Abstract
DNA double-strand breaks (DSBs), which are generated by ionizing radiation (IR) and a range of other DNA damaging agents, are repaired by homologous recombination (HR) or non-homologous end-joining (NHEJ). Previous studies have shown that NHEJ in Saccharomyces cerevisiae requires the Yku70p–Yku80p heterodimer and a complex consisting of DNA Ligase IV, Lif1p and Nej1p. Here, we report that Nej1p is phosphorylated in response to DNA damage in a manner that relies on the DNA damage checkpoint kinases Mec1p, Rad53p and Dun1p. By using a mutational approach, we have identified a consensus Dun1p phosphorylation site in Nej1p, and mutation of conserved serine residues within it leads to decreased NHEJ efficiency. These data, together with previous findings that Rad55p – a protein involved in HR – is phosphorylated analogously, point to there being a broad signalling network connecting DNA damage checkpoint responses with the regulation of DNA DSB repair activities.
Keywords: S. cerevisiae, Checkpoint, DNA damage, DUN1, NHEJ, Phosphorylation
INTRODUCTION
The repair of DNA double-strand breaks (DSBs) is essential for all living organisms, as even one single unrepaired DSB can be lethal for a cell. DSBs and other DNA lesions are known to trigger a cellular response known as the DNA damage response (DDR), which in large part is conserved from yeast to man [1]. In Saccharomyces cerevisiae, DNA lesions lead to activation of the protein kinases Mec1p and Tel1p that then trigger the subsequent activation of the effector kinases Rad53p, Chk1p and Dun1p, resulting in cell cycle arrest, induction of transcription and enhancement of repair [2]. While it is quite well established how the DDR impacts on cell cycle progression [3-5], its regulatory functions on repair activities remain largely unclear [6].
Eukaryotic cells possess two principle pathways to repair DSBs: homologous recombination (HR) and non-homologous end-joining (NHEJ). Work over the past decade has discovered a set of proteins that are required for NHEJ [7]. At the centre of the NHEJ machinery is the DNA ligase IV (Dnl4p) - Lif1p complex, which provides the enzymatic activity to religate the two broken DNA ends [8,9]. In addition, the DNA end-binding Yku70p-Yku80p heterodimer is crucial for NHEJ, most likely by providing a recruitment platform through its ability to recognize and stabilize double-stranded DNA ends [10]. Recent studies in yeast have implicated another protein, Nej1p, in NHEJ [11-14]. Nej1p interacts with Lif1p and is required for efficient NHEJ in vivo. Mapping studies have shown that the C-terminal 192 residues of Nej1p are required for its interaction with Lif1p [14]. However, by using a yeast-two-hybrid approach, it was shown that Nej1p is not required for the binding of Lif1p to Dnl4p; and vice versa, Dnl4p is not required for the Nej1p–Lif1p interaction [14]. Notably, the expression of NEJ1 is regulated by the MATa/α repressor, with Nej1p being down-regulated in diploid cells – a state in which HR is the highly preferred pathway of DSB repair – thus leading to a model in which Nej1p acts to control NHEJ depending on cell ploidy [11-14].
The mechanism(s) by which Nej1p operates is still far from certain. By employing a plasmid –based DSB repair assay and by introducing a DSB in the yeast genome with the HO endonuclease, nej1 mutants were found to exhibit a profound repair defect [11-13]. Nevertheless, nej1 mutants were only partly defective in suicide deletion assays where the repair of I-SceI induced DSBs was monitored , a phenotype that contrasts with the severe end-joining defects of yku70, yku80, lif1 and dnl4 mutants in such an assay [15]. Taken together, these findings suggest as yet unexplored regulatory roles of Nej1p in haploid cells. Moreover, the antithetical finding that Nej1p acts to prevent Dnl4p dependent chromosomal fusions in the absence of telomerase [16] lends further support for more complex models of Nej1p function. Notably, it has been pointed out that Nej1p has two potential transmembrane helices, and it has been shown that in diploid yeast cells Lif1p partly mis-localizes to the cytoplasm if Nej1p is absent [12]. However, it remains unclear if such an effect directly depends on Nej1p function, as another study did not detect an effect of Nej1p on Lif1p nuclear localization in haploid cells [11]. Moreover, indirect immunofluorescence analysis of Nej1p did not provide any indication that the protein is associated with the nuclear membrane [11]. It therefore remains unclear how Nej1p facilitates NHEJ and whether it actively participates in the repair process or carries out more regulatory functions.
Recent work in S. cerevisiae has established that HR and DDR proteins are recruited to sites of DSBs in an orchestrated and temporally coordinated manner [17]. However, it is still unclear to what extent the NHEJ machinery is subject to such DDR controlled orchestration and whether NHEJ activities are modulated under various physiological circumstances. A variety of recent studies have revealed the existence of alternative end-joining pathways (or subpathways) and additional end-processing steps, suggesting that there might be mechanisms to regulate the usage of various processing enzymes and Dnl4p independent end-joining events [18-22]. Moreover, it has been shown that the transient stability of DSB ends is closely connected to the temporal orchestration of both HR and NHEJ [23], further strengthening the idea that overall regulation of repair and DDR responses has to occur to ensure that DSBs are repaired efficiently. We therefore became interested in the possibility that Nej1p, with its ploidy related function in S. cerevisiae, might also be part of a regulatory network to modulate end-joining in haploid yeast cells. Indeed, we show here that Nej1p is a target of the Mec1p- Rad53p- and Dun1p-dependent DNA damage signalling pathway and provide data indicating that such DNA damage dependent regulation is required for efficient NHEJ mediated repair of DNA DSBs.
MATERIALS AND METHODS
Strains and plasmids
All strains used in this study are described in Table 1. All experiments were performed with isogenic strains. 13-myc tagged NEJ1 (pNej1) was cloned under its own promoter in the low copy, centromeric plasmid pRS415 (Stratagene) from a template provided by S. Marcand. pRS416 containing RAD53 expressed under its own promoter (pRad53) was provided by J. Rouse. pGAP-DUN1-HA, a 2μ plasmid expressing HA-tagged Dun1p under the GAP promoter was created for this study from reagents provided by D. Durocher. pUG36-MET25-EGFP-Lif1 (pLif1-EGFP) was provided by P. Schär. Nej1p internal deletion constructs were created with pNej1 as template in a PCR based deletion approach [24].
Table 1.
S. cerevisiae strains used in this study
| Strain | Relevant genotype | Source |
|---|---|---|
| W303a | MATa, ade2-1, leu2-3, 112 his3-11, 15 trp1 ura3-1 can1-100, rad5-535 | R. Rothstein |
| mec1Δ | W303a; mec1Δ::trp1 | D. D’Amours |
| tel1Δ | W303a; tel1Δ::his3 | D. D’Amours |
| chk1Δ | W303a; chk1Δ::trp1 | D. Durocher |
| dun1Δ | W303a; dun1Δ::his3 | D. Durocher |
| rad53Δ | W303a; rad53Δ::his3 | R. Rothstein |
| rad53-1 | W303a; rad53Δ::rad53-1 | N. Lowndes |
| ku80Δ | W303a; ku80Δ::kanMX | R. Roy |
| lif1Δ | W303a; lif1Δ::ura3 | S. Teo |
| PA001 | W303a; nej1Δ::kanMX | this study |
| PA002 | W303a; nej1Δ::nej1-13myc::kanMX | this study |
| PA006 | W303a; nej1Δ::kanMX; dun1Δ::his3 | this study |
| PA007 | W303a; nej1Δ::kanMX; lif1Δ::ura3 | this study |
Antibodies, Western blotting and immunoprecipitation
13-myc tagged Nej1p was detected with a mouse monoclonal anti-Myc antibody provided by Cancer Research UK. Rad53p was detected with a rabbit anti-Rad53p antibody provided by N. Lowndes. Lif1p-EGFP was detected with a mouse monoclonal anti-GFP antibody (Cancer Research UK). Tubulin was detected with a rat monoclonal anti-tubulin antibody (Abcam, ab6161). Orc2p was detected with a mouse monoclonal anti-Orc2p antibody (Oncogene Research Products, NA38). Detection of the Nej1p mobility shift was done by 10% SDS-PAGE, 120 V for 1 h, 180 V for 2 h and western blotting under standard conditions. Immunoprecipitations were carried out under standard conditions (50 mM Tris-HCl pH 7.4, 120 mM NaCl2, 0.5% NP-40) from 200 μg of yeast whole cell extract with equal amounts of polyclonal anti-Rad53p (rabbit; N. Lowndes) and anti-Dun1p (goat; Santa Cruz, sc6750) antibodies. Antibody-kinase complexes were precipitated with Protein-G sepharose (Cancer Research UK) and washed three times with buffer before analysis.
Cell culture and synchronization
S. cerevisiae cultures were grown under standard conditions in complete or selective medium. For induction of DNA damage, cells were grown overnight, diluted to an OD600 of 0.08 and re-grown to an OD600 of 0.2. Drugs were added to the culture and incubated as indicated (unless stated otherwise, working concentrations were: 1 μg/ml of 4-NQO, 25 μg/ml of phleomycin, 0.0125% of MMS). Cultures were arrested in G1 with 5 μg/ml of α-factor or in G2/M with 15 μg/ml of nocodazole. Arrests were confirmed by light microscopy and FACS analysis. When released, cells were filtered with a Millipore cell filtration unit with excess medium to wash away residual α-factor.
Site-directed mutagenesis
Site-directed mutagenesis of NEJ1 tagged with 13-myc and cloned in pRS415 was carried out by the Stratagene QuickChangeTM procedure according to the manufacturer’s instructions.
In vitro phosphorylation
Dun1p or Rad53p was precipitated with 0.1 μg of the respective polyclonal antibody from 200 μg of yeast whole cell extract. Extracts were prepared from untreated cycling cells or cells treated with 0.0125% MMS for 2 h to induce the DDR. Protein G-antibody bound kinase was incubated with 1 μg of recombinant substrate in the presence of 32P ATP (190 Bq; 50 mM Tris-HCl pH 7.4, 20 mM MgCl2, 1 mM DTT, 0.025 mM ATP) for 30 min at 30°C before the addition of 2 × SDS sample buffer to stop the reaction. SDS-PAGE was performed and autoradiography was carried out with a Fujifilm Phosphorimager (FLA-5000) and Fujifilm quantitative software (Mac-Bas).
Plasmid repair
Plasmid repair assays were performed essentially as described before [10]. pBTM116 or pRS416 was digested with the indicated restriction enzymes to completion and transformed with lithium-acetate. Undigested plasmid was transformed to equalize for transformation efficiency. Cells were quantitatively plated with a spiral logarithmic cell plater (DU scientific) and transformed colonies on selective medium were counted. Error bars represent the mean and standard error of at least three independent experiments.
Phleomycin survival
Early log-phase cells were treated with indicated doses of Phleomycin for 4 h and afterwards washed once with drug-free medium. Cells were quantitatively plated with a spiral logarithmic cell plater (DU scientific) and surviving colonies were counted after three days. Data shown represents the mean and error of at least three independent experiments.
GFP microscopy
GFP staining was analyzed on a fluorescent microscope (BioRad/Nikon 027034, Nikon Plan 100/1.25 oil objective). Cells were grown as described above, harvested by centrifugation and washed twice with PBS. The cell pellet was resuspended in 70% ethanol, sonicated briefly and incubated overnight at 4°C. Fixed cells were re-hydrated for 1 h in cold PBS and mounted on slides using 35% glycerol containing 0.15 μg/ml of DAPI.
Chromatin fractionation
Chromatin fractionation was performed as described by Frei and Gasser [25]. Briefly, cultures were washed twice with cold TBS, incubated in 0.1M Tris-HCl pH 9.4, 20 mM DTT for 10 min, washed twice with HBS (50 mM Hepes pH 7.4, 1.2 M sorbitol), treated with 20 μg oxalyticase in 1 ml HBS at 30°C for 30 min. Spheroplasting was confirmed by light microscopy. Spheroplasts were washed twice and resuspended in TWB (5 mM Tris-HCl pH 7.4, 20 mM KCl, 2 mM EDTA, 0.4 M sorbitol, 1% thiodiglycol 0.5 mM PMSF). 10% v/v Triton X-100 was added and cells were lysed on ice for 5 min (WCE). Samples were centrifuged at 13500 rpm at 4°C for 15 min and supernatant was removed as soluble fraction (S). Pellets were resuspended in 75μl of DB (50 mM Tris-HCl pH 7.4, 125 mM KCl, 7.5 mM MgCl2, 0.5% Triton X-100, 50 μg/ml DNaseI, 0.5 mM PMSF) and incubated for 20 min at 4°C. Samples were centrifuged for 5 min at 13500 rpm at 4°C and supernatant was removed as the soluble chromatin fraction (SC). The remaining pellet was resuspended in 75 μl of DB (ISC). Gels were loaded so that ratios correspond to equal cell input numbers.
RESULTS
Nej1p is rapidly phosphorylated in response to DNA damage
To explore a putative regulatory role of Nej1p in haploid cells, we were interested to see whether Nej1p is subject to post-translational modification in response to DNA damage. Therefore, 13-myc tagged Nej1p was expressed from either its endogenous genomic locus (nej1Δ::nej1-13myc::kanMX) or from a pRS415 vector under its endogenous promotor (expression levels were found to be equivalent in both cases; data not shown). We found that whereas 13-myc tagged Nej1p normally migrates as a single species in SDS-polyacrylamide gels, a proportion of it is converted into a distinct, slower migrating form (*) when cells are treated with the DNA base-damaging drug 4-nitro-quinoline oxide (4-NQO) or the DSB-generating agent, phleomycin (Fig. 1A). This modified form reflects Nej1p phosphorylation, because it was lost when extracts from damaged cells were incubated with λ-phosphatase, but not when the phosphatase inhibitor EDTA was included in addition to λ-phosphatase (Fig. 1B; top and bottom panels). Phosphorylation of Nej1p appears to be a common response to DNA DSB inducing agents, as comparable mobility shifts were observed following treatment of cells with methyl methane sulphonate (MMS), hydroxyurea (HU), 4-NQO, phleomycin or ionizing radiation (Fig. 1C). Nej1p phosphorylation following DNA damage is unlikely to be caused by the accumulation of a phosphorylated population of Nej1p at a specific stage of the cell cycle, as we were unable to detect the slower-migrating forms of Nej1p when we analyzed cell populations at various stages of the cell cycle in the absence of a DNA damaging agent (Fig. 1D). Furthermore, through time-course analyses, we found that Nej1p phosphorylation was detectable within 5 minutes of treating cells with 4-NQO and that this phosphorylation persisted for more than 90 minutes (Fig. 1E, left). When using phleomycin we observed slightly slower kinetics but consistently found Nej1p to be phosphorylated after 60 minutes of treatment (Fig. 1E, right). Interestingly, Nej1p was also readily phosphorylated in cells that were arrested in G1 or at the G2/M boundary (Fig. 1F), indicating that phosphorylation of Nej1p in response to DNA damage is not restricted to a specific stage of the cell cycle. Taken together, these results show that Nej1p phosphorylation takes place rapidly in response to DNA damage.
Figure 1. Nej1p is rapidly phosphorylated in response to DNA damage.
(A,B,D-F); Extracts prepared from the indicated strains were run on 10% SDS-gels and analyzed by Western blotting with a mouse anti-myc monoclonal antibody to detect Nej1p. The asterisk represents the phosphorylated, slower migrating form of Nej1p generated in response to DNA damage, while “Loading” represents an unspecific band (~50 kDa) recognized by the mouse anti-Myc antibody in the absence or presence of 13-myc-Nej1p. A nej1Δ cells were transformed with pNej1 or empty vector (pRS415) and treated with phleomycin or 4-NQO for 2 h. B Native protein extracts (80 μg) prepared from untreated cells or cells treated with 4-NQO (top) or phleomycin (bottom), were treated with or without 800 U of λ-phosphatase (NEB) and with or without 50 mM EDTA as indicated. C Asynchronously growing cells transformed with pNej1 untreated or treated with 0.075% of MMS, 90 min; 100 mM of HU, 90 min; 1μg/ml of 4-NQO, 90 min; 25 μg/ml of phleomycin , 90 min; 230 Gy of IR; 100 J/m2 of UV. Extracts were prepared and resolved on a 8% SDS-PAGE before immunoblotting with mouse anti-myc antibody. D G1 arrested cells were released into the cell cycle. Samples were taken and phosphorylation was analyzed by Western blotting (upper panel). As a control, cycling cells were treated with 4-NQO. Progression through the cell cycle was monitored by light microscopy (lower panel). E Nej1-13myc cells were grown asynchronously and extracts prepared at indicated time intervals after drug addition. F nej1Δ cells were transformed with pNej1 and either grown asynchronously, arrested at G2/M with 15 μg/ml of nocodazole, or arrested in G1 with 5 μg/ml of α-factor. Cycling and arrested cultures were treated with 4-NQO for 90 min before extract preparation.
Nej1p phosphorylation requires Mec1p, Rad53p and Dun1p
The DDR in yeast is controlled by a number of protein kinases that become rapidly activated when cells are challenged with DNA damaging agents [1-6]. The above findings suggested a close correlation between Nej1p phosphorylation and the activation of the yeast DNA damage checkpoint kinase cascade, thereby prompting us to investigate the possible genetic dependency of Nej1p phosphorylation on the main kinases of the DDR. As shown in Fig. 2A, Nej1p phosphorylation in response to 4-NQO (or phleomycin; data not shown) requires Mec1p, the central DNA damage checkpoint kinase in S. cerevisiae, as no phosphorylated Nej1p was observed in cells lacking Mec1p. A key function of Mec1p is the activation of the downstream DDR kinase Rad53p. Consistent with a model in which Nej1p is a downstream target of the DDR response, we found that Rad53p kinase activity is also required for Nej1p phosphorylation (Fig. 2B). Thus, the changes in Nej1p electrophoretic mobility in response to DNA damage were not observed in a rad53 deletion mutant nor in cells harbouring kinase-defective Rad53p (rad53-1, Fig. 2B). Introduction of a plasmid bearing wild-type RAD53 into the rad53 deleted strain reconstituted the DNA damage induced Nej1p mobility shift (Fig. 2B).
Figure 2. Genetic dependencies of Nej1p phosphorylation.
(A-E); Extracts prepared from the indicated strains were run on 10% SDS-gels and analyzed by Western blotting with anti-Myc antibody to detect Nej1p. The asterisk represents the phosphorylated, slower migrating form of Nej1p generated in response to DNA damage. Nej1p phosphorylation depends on the DNA damage checkpoint kinases Mec1p, Rad53p and Dun1p. A W303a, mec1Δ::trp1, tel1Δ::his3 or chk1Δ::trp1 strains were transformed with pNej1. Cultures were treated with drug for 2 h as indicated. Loading represents an unspecific band (~50 kDa) recognized by the anti-Myc antibody in the absence or presence of 13-myc-Nej1p. B W303a, rad53Δ::his3 and rad53Δ::rad53-1 and rad53Δ::his3 + pRad53 strains were transformed with pNej1. Cultures were treated with drug for 2 h as indicated. C dun1Δ::his3 nej1Δ::kanMX cells were transformed with pNej1 and pDun1 as indicated. Nej1-13myc cells were used as control. Cultures were treated with drug for 90 min as indicated. D Nej1p phosphorylation is independent of the NHEJ machinery. nej11Δ::kanMX, lif11Δ::ura3 and yku80Δ::kanMX cells were transformed with pNej1. Cultures were treated with drug for 90 min as indicated. Extracts were prepared, resolved by 10% SDS-PAGE and analyzed by western blotting with a mouse anti-Myc antibody to detect Nej1p. E Nej1p phosphorylation is enhanced in the absence of function HR. nej1Δ::kanMX (W303) or rad52Δ::trp1 strains were transformed with pNej1. Cells were grown as indicated in the absence or presence of 4-NQO. Extracts were run on SDS-gels and analyzed as described above. Loading represents an unspecific band (~50 kDa) recognized by the anti-Myc antibody in the absence or presence of 13-myc-Nej1p.
One target of Rad53p in the DDR is the related protein kinase Dun1p. Dun1p has been shown to control the transcriptional induction of certain genes in response to DNA damage [26,27], to mediate phosphorylation of Sml1p to control dNTP levels after DNA damage [28], and to bring about phosphorylation of the HR protein, Rad55p [29]. Notably, we found that Nej1p was not detectably phosphorylated following DNA damage in cells lacking Dun1p, whereas its phosphorylation was restored by introducing a plasmid expressing functional Dun1p in these cells (Fig. 2C). Similar to other NHEJ proteins in S. cerevisiae, Nej1p itself is not required for checkpoint activation, as phosphorylation of Rad53p occurs normally in response to both phleomycin and 4-NQO in the absence or presence of Nej1p (data not shown). Taken together, these data indicate that Nej1p phosphorylation after DNA damage requires Mec1p, Rad53p and Dun1p; a situation highly analogous to the phosphorylation of Rad55p in response to DNA damage [29].
Other NHEJ proteins are not required for Nej1p phosphorylation but Nej1p phosphorylation is enhanced in the absence of HR
As Nej1p is required for NHEJ and interacts with Lif1p, we tested whether Nej1p phosphorylation is affected in cells with impaired NHEJ or HR. We found that Nej1p phosphorylation in response to DNA damage was normal in cells deleted for the core NHEJ proteins Yku80p or Lif1p, indicating that phosphorylation of Nej1p does not require the assembly of a functional NHEJ complex at sites of DNA damage (Fig. 2D). However, in the absence of the core HR protein, Rad52p, Nej1p phosphorylation was increased and could even be observed in the absence of exogenous DNA damaging agents (Fig. 2E). This suggests that the absence of HR in cycling yeast cells - when low levels of endogenous DNA damage are sufficient to induce Rad53p kinase activity [30] - causes subsequent phosphorylation of Nej1p. Additionally, in the absence of HR cells have to rely on alternate DSB repair pathways and therefore NHEJ-specific regulatory events might become more important.
Mutation of Ser-297/8 abolishes the phosphorylation-induced mobility shift of Nej1p
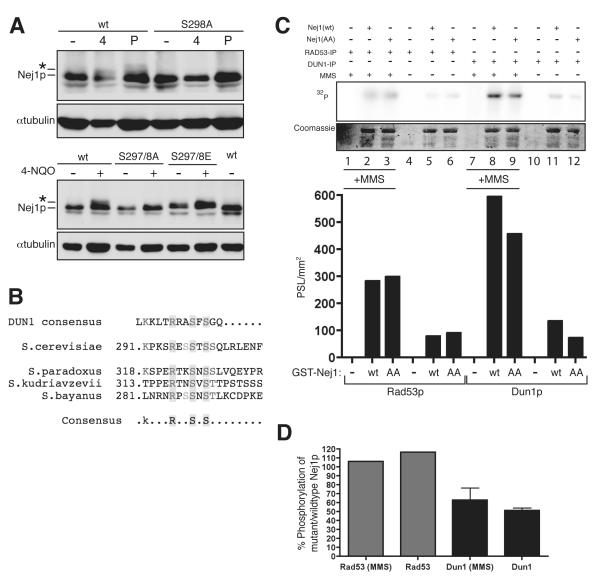
We identified a region in Nej1p that shows homology to a previously defined Dun1p consensus sequence [31]. Indeed, mutation of Ser-298 to an unphosphorylatable Ala residue or both Ser-297 and Ser-298 to Ala caused a complete loss of the DNA-damage induced mobility shift of Nej1p (Fig. 3A and data not shown) suggesting that phosphorylation of these residues might be responsible for the altered migration properties of Nej1p in response to DNA-damage. Moreover, when both Ser-297 and Ser-298 were mutated to a potentially phospho-mimicking Glu residue, we found mutated Nej1p to constitutively migrate slower than wild-type Nej1p (Fig. 3A). The mobility shift of Nej1p was still observed when we mutated a range of other potential DNA damage kinase target residues to Ala (Ser-58, Thr-60, Ser-63, Ser-68 and Ser-280; data not shown). Ser-297/8 resembles the core characteristics of a Dun1p consensus phosphorylation site as described by Sanchez et al. (Fig. 3B; [31]). Interestingly, analogous sites are conserved in other fungal Nej1p homologues even though the Nej1p C-terminus shows considerable sequence divergence (Fig. 3B).
Figure 3. Nej1p serines 297 and 298 are involved the DNA damage dependent phosphorylation of Nej1p.
A Site-directed mutagenesis of potential phosphorylation sites in Nej1p. nej11Δ::kanMX cells were complemented with pNej1(wt, W303) or pNej1 plasmids harbouring the amino acid substitutions Ser-298 to Ala, Ser-297/Ser-298 to Ala, or Ser-297/Ser-298 to Glu. Transformed strains were treated with drug for 90 min with phleomycin or 4-NQO and extracts were resolved by 10% SDS-PAGE and western blotting with anti-Myc antibody to detect Nej1p. Tubulin was detected with a rat anti-tubulin monoclonal antibody. The asterisk represents the phosphorylated, slower migrating form of Nej1p generated in response to DNA damage. B Alignment of Nej1p homologues of different yeast species to the Dun1p consensus peptide [31]. C In vitro phosphorylation reactions with wild-type Nej1p (wt) and Nej1p mutated on Ser residues 297 and 298 (AA) expressed as GST fusion proteins. Phosphorylation of Nej1p was monitored by autoradiography (upper panel 32P; Coomassie shows the loading of GST-Nej1p) and quantified with a phosphorimager (histogram in lower panel). D Substrate specificity of Rad53p and Dun1p. Ratio (in %) of phosphorylated Nej1p mutated at Ser residues 297 and 298 to wild-type Nej1p obtained with quantification data from in vitro phosphorylation reactions. Error bars indicate the mean and standard deviation of two independent experiments.
Taken together with our findings on the genetic dependency of Nej1p phosphorylation, the above data strongly suggested that Ser-297/8 of Nej1p are directly phosphorylated by Dun1p following DNA damage. To test this, we immunoprecipitated Rad53p or Dun1p from yeast whole cell extracts prepared either from untreated cells (Fig. 3C, lanes 4-6 and 10-12) or cells treated with MMS (Fig. 3C, lanes 1-3 and 7-9). The precipitates were then incubated in the presence of 32P-ATP with purified GST-Nej1p or with GST-Nej1p in which both Ser-297 and Ser-298 were mutated to Ala (AA). As shown in Fig. 3C, Dun1p precipitates were able to phosphorylate Nej1p in vitro (Fig. 3C, lanes 8 and 11), and by quantitating the amount of incorporated 32P-ATP we found that the phosphorylation site mutant was less efficiently phosphorylated as compared to wild-type Nej1p (Fig. 3C, compare lane 8 with lane 9 and lane 11 with lane 12; see histogram in bottom panel for quantitation). By contrast, phosphorylation of Nej1p by Rad53p precipitates was not significantly affected by mutating the Ser residues (Fig. 3C, lanes 2,3 and 5,6). To test the specificity of the precipitation we immunoprecipitated from wild-type and dun1 deleted cells in parallel. In agreement with our previous findings, Dun1p precipitates only promoted phosphorylation of GST-Nej1p when Dun1p was present in the cells (Supplementary Figure 1). Both Rad53p and Dun1p are markedly stimulated by DNA damage (such as induced by MMS; [32]), and we found that Dun1p precipitates from cells treated with MMS phosphorylated wild-type Nej1p more efficiently than the derivative mutated at Ser-298 and Ser-297 (Fig. 3C, compare lanes 8 and 9 with lanes 11 and 12; also see Fig. 3D). By contrast, Rad53p precipitates failed to display any appreciable difference in their ability to phosphorylate the two Nej1p variants (Fig. 3B and 3C). Although we cannot rule out that Nej1p is subject to phosphorylation by both Rad53p and Dun1p in vivo, targeted by a third kinase that is co-precipitated or indeed that Dun1p phosphorylates multiple sites on Nej1p, these data support a model in which Dun1p directly phosphorylates Nej1p on Ser-297/8 in response to DNA damage and this phosphorylation event is responsible for the change of Nej1p mobility on SDS-PAGE in response to DNA damage.
Nej1p Ser-297/8 is required for full NHEJ activity in vivo
We next tested whether Nej1p Ser-297 and Ser298 were required for efficient NHEJ. Thus, we carried out quantitative in vivo plasmid repair assays to evaluate NHEJ activity in cells harbouring various Nej1p mutants. As described before, NHEJ is severely impaired in the absence of Nej1p (Fig. 4A, pRS415). Consistent with our findings that Nej1p is phosphorylated in response to DNA damage, mutation of Ser-298 to an unphosphorylatable Ala led to a consistent decrease in NHEJ activity (Fig. 4A, S298A), and mutation of both Ser-297 and Ser-298 to Ala lead to a further decrease in NHEJ activity (Fig. 4A, S297/8A). By contrast, mutation of Ser-298 to a potentially phospho-mimicking negatively charged Glu residue allowed wild-type levels of repair (Fig. 4A, S298E). No decrease in NHEJ activity was observed, however, when other putative DDR kinase phosphorylation sites in Nej1p were mutated to unphosphorylatable residues (Ser 280 to Gly; Ser 58,63,68 and Thr 60 to Ala; Fig. 4A, S280G and STSS, respectively). Notably all these mutants also retain the migration shift of Nej1p in response to treatment with DNA damaging agents (data not shown). Consistent with the finding that Ser-297/8 may be a Dun1p consensus site, mutation of Arg-294 (at position -3 to Ser-298), a residue crucial to Dun1p dependent phosphorylation in enzymatic studies of Dun1p kinase activity [31], impaired NHEJ activity, and indeed did this to an extent comparable to that obtained when Dun1p itself was deleted (Fig. 4A, R294A and Δdun1, respectively). Previous studies have suggested that the C-terminus of Nej1p is required for its interaction with Lif1p and it has been hypothesized that this interaction may be required for the function of Nej1p in NHEJ [14]. Consistent with these findings, deletion of amino acid residues 243 to 323 in the Nej1p C-terminus impaired NHEJ activity to levels comparable to those caused by total loss of Nej1p (Fig. 4A, Δ243-323 and pRS415, respectively). Taken together, these data suggest that phosphorylation of Nej1p by Dun1p in response to DNA damage is required for fully functional NHEJ in S. cerevisiae, and confirms earlier reports that NHEJ is compromised in the absence of a functional DDR [33].
Figure 4. Nej1p serines 297 and 298 are required for efficient NHEJ and survival following acute phleomycin treatment.
A Plasmid repair assay. nej1::kanMX (Δnej1) cells complemented with full-length Nej1p (pNej1), empty vector (pRS415), Nej1p point mutants (S298A, S298E, S297/8A, R294A, S280G, S58G/T60G/S63G/S68G STSS), Nej1p deleted for amino acids 243-323, or dun1::HIS3 cells were transformed with 0.5 μg of pBTM116. Plasmid substrate was linearized with EcoRI and equal amounts of circular plasmid were transformed in parallel to correct for differences in transformation efficiency. Error bars indicate the mean and standard deviation of three independent experiments. B End joining of different DSB end substrates. nej1::kanMX (Δnej1) cells complemented with full-length Nej1p (pNej1), empty vector (pRS415) or Nej1p S298A point mutants were transformed with 0.5 μg pRS416 digested with either EcoRI (5′-overhang, MCS), SmaI (blunt end, MCS) or NcoI (5′-overhang, outside MCS). Equal amounts of circular plasmid were transformed in parallel to correct for differences in transformation efficiency. The NHEJ rate in cells complemented with wild-type Nej1p was set to 100% as a reference. Error bars indicate mean and standard deviation of three independent experiments. C Phleomycin survival assay. Nej11Δ::kanMX cells were complemented with pNej1 or pNej1 plasmids harbouring the amino acid substitutions Ser-298 to Ala, Ser-297/Ser-298 to Ala, Ser-298 to Glu or Ser-297/Ser-298 to Glu. Early log phase cells were grown in the presence of 5 μg/ml, 10 μg/ml or 20 μg/ml phleomycin for 4 h and plated. Surviving colonies were counted after three days. Data represent % surviving colonies after treatment compared to untreated cells.
It has been shown previously that NHEJ efficiency in S. cerevisiae is strongly governed by the availability of cohesive DNA ends [10]. To test if the repair of DSB ends with different conformations is modulated by Nej1p and/or regulated through DDR dependent phosphorylation of Nej1p, we performed plasmid repair assays by introducing DSBs with EcoRI in the multiple cloning site (MCS; producing complementary 5′-overhanging ends), SmaI (producing blunt ends) or NcoI (producing complementary 5′-overhanging ends outside the MCS). Interestingly, Nej1p mutation appeared to have an equivalent impact on each of the different types of end-configuration tested (Fig. 4B). This suggests that phosphorylation of Nej1p does not specifically enhance certain types of joining event, such as those involving non-cohesive ends, nor does it specifically mediate NHEJ through annealing microhomologies or promote NHEJ in the absence of microhomologies (outside the MCS). These findings are consistent with our observation that NHEJ in the absence of Nej1p, or when Nej1p was mutated at Ser-297/8 to Ala, was still precise, as 100% of vectors sequenced after plasmid rejoining showed accurate restoration of the EcoRI restriction site (data not shown).
While characterizing strains bearing the Nej1p mutations, we found that mutation of Ser-297 and Ser-298 to Ala increased the sensitivity of cells to acute treatment with high does of phleomycin to a level equivalent to that seen with cells lacking Nej1p (Fig. 4C). Furthermore, and consistent with a model in which regulatory phosphorylation of Nej1p enhances DSB repair in response to DNA damage, mutation of Ser-297 and Ser-298 to phospho-mimicking Glu residues slightly increased the resistance of cells to phleomycin as compared to cells expressing wild-type Nej1p (Fig 4C). However, when we treated cells chronically with lower doses of phleomycin, no significant difference in survival between cells expressing none, mutant or wild-type Nej1p was detected (data not shown). Taken together, these results suggest that phosphorylation of Nej1p through the DNA damage checkpoint kinase cascade is likely to provide a regulatory function to enhance the repair of DNA DSBs through NHEJ. However, due to the small contribution of NHEJ to the overall repair of DNA DSBs in S. cerevisiae, impairing such regulatory events only impacts directly on cellular survival when cells are severely challenged by high doses of DNA damaging agent.
Nej1p is not required for the nuclear localization of Lif1p, or for Lif1p association with chromatin in haploid yeast cells
To understand how Dun1p dependent phosphorylation of Nej1p might serve to regulate NHEJ activity, we investigated the proposed requirement of Nej1p for nuclear localization of Lif1p [12]. However, when we complemented LIF1 disrupted cells with EGFP-Lif1p we discovered that a fully functional EGFP-Lif1p fusion protein [12] was consistently localized to the nucleus, and that this localization was independent of the presence or absence of Nej1p in haploid yeast cells (Fig. 5A). In some cases EGFP-Lif1p appeared to localize to and/or accumulate in distinct subnuclear regions irrespective of DNA damage (Fig. 5A first top panel and data not shown). Intriguingly, similar patterns of localization have also been observed for XRCC4 in various mammalian cell lines (PA unpublished results) and future studies may be able to address what such patterns of subnuclear localization represent. In addition, we monitored the localization of Lif1p in the presence or absence of DNA damage but were unable to observe any change in the nuclear localization (data not shown). By using chromatin fractionation [25], we found that EGFP-Lif1p was not only localized to the nucleus but was also very tightly associated with the chromatin fraction (ISC; Fig. 5B). In addition, this chromatin association of Lif1p was independent of Nej1p and was unchanged in response to DNA damage (Fig. 5B). By contrast, Nej1p was largely found in the nucleoplasmic fraction (S) with only a small proportion of it being associated with chromatin (SC and ISC; Fig. 5B). It therefore appears that, at least in haploid yeast cells, Nej1p does not markedly affect the subcellular localization of EGFP-Lif1p and, consequently, Dun1p-dependent phosphorylation of Nej1p is unlikely to affect NHEJ by modulating Lif1p localization to the nucleus.
Figure 5. Lif1p nuclear localization and chromatin binding are not abolished in the absence of Nej1p.
A Localization of EGFP-Lif1p. nej1Δ::kanMX lif1Δ::ura3 cells (Δnej1 Δlif1) were transformed with both pNej1 and pLif1-EGFP in parallel or separately. The GFP signal was monitored by fluorescence microscopy. B Δnej1 Δlif1 cells were either transformed with pNej1 and pLif1 in parallel or separately. Cultures were grown and treated with 0.0125% of MMS as described above. As a control, whole cell extract (WCE) is shown. Cells were fractionated to generate a soluble pool that contains the combined soluble nuclear and cytoplasmic proteins (S), a soluble chromatin pool that contains weakly chromatin-bound proteins (SC), and an insoluble chromatin fraction (ISC) containing tightly chromatin-bound proteins. Nej1p was detected with an anti-Myc antibody, EGFP-Lif1p was detected with an anti-GFP antibody; tubulin as a control for the soluble fraction was detected with an anti-tubulin antibody. Orc2p as a control for the chromatin fractions, was detected with an anti-Orc2p antibody. Phosphorylation of Nej1p observed in these experiments in the absence of DNA damage was found to be due to background level activation of the Rad53p kinase cascade by oxalyticase treatment.
DISCUSSION
It is well established that Nej1p interacts with the Lif1p-Dnl4p complex in S. cerevisiae, and is not only required for NHEJ but is also responsible for the diploid-haploid regulation of NHEJ activity in yeast cells [11-14]. However, it remains thus far unclear precisely how Nej1p functions. We found that Nej1p is rapidly phosphorylated in response to DNA damage and that the DNA damage checkpoint kinase cascade mediates this phosphorylation, thus establishing Nej1p as a new target of the DDR in yeast. In addition, we have provided evidence that Dun1p phosphorylates Nej1p on Ser-297/8 and that this phosphorylation event is required for full NHEJ activity in yeast cells. In our attempts to show directly that Ser-297/8 of Nej1p is phosphorylated in vivo, we raised phospho-specific antibodies against a peptide containing phosphorylated Ser-298. However, these reagents were unable to recognize phosphorylated Nej1p in yeast whole cell extracts or in Nej1p immunoprecipitates (data not shown). In addition, we are unable to exclude the possibility that different residues in Nej1p might also be targets of phosphorylation by Dun1p or by the other checkpoint kinases. Nevertheless, our analyses clearly establish that Ser-297/8 is required for the phosphorylation-induced DNA damage dependent migration shift of Nej1p, and for efficient NHEJ as well as normal cellular resistance towards acute treatment with phleomycin.
In conjunction with previous work, our data support a model in which Nej1p acts in various ways, and in a regulated manner, to promote DSB repair by NHEJ. The primary function of Nej1p appears to be fundamental to the NHEJ process and this function most likely requires its interaction with Lif1p. Supporting this idea, we have demonstrated that in the absence of Nej1p – or when a large part of the Lif1p interacting region of Nej1p is deleted –NHEJ activity is severely curtailed. Moreover, our analyses have indicated another level by which Nej1p acts to control NHEJ, which appears to be controlled by the DNA damage checkpoint kinases Mec1p, Rad53p and Dun1p.
Consistent with previous findings demonstrating an end-joining defect of cells lacking Dun1p [33], we found that cells unable to phosphorylate Nej1p on Ser-297/8, a phosphorylation event most likely to be carried out directly by Dun1p, display a significant decrease in NHEJ activity. Notably, Bashkirov et al. [29] showed that Dun1p also mediates the phosphorylation of S. cerevisiae Rad55p, a protein that plays a key role in HR. Although Rad55p phosphorylation has not so far been shown to directly influence HR activity, we speculate that Dun1p-mediated phosphorylation of Rad55p and Nej1p might constitute a mechanism to regulate and co-ordinate the activities of both major pathways of DSB repair and thus ensure that their activities are orchestrated correctly in the context of DNA damage checkpoint signalling. Analogously to the Nej1p - Lif1p interaction, phosphorylation of Rad55p might control its interaction with Rad57p and/or Rad51p as a regulatory mechanism in response to DNA DSBs. Further studies on these interactions and on other putative DDR targets involved in DSB repair will clarify whether more components of the NHEJ and HR machineries are targets of the checkpoint kinase cascade, and what effects such DDR controlled phosphorylations might have on DSB repair events.
In diploid yeast cells, Nej1p has been reported to be involved in the nuclear localization of Lif1p, and it was proposed that Nej1p could be required for the nuclear import or the stabilization of nuclear Lif1p [12]. However, consistent with data from a previous study [11], our results indicate that both the nuclear localization as well as the chromatin association of EGFP-Lif1p do not depend on the presence of Nej1p in haploid yeast cells. Consequently, DNA damage dependent phosphorylation of Nej1p is also unlikely to affect the localization or nuclear recruitment of Lif1p. More detailed analysis of Nej1p function will be required to elucidate precisely how Nej1p is able to positively and/or negatively stimulate Dnl4p dependent end-joining events and what aspect of such functions may be regulated by phosphorylation.
It is apparent from our data that only a proportion of cellular Nej1p is phosphorylated even after extended periods of treatment with a DNA damaging agent, and that the amount of phosphorylated Nej1p appears to be less in G1 cells than in cycling cells. These findings, together with our observation that impairing Nej1p phosphorylation decreases but does not prevent NHEJ, suggest that Nej1p phosphorylation might play a particularly important role in the repair of a subset of DNA lesions. Such lesions might be more prevalent or particularly toxic at certain cell cycle stages or when cells are under additional stress. Such an hypothesis is substantiated by our finding that mutation of Ser297/8 only detectably impacts on survival when cells are challenged with high doses of DNA damaging agents. In addition, we consistently observed that most Nej1p in the cell partitions into the soluble nuclear fraction, while most of its interacting partner, Lif1p, is strongly bound to chromatin. This could not only explain why several groups were unable to immunoprecipitate Lif1p and Nej1p ([13]; PA unpublished data) but also strengthens the idea of Nej1p being a regulatory factor [15]. A number of recent studies have linked phosphorylation of DNA repair and DDR factors to the regulation of protein-protein interactions [19,34,35]. Considering that the Lif1p–Nej1p interaction is most likely mediated by a domain encompassing Nej1p amino acid residues 243 to 323 ([14], PA unpublished data) and that the Dun1p phosphorylation site (Ser-297/8) we report here lies within this region, it is tempting to speculate that Nej1p phosphorylation in response to DNA damage could facilitate or impair the Nej1p–Lif1p interaction. This might help to regulate the recruitment of the Ligase IV complex to the site of a DSB, increase the transient stability of aligned ends or improve dissociation of Dnl4p from ends that are not yet suitable for ligation. However, the phospho-epitope encompassing Ser298 is not sufficient to mediate the interaction between Lif1p and Nej1p, as we have been unable to observe an interaction between phosphorylated or unphosphorylated peptides corresponding to Nej1p amino acid residues 291 to 302 and Lif1p (data not shown). Furthermore, phosphorylation of Nej1p might also help to regulate the recruitment and/or activities of various enzymes that have been implicated in DSB processing. Interfering with such processes is expected to reduce NHEJ activity without completely preventing this mechanism of DSB repair. Such a model would therefore fit our observation that Dnl4p dependent NHEJ still occurs, albeit at lower levels, when Nej1p is not phosphorylatable.
Notably, XLF (also called Cernunnos), a recently discovered mammalian protein that associates with XRCC4 and is required for NHEJ in human cells [36,37], has been proposed to be a Nej1p orthologue [38]. Similar to our findings with Nej1p, only a fraction of XLF seems to associate with the Ligase IV complex in vivo, thus raising the possibility that it may associate with the Ligase IV complex in a regulated manner [36]. It will therefore be intriguing to study whether XLF, like Nej1p, is a target of DDR kinases in response to DNA damage and whether such posttranslational modification of XLF regulates NHEJ in mammalian cells.
Supplementary Material
Supplementary Figure 1: Immunoprecipitation with anti-Dun1 antibody specifically precipitates a Dun1p dependent kinase activity.
In vitro phosphorylation reactions using wild-type GST-Nej1p. Purified protein was incubated with Dun1p precipitates from extracts either prepared from W303a cells or cells lacking Dun1p (W303a; dun1Δ::his3) Phosphorylation of Nej1p was monitored by autoradiography.
ACKNOWLEDGEMENTS
We thank D. D’Amours, D. Durocher, N. Lowndes, S. Marcand, R. Rothstein, J. Rouse, R. Roy and P. Schär for kindly supplying strains and plasmids. We also thank all past and present members of the Jackson Laboratory, in particular J. Rouse, M. Grenon, S. Gravel and M. Stucki for helpful discussions. In addition, we are grateful to S. Gravel and A. Sartori for critical reading of the manuscript. This study was supported by Cancer Research UK and was made possible by core infrastructure funding from Cancer Research UK and the Wellco Trust.
REFERENCES
- [1].Kolodner RD, Putnam CD, Myung K. Maintenance of genome stability in Saccharomyces cerevisiae. Science. 2002;297:552–557. doi: 10.1126/science.1075277. [DOI] [PubMed] [Google Scholar]
- [2].Rouse J, Jackson SP. Interfaces between the detection, signaling, and repair of DNA damage. Science. 2002;297:547–551. doi: 10.1126/science.1074740. [DOI] [PubMed] [Google Scholar]
- [3].Sancar A, Lindsey-Boltz LA, Unsal-Kaccmaz K, Linn S. Molecular Mechanisms of Mammalian DNA Repair and the DNA Damage Checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- [4].Longhese MP, Foiani M, Muzi-Falconi M, Lucchini G, Plevani P. DNA damage checkpoint in budding yeast. EMBO Journal. 1998;17:5525–5528. doi: 10.1093/emboj/17.19.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Melo J, Toczyski D. A unified view of the DNA-damage checkpoint. Current Opinion in Cell Biology. 2002;14:237–245. doi: 10.1016/s0955-0674(02)00312-5. [DOI] [PubMed] [Google Scholar]
- [6].Carr AM. DNA structure dependent checkpoints as regulators of DNA repair. DNA Repair (Amst) 2002;1:983–994. doi: 10.1016/s1568-7864(02)00165-9. [DOI] [PubMed] [Google Scholar]
- [7].Dudasova Z. A. Dudas and M. Chovanec Non-homologous end-joining factors of Saccharomyces cerevisiae. FEMS Microbiol Rev. 2004;28:581–601. doi: 10.1016/j.femsre.2004.06.001. [DOI] [PubMed] [Google Scholar]
- [8].Herrmann G, Lindahl T, Schar P. Saccharomyces cerevisiae LIF1: a function involved in DNA double-strand break repair related to mammalian XRCC4. EMBO Journal. 1998;17:4188–4198. doi: 10.1093/emboj/17.14.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schar P, Herrmann G, Daly G, Lindahl T. A newly identified DNA ligase of Saccharomyces cerevisiae involved in RAD52-independent repair of DNA double-strand breaks. Genes and Development. 1997;11:1912–1924. doi: 10.1101/gad.11.15.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boulton SJ, Jackson SP. Saccharomyces cerevisiae Ku70 potentiates illegitimate DNA double-strand break repair and serves as a barrier to error-prone DNA repair pathways. Embo Journal. 1996;15:5093–5103. [PMC free article] [PubMed] [Google Scholar]
- [11].Kegel A, Sjostrand JOO, Astrom SU. Nej1p, a cell type-specific regulator of nonhomologous end joining in yeast. Current Biology. 2001;11:1611–1617. doi: 10.1016/s0960-9822(01)00488-2. [DOI] [PubMed] [Google Scholar]
- [12].Valencia M, Bentele M, Vaze MB, Herrmann G, Kraus E, Lee SE, Schar P, Haber JE. NEJ1 controls non-homologous end joining in Saccharomyces cerevisiae. Nature. 2001;414:666–669. doi: 10.1038/414666a. [DOI] [PubMed] [Google Scholar]
- [13].Frank-Vaillant M, Marcand S. NHEJ regulation by mating type is exercised through a novel protein, Lif2p, essential to the ligase IV pathway. Genes and Development. 2001;15:3005–3012. doi: 10.1101/gad.206801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ooi SL, Shoemaker DD, Boeke JD. A DNA microarray-based genetic screen for nonhomologous end-joining mutants in Saccharomyces cerevisiae. Science. 2001;294:2552–2556. doi: 10.1126/science.1065672. [DOI] [PubMed] [Google Scholar]
- [15].Wilson TE. A genomics-based screen for yeast mutants with an altered recombination/end-joining repair ratio. Genetics. 2002;162:677–688. doi: 10.1093/genetics/162.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liti G, Louis EJ. NEJ1 prevents NHEJ-dependent telomere fusions in yeast without telomerase. Molecular Cell. 2003;11:1373–1378. doi: 10.1016/s1097-2765(03)00177-1. [DOI] [PubMed] [Google Scholar]
- [17].Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- [18].Budman J, Chu G. Processing of DNA for nonhomologous end-joining by cell-free extract. Embo J. 2005;24:849–860. doi: 10.1038/sj.emboj.7600563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Koch CA, Agyei R, Galicia S, Metalnikov P, O’Donnell P, Starostine A, Weinfeld M, Durocher D. Xrcc4 physically links DNA end processing by polynucleotide kinase to DNA ligation by DNA ligase IV. Embo J. 2004;23:3874–3885. doi: 10.1038/sj.emboj.7600375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ma Y, Lu H, Tippin B, Goodman MF, Shimazaki N, Koiwai O, Hsieh CL, Schwarz K, Lieber MR. A Biochemically Defined System for Mammalian Nonhomologous DNA End Joining. Mol Cell. 2004;16:701–713. doi: 10.1016/j.molcel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- [21].Ma JL, Kim EM, Haber JE, Lee SE. Yeast Mre11 and Rad1 proteins define a Ku-independent mechanism to repair double-strand breaks lacking overlapping end sequences. Mol Cell Biol. 2003;23:8820–8828. doi: 10.1128/MCB.23.23.8820-8828.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Riballo E, Kuhne M, Rief N, Doherty A, Smith GC, Recio MJ, Reis C, Dahm K, Fricke A, Krempler A, Parker AR, Jackson SP, Gennery A, Jeggo PA, Lobrich M. A Pathway of Double-Strand Break Rejoining Dependent upon ATM, Artemis, and Proteins Locating to gamma-H2AX Foci. Mol Cell. 2004;16:715–724. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- [23].Frank-Vaillant M, Marcand S. Transient stability of DNA ends allows nonhomologous end joining to precede homologous recombination. Molecular Cell. 2002;10:1189–1199. doi: 10.1016/s1097-2765(02)00705-0. [DOI] [PubMed] [Google Scholar]
- [24].Grawunder U, Zimmer D, Kulesza P, Lieber MR. Requirement for an interaction of XRCC4 with DNA ligase IV for wild type V(D)J recombination and DNA double strand break repair in vivo. Journal of Biological Chemistry. 1998;273:24708–24714. doi: 10.1074/jbc.273.38.24708. [DOI] [PubMed] [Google Scholar]
- [25].Frei C, Gasser SM. The yeast Sgs1p helicase acts upstream of Rad53p in the DNA replication checkpoint and colocalizes with Rad53p in S-phase-specific foci. Genes and Development. 2000;14:81–96. [PMC free article] [PubMed] [Google Scholar]
- [26].de la Torre Ruiz M-A, Lowndes NF. DUN1 defines one branch downstream of RAD53 for transcription and DNA damage repair in Saccharomyces cerevisiae. FEBS Letters. 2000;485:205–206. doi: 10.1016/s0014-5793(00)02198-0. [DOI] [PubMed] [Google Scholar]
- [27].Zhou Z, Elledge SJ. Dun1 encodes a protein kinase that controls the DNA damage response in yeast. Cell. 1993;75:1119–1127. doi: 10.1016/0092-8674(93)90321-g. [DOI] [PubMed] [Google Scholar]
- [28].Zhao X, Rothstein R. The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor SmI1. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3746–3751. doi: 10.1073/pnas.062502299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bashkirov VI, King JS, Bashkirova EV, Schmuckli-Maurer J, Heyer W-D. DNA repair protein Rad55 is a terminal substrate of the DNA damage checkpoints. Molecular and Cellular Biology. 2000;20:4393–4404. doi: 10.1128/mcb.20.12.4393-4404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vaze MB, Pellicioli A, Lee SE, Ira G, Liberi G, Arbel-Eden A, Foiani M, Haber JE. Recovery from checkpoint-mediated arrest after repair of a double-strand break requires Srs2 helicase. Molecular Cell. 2002;10:373–385. doi: 10.1016/s1097-2765(02)00593-2. [DOI] [PubMed] [Google Scholar]
- [31].Sanchez Y, Zhou Z, Huang M, Kemp BE, Elledge SJ. Analysis of budding yeast kinases controlled by DNA damage. Methods in Enzymology. 1997;283:398–410. doi: 10.1016/s0076-6879(97)83033-9. [DOI] [PubMed] [Google Scholar]
- [32].Allen JB, Zhou Z, Siede W, Friedberg EC, Elledge SJ. The Sad1/Rad53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes and Development. 1994;8:2401–2415. doi: 10.1101/gad.8.20.2401. [DOI] [PubMed] [Google Scholar]
- [33].de la Torre-Ruiz MA, Lowndes NF. The Saccharomyces cerevisiae DNA damage checkpoint is required for efficient repair of double strand breaks by non-homologous end joining. FEBS Letters. 2000;467:311–315. doi: 10.1016/s0014-5793(00)01180-7. [DOI] [PubMed] [Google Scholar]
- [34].Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123:1213–1226. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- [35].Esashi F, Christ N, Gannon J, Liu Y, Hunt T, Jasin M, West SC. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature. 2005;434:598–604. doi: 10.1038/nature03404. [DOI] [PubMed] [Google Scholar]
- [36].Ahnesorg P, Smith P, Jackson SP. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell. 2006;124:301–313. doi: 10.1016/j.cell.2005.12.031. [DOI] [PubMed] [Google Scholar]
- [37].Buck D, Malivert L, de Chasseval R, Barraud A, Fondaneche MC, Sanal O, Plebani A, Stephan JL, Hufnagel M, le Deist F, Fischer A, Durandy A, de Villartay JP, Revy P. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell. 2006;124:287–299. doi: 10.1016/j.cell.2005.12.030. [DOI] [PubMed] [Google Scholar]
- [38].Callebaut I, Malivert L, Fischer A, Mornon JP, Revy P, de Villartay JP. Cernunnos interacts with the XRCC4/DNA-ligase IV complex and is homologous to the yeast nonhomologous end-joining factor NEJ1. J Biol Chem. 2006 doi: 10.1074/jbc.C500473200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Immunoprecipitation with anti-Dun1 antibody specifically precipitates a Dun1p dependent kinase activity.
In vitro phosphorylation reactions using wild-type GST-Nej1p. Purified protein was incubated with Dun1p precipitates from extracts either prepared from W303a cells or cells lacking Dun1p (W303a; dun1Δ::his3) Phosphorylation of Nej1p was monitored by autoradiography.