Ethanol-Acetic acid fixative (EtOH/AA) known to preserve extracellular matrix integrity, including Heparan Sulfates (Gritli-Linde et al., 2001; Tuckett and Morriss-Kay, 1988). In these experiments, Hh protein was not only detected in the P compartment but also as a punctate staining at the apical pole of Hh receiving cells, located even at distance from the AP boundary, as assessed by double staining with Col (Fig. 6A and C,C’). Interestingly, a detailed examination of Hh distribution revealed a previously undescribed Hh protein pattern. Indeed, Hh staining was invariably lowered in two stripes parallel to the AP boundary and in a domain along the DV boundary (Fig. 6A and C). To ascertain that this pattern did not result from an artefact due to the fixation procedure, we constructed a Horseradish Peroxidase (HRP) tagged version of Hh (HhHRP) that can be used to detect the morphogen in living tissues. HhHRP was expressed in the Hh producing cells using the engrailed-Gal4 driver. In these larvae, a strong reduction of HRP activity was clearly noticeable in two stripes, parallel to the AP boundary, as well as one stripe along the DV boundary (Fig. 6B). This surprising observation indicated that instead of forming a linear decreasing gradient of concentration at the apical pole of disc cells along the AP axis, Hh distribution was interrupted by gaps of lower concentration at precise AP positions. Comparison of Hh immunostaining with Col showed that these stripes of lower Hh staining were located on either side of Col-expressing domain, as quantified by fluorescence (Fig. 6C,C’ and C”). Together, these results indicated that Hh concentration at the apical pole of provein cells was reduced, in particular in L3 and L4 provein domains, i.e. in domains where Dsulf1 is highly expressed. To test the possible role of DSulf1 in regulating Hh apical distribution, we analyzed Hh protein pattern in sulf1ΔP1 mutant discs. Hh was still detected at the apical pole of cells in both A and P compartments. However, the staining appeared more diffuse and the decreased Hh immunoreactivity in L1, L3 and L4 provein domains was no longer detected (Fig. 6D and D’). Measurement of fluorescence intensity in sulf1ΔP1 mutant discs confirmed that Hh staining decreased linearly in L3 and L4 provein domains in this genetic context (Fig. 6D”). Thus, in the absence of DSulf1 activity, decreasing gradient of Hh apical protein in the A compartment is linear and Hh apical distribution in producing cells is also homogenous, suggesting a local action of this enzyme in modulating Hh distribution. To further confirm that DSulf1 was able to reduce Hh apical distribution, we analysed Hh localisation when DSulf1 was over-expressed in the D compartment using the apterous-Gal4 driver. We first verified that Dsulf1 ectopic expression did not affect Hh transcription in P cells (data not shown). In these discs, we observed that Hh apical detection was strongly reduced in the entire D compartment over-expressing Dsulf1, while its distribution was not modified in its ventral (V) counterpart compared to wt (Fig. 6E,E’ and F), showing that Dsulf1 over-expression triggers Hh protein removal from the apical side of wing disc cells. Moreover, the wt pattern of Hh distribution observed in the V compartment indicates that DSulf1 activity did not spread at distance from Dsulf1 producing cells, supporting a local activity for this secreted sulfatase.
Figure 6. Hh apical localisation is disrupted in Dsulf1-expressing cells.
(A) Confocal imaging showing Hh immunostaining done in wt wing disc fixed with EtOH/AA. The focus has been made on the D compartment, at the apical surface of disc cells. (B) Confocal imaging of wing disc expressing a Hh-HRP fusion protein in the P compartment (en>HhHRP). HRP enzymatic activity was detected prior to fixation on living tissue. Note in A and B, a reduction of staining intensity in two domains parallel to the AP boundary (white arrowheads) and in one longitudinal domain at the wing margin (arrow). (C-D) Double stainings of Col (red) and Hh (green) in EtOH/AA fixed wt (C) and Dsulf1ΔP1 (D) discs. (C-C’) In wt, gaps of lowered Hh apical concentration (white arrowheads) are positioned on both sides of the Col-expressing domain. (C”) Apical Hh (upper panel) and Col (lower panel) fluorescence intensities have been measured along the AP axis, the AP boundary being positioned by the dotted line. (D-D’) In Dsulf1ΔP1 mutant discs, Hh apical staining is homogeneous in the P compartment and decreases linearly in the A compartment as confirmed by measurement of fluorescent intensities of apical Hh (upper panel) and Col (lower panel) stainings. (E-E’) Confocal imaging showing apical Hh (E, green) and Col (E, red) immunostainings in wing discs over-expressing Dsulf1 in the D compartment (ap>Sulf1). Note the marked reduction of Hh immunoreactivity at the apical pole of wing cells in the D compartment over-expressing Dsulf1 compared to its V counterpart. (F) Confocal analysis of a z section showing that Hh apical staining is strongly reduced at the apical surface of dorsal (D) Dsulf1-over-expressing cells. Arrowheads in E and F point to the DV boundary.
Together, these results showed that DSulf1 contributes to shape Hh distribution in the wing imaginal disc by reducing the level of Hh protein at the apical pole of the cells expressing the enzyme in both Hh responding and producing cells.
Dsulf1 expression in L3 and L4 provein domains is established under the control of Hh-dependant EGFR signalling activation
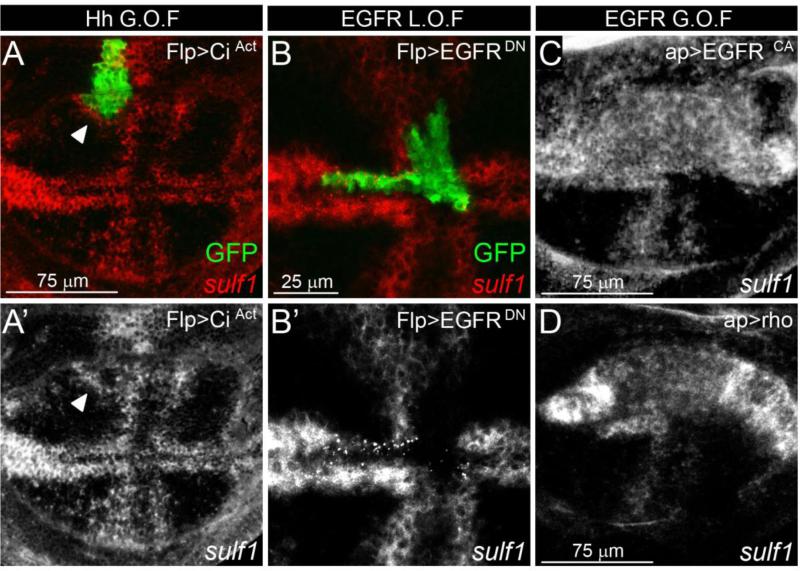
We subsequently questioned the regulation of Dsulf1 expression. It has recently been reported that Wg signalling at the DV boundary regulates Dsulf1 expression in the wing disc (Kleinschmit et al., 2010). However, our results showing that Dsulf1 was initially detected in the central region of the wing pouch at early 3rd instar stage, led us to hypothesize that its expression may also depend on signals involved in AP patterning. We first asked whether DSulf1 expression depends on Hh signalling. To test this, we generated clones of cells expressing a constitutively active form of Cubitus interruptus (Ciact) to autonomously activate the Hh signalling pathway (Méthot and Bassler, 2000). We observed that Dsulf1 was not detected in Ciact-expressing cells but was invariably up-regulated in adjacent cells (Fig. 7A and A’). These data showed that Dsulf1 is not a direct target of Ci and suggested that its expression depends on a Hh-dependant secondary signal. We therefore turned to EGFR signalling known to be regulated by Hh and involved in early development of proveins (Blair, 2007; Crozatier et al., 2002). To test whether Dsulf1 was regulated by EGFR signalling pathway, we ectopically expressed a dominant negative form of the EGF receptor (EGFRDN) at random locations throughout the wing disc (Ito et al., 1997). Our results clearly showed that Dsulf1 expression was cell autonomously down-regulated in EGFRDN-expressing cells, whatever their location in the A or P compartment, or in the L1 provein domain (Fig. 7B and B’ and data not shown). Conversely, we generated a cell-autonomous up-regulation of the EGFR pathway by expressing a constitutive active form of the receptor (EGFRCA) or Rhomboïd (Rho), a downstream effector of this pathway in the dorsal compartment (Guichard et al., 1999; Urban, 2006). This resulted in cell autonomous ectopic activation of Dsulf1 in the whole compartment (Fig. 7C and D). From these results, we concluded that Dsulf1 expression is under the control of the EGFR pathway in the 3rd instar wing imaginal disc.
Figure 7. Dsulf1 expression is regulated by EGFR signalling.
(A, A') Dsulf1 expression (red in A) detected in late 3rd instar wing disc containing clonal cells (green) over-expressing an active form of Ci (CiAct). Note that Dsulf1 expression is not detected within the clone but is activated ectopically, in cells adjacent to Flp>CiAct cells (arrowhead in A and A’). (B, B') Dsulf1 expression (red in B) detected in late 3rd instar wing disc containing a clone of cells expressing a dominant-negative form of EGFR, marked by GFP (Flp>EGFRDN, green). Note the repression of endogenous Dsulf1 expression within Flp>EGFRDN clones. (C-D) Wing discs expressing a constitutively activated form of the EGF receptor (ap>EGFRCA, C) or Rhomboïd (ap>Rho, D) in the dorsal compartment display an autonomous ectopic up-regulation of Dsulf1 expression.
Uur data provide evidence that Dsulf1 expression is under the control of the EGFR signalling pathway, a Hh-dependant secondary signal in the central region of the wing pouch, highlight a novel Hh-regulatory feedback loop during wing development.
Genetic interactions between Dsulf1 and glypican genes
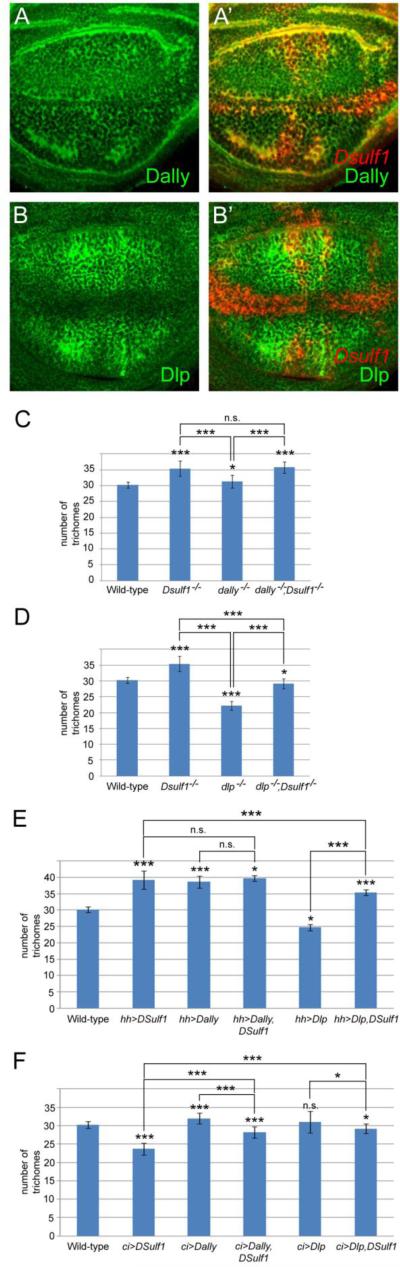
The two glypicans, Dally and Dallylike (Dlp), that have been shown to regulate apical Hh levels in the wing pouch (Ayers et al., 2010; Gallet et al., 2008; Glise et al., 2005; Han et al., 2004a), were good candidates for being DSulf1 substrates. To assess this possibility, we first positioned Dally and Dlp expression domains relative to Dsulf1 in late 3rd instar wing imaginal discs. Immunostaining of Dally, even heterogeneous, was observed all over the wing pouch and clearly overlapped Dsulf1 expression domains both in the A and P compartments (Fig. 8A,A’). Dlp was also detected both in A and P compartments with higher expression levels in the central region of the wing pouch but was excluded from the margin region (Fig. 8B). Therefore, Dlp overlaps Dsulf1 expression domains except at the margin (Fig. 8B,B’). The patterns of Dally and Dlp expression were consistent with their potential role in mediating DSulf1 function with respect to Hh signalling and were also in agreement with the recent proposal that DSulf1 regulates Wg signalling for DV patterning mainly by modulating Dally but not Dlp activity (Kleinschmit et al., 2010; You et al., 2011).
Figure 8. Genetic interactions between Dsulf1, dally and dlp.
(A-B) Immunodetection of Dally (A) and Dlp (B) in wt late 3rd instar wing discs. (A’-B’) Double detections of Dsulf1 (red) and Dally (green, A’) or Dlp (green, B’) show that Dsulf1 expression overlaps with both Dally and Dlp except at the margin where Dlp is not detected. (C-F) Phenotypic analysis of adult wings by quantifying the number of trichomes between L3 and L4 veins, at the level of the posterior crossvein. (C-D) Comparison of Dsulf1 (Dsulf1-/-), dally (dally-/-, C) or dlp (dlp-/-, D) simple mutants with dally/Dsulf1 (dally-/-;Dsulf1-/-, C) and dlp/Dsulf1 (dlp-/-;Dsulf1-/-,D) double mutant adult wings. (E-F) Over-expression of Dsulf1, dally or dlp alone or in combination in the P compartment using a hh-Gal4 driver (E) or in the A compartment using a ci-Gal4 driver (F). Results are expressed as the mean number of trichomes ± standard deviation. Statistical significance compared to wt is indicated on top of each bar. Other comparisons are marked by horizontal bars, and statistical significance is indicated (***=P<0.0005, *=P<0.05, n.s. for non significant difference).
We then tested the genetic interactions between Dsulf1 and dally or dlp by analysing the L3-L4 spacing in adult wings of simple and double mutant flies. While not providing clear information for Dally, our results suggested that Dlp may be a substrate for DSulf1 activity in Hh signalling. Indeed, in both Dsulf1 (sulf1ΔP1) and dally mutant wings, we observed an enlargement of the L3-L4 spacing, even milder in dally mutants, as compared to wt (Fig. 8C). In dally-Dsulf1 double mutant wings, a similar phenotype than in Dsulf1 mutant wings was found (Fig. 8C). Due to the lack of synergistic effect in this experiment, it was not possible to define whether they can work together or not but indicated that if Dally is indeed a substrate for DSulf1, this enzyme also acts through other substrate to modulate Hh signalling. We also examined the genetic interaction of dlp with Dsulf1. In dlp mutant wings, L3-L4 spacing was reduced compared to wt (Fig. 8D). In dlp-Dsulf1 mutant wings, the Dsulf1 mild Hh gain-of function phenotype was partially rescued, suggesting that Dlp is the main but not the only substrate for DSulf1 activity in regulating Hh signalling (Fig. 8D).
To further analyse the substrate specificity of DSulf1, we examined the effect of Dsulf1 on dally and dlp over-expression phenotypes. As expected, over-expression of dally in the P compartment, using hh-Gal4 driver, increased Hh activity as assessed by enlargement of the L3-L4 spacing in adult wings (Fig. 8E) (Ayers et al., 2010). In contrast, dlp over-expression in the P compartment led to an opposite phenotype, i.e. narrowing of the L3-L4 spacing (Fig. 8E). This indicated that Dlp activity, instead of favouring Hh movement from the P compartment as reported for Dally, rather restricts Hh movement from its source. To determine whether Dsulf1 modifies these phenotypes, we co-expressed Dsulf1 with dally and dlp in the P compartment. While co-expression of Dsulf1 with dally resulted in a phenotype similar to that observed for dally, co-expression of Dsulf1 with dlp partially rescued the Hh loss-of-function phenotype observed with dlp (Fig. 8E). Similar experiments were performed in the Hh receiving field using a ci-Gal4 driver. When over-expressed in the A compartment, dally led to a mild activation of the Hh signalling while dlp did not change the L3-L4 spacing in the adult wings (Fig. 8F). Co-expression of Dsulf1 with dally resulted in a reduction of L3-L4 spacing compared to both dally and wt situations but in an enlargement of the L3-L4 spacing compared to wings over-expressing Dsulf1 in the A compartment (Fig. 8F). When Dsulf1 was co-expressed with dlp, a slight reduction of L3-L4 spacing was observed compared to dlp over-expression, but this reversed the Hh loss-of-function phenotype obtained when Dsulf1 was over-expressed in the A compartment (Fig. 8F).
Together, our data supported the view that DSulf1 modulates both Dally and Dlp activities in the Hh signalling compartment and provided evidence for Dlp being the major substrate for Dsulf1 in the Hh producing compartment.
Discussion
DSulf1 is a novel regulator of Hh signalling pathway
Here, we have analysed the function of the 6-O-endosulfatase encoding gene, Dsulf1, in Drosophila. We found that Dsulf1 is a modulator of Hh signalling during wing development. Indeed, sulf1ΔP1 null mutants display wing patterning defects along the AP axis which are characteristic of a mild increased Hh activity. Furthermore, our analysis of Hh protein distribution in wt shows that Hh apical localisation along the AP axis displays a non linear pattern that depends upon DSulf1 activity in the wing imaginal disc. By complementary gain-of-function approach, we found that DSulf1 is sufficient to disrupt Hh apical localisation in either Hh receiving or producing compartments. Altogether, these results allow us to propose that DSulf1 modulates Hh/HSPGs interactions, thus promoting Hh release from the cell surface of wing disc cells. Sulf proteins, by their endosulfatase activity, have been shown to modify HSPG by removing 6-O-sulfate from HS chains (Ai et al., 2006; Ai et al., 2003; Dhoot et al., 2001; Kleinschmit et al., 2010; Morimoto-Tomita et al., 2002; Viviano et al., 2004). Interaction of lipid-modified Hh with HSPGs, such as the glypicans Dally and Dlp, has been reported to play a major role in Hh pathway activation as well as in Hh spreading throughout the extracellular matrix (Callejo et al., 2006; Gallet et al., 2008; Lin and Perrimon, 2002). Our results showing genetic interactions between dally, dlp and Dsulf1, even not providing definitive evidence, strongly support that these molecules are mediators of DSulf1 AP patterning activity in the wing disc. Moreover, impairment of Hh signalling in sulfateless mutants, in which N-, 6-O-, and 2-O- sulfates are absent from disaccharide units, indicated a key role of HS sulfation state in regulating this pathway (Han et al., 2004b; Toyoda et al., 2000). Biochemical analysis, showing that high HS sulfation levels enhance binding affinity of HS chains for members of the Hh family, further support the role of sulfation in HS/Hh interaction (Zhang et al., 2007). Involvement of DSulf1 in modulating Hh signalling confirms and extends the importance of HSPG and their sulfation state and points out the specific role of 6-O-sulfation state in regulating Hh diffusion and activity during wing development.
Surprisingly, although Dsulf1 was expressed at embryonic stages (Bowler et al., 2006), we did not observed embryonic lethality in sulf1ΔP1 mutant, indicating that DSulf1 does not play a major role in regulating Hh signalling in embryo. Similar observations have been done for Shifted, a secreted factor involved in regulating Hh distribution (Glise et al., 2005; Gorfinkiel et al., 2005). In agreement with these data, it has been recently reported that, in the embryo, Dlp regulates Hh signalling independently of HS chains (Williams et al., 2010).
In the wing disc, it has been recently proposed that Hh signalling regulates expression of its different target genes through two distinct routes. Long-range signalling, leading to up-regulation of the low-threshold target gene dpp, has been attributed to apical Hh, while short-range signalling, responsible for up-regulation of the high-threshold target gene en, is regulated by baso-lateral Hh (Ayers et al., 2010). Here, we provide evidences that DSulf1 activity is involved in regulating all Hh target genes indicating that HSPG 6-O-sulfation level controls both long- and short-range Hh signalling. Our data clearly involve DSulf1 in the control of Hh distribution at the apical pole of wing disc cells but we do not exclude that DSulf1 also acts by regulating baso-lateral Hh signalling in its receiving field.
Finally, our loss- and gain-of-function data indicate that regulation of apical Hh distribution by DSulf1 is limited to cells expressing the enzyme. Similar conclusion has been done for modulation of Wg extracellular levels by DSulf1 (Kleinschmit et al., 2010). Thus, results obtained in the Drosophila wing disc are in agreement with previous proposals that Sulf enzymes acts cell-autonomously, either at the cell surface or in the Golgi apparatus of their producing cells (Ai et al., 2006; Kleinschmit et al., 2010).
DSulf1 differentially regulates Hh signalling in producing and receiving compartments
The novelty with the characterization of DSulf1 as a regulator of Hh signalling in the wing disc is that the same factor regulates this pathway in opposite ways in Hh producing or receiving fields. Indeed, our Dsulf1 gain- or loss-of-function experiments undoubtedly show that DSulf1 positively regulates Hh signalling in the P compartment and negatively regulates Hh signalling in the A compartment (Fig. S3). The distribution of Hh in wt discs, showing gaps of lower detection in L3 and L4, a pattern lost in sulf1ΔP1 mutant and in Dsulf1 over-expressing discs, strongly supports that DSulf1 enhances Hh release from the cell surface in both compartments. This similar activity might allow DSulf1 to fulfil a positive function on Hh signalling by its contribution for providing more Hh from its source and a negative regulation of Hh signalling by reducing the amount of Hh in the target field. Thus, DSulf1 by participating in a balance between Hh release from the source and Hh availability for its target cells contributes to the precision of Hh patterning activity (Fig. S3). Again, the glypicans, Dally and Dlp, have been involved in Hh transport and stability, contributing to the precision of Hh positional specification (Irons et al., 2010). In the P compartment, Dally has been reported to positively regulate Hh movement (Ayers et al., 2010), suggesting that Dally is a good candidate for being DSulf1 substrate in Hh producing cells. This is further supported by recent reports showing that DSulf1 restricts Wg signalling by modulating Dally activity in this tissue (Kleinschmit et al., 2010; You et al., 2011). However, we found similar phenotypes in dally and Dsulf1 mutant wings as well as in wings over-expressing dally or Dsulf1 in the P compartment, supporting the view that both molecules promote Hh movement. Thus, DSulf1 function on Hh release from producing cells cannot be simply explained by its ability to lower Hh/Dally interaction. In contrast, although Dlp is not recognized as a regulator of Hh activity in P cells (Gallet et al., 2008), our data, showing that Dsulf1 or dlp over-expression in the P compartment result in opposite phenotypes and that these genes genetically interact, suggest that Dlp may be the preferential DSulf1 substrate for regulating the release of Hh from its source. However, as mentioned above, our results support the view that both Dally and Dlp may contribute to mediate DSulf1 function. One possibility could be that in Hh producing cells, DSulf1 by lowering Hh/Dlp interaction favour binding of Hh to Dally which will subsequently promotes Hh movement into the A compartment. Alternative mechanisms, like stimulation of local endocytosis and/or Hh degradation, might also explain the effect of DSulf1 activity in lowering Hh apical concentration (Bornemann et al., 2004). However, according to this hypothesis over-expression of DSulf1 in P cells would lead to down-regulation of Hh signalling instead of over-activation of the pathway. In the anterior Hh responding cells, Dlp endocytosis from the apical surface of Hh-receiving cells has been shown to stimulate the internalization of Hh bound to its receptor Ptc which is necessary for full-strength Hh signalling (Gallet et al., 2008). Thus, the contribution of DSulf1 in lowering Hh response in A cells may be explained by its involvement in locally reducing the formation of Hh/Dlp/Ptc interaction. Our data support the possibility that Dally may also mediate DSulf1 function in Hh receiving cells. However, this remains to be clarified since Dally has not been reported to be a modulator of Hh signalling in the A compartment (Ayers et al., 2010).
Dsulf1 expression is temporally regulated during wing disc patterning
In this study, we report that Dsulf1 displays a temporally regulated pattern of expression controlled by EGFR signalling, a Hh-dependent secondary signal. We provide evidence that Dsulf1 initial expression, concomitant to up-regulation of dpp and Col, is restricted to the A compartment. It secondarily extends in Hh producing cells of the P compartment and is further patterned in stripes of high expression in L3 and L4 provein domains. This timely regulated expression of Dsulf1, first restricted to Hh receiving field, indicates that, at the onset of Hh target gene up-regulation, the enzyme is involved in limiting their range of activation due to its negative regulatory activity in this compartment. The anterior extension of Hh target gene expression in sulf1ΔP1 discs agrees with the loss of DSulf1 regulatory activity during the period of their up-regulation. Thereafter, from mid-late 3rd instar stage, Dsulf1 was upregulated in Hh producing cells. We propose that, at these stages, DSulf1 by favouring the release of Hh from P cells located at the AP boundary, helps in providing higher amounts of Hh to A cells. Accordingly, this correlates with upregulation of engrailed, a high level Hh target gene that has been shown to occur only at late 3rd instar stage (Blair, 1992). The Hh gain-of-function we observed in sulf1ΔP1 mutant also supports a temporal delay between negative and positive activities since it allows explaining why the positive regulatory function of DSulf1 is not epistatic to its negative regulatory activity. Moreover, we know that maintenance of the Hh target genes dpp and en in the wing disc requires sustained Hh signalling (Strigini and Cohen, 1997). Then, the sharpening of both dpp and Col domains and failure in En up-regulation in discs lacking Dsulf1 in the P compartment, support the view that posterior DSulf1 contributes both to maintain Hh target gene expression and to induce expression of a high-level Hh target gene in cells adjacent to the AP boundary. Concomitantly to up-regulation of Dsulf1 in Hh producing cells, its expression in the receiving compartment is reinforced in a domain abutting the anterior limit of the Col domain. This correlates with the stabilization of dpp and Col domains strongly arguing in favour of DSulf1 involvement in fixing Hh target gene expression boundaries in the A compartment. The function of DSulf1 in stabilizing the anterior boundary of Col can be simply explained by its role in lowering Hh apical concentration in the adjacent L3 provein domain. However, how DSulf1 contributes to define the anterior boundary of dpp remains unclear. An attractive possibility could be that DSulf1, at late 3rd instar stage, in addition to Hh, positively regulates Dpp signalling in the L3 provein domain. The Dpp pathway is strongly activated in L3 and L4 provein domains as assessed by high level of pMad, the phosphorylated form of mother against dpp, in these cells, a process depending on HSPGs (Belenkaya et al., 2004; Fujise et al., 2003; Teleman and Cohen, 2000). Moreover, in DSulf1 gain-of-function experiments Kleinschmit and collaborators showed that pMad staining was lost in Dpp receiving cells, but was higher in the Dpp-expressing domain (Kleinschmit et al., 2010). Finally, a recent report showed that dpp expression is retained, even at low level, in late 3rd instar discs when Hh signalling has been disrupted at mid 3rd instar stage, indicating that at late stages of disc development dpp expression is maintained by a Hh independent signal, probably by Dpp signalling itself (Nahmad and Statopoulos, 2009), which might be positively regulated by DSulf1.
With the characterization of DSulf1, we then evidence a novel regulatory feedback loop that regulates the dynamic of Hh activity and is necessary to accurately position Hh target gene expression boundaries during wing imaginal disc development.
DSulf1, a single regulator coordinating distinct morphogen activities
As already mentioned, Sulfs regulate several signalling pathways in vertebrates (Lamanna et al., 2007; Rosen and Lemjabbar-Alaoui, 2010). Moreover, these enzymes have been recently reported to simultaneously regulate the balance between two distinct signalling pathways, BMP and FGF, within the same tissue (Otsuki et al., 2010). Similarly, in the Drosophila wing disc, DSulf1 activity is not specific for the regulation of a single morphogen but is rather involved in regulating different signalling pathways within the same tissue. Indeed, DSulf1 is known to be a regulator of Wg signalling (Kleinschmit et al., 2010; You et al., 2011) and our work also involves this enzyme in regulating Hh signalling. Moreover, deregulation of the Dpp signalling pathway was also observed (present work and Kleinschmit et al., 2010). A common trait of DSulf1 activity is that it negatively regulates morphogen signalling when expressed in their receiving fields. However, DSulf1-dependant regulation of Hh and Dpp pathways, in contrast to Wg, is further complicated by the expression of Dsulf1 in cells producing the morphogen factors.
We showed that the complex expression of Dsulf1 in the wing imaginal disc is under the control of EGFR signalling in all provein domains, including the L1 territory. Interestingly, it has been shown that EGFR activity is itself patterned by the different morphogen factors (Crozatier et al., 2004; Sturtevant et al., 1993). Therefore, DSulf1 might be viewed as an integrator of morphogen signalling pathways involved in regulatory feedback loops modulating the activity of these three morphogens. Altogether, our data suggest that DSulf1 activity might be important for the general precision of the positional information displayed in the wing disc.
This work brings new arguments supporting the current view that tissue patterning by Hh is highly regulated in space and time, through the requirement of feedbacks (Dessaud et al., 2008; Irons et al., 2010; Jaeger et al., 2008; Kutejova et al., 2009; Nahmad and Stathopoulos, 2009). Finally, our study raises the importance of HSPG sulfation state in a spatial point of view, i.e. both in morphogen producing and receiving fields.
Supplementary Material
Research highlights.
> DSulf1 is a regulator of Hedgehog signalling during wing development. > DSulf1 is expressed both in Hh producing and receiving cells. > DSulf1 positively regulates Hh signalling in the source of the morphogen. > DSulf1 negatively regulates Hh signalling in its target field. > Our data evidence a novel Hh regulatory feedback loop, involving DSulf1.
Acknowledgments
We thank Drs. D.L. Cribbs, L.Dubois, M. Freeman, A. Gallet, P.W. Ingham, D. Strutt, P.P. Thérond, A. Vincent, J.P. Vincent, the Bloomington Stock Center and Developmental Studies Hybridoma Bank for providing fly strains and reagents; the Drosophila Genomics Resource Center for clones; the FlyBase database and the Berkeley Drosophila Genome Project for DNA sequences. We thank the Toulouse RIO Imaging platform for assistance with confocal microscopy. We are grateful to Drs. M. Crozatier, C. Danesin, L. Dubois, C. Monod-Wissler, A. Vincent and members of the C.S. group for critical reading of the manuscript and fruitful discussions. This work is supported by grants from the “Agence Nationale pour la Recherche” (ANR) and the Université Paul-Sabatier, CNRS. A.W. is supported by a graduate fellowship from the French “Ministère de l'enseignement supérieur et de la recherche”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai X, Do AT, Kusche-Gullberg M, Lindahl U, Lu K, Emerson CP., Jr. Substrate specificity and domain functions of extracellular heparan sulfate 6-O-endosulfatases, QSulf1 and QSulf2. J Biol Chem. 2006;281:4969–4976. doi: 10.1074/jbc.M511902200. [DOI] [PubMed] [Google Scholar]
- Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP., Jr. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J Cell Biol. 2003;162:341–351. doi: 10.1083/jcb.200212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers KL, Gallet A, Staccini-Lavenant L, Therond PP. The long-range activity of Hedgehog is regulated in the apical extracellular space by the glypican Dally and the hydrolase Notum. Dev Cell. 2010;18:605–620. doi: 10.1016/j.devcel.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, Lin X. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 2004;119:231–244. doi: 10.1016/j.cell.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Blackman RK, Sanicola M, Raftery LA, Gillevet T, Gelbart WM. An extensive 3' cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-beta family in Drosophila. Development. 1991;111:657–666. doi: 10.1242/dev.111.3.657. [DOI] [PubMed] [Google Scholar]
- Blair SS. Engrailed expression in the anterior lineage compartment of the developing wing blade of Drosophila. Development. 1992;115:21–33. doi: 10.1242/dev.115.1.21. [DOI] [PubMed] [Google Scholar]
- Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol. 2007;23:293–319. doi: 10.1146/annurev.cellbio.23.090506.123606. [DOI] [PubMed] [Google Scholar]
- Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development. 2004;131:1927–1938. doi: 10.1242/dev.01061. [DOI] [PubMed] [Google Scholar]
- Bowler T, Kosman D, Licht JD, Pick L. Computational identification of Ftz/Ftz-F1 downstream target genes. Dev Biol. 2006;299:78–90. doi: 10.1016/j.ydbio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Callejo A, Torroja C, Quijada L, Guerrero I. Hedgehog lipid modifications are required for Hedgehog stabilization in the extracellular matrix. Development. 2006;133:471–483. doi: 10.1242/dev.02217. [DOI] [PubMed] [Google Scholar]
- Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- Crozatier M, Glise B, Khemici V, Vincent A. Vein-positioning in the Drosophila wing in response to Hh; new roles of Notch signaling. Mech Dev. 2003;120:529–535. doi: 10.1016/s0925-4773(03)00041-8. [DOI] [PubMed] [Google Scholar]
- Crozatier M, Glise B, Vincent A. Connecting Hh, Dpp and EGF signalling in patterning of the Drosophila wing; the pivotal role of collier/knot in the AP organiser. Development. 2002;129:4261–4269. doi: 10.1242/dev.129.18.4261. [DOI] [PubMed] [Google Scholar]
- Crozatier M, Glise B, Vincent A. Patterns in evolution: veins of the Drosophila wing. Trends Genet. 2004;20:498–505. doi: 10.1016/j.tig.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Danesin C, Agius E, Escalas N, Ai X, Emerson C, Cochard P, Soula C. Ventral neural progenitors switch toward an oligodendroglial fate in response to increased Sonic hedgehog (Shh) activity: involvement of Sulfatase 1 in modulating Shh signaling in the ventral spinal cord. J Neurosci. 2006;26:5037–5048. doi: 10.1523/JNEUROSCI.0715-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis JF, Bray S, Garcia-Bellido A. Notch signalling regulates veinlet expression and establishes boundaries between veins and interveins in the Drosophila wing. Development. 1997;124:1919–1928. doi: 10.1242/dev.124.10.1919. [DOI] [PubMed] [Google Scholar]
- Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–1666. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- Dubois L, Enriquez J, Daburon V, Crozet F, Lebreton G, Crozatier M, Vincent A. Collier transcription in a single Drosophila muscle lineage: the combinatorial control of muscle identity. Development. 2007;134:4347–4355. doi: 10.1242/dev.008409. [DOI] [PubMed] [Google Scholar]
- Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Eugster C, Panakova D, Mahmoud A, Eaton S. Lipoprotein-heparan sulfate interactions in the Hh pathway. Dev Cell. 2007;13:57–71. doi: 10.1016/j.devcel.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Freeman SD, Moore WM, Guiral EC, Holme AD, Turnbull JE, Pownall ME. Extracellular regulation of developmental cell signaling by XtSulf1. Dev Biol. 2008;320:436–445. doi: 10.1016/j.ydbio.2008.05.554. [DOI] [PubMed] [Google Scholar]
- Fujise M, Takeo S, Kamimura K, Matsuo T, Aigaki T, Izumi S, Nakato H. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development. 2003;130:1515–1522. doi: 10.1242/dev.00379. [DOI] [PubMed] [Google Scholar]
- Gallet A, Staccini-Lavenant L, Therond PP. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev Cell. 2008;14:712–725. doi: 10.1016/j.devcel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Glise B, Miller C, Crozatier M, Halbisen M, Wise S, Olson D, Vincent A, Blair S. Shifted, the Drosophila ortholog of Wnt Inhibitory Factor-1, controls the distribution and movement of Hedgehog. Dev Cell. 2005;8:255–266. doi: 10.1016/j.devcel.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Sierra J, Callejo A, Ibañez C, Guerrero I. The Drosophila Ortholog of the Human Wnt Inhibitor Factor Shifted Controls the Diffusion of Lipid-Modified Hedgehog. Dev Cell. 2005;8:241–253. doi: 10.1016/j.devcel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Gorsi B, Stringer SE. Tinkering with heparan sulfate sulfation to steer development. Trends Cell Biol. 2007;17:173–177. doi: 10.1016/j.tcb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- Guichard A, Biehs B, Sturtevant MA, Wickline L, Chacko J, Howard K, Bier E. rhomboid and Star interact synergistically to promote EGFR/MAPK signaling during Drosophila wing vein development. Development. 1999;126:2663–2676. doi: 10.1242/dev.126.12.2663. [DOI] [PubMed] [Google Scholar]
- Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Khodoun M, Tauchi M, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004a;131:1563–1575. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Wang B, Lin X. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development. 2004b;131:601–611. doi: 10.1242/dev.00958. [DOI] [PubMed] [Google Scholar]
- Irons DJ, Wojcinski A, Glise B, Monk NA. Robustness of positional specification by the hedgehog morphogen gradient. Dev Biol. 2010 doi: 10.1016/j.ydbio.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Irons D, Monk N. Regulative feedback in pattern formation: towards a general relativistic theory of positional information. Development. 2008;135:3175–3183. doi: 10.1242/dev.018697. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Grenier JK, Scott MP. patched overexpression alters wing disc size and pattern: transcriptional and post-transcriptional effects on hedgehog targets. Development. 1995;121:4161–4170. doi: 10.1242/dev.121.12.4161. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Koyama T, Habuchi H, Ueda R, Masu M, Kimata K, Nakato H. Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. J Cell Biol. 2006;174:773–778. doi: 10.1083/jcb.200603129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmit A, Koyama T, Dejima K, Hayashi Y, Kamimura K, Nakato H. Drosophila heparan sulfate 6-O endosulfatase regulates Wingless morphogen gradient formation. Dev Biol. 2010;345:204–214. doi: 10.1016/j.ydbio.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutejova E, Briscoe J, Kicheva A. Temporal dynamics of patterning by morphogen gradients. Curr Opin Genet Dev. 2009;19:315–322. doi: 10.1016/j.gde.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lamanna WC, Kalus I, Padva M, Baldwin RJ, Merry CL, Dierks T. The heparanome--the enigma of encoding and decoding heparan sulfate sulfation. J Biotechnol. 2007;129:290–307. doi: 10.1016/j.jbiotec.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Lin X, Perrimon N. Developmental roles of heparan sulfate proteoglycans in Drosophila. Glycoconj J. 2002;19:363–368. doi: 10.1023/A:1025329323438. [DOI] [PubMed] [Google Scholar]
- Méthot N, Bassler K. Suppressor of fused opposes Hedgehog signal transduction by impeding nuclear accumulation of the activator form of Cubitus interruptus. Development. 2000;127:4001–4010. doi: 10.1242/dev.127.18.4001. [DOI] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmad M, Stathopoulos A. Dynamic interpretation of hedgehog signaling in the Drosophila wing disc. PLoS Biol. 2009;7:e1000202. doi: 10.1371/journal.pbio.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development. 1997;124:871–880. doi: 10.1242/dev.124.4.871. [DOI] [PubMed] [Google Scholar]
- Ori A, Wilkinson MC, Fernig DG. The heparanome and regulation of cell function: structures, functions and challenges. Front Biosci. 2008;13:4309–4338. doi: 10.2741/3007. [DOI] [PubMed] [Google Scholar]
- Otsuki S, Hanson SR, Miyaki S, Grogan SP, Kinoshita M, Asahara H, Wong CH, Lotz MK. Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways. Proc Natl Acad Sci U S A. 2010;107:10202–10207. doi: 10.1073/pnas.0913897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan AM, Ghabrial A, Schupbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124:3871–3880. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- Rosen SD, Lemjabbar-Alaoui H. Sulf-2: an extracellular modulator of cell signaling and a cancer target candidate. Expert Opin Ther Targets. doi: 10.1517/14728222.2010.504718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SD, Lemjabbar-Alaoui H. Sulf-2: an extracellular modulator of cell signaling and a cancer target candidate. Expert Opin Ther Targets. 2010 doi: 10.1517/14728222.2010.504718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development. 1997;124:4697–4705. doi: 10.1242/dev.124.22.4697. [DOI] [PubMed] [Google Scholar]
- Sturtevant MA, Roark M, Bier E. The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes & Development. 1993;7:961–973. doi: 10.1101/gad.7.6.961. [DOI] [PubMed] [Google Scholar]
- Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Nakano Y, Mohler J, Ingham PW. Contrasting distributions of patched and hedgehog proteins in the Drosophila embryo. Mech Dev. 1993;42:89–96. doi: 10.1016/0925-4773(93)90101-3. [DOI] [PubMed] [Google Scholar]
- Teleman AA, Cohen SM. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 2000;103:971–980. doi: 10.1016/s0092-8674(00)00199-9. [DOI] [PubMed] [Google Scholar]
- The I, Bellaiche Y, Perrimon N. Hedgehog movement is regulated through tout velu-dependent synthesis of a heparan sulfate proteoglycan. Mol Cell. 1999;4:633–639. doi: 10.1016/s1097-2765(00)80214-2. [DOI] [PubMed] [Google Scholar]
- Torroja C, Gorfinkiel N, Guerrero I. Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development. 2004;131:2395–2408. doi: 10.1242/dev.01102. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Kinoshita-Toyoda A, Fox B, Selleck SB. Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu. Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. J Biol Chem. 2000;275:21856–21861. doi: 10.1074/jbc.M003540200. [DOI] [PubMed] [Google Scholar]
- Tuckett F, Morriss-Kay G. Alcian blue staining of glycosaminoglycans in embryonic material: effect of different fixatives. Histochem J. 1988;20:174–182. doi: 10.1007/BF01746681. [DOI] [PubMed] [Google Scholar]
- Urban S. Rhomboid proteins: conserved membrane proteases with divergent biological functions. Genes Dev. 2006;20:3054–3068. doi: 10.1101/gad.1488606. [DOI] [PubMed] [Google Scholar]
- Vervoort M, Crozatier M, Valle D, Vincent A. The COE transcription factor Collier is a mediator of short-range Hedgehog-induced patterning of the Drosophila wing. Curr Biol. 1999;9:632–639. doi: 10.1016/s0960-9822(99)80285-1. [DOI] [PubMed] [Google Scholar]
- Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S. Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J Biol Chem. 2004;279:5604–5611. doi: 10.1074/jbc.M310691200. [DOI] [PubMed] [Google Scholar]
- Wang S, Ai X, Freeman SD, Pownall ME, Lu Q, Kessler DS, Emerson CP., Jr. QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc Natl Acad Sci U S A. 2004;101:4833–4838. doi: 10.1073/pnas.0401028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EH, Pappano WN, Saunders AM, Kim MS, Leahy DJ, Beachy PA. Dally-like core protein and its mammalian homologues mediate stimulatory and inhibitory effects on Hedgehog signal response. Proc Natl Acad Sci U S A. 2010;107:5869–5874. doi: 10.1073/pnas.1001777107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Belenkaya T, Lin X. Sulfated is a negative feedback regulator of wingless in Drosophila. Dev Dyn. 2011;240:640–648. doi: 10.1002/dvdy.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, McLellan JS, Ayala AM, Leahy DJ, Linhardt RJ. Kinetic and structural studies on interactions between heparin or heparan sulfate and proteins of the hedgehog signaling pathway. Biochemistry. 2007;46:3933–3941. doi: 10.1021/bi6025424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.