Abstract
Prion diseases are diagnosed by the detection of their proteinase K-resistant prion protein fragment (PrPSc). Various biochemical protocols use different detergents for the tissue preparation. We found that the resistance of PrPSc against proteinase K may vary strongly with the detergent used. In our study, we investigated the influence of the most commonly used detergents on eight different TSE agents derived from different species and distinct prion disease forms. For a high throughput we used a membrane adsorbtion assay to detect small amounts of prion aggregates, as well as Western blotting. Tissue lysates were prepared using DOC, SLS, SDS or Triton X-100 in different concentrations and these were digested with various amounts of proteinase K. Detergents are able to enhance or diminish the detectability of PrPSc after proteinase K digestion. Depending on the kind of detergent, its concentration - but also on the host species that developed the TSE and the disease form or prion type - the detectability of PrPSc can be very different. The results obtained here may be helpful during the development or improvement of a PrPSc detection method and they point towards a detergent effect that can be additionally used for decontamination purposes. A plausible explanation for the detergent effects described in this article could be an interaction with the lipids associated with PrPSc that may stabilize the aggregates.
Keywords: prion protein, scrapie, BSE, chronic wasting disease, proteinase resistance, detergent
1. Introduction
Chronic wasting disease (CWD) in cervids, scrapie in sheep and goats, bovine spongiform encephalopathy (BSE) in cattle and Creutzfeldt-Jakob disease in humans (CJD) are fatal transmissible spongiform encephalopathies (TSEs), also called prion diseases. These neurogenerative disorders are characterized by aggregates of a partly protease-resistant, self-replicating protein called “proteinacous infectious particle” (hereafter referred to as “prion”) that are detectable in the central nervous system (CNS). According to the prion hypothesis, the disease-associated prion protein (PrPSc) is the principal or only constituent of the infectious agent (Prusiner, 1982). Prion infectivity, prion protein aggregation and PrPSc seem to be closely interrelated (Beekes et al., 1996; Prusiner et al., 1983). The physiological isoform, a cell surface protein (PrPc), is expressed not only in the CNS, but also in a number of extracerebral tissues. Both isoforms share the same amino acid sequence, three glycosylation forms and a lipid anchor, but differ in their protein conformation. Conformational differences are reflected e.g. by the insolubility of the aggregating PrPSc in aqueous buffers and its tendency to form fibrils (Meyer et al., 1986). The proteinase K digestion of PrPSc results in an N-terminal truncation up to amino acids 82 or 97 in human CJD (Parchi et al., 2000) amino acids 81 - 89 in scrapie, amino acids 96 - 97 in classical BSE (Hayashi et al., 2005) and amino acids 82 and 78 in CWD (Xie et al., 2006). The detection of the proteinase K-resistant form of PrPSc is used as a diagnostic tool and marker for prion diseases (Bolton et al., 1982). The partial protease resistance of PrPSc has been widely accepted as the biochemical basis for distinguishing between PrPc and PrPSc. It can be influenced by many factors like the interaction time of enzyme and PrPSc, the enzyme concentration (McKinley et al., 1983) or the addition of chemicals: Urea, GdnSCN and detergents like sodium dodecyl sulfate (SDS) are known to decrease the proteinase K resistance of PrPSc (Madec et al., 1997; Oesch et al., 1994). Whereas chaotropic salts destabilize proteins by destroying hydrogen bonds, detergents affect the tertiary structure of proteins by interacting with hydrophilic and hydrophobic areas of the molecule (Hörnlimann et al., 2007). Detergents are commonly used in tissue homogenisation and pK digestion buffers. Concentration and combination of the detergents vary considerably. It is further known that PrPSc of some TSEs are more resistant to proteinase K digestion than others (Kuczius and Groschup, 1999). This can raise problems when a diagnostic method is not suitable for detecting prion diseases with comparably low protease resistance. Unlike PrPSc of classical scrapie, PrPSc of atypical/Nor98 scrapie is completely diminished when a stringent pK digestion is performed (Buschmann et al., 2004; Everest et al., 2006; Klingeborn et al., 2006). This was one of the reasons why atypical/Nor98 scrapie was identified as a type of sheep scrapie only some years ago (Benestad et al., 2003). Classical scrapie and chronic wasting disease share many features such as the neuropathological PrPSc deposition pattern and the horizontal transmissibility (Williams, 2003). CWD has been considered a disease entity, but differences have been reported between CWD of different susceptible species upon transmission to rodents. These findings suggest the existence of strains like those known for other TSEs such as classical scrapie (Sigurdson, 2008; Williams, 2005). While working with CWD of elk and white-tailed deer, it became clear that the effect of a particular detergent on the stability of PrPSc against proteinase K had not been investigated systematically. Our approach was therefore to explore different detergents in homogenisation buffer regarding their influence on the resistance of PrPSc against pK digestion under common routine conditions.
2. Materials and methods
We investigated the influence of the most commonly used detergents on eight different TSE agents derived from different species and distinct prion disease forms:
2.1. Animals
CNS tissues were taken from German (classical scrapie, atypical/Nor98 scrapie, negative control) and Norwegian sheep (atypical/Nor98 scrapie) and bovine CNS tissue (classical- and L-type BSE) from German BSE cases. Brain tissue from Rocky Mountain elk (Cervus elaphus nelsoni) was kindly provided by the CFIA, Lethbridge Laboratory, Canada and brain tissue from white-tailed deer (Odocoilus virginianus) was obtained from Dr. Jürgen A. Richt, College of Veterinary Medicine, Kansas State University, USA. We tested three different cases of each TSE except for atypical BSE (4 BSE cases in total) and CWD in white-tailed deer (4 cases of CWD in total)
2.2. Sporadic CJD cases
CNS tissue came from patients with a diagnosis of sporadic CJD who were included in the National CJD Surveillance Study in Germany (approved by the ethics committee of the medical faculty of the University of Göttingen). The methionine/valine (M/V) polymorphism at codon 129 of the PRNP was determined using a standard protocol according to Windl et al. (1999). Tissues from frontal, temporal, parietal and occipital cortices as well as cerebellum were used to determine the PrPSc type according to the Parchi classification, which was chosen for its reproducibility in different laboratories. Alternative classifications do not take into account possible inconsistent pH-conditions during proteinase K-digestion, which result in band shifting during PrPSc typing (Notari et al., 2004). Brain tissue was taken from heterozygous CJD type 1 (MV1) and heterozygous CJD type 2 (MV2).
2.3. Preparation of tissue homogenates
Different homogenates of each TSE case were prepared as follows: 500mg tissue blocks were removed from the frontal cortex (cruciate sulcus region), the cerebellar cortex or the pons of the brain stem. Two procedures were used to prepare the lysates: The tissue was either fragmented with a sterile scalpel into equal amounts homogenized in phosphate-buffered saline (PBS, pH 7.5) containing 0.5 % or 1 % DOC (sodium deoxycholate, Fluka, Buchs, Switzerland, anionic detergent), 1 % or 5 % Triton-X-100 (Roth, Karlsruhe, Germany, nonionic detergent), 2 % or 5 % SLS (N-sodium lauroyl sarcosine, Sigma, Steinheim, Germany, anionic detergent), 1 % or 5 % SDS (sodium dodecyl sulfate, Roth, anionic detergent) or in plain PBS, depending on the experimental setting, or homogenized in phosphate-buffered saline and diluted afterwards with the required detergent to a concentration of 1:10. Both methods showed the same results. The tools and gloves were changed after preparation of each sample. All experiments were done using these stock homogenates. The homogenates were prepared at different timepoints and with fresh detergent solutions. The detergents were chosen according to commonly used homogenization buffers as published in the prion literature.
2.4. Western blot analysis
The brain homogenates were adjusted to room temperature. Protease digestion was performed with a final concentration of 12.5 to 800 μg/ml proteinase K [Sigma-Aldrich, Missouri, USA, (pK stock 50 mg/ml in 1M Tris-HCl pH 7.8 and 5 M NaCl + 10 % MgCl2)] in 4 × PBS, (pH 7.5; see Notari et al., 2004) for 1 hour at 37 °C. Samples were mixed 1:4 with sample buffer (TRIS-buffer containing 12 % SDS, 30 % glyerine, 0.02 % bromophenol blue and 5 % mercaptoethanol, pH 6.8) and boiled for 15 minutes. The equivalent of 119 μg brain wet weight per lane was loaded on homemade 15 % acrylamide gels and, after electrophoresis, semidry blotting was carried out using 0.45 μm nitrocellulose membranes (Bio-Rad, Munich, Germany). Membranes were decontaminated using 4 M guanidine (iso)-thiocyanate (GdnSCN, 30 minutes). After rinsing in TRIS-buffered saline pH 7.8 (TBS) and blocking with 0.2 % casein in PBS for 30 minutes, the primary antibody P4 (R-Biopharm, Darmstadt, Germany) diluted 1:3000 in TBS and 0.02 % casein was applied and incubated overnight at 4 °C. After incubation with a horseradish peroxidaseconjugated goat anti-mouse antibody (Dako, Carpinteria, CA, USA) for 1 hour, the immunoreaction was visualized with Super Signal Femto West Maximum Sensitivity Substrate (Perbio, Erembodegem, Belgium) on X-ray film.
2.5. Membrane adsorption assay
Brain homogenates were prepared in PBS with the respective detergent and then treated with proteinase K (concentration depending on the experimental setting) for 30 min at 37 °C. Samples were diluted in TBS containing 5 mM EDTA and subjected to a commercially available dot blot device (Bio-Rad), run by a diaphragm pump. Samples were drawn through a nitrocellulose membrane (0.45 μm, Bio-Rad), following the assay by Winklhofer et al. (2001) with modifications (Wemheuer et al., 2009). During suction, proteins adsorb to the membrane which had been prepared with 10 % Roti Block (Roth, Karlsruhe, Germany) for 30 minutes. Each dot was rinsed with 200 μl 0.1 % DOC before and after the samples were applied to the membrane that was treated as described above (2.4. Western blot analysis), but with an additional blocking step (0.3 % H2O2, 15 min). A combination of mAb P4, (epitope of amino acids 89—104; R-Biopharm, Darmstadt, Germany) and mAb 3F4, (epitope of amino acids 108—113, kindly provided by PD Dr. Beekes, RKI, Berlin, Germany), was used (both diluted 1:3000) for cervid, ovine and human samples. For blots with bovine, ovine and human samples a combination of mAb P4, (1:3000) and mAb 12F10, (epitope of amino acids 153—163,1:10 000, kindly provided by Dr. Dirk Motzkus, German Primate Center, Göttingen, Germany) was applied. Those anti-prion antibodies were chosen which were used frequently for diagnostic purposes. In a first step the membrane adsorption assay was carried out without any detergent and the quantities of homogenates for loading were adjusted to get the same amounts of PrPSc for the following comparisons. Brain equivalents applied to the membrane reached from 1.19 μg (25 μl of 10−5 dilution) to 857 μg (180 μl of 10−3 dilution). For all blots, two negative controls (a scrapie-negative sheep sample prepared with the particular detergent in use and a human sample with a non-prion brain disease prepared in 0.5 % DOC) and a positive control (a brain sample of CWD-infected elk prepared in 0.5 % DOC) were included. Because of its exceptional protease sensitivity, atypical/Nor98 scrapie samples were digested with lower proteinase K concentrations (final concentration of 6.5 to 50 μg/ml) in order to test the detergent effects.
2.6. Densitometric analysis
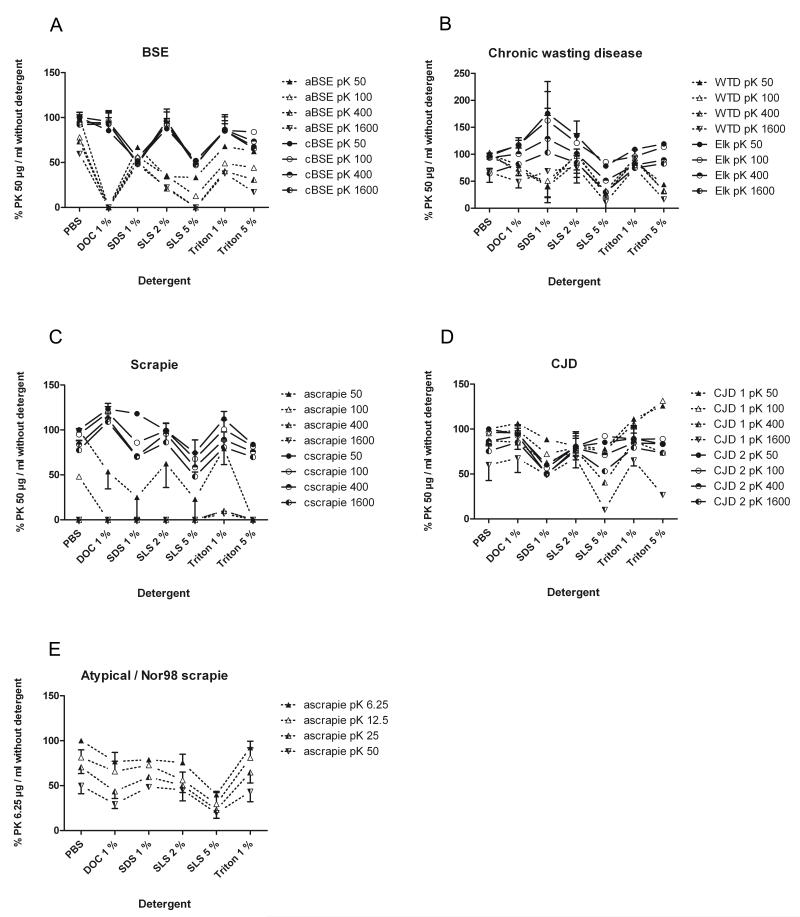
The intensity of the dots on the X-ray films (membrane adsorption assay) was analysed via “Chemi Doc” (Bio Rad, Munich, Germany) using the software “Quantitiy One” version 4.4.0 from Bio Rad. The 96 well volume array tool was used to exclude influences of the dot size. For standardization, the same sample of CWD in elk homogenized with 0.5 % DOC was included in each blot to ensure comparability. Each dot used for densitometric analysis was corrected with the value of the standardization sample on the respective blot. In the following the influence of detergents and proteinase K concentration on the detectability of PrPSc is calculated as a percentaged variation to the reference. The treatment of the respective TSE sample with 50 μg/ml proteinase K in the absence of any detergent serves as reference. For each sample with the respective detergent the mean value of the two dilutions 10−3 and 10−4 was calculated to reduce blotting artefacts. For the graphs shown in fig. 5 the mean value of repeated blots per case and 1-3 cases per TSE were calculated.
Fig. 5. Densitometric analysis of the effect of detergents on the proteinase K resistance in various TSEs.
Referenced to the digestion of the respective TSE by 50mg/ml proteinase K without detergent, the influence of 1% DOC, SDS, Triton 100, 2% SLS, 5% SDS and 5% Triton 100 on the digestion of PrPSc of BSE, scrapie, CWD and CJD with a concentration series of proteinase K is shown. It is obvious that the detergent effect depends on the respective TSE, the prion type or whether classical or atypical TSEs were investigated. WTD and elk prions differ remarkably regarding the influence of the same detergent. Atypical/Nor98 scrapie was shown separately, digested with a proteinase K concentration series of 6.25 to 50mg/ml. The signal intensities are referenced to digestion with pK by 6.25mg/ml without detergent. Abbreviations: aBSE - atypical BSE; cBSE - classical BSE; ascrapie - atypical scrapie; cscrapie - classical scrapie; CJD 1 - CJD type 1; CJD 2 - CJD type 2 (according to Parchi et al., 2000 and Wemheuer et al., 2009).
3. Results
3.1. The detergent influences the proteinase K resistance of PrPSc
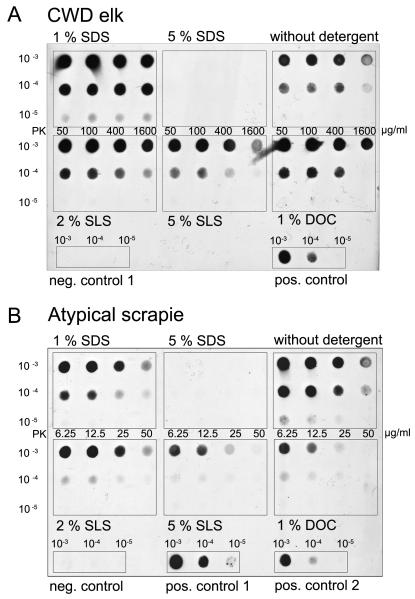
PrPSc in TSE shows in general a partial resistance to proteinase K. Proteinase K is used for the unmasking of formerly hidden epitopes by loosening the structure of protein aggregates. With increasing pK concentrations, the amount of detectable aggregate residues decreases (McKinley et al., 1983). PK resistance, however, is additionally affected by the detergent in the homogenate. While this is apparent using SDS, it is less obvious with SLS and DOC, and no additional destabilizing detergent effect on PrPSc can be seen with Triton X-100 at low concentration (see fig. 1). The detergent strongly influences the detectability of the proteinase K-resistant prion protein fragment as shown with the membrane adsorbtion assay. When different detergents of a low (1-2 %) concentration were added to tissue homogenates from the same TSE sample, differences in the detectability of PrPSc could be observed (see fig. 2). Differences in the detergent effect became more evident at higher concentrations. Five percent SDS had the strongest destabilizing effect on PrPSc of all tested TSEs, whereas 5 % SLS and Triton X-100 affected the prion aggregates to a lesser degree (fig. 3, 4 and 5).
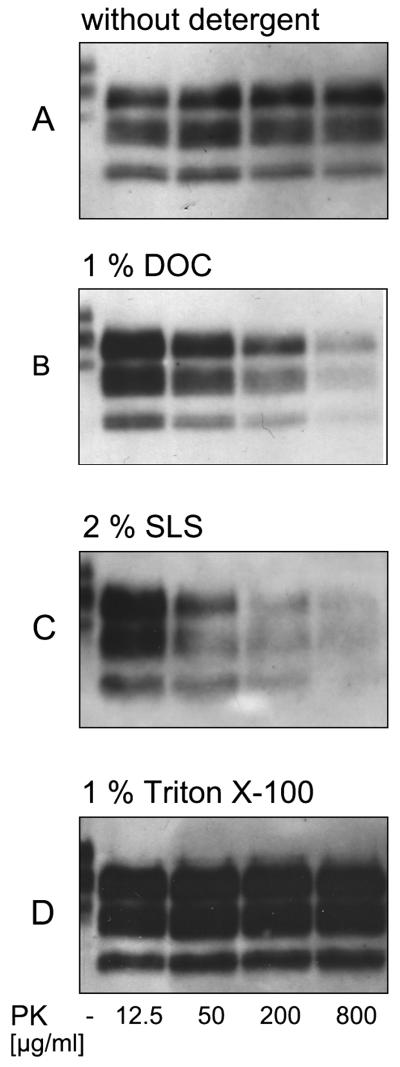
Fig. 1. Detergents in the homogenisation buffer influences pK resistance and strength of the PrPSc signal.
The typical three-banded prion protein pattern in the brain homogenate from elk infected with chronic wasting disease shows a decrease with ascending concentrations of proteinase K (B and C) in the presence of DOC or SLS, but an enhanced signal when Triton X-100 is used as detergent (D). A) Reference sample after pK digestion without any detergent. B) In comparison to digestion in plain PBS, 1 % DOC enhances the signal after digestion using 12.5 μg/ml pK, whereas aggregates were degradable at higher pK concentrations. C) 2 % SLS show in principle the same effect on aggregates of PrPSc of CWD in elk as 1 % DOC. D) The use of 1 % Triton X-100 enhances the PrPSc signal after treatment of 12.5 to 800 μg/ml pK. (mAb P4 1:2000).
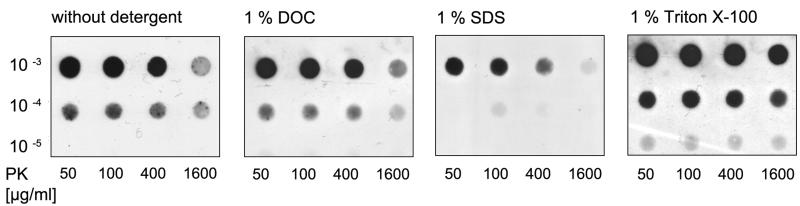
Fig. 2. Various detergents in low concentration have different effects on the pK stability of same TSE sample.
One percent DOC has the same effect on pK digestion of CWD in white-tailed deer as PBS, while SDS weakens the signal and Triton X-100 slightly enhances it (mAb P4 1:3000). Brain homogenate from white-tailed deer infected with chronic wasting disease was prepared in PBS, 1 % DOC, 1 % SDS or 1 % Triton X-100, digested with proteinase K (final concentration of 50 to 1600 μg/ml) for 1 hour at 37 °C, diluted in TBS up to 1:105 and sucked through a nitrocellulose membrane using a diaphragm pump (membrane adsorption assay).
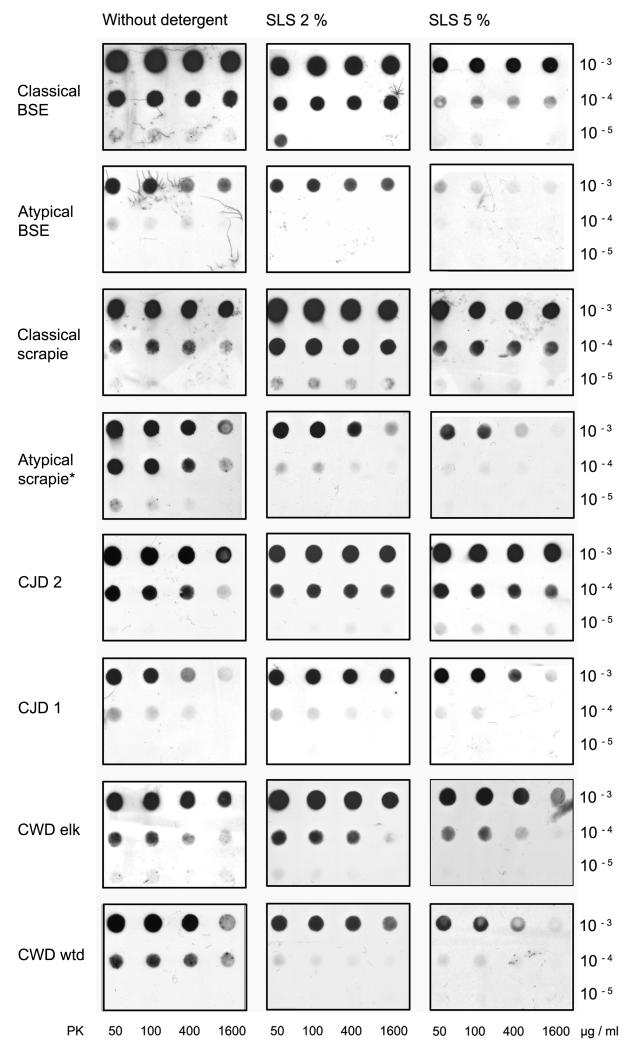
Fig. 3. TSE agents react differently to the same treatment.
Whereas classical BSE, classical scrapie, CJD and CWD in elk remain relatively stable, 5 % SLS considerably destabilizes aggregates of atypical BSE, atypical/Nor98 scrapie and CWD in white-tailed deer for pK digestion. Brain samples were prepared in PBS either with or without 2 % or 5 % of SLS. Proteinase K digestion was performed for 30 minutes at a final concentration of 50 to 1600 μg/ml pK with the exception of atypical/Nor98 scrapie, where a graded series of 6.25 to 50 μg/ml pK was used (see also fig. 4/B). Monoclonal antibody P4 1:3000, mAb 3F4 1:3000, mAb 12F10 1:10 000.
Fig. 4. Detergent effects are different in distinct TSE.
Homogenates of CWD in elk or atypical/Nor98 scrapie were prepared using different detergents. A) CWDelk: 1 % SDS, 2 % SLS and 1 % DOC enhance the PrPSc signal while 5 % SLS slightly reduces it compared to digestion without detergents. B) Atypical/Nor98 scrapie: Preparation in PBS results in the strongest PrPSc signal and all detergents tested on this blot reduce it. Proteinase K in the presence of the mild detergent DOC degrades PrPSc of atypical/Nor98 scrapie more than 2 % SLS or 1 % SDS do (mAb P4 1:2000).
3.2. Detergents can enhance or diminish PrPSc signal detection
By affecting the proteinase K resistance of PrPSc, different detergents in commonly used concentrations can enhance or decrease the detectability of PrPSc with Western blot or filtration techniques (see fig. 1). We demonstrate these effects for homogenization buffers which usually contain detergents in a concentration of up to 5 %. Which of these effects takes place depends on the detergent, its concentration and the species, as well as the form of the tested TSE. In summary, low concentrations of DOC, SLS, SDS and Triton X-100 enhance the PrPSc signal of some TSEs in combination with a mild proteinase K digestion of 12.5 μg/ml (or at higher pK concentrations in some instances), but in other TSEs they decrease the PrPSc detectability, even at low pK concentrations (fig. 3, 4, 5).
3.3. Influence of the detergent concentration
Higher detergent concentrations resulted in weaker signals of PrPSc after pK digestion, especially when the proteinase K concentration was increased. Five percent SDS had the strongest destabilizing effect on PrPSc of all tested TSEs, whereas 5 % SLS and Triton X-100 affected the prion aggregates to a lesser degree (fig. 3, 4, 5,). Adding 5 % SDS to the homogenization buffer resulted in an extensive pK degradation of all tested TSEs. They were no longer detectable by membrane adsorption assay or Western blot. SLS in a concentration of 5 % resulted in a considerable degradation and PrPSc signal reduction in several of the tested TSEs e.g. atypical BSE, atypical/Nor98 scrapie and CWD in white-tailed deer (see fig. 3). When the concentration of Triton X-100 was increased from 1 % to 5 %, its destabilizing features abolished the stabilizing effect of low Triton X-100 on the pK-treated prion protein aggregates (fig. 5). Five percent Triton X-100 reduced the PrPSc signal in most tested TSEs, but less so than did SLS and SDS at the same concentration.
3.4. Prion type, disease form and species as factors that influence the effect of detergents on proteinase K digestion
In our experiments we observed that the same treatment had different effects on the TSE of different species. Detergents in low concentrations of one to two percent had a slight up to a considerable enhancing effect on the PrPSc signal of CJD, classical scrapie and CWD in elk. The use of these detergents in the mentioned low concentration weakened the signal of atypical/Nor98 scrapie, atypical BSE and CWD in white-tailed deer (fig. 3, 5). The use of 1 % Triton X-100 did not destabilize and sometimes enhanced the PrPSc signal of almost all of the tested TSEs (fig. 1, 2, 5). TSE agents showed a remarkable variation regarding the sensitivity to detergent treatment preceding pK digestion. The vulnerability depends on the species and the prion type or disease form. In our experiments we saw that the PrPSc signal of classical scrapie, classical BSE and CJD and CWD in elk could be enhanced by the use of mild detergents in low concentrations, while the detectability of atypical (L-type) BSE, atypical/Nor98 scrapie and CWD in white-tailed deer could be affected even by the mild nonionic sodium desoxycholate (DOC; fig. 4, 5). Within a given species, the atypical TSE forms or human prion type 1 are easier to affect than the classical TSE forms or human prion type 2 (fig. 5). In chronic wasting disease, the stability depends on which species was investigated, although it has not yet been shown that the different species suffer from distinct TSE forms (fig. 5). CWD in elk and in white-tailed deer both resemble classical scrapie in their histopathologic outcome, including the aggregation forms of PrPSc deposits when stained by immunohistochemistry (Williams, 2005).
4. Discussion
Our results stress the fact that the detergents commonly used in diagnostic tests influence the partial proteinase K resistance of PrPSc. Detergents can have different effects on partial proteinase K-resistant PrPSc; they can enhance or diminish its detectability. The factors that influence detectability are the kind of detergent, its concentration, the species that developed the TSE and the form of the disease or the prion type.
In TSEs that are stable against denaturation, like type 2 prions (e.g. classical scrapie or CJD type 2 (Wemheuer et al., 2009), the proteinase K resistance can hardly be diminished by the use of mild detergents in low concentrations. In contrast, the addition of detergents can enhance the PrPSc signal. Type 2 prions are characterized by their complex form and the larger size of their aggregates (Kobayashi et al., 2005). The explanation for their detergent-related proteinase K resistance may be that detergents are helpful in de-masking antigenic structures formerly hidden inside the aggregates by affecting the tertiary protein structure and loosening the hydrophobic protein core of PrPSc (Hörnlimann et al., 2007).
In TSEs like atypical/Nor98 scrapie and atypical BSE, the detectability after pK digestion can easily be diminished even by the use of small amounts of detergent. The explanation may be that these small, synaptic aggregates (Lombardi et al., 2008; Wemheuer et al., 2009) with an accordingly larger surface are easier to destabilize by detergents. For atypical/Nor98 scrapie the high sensitivity to pK digestion is well known (Buschmann et al., 2004; Everest et al., 2006; Klingeborn et al., 2006) and was observed in our experiments even when the mild non-denaturating anionic detergent sodium deoxycholate (DOC) was used in low concentrations.
Although non-ionic and non-denaturating anionic detergents fail to inactivate PrPSc without pK digestion (Prusiner et al., 1980), and PrPSc is known to be insoluble in mild detergents, we found DOC and Triton X-100 to influence the partial pK resistance of PrPSc. This effect may be due to a partial loosening of the tertiary protein structure, providing easier access for proteinase K to suitable regions of the molecule or an effect on a non-protein component that is associated with the prion protein aggregates. Lipids that may be associated with prions are known to provide a certain protection of the protein aggregates (Appel et al., 2001). A removal of such lipids could therefore enhance the signal by unmasking epitopes in a first step. However, as a consequence also the resistance of the molecule to proteases would decrease when protein-associated lipids were dissolved. These results are consistent with those of Wang et al. (2007, 2010) who demonstrated a lipid effect on gaining proteinase K resistance through the conformational change of the cellular to the scrapie-like protein.
Differences in pK stability between CJD subtypes are well known and may be additionally influenced by the methionine/valine polymorphism at codon 129 (Notari et al., 2004). For excluding this variable, we tested MV1 and MV2 and found CJD type 2 in general to be more resistant to pK digestion than CJD type 1 as described by Notari et al. (2004).
Variations in the detectability of pK-resistant prion protein may depend on the antibody used. Unfortunately, no antibody is available that detects all TSEs from different species with the same sensitivity. We utilized combinations of the most common antibodies in use, P4 and 3F4 or P4 and 12F10. By reprobing human samples, we found no indication that the antibody combination interferes with the detectability of the detergent effect on the pK stability of the prion aggregates. The differences in the detergent sensitivities between the prion types, i.e. classical and atypical TSE forms, were seen regardless of what antibodies were used.
The destabilizing effect of detergents on protein molecules is also used for prion decontamination purposes. The denaturing anionic detergent sodium dodecyl sulfate (SDS) seems to be the most effective. When used under mild acid conditions, SDS seems to be a highly potent reagent for inactivation of prions (Peretz et al., 2006), but exhibits only a modest ability to inactivate prions under neutral pH. Five percent SDS alone led to a prion titre reduction of 2 to 5 logs, depending on the incubation temperature, but failed to completely degrade PrPSc of classical scrapie until NaOH was added (Lemmer et al., 2008). We can confirm the destabilizing effect of SDS, as we were not able to detect any PrPSc signal from TSE agents prepared in 5 % SDS. The stabilizing and signal-enhancing detergent effect can be of practical use in prion diagnostics and may help to detect small amounts of PrPSc. The detergent effect should also be carefully considered when instable forms of TSE agents are investigated. If difficulties occur during PrPSc detection, the use of a non-ionic and non-denaturating detergent like Triton X-100, sample preparation without detergents or a lower proteinase K concentration might be useful. Higher detergent concentrations hinder PrPSc detection, as PrPSc is apparently becoming destabilized instead of epitopes becoming demasked. Furthermore, samples are more difficult to prepare with higher detergent concentrations due to foam development.
The results of this study provide valuable information on the effect that different and commonly used detergents have on the detection of PrPSc. The investigated effects of several detergents on different TSEs with varying concentrations of proteinase K are useful for both prion diagnostics as well as for prion decontamination procedures.
Acknowledgments
We would like to thank Manuela Becker for her skilful technical assistance and Niels Kruse for his help in densitometric analyses. Antibodies were kindly provided by Dr. Michael Beekes, RKI Berlin and Dr. Dirk Motzkus, DPZ Göttingen. The work was supported by a grant from the Alberta Prion Research Institute to WJSS, and Stefanie Czub, Head of the National & OIE BSE Reference Laboratories, CFIA, Lethbridge, Alberta and by the NIAID-NIH PO1 AI 77774-01 “Pathogenesis, Transmission and Detection of Zoonotic Prion Diseases”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appel T, Wolff M, von RF, Heinzel M, Riesner D. Heat stability of prion rods and recombinant prion protein in water, lipid and lipid-water mixtures. J. Gen. Virol. 2001;82:465–473. doi: 10.1099/0022-1317-82-2-465. [DOI] [PubMed] [Google Scholar]
- Beekes M, Baldauf E, Diringer H. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J. Gen. Virol. 1996;77:1925–1934. doi: 10.1099/0022-1317-77-8-1925. [DOI] [PubMed] [Google Scholar]
- Benestad SL, Sarradin P, Thu B, Schonheit J, Tranulis MA, Bratberg B. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet. Rec. 2003;153:202–208. doi: 10.1136/vr.153.7.202. [DOI] [PubMed] [Google Scholar]
- Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Buschmann A, Biacabe AG, Ziegler U, Bencsik A, Madec JY, Erhardt G, Luhken G, Baron T, Groschup MH. Atypical scrapie cases in Germany and France are identified by discrepant reaction patterns in BSE rapid tests. J. Virol. Methods. 2004;117:27–36. doi: 10.1016/j.jviromet.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Everest SJ, Thorne L, Barnicle DA, Edwards JC, Elliott H, Jackman R, Hope J. Atypical prion protein in sheep brain collected during the British scrapie-surveillance programme. J. Gen. Virol. 2006;87:471–477. doi: 10.1099/vir.0.81539-0. [DOI] [PubMed] [Google Scholar]
- Hayashi HK, Yokoyama T, Takata M, Iwamaru Y, Imamura M, Ushiki YK, Shinagawa M. The N-terminal cleavage site of PrPSc from BSE differs from that of PrPSc from scrapie. Biochem. Biophys. Res. Commun. 2005;328:1024–1027. doi: 10.1016/j.bbrc.2005.01.065. [DOI] [PubMed] [Google Scholar]
- Hörnlimann B, Schulz-Schaeffer WJ, Roth K, Yan Z, Müller H, Oberthür RC, Rieser D. Chemical Disinfection and Inactivation of Prions. In: Hörnlimann B, editor. Prions in Humans and Animals. De Gruyter; New York: 2007. pp. 504–514. [Google Scholar]
- Klingeborn M, Wik L, Simonsson M, Renstrom LH, Ottinger T, Linne T. Characterization of proteinase K-resistant N- and C-terminally truncated PrP in Nor98 atypical scrapie. J. Gen. Virol. 2006;87:1751–1760. doi: 10.1099/vir.0.81618-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Satoh S, Ironside JW, Mohri S, Kitamoto T. Type 1 and type 2 human PrPSc have different aggregation sizes in methionine homozygotes with sporadic, iatrogenic and variant Creutzfeldt-Jakob disease. J. Gen. Virol. 2005;86:237–240. doi: 10.1099/vir.0.80389-0. [DOI] [PubMed] [Google Scholar]
- Kuczius T, Groschup MH. Differences in proteinase K resistance and neuronal deposition of abnormal prion proteins characterize bovine spongiform encephalopathy (BSE) and scrapie strains. Mol. Med. 1999;5:406–418. [PMC free article] [PubMed] [Google Scholar]
- Lemmer K, Mielke M, Kratzel C, Joncic M, Oezel M, Pauli G, Beekes M. Decontamination of surgical instruments from prions. II. In vivo findings with a model system for testing the removal of scrapie infectivity from steel surfaces. J. Gen. Virol. 2008;89:348–358. doi: 10.1099/vir.0.83396-0. [DOI] [PubMed] [Google Scholar]
- Lombardi G, Casalone C, D’ Angelo A, Gelmetti D, Torcoli G, Barbieri I, Corona C, Fasoli E, Farinazzo A, Fiorini M, Gelati M, Iulini B, Tagliavini F, Ferrari S, Caramelli M, Monaco S, Capucci L, Zanusso G. Intraspecies transmission of BASE induces clinical dullness and amyotrophic changes. PLoS. Pathog. 2008;4:e1000075. doi: 10.1371/journal.ppat.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madec JY, Vanier A, Dorier A, Bernillon J, Belli P, Baron T. Biochemical properties of protease resistant prion protein PrPsc in natural sheep scrapie. Arch. Virol. 1997;142:1603–1612. doi: 10.1007/s007050050183. [DOI] [PubMed] [Google Scholar]
- McKinley MP, Bolton DC, Prusiner SB. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983;35:57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Meyer RK, McKinley MP, Bowman KA, Braunfeld MB, Barry RA, Prusiner SB. Separation and properties of cellular and scrapie prion proteins. Proc. Natl. Acad. Sci. 1986;83:2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari S, Capellari S, Giese A, Westner I, Baruzzi A, Ghetti B, Gambetti P, Kretzschmar HA, Parchi P. Effects of different experimental conditions on the PrPSc core generated by protease digestion: implications for strain typing and molecular classification of CJD. J. Biol. Chem. 2004;279:16797–16804. doi: 10.1074/jbc.M313220200. [DOI] [PubMed] [Google Scholar]
- Oesch B, Jensen M, Nilsson P, Fogh J. Properties of the scrapie prion protein: quantitative analysis of protease resistance. Biochemistry. 1994;33:5926–5931. doi: 10.1021/bi00185a033. [DOI] [PubMed] [Google Scholar]
- Parchi P, Zou W, Wang W, Brown P, Capellari S, Ghetti B, Kopp N, Schulz-Schaeffer WJ, Kretzschmar HA, Head MW, Ironside JW, Gambetti P, Chen SG. Genetic influence on the structural variations of the abnormal prion protein. Proc. Natl. Acad. Sci. 2000;97:10168–10172. doi: 10.1073/pnas.97.18.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz D, Supattapone S, Giles K, Vergara J, Freyman Y, Lessard P, Safar JG, Glidden DV, McCulloch C, Nguyen HO, Scott M, DeArmond SJ, Prusiner SB. Inactivation of prions by acidic sodium dodecyl sulfate. J. Virol. 2006;80:322–331. doi: 10.1128/JVI.80.1.322-331.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner SB, Groth DF, Cochran SP, Masiarz FR, McKinley MP, Martinez HM. Molecular properties, partial purification, and assay by incubation period measurements of the hamster scrapie agent. Biochemistry. 1980;19:4883–4891. doi: 10.1021/bi00562a028. [DOI] [PubMed] [Google Scholar]
- Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, Groth DF, Glenner GG. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983;35:349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ. A prion disease of cervids: chronic wasting disease. Vet. Res. 2008;39:41. doi: 10.1051/vetres:2008018. [DOI] [PubMed] [Google Scholar]
- Wang F, Yang F, Hu Y, Wang X, Wang X, Jin C, Ma J. Lipid interaction converts prion protein to a PrPSc-like proteinase K-resistant conformation under physiological conditions. Biochemistry. 2007;46:7045–7053. doi: 10.1021/bi700299h. [DOI] [PubMed] [Google Scholar]
- Wang F, Yin S, Wang X, Zha L, Sy MS, Ma J. Role of the highly conserved middle region of prion protein (PrP) in PrP-lipid interaction. Biochemistry. 2010;49:8169–8176. doi: 10.1021/bi101146v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemheuer WM, Benestad SL, Wrede A, Schulze-Sturm U, Wemheuer WE, Hahmann U, Gawinecka J, Schutz E, Zerr I, Brenig B, Bratberg B, Andreoletti O, Schulz-Schaeffer WJ. Similarities between forms of sheep scrapie and Creutzfeldt-Jakob disease are encoded by distinct prion types. Am. J. Pathol. 2009;175:2566–2573. doi: 10.2353/ajpath.2009.090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ES. Scrapie and chronic wasting disease. Clin. Lab. Med. 2003;23:139–159. doi: 10.1016/s0272-2712(02)00040-9. [DOI] [PubMed] [Google Scholar]
- Williams ES. Chronic wasting disease. Vet. Pathol. 2005;42:530–549. doi: 10.1354/vp.42-5-530. [DOI] [PubMed] [Google Scholar]
- Windl O, Giese A, Schulz-Schaeffer W, Zerr I, Skworc K, Arendt S, Oberdieck C, Bodemer M, Poser S, Kretzschmar HA. Molecular genetics of human prion diseases in Germany. Hum. Genet. 1999;105:244–252. doi: 10.1007/s004399900124. [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Hartl FU, Tatzelt J. A sensitive filter retention assay for the detection of PrP(Sc) and the screening of anti-prion compounds. FEBS Lett. 2001;503:41–45. doi: 10.1016/s0014-5793(01)02692-8. [DOI] [PubMed] [Google Scholar]
- Xie Z, O’Rourke KI, Dong Z, Jenny AL, Langenberg JA, Belay ED, Schonberger LB, Petersen RB, Zou W, Kong Q, Gambetti P, Chen SG. Chronic wasting disease of elk and deer and Creutzfeldt-Jakob disease: comparative analysis of the scrapie prion protein. J. Biol. Chem. 2006;281:4199–4206. doi: 10.1074/jbc.M509052200. [DOI] [PMC free article] [PubMed] [Google Scholar]