Abstract
In reflex epilepsies, alteration of gamma oscillations may mediate transition between interictal and ictal states. Here we explored a patient having seizures triggered by syrup intake. From intracranial electroencephalography combined with functional magnetic resonance imaging, the overlap of the gustatory cortex and of the preictal and ictal onset zones, as defined by early gamma changes, motivated the successful resective surgery of the middle short gyrus of the right insula. This case provides a rare demonstration from human gamma activity that the route to seizure may be supported by the interplay between physiological and epileptogenic networks.
Keywords: Brain Mapping; Brain Waves; physiology; Cerebral Cortex; physiopathology; surgery; Dominance, Cerebral; physiology; Electroencephalography; Epilepsy, Reflex; diagnosis; physiopathology; surgery; Female; Frontal Lobe; physiopathology; surgery; Hemangioma, Cavernous, Central Nervous System; surgery; Humans; Image Processing, Computer-Assisted; Magnetic Resonance Imaging; Nerve Net; physiopathology; surgery; Postoperative Complications; diagnosis; physiopathology; surgery; Young Adult
Keywords: Case Report, Eating seizures; Functional MRI; Gamma oscillations; Gustatory Cortex; Insula; Reflex Epilepsy; Stereo-Electroencephalography
I. INTRODUCTION
Fast oscillations (gamma, >30 Hz) are particularly important for brain function. They may remain focal [1], and are tightly related to the blood oxygen level dependent (BOLD) contrast measured in functional magnetic resonance imaging (fMRI) [2]. In epilepsy, high frequency oscillations are supposed to support the rapid transitions between interictal and ictal states [3]. This is specially likely for reflex seizures, i.e. triggered by a stimulus [4]. Though this hypothesis is interesting, more data are needed to demonstrate common neuroanatomical underpinnings of physiological and pathological gamma oscillations.
Here, we report the case of a woman presenting refractory partial epilepsy triggered by alimentation, and most easily by strawberry syrup intake. Multimodal exploration using stereotaxic electroencephalography (SEEG), taste fMRI and interictal simultaneous fMRI/EEG, associated to resective surgery, allowed us to characterize extensively the interactions between gustatory and epileptic networks.
II. METHODS
Experiments were approved by the ethics committee of the Grenoble University Hospital. The patient gave her written informed consent.
II.1. Case history
A woman was treated surgically at 19 yo for a right operculo-insular cavernous malformation. Nine years after surgery, refractory partial seizures occurred, which could be spontaneous but were the most often triggered by alimentation, with an unpleasant taste as initial symptom. Structural MRI was normal, except the post-operative cavity. During most recent video-EEG monitoring, the patient experienced two events of dysgeusia secondary to food intake with no EEG modification. A complete seizure was recorded during breakfast with staring, loss of consciousness, oro alimentary automatism, salivation and left hand dystonia. It was then decided to perform a SEEG with extensive sampling of the right insular, opercular and temporal cortices, and to map the gustatory cortex using fMRI.
II.2. Data acquisition
II.2.1. Stereo EEG
168 electrode contacts, with locations reported in the Montreal Neurological Institute (MNI) space, allowed video SEEG recording with a sampling rate of 512 Hz of the right insula and connected regions (Figure 1A, left).
Figure 1.
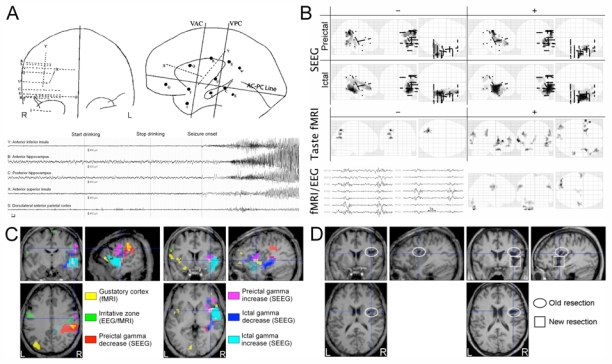
A) Left: Coronal and lateral representation of the stereotaxic implantation scheme. Right: Reflex seizure showing ictal onset in the right insula and secondary spreading in the hippocampus.
B) SEEG: Maximum intensity projections of changes (decrease: −; increase: +) in gamma power during preictal and early ictal states, as compared to baseline level (two-sample t-test, p<0.05, FWE corrected). The two seizures have been pooled together using a fixed-effect analysis. Black dots indicate electrode positions. Regions showing the strongest effects (in dark) are: dorsolateral parietal cortex (preictal −), hippocampus (ictal −), primary sensorimotor cortex (preictal +), insula (ictal +). Taste fMRI: BOLD activations and deactivations (p<0.005, uncorrected) observed during strawberry syrup intake. Note the activation of the insula bilaterally and of the right frontal dorsolateral cortex. fMRI/EEG: fMRI/EEG showed bilateral BOLD changes (p<0.05, FWE corrected) in the lower part of the primary sensorymotor cortex, which were correlated to bilateral brief paroxystic EEG activity.
C) Overlay of fMRI and SEEG results using the same statistical thresholds as in B). Left: Crosshairs on the dorsolateral fronto-parietal region. Right: Crosshairs on the anterior insula.
D) Left: Preoperative T1 MRI showing the scar of the resection of the cavernoma (ellipse). Right: Postoperative T1 MRI showing limited resection in the middle short gyrus of the insula (square).
II.2.2. Functional MRI
3T fMRI was performed a few weeks after SEEG. In the first session, simultaneous EEG/fMRI were recorded at rest during 30 minutes [5]. In the second session, the same syrup as the one that elicited seizures during SEEG was contrasted against water intake to map the gustatory cortex using 8 repetitions of 3 water injections and 3 syrup injections. Each injection of 1ml of liquid was preceded by a visual warning and occurred every 12 seconds. Six seconds after the injection, the patient was instructed to swallow.
Functional images were obtained using gradient echo planar imaging (TR=3s, voxel size: 3×3×3.5mm). A T1-weighted image was used for anatomical localisation. EEG data during rest fMRI were acquired at 1024 Hz using 17 electrodes.
II.3. Data analysis
II.3.1. Stereo EEG
SEEG power around seizure onset between 30 and 210 Hz was obtained using a Morlet wavelet transform with a time/frequency resolution of 0.5 s/1 Hz. Power was normalized for each frequency and was averaged across frequencies to obtain a single time series of gamma activity. Finally, SEEG power was linearly interpolated in space using a 4 mm isotropic kernel. Similarly to fMRI, statistical analyses were then performed in Statistical Parametric Mapping software (SPM5, Wellcome Department of Imaging Neuroscience, www.fil.ion.ucl.ac.uk/spm) on 3D images of gamma power [6].
Three periods were defined in peri-onset time: (i) baseline consisted of the 20s before the first beverage intake; (ii) preictal period was defined in between the last beverage intake and the seizure onset; (iii) ictal period consisted of the first 10 s of the seizure. Statistical inference between the different states was performed using two-sample t-tests between baseline and ictal period, and between baseline and preictal period.
II.3.2. Functional MRI
FMRI preprocessing (motion and drift correction, MNI normalisation, spatial smoothing) was performed using SPM5.
Activation maps of taste were obtained using the standard procedure, in which the stimulus function is convolved with a canonical hemodynamic response and used as a regressor to construct maps of the associated t-statistics.
FMRI/EEG activations were obtained using an approach sensitive to putative hemodynamic variability of epileptic networks [5], in which EEG power in the frequency band of observed interictal epileptic discharges (i.e. between 2 and 8 Hz) was estimated after artefact correction [7] and used as a regressor. A standard EEG referential montage was used, with the reference (G2) taken at the vertex.
III. RESULTS
III.1. Electroclinical characterisation
Numerous interictal spikes were recorded mainly in the anterior hippocampus (irritative zone). More infrequent asynchronous spikes were also detected in the anterior insula.
Two reflex seizures to strawberry syrup intake were recorded, which started with loss of consciousness, followed by facial flushing, oro-alimentary automatisms, repeated swallowing and sialorhea, and ended with postictal confusion and amnesia. From visual analysis of SEEG (Figure 1A, right), seizures began in the anterior inferior portion of the insula with a high amplitude spike followed by a low voltage high frequency discharge with secondary spreading to the hippocampus and then to the temporal neocortex. The spatio-temporal evolution of the discharges was thus in agreement with the clinical findings that suggested an ictal onset in the insula (gustatory aura) with secondary spread to the hippocampus (temporal lobe semiology).
III.2. Statistical mapping
Figure 1B shows statistical analyses of brain responses.
In SEEG (p<0.05, FWE corrected), early ictal activity was characterised by an increase of gamma power in the insular anterior inferior portion, which was concomitant with a hippocampal power decrease. Preictal activity showed a power increase in the mouth primary sensorimotor area and in the anterior superior portion of the insula, and a more widespread power decrease in the post-central gyrus.
During fMRI acquisition, EEG was continuously monitored and allowed us to verify that no seizure occurred. The patient did not report any aura as well. FMRI mapping of the gustatory cortex showed significant (p<0.005, uncorrected) activations primarily in both insulas, but also in the right dorsolateral frontal cortex and in the right dorsolateral parietal cortex. Bilateral EEG abnormalities detected during rest fMRI/EEG were correlated with bilateral BOLD changes (p<0.05, FWE corrected) in the mouth area of the primary sensorimotor cortex.
III.3. Summary and surgery
Three main findings emerged (Figure 1C):
The dorsolateral anterior parietal cortex showed preictal gamma power decrease (red) and belonged to the fMRI gustatory network (yellow). In the vicinity, the dorsolateral posterior frontal cortex showed preictal gamma power increase (magenta) and interictal fMRI/EEG activations (green).
Early ictal gamma power increase (cyan) showed up in the inferior/middle part of the anterior insula, which also responded to taste fMRI (yellow). A preictal increase in gamma power (magenta) was observed in the upper part of the anterior insula.
Hippocampus showed early ictal decrease of gamma activity (blue).
We thus identified interconnections between the gustatory cortex and an insulo-hippocampal epileptogenic circuit. This motivated the resective surgery of the middle short gyrus of the insula (Figure 1D). The patient is now seizure free with a follow-up of 2 years.
IV. DISCUSSION
This rare case of eating reflex seizure was an opportunity to investigate, using neuroimaging techniques, the putative interactions between physiological and pathological gamma oscillations at seizure onset. We identified overlaps between the gustatory cortex, involving insular, parietal and frontal regions [8], and a fronto-insulo-hippocampal epileptogenic network. Resective surgery of a small part of the insula rendered the patient seizure-free. Such focal resection disrupted local neural circuitry that may have supported pathological gamma activity, the emergence of which was facilitated by functional (taste) gamma activity. To our knowledge, this is the first case of intracerebral recordings of eating seizures arising in the insula.
FMRI co-localization of functional and epileptic networks was also recently reported in musicogenic [9] and reading epilepsy [10]. Other studies focused on the correspondence between BOLD and gamma responses during sensory stimulation or cognitive tasks [2, 11, 12]. Here, using a new representation of SEEG data as 3D images, we found striking overlap between gamma SEEG and fMRI. Though a positive BOLD activation is often found in regions showing an increase of gamma activity [2, 12, 13], the opposite finding has also been reported [14]. Here, using non simultaneous measures, we found both possibilities regarding preictal changes that were assumed to occur within physiological networks: a positive correlation of the sign of BOLD and gamma activity in the prefrontal cortex and a negative correlation in parietal regions.
We also analysed SEEG frequencies below 30Hz. They showed more diffused and less interpretable statistical maps regarding fMRI results and patient’s semiology (results not shown). This speaks for a highly segregated functional role of gamma oscillations [1]. This clinical case is thus a rare illustration in human that gamma activity may be crucial for brain function and dysfunction. Conceptually, two overlapping neuronal networks, physiological and epileptogenic, may have supported the route to reflex seizures. Whether this is a common rule remains a crucial issue.
Acknowledgments
This study was funded by the Inserm.
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
None of the authors has any conflict of interest to disclose.
COPYRIGHT LICENCE STATEMENT
I Olivier David The Corresponding Author of this article (the Contribution”) has the right to grant on behalf of all authors and does grant on behalf of all authors, a licence to the BMJ Publishing Group Ltd and its licensees, to permit this Contribution (if accepted) to be published in Journal of Neurology, Neurosurgery and Psychiatry (JNNP) and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence set out at: (http://jnnp.bmj.com/site/about/licence.pdf)
References
- 1.Jerbi K, Ossandon T, Hamame CM, Senova S, Dalal SS, Jung J, et al. Task-related gamma-band dynamics from an intracerebral perspective: review and implications for surface EEG and MEG. Hum Brain Mapp. 2009 Jun;30(6):1758–71. doi: 10.1002/hbm.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, et al. Relationship between task-related gamma oscillations and BOLD signal: New insights from combined fMRI and intracranial EEG. HumBrain Mapp. 2007 doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129(Pt 6):1593–608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- 4.Zifkin B, Guerrini R, Plouin P. Reflex seizures. Lippincott Williams and Wilkins; 2008. [Google Scholar]
- 5.Grouiller F, Vercueil L, Krainik A, Segebarth C, Kahane P, David O. Characterisation of the hemodynamic modes associated with interictal epileptic activity using a deformable model-based analysis of combined EEG and functional MRI recordings. Human Brain Mapp. doi: 10.1002/hbm.20925. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiebel SJ, Tallon-Baudry C, Friston KJ. Parametric analysis of oscillatory activity as measured with EEG/MEG. HumBrain Mapp. 2005;26(3):170–7. doi: 10.1002/hbm.20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grouiller F, Vercueil L, Krainik A, Segebarth C, Kahane P, David O. A comparative study of different artefact removal algorithms for EEG signals acquired during functional MRI. Neuroimage. 2007;38(1):124–37. doi: 10.1016/j.neuroimage.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, et al. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999 Jan 18;10(1):7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- 9.Marrosu F, Barberini L, Puligheddu M, Bortolato M, Mascia M, Tuveri A, et al. Combined EEG/fMRI recording in musicogenic epilepsy. Epilepsy Res. 2009 Mar;84(1):77–81. doi: 10.1016/j.eplepsyres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Salek-Haddadi A, Mayer T, Hamandi K, Symms M, Josephs O, Fluegel D, et al. Imaging seizure activity: a combined EEG/EMG-fMRI study in reading epilepsy. Epilepsia. 2009 Feb;50(2):256–64. doi: 10.1111/j.1528-1167.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 11.Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007 Aug 7;17(15):1275–85. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 12.Zaehle T, Frund I, Schadow J, Tharig S, Schoenfeld MA, Herrmann CS. Inter- and intra-individual covariations of hemodynamic and oscillatory gamma responses in the human cortex. Front Hum Neurosci. 2009;3:8. doi: 10.3389/neuro.09.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilner JM, Mattout J, Henson R, Friston KJ. Hemodynamic correlates of EEG: a heuristic. Neuroimage. 2005 Oct 15;28(1):280–6. doi: 10.1016/j.neuroimage.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Muthukumaraswamy SD, Singh KD. Functional decoupling of BOLD and gamma-band amplitudes in human primary visual cortex. Hum Brain Mapp. 2009 Jul;30(7):2000–7. doi: 10.1002/hbm.20644. [DOI] [PMC free article] [PubMed] [Google Scholar]


