Abstract
The aim of the present study was to assess quercetin’s mechanism of action in rat pial microvessels during transient bilateral common carotid artery occlusion (BCCAO) and reperfusion. Rat pial microcirculation was visualized using fluorescence microscopy through a closed cranial window. Pial arterioles were classified in five orders of branchings. In ischemic rats, 30 min BCCAO and 60 min reperfusion caused arteriolar diameter decrease, microvascular leakage, leukocyte adhesion in venules, and reduction of capillary perfusion. Quercetin highest dose determined dilation in all arteriolar orders, by 40 ± 4% of baseline in order 2 vessels, and prevented microvascular permeability [0.15 ± 0.02 normalized gray levels (NGL)], leukocyte adhesion, and capillary failure. Protein kinase C (PKC) inhibition exerted by chelerythrine prior to quercetin attenuated quercetin-induced effects: order 2 arterioles dilated by 19.0 ± 2.4% baseline, while there was an increase in permeability (0.40 ± 0.05 NGL) and leukocyte adhesion with a marked decrease in capillary perfusion. Tyrosine kinase (TK) inhibition by tyrphostin 47 prior to quercetin lessened smaller pial arterioles responses, dilating by 20.7 ± 2.5% of baseline, while leakage increased (0.39 ± 0.04 NGL) sustained by slight leukocyte adhesion and ameliorated capillary perfusion. Inhibition of endothelium nitric oxide synthase (eNOS) by NG-nitro-L-arginine-methyl ester (L-NAME) prior to PKC or TK reduced the quercetin’s effects on pial arteriolar diameter and leakage. eNOS inhibition by L-NAME reduced quercetin effects on pial arteriolar diameter and leakage. Finally, combined inhibition of PKC and TK prior to quercetin abolished quercetin-induced effects, decreasing eNOS expression, while blocking ATP-sensitive potassium (KATP) channels by glibenclamide suppressed arteriolar dilation. In conclusion, the protective effects of quercetin could be due to different mechanisms resulting in NO release throughout PKC and TK intracellular signaling pathway activation.
Keywords: bilateral common carotid artery occlusion, reperfusion, pial microcirculation, quercetin, nitric oxide, protein kinase C, tyrosine kinase
Introduction
The cerebral transient hypoperfusion induced in rats by bilateral common carotid artery occlusion (BCCAO) and followed by reperfusion has been shown to induce a decrease in pial arteriolar diameter, disruption of the blood–brain barrier, leukocyte adhesion, and a reduction in capillary perfusion. These microvascular responses were significantly affected by quercetin, a natural flavonoid, effective in preventing arteriolar diameter decrease and macromolecular leakage, in reducing leukocyte adhesion, and facilitating capillary perfusion (Lapi et al., 2012). These protective quercetin effects have been suggested to be related to nitric oxide release, throughout endothelium nitric oxide synthase (eNOS) activation to increase as expression and activity detected by Western blotting (Lapi et al., 2012). Conversely, nitric oxide (NO) increase was not related to activation of neuronal nor inducible NOS. Moreover, the protection exerted by quercetin, especially on leukocyte adhesion, has been correlated to decrease in formation of reactive oxygen species (ROS), as reported in the joined paper (Huk et al., 1998; Rice-Evans, 2001; Lapi et al., 2012). However, several studies have suggested that quercetin can modulate vascular tone by protein kinase C (PKC) or cAMP- or cGMP-phosphodiesterase or tyrosine kinase (TK) intracellular signaling pathways (Picq et al., 1989; Cogolludo et al., 2007; Negash et al., 2007; Chiwororo and Ojewole, 2010). To date, quercetin action mechanism remains partially unexplained.
According to our results in the rat pial microcirculation, we hypothesized pial arterioles dilation, prevention of leakage, and preservation of capillary perfusion during BCCAO and reperfusion may be due to activation of PKC or/and TK intracellular signaling pathway, with consequent increase in NO production and release. In several experimental models of preconditioning in different organs the protection is exerted through activation of PKC and TK signaling pathways (Fryer et al., 1999; Pagliaro et al., 2001; Sakamoto et al., 2005). The present study was aimed to investigate the quercetin’s mechanism of action in rat pial microcirculation during BCCAO and reperfusion to gain new insight in prevention of ischemic injury.
We assessed the role of PKC and TK intracellular signaling pathways in the protection exerted by quercetin. We inhibited, indeed, PKC by chelerythrine or/and TK by tyrphostin 47; moreover, either inhibition was coupled to eNOS blockade by NG-nitro-l-arginine-methyl ester (l-NAME) to clarify the quercetin’s mechanism of action. Furthermore, potassium channels have been suggested to be activated during ischemia reperfusion injury and preconditioning in experimental models through PKC and TK signaling pathway stimulation (Pagliaro et al., 2001). Therefore, we assessed the role of potassium channels in the dilation induced by quercetin, blocking ATP-sensitive potassium (KATP) channels by glibenclamide. Finally, we investigated the effects of combined PKC and TK inhibition on eNOS expression.
We used in vivo fluorescence technique to visualize rat pial microcirculation to determine changes in pial arteriole diameter, permeability increase, leukocyte adhesion, and capillary perfusion, as previously reported (Lapi et al., 2012). These data might be important to clarify the mechanisms effective in brain damage during hypoperfusion and reperfusion and to improve strategies against brain injury.
Materials and Methods
Experimental groups
Male Wistar rats weighing 250–300 g (Harlan, Italy) were randomly assigned to eight group: (1) The first group was composed by the animals not subjected to BCCAO and reperfusion [Sham-Operated (S) group, n = 14], as indicated in the previous paper (Lapi et al., 2012). Moreover, sham-operated rats received intravenously (i.v.) chelerythrine, 3.0 mg/Kg b.w. (group SC, n = 5) or i.v. tyrphostin 47, 2.2 mg/Kg b.w. (group ST, n = 5), or i.v. glibenclamide, 1.0 mg/100 g b.w. (group SG, n = 5). (2) Ischemic rats (group I, n = 20) were treated with 1.5 ml vehicle (physiological saline solution), i.v. injected, and subjected to 30 min of BCCAO and 60 min of reperfusion, as reported in the previous manuscript (Lapi et al., 2012). (3) The third group (Q3 group, n = 14) was treated with i.v. quercetin 5.0 mg/Kg b.w. (4) The fourth group of rats (CQ group, n = 9) received i.v. chelerythrine (3.0 mg/Kg b.w.) prior to i.v. quercetin (5.0 mg/Kg b.w.). (5) The fifth group of animals (group TQ, n = 9) was subjected to i.v. treatment with tyrphostin 47 (2.2 mg/Kg b.w.) before quercetin (5.0 mg/Kg b.w.). (6) The sixth group of rats (LCQ group, n = 9) was treated with i.v. l-NAME (10.0 mg/Kg b.w.) before i.v. chelerythrine (3.0 mg/Kg b.w.) and i.v. quercetin (5.0 mg/Kg b.w.) with an interval of 10 min among each drug administration. (7) The seventh group of rats (group LTQ, n = 9) was treated with i.v. l-NAME (10.0 mg/Kg b.w.) prior to i.v. tyrphostin 47 (2.2 mg/Kg b.w.) and i.v. quercetin (5.0 mg/Kg b.w.) with an interval of 10 min after each drug administration. (8) The eighth group of animals (CTQ group, n = 14) was treated with i.v. chelerythrine (3.0 mg/Kg b.w.) prior to i.v. tyrphostin 47 (2.2 mg/Kg b.w.) and i.v. quercetin (5.0 mg/Kg b.w.) with an interval of 10 min after each drug administration. Nine animals were utilized for microvascular studies, five to evaluate the eNOS expression by Western blotting. (9) The ninth group of rats (group GQ, n = 9) was treated with i.v. glibenclamide (1.0 mg/100 g b.w.) prior to quercetin (5.0 mg/Kg b.w.). (10) Finally, the last group of rats of rats received chelerythrine (3.0 mg/Kg b.w.) or tyrphostin 47 (2.2 mg/Kg b.w.) or glibenclamide (1.0 mg/100 g b.w.; group C, T, and G, n = 9, respectively), i.v. injected 10 min before BCCAO and at the beginning of reperfusion.
Drugs administration
Quercetin solutions were obtained dissolving 5.0 mg/Kg b.w. in 0.5 ml of saline solution and i.v. infused (3 min) to the rats 10 min before BCCAO and at the beginning of reperfusion (Cho et al., 2006).
Chelerythrine (3.0 mg/Kg b.w.; Colantuoni et al., 2005), tyrphostin 47 (2.2 mg/Kg b.w.), glibenclamide (1.0 mg/100 Kg b.w.), and l-NAME (10.0 mg/Kg b.w.; Xu et al., 2002; Lapi et al., 2008), were dissolved in 0.5 ml of saline solution, respectively. Each substance was i.v. administered 10 min prior to quercetin (5.0 mg/Kg b.w.) before BCCAO and at the beginning of reperfusion (Lapi et al., 2012). The drugs were purchased by Sigma Chemical, St. Louis, MO, USA.
In previous experiments i.v. infusion of l-NAME at the dosage of 10 mg/Kg b.w., chosen for the present study, abolished vasodilation due to topical application of acetylcholine, 100 μM (n = 10), while the diameter increase was by 42.3 ± 3.2% of baseline in sham-operated animals treated with acetylcholine, n = 10 (Oriji, 1999).
Animal preparation
Anesthesia was induced with α-chloralose (50 mg/Kg b.w., i.p.) plus urethane (600 mg/Kg b.w., i.p.) and maintained with urethane alone (100 mg/Kg b.w., i.v. every hour). Rats were tracheotomized, paralyzed with tubocurarine chloride (1 mg/Kg·h, i.v.) and mechanically ventilated with room air and supplemental oxygen, as previously reported (Lapi et al., 2012). Briefly, the right and left common carotid arteries were isolated for successive clamping. A catheter was placed in the left femoral artery, to measure arterial blood pressure, and in the right femoral vein for fluorescent tracers injection [fluorescein isothiocyanate bound to dextran, molecular weight 70 KDa (FD 70), 50 mg/100 g b.w., i.v. as 5% wt/vol solution, and rhodamine 6G, 1 mg/100 g b.w. in 0.3 ml, as a bolus with supplemental injection throughout BCCAO and reperfusion (final volume 0.3 ml·100 g−1·h−1) to label leukocytes for adhesion evaluation] and of drugs. Blood gas measurements were carried out on arterial blood samples (ABL5; Radiometer, Copenhagen, Denmark). Throughout all experiments, mean arterial blood pressure, heart rate, respiratory CO2, and blood gases values were recorded and stable settled within physiological ranges. Rectal temperature was monitored and preserved at 37.0 ± 0.5°C, as previously reported (Lapi et al., 2012).
To observe the pial microcirculation, a closed cranial window (4 mm × 5 mm) was implanted above the left frontoparietal cortex (posterior 1.5 mm to bregma; lateral, 3 mm to the midline; Chau et al., 2002). The dura mater was gently removed and a 150-μm-thick quarz microscope coverglass was sealed to the bone with dental cement. The brain parenchyma was continuously superfused with artificial cerebrospinal fluid (aCSF; Ngai et al., 1988; Moreau et al., 1995). The rate of superfusion was 0.5 ml/min controlled by a peristaltic pump. The composition of the aCSF was: 119.0 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4·7H2O, 1.0 mM NaH2PO4, 26.2 mM NaHCO3, 2.5 mM CaCl2, and 11.0 mM glucose (equilibrated with 10.0% O2, 6.0% CO2, and 84.0% N2; pH 7.38 ± 0.02). The temperature was settled at 37.0 ± 0.5°C.
Cerebral blood flow reduction was produced by placement of two atraumatic microvascular clips for 30 min on common carotid arteries, previously isolated. After removing the clamp, the pial microcirculation was observed for 60 min (reperfusion period).
All experiments conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and to institutional rules for the care and handling of experimental animals. The protocol was approved by the “Federico II” University of Naples Ethical Committee.
Intravital microscopy and microvascular parameter evaluation
Observations of pial vessels were conducted by a fluorescence microscope (Leitz Orthoplan), as previously described (Lapi et al., 2012). Epiillumination was provided by a 100-W mercury lamp using the appropriate filters for FITC, for rhodamine 6G, and a heat filter (Leitz KG1). The pial microcirculation was televised with a DAGE MTI 300RC low-light level digital camera and recorded by a computer based frame grabber (Pinnacle DC 10 plus, Avid Technology, MA, USA).
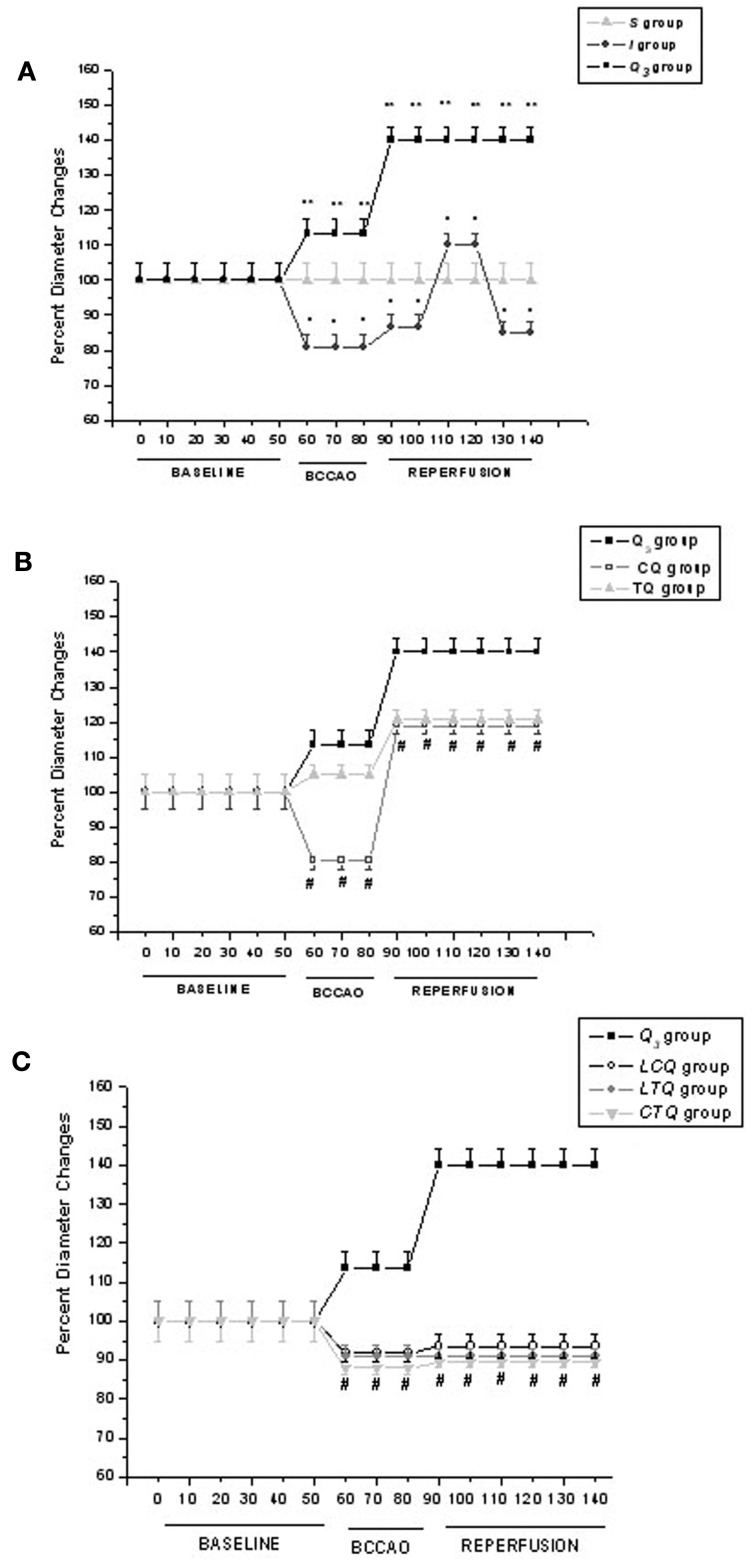
Microvascular measurements were made off-line using a computer-assisted imaging software system (MIP Image, CNR Institute of Clinical Physiology, Pisa, Italy). We reported data under baseline conditions, at the end of BCCAO and at the end of reperfusion (RE). In Figure 1 we showed the time-dependent changes in arteriolar diameters to clarify the time-dependent pattern of order 2 arteriole response.
Figure 1.
Time course plots of diameter changes in the experimental groups. (A) Diameter changes of order 2 arterioles, expressed as percent of baseline, under baseline conditions and during BCCAO and reperfusion in sham-operated group (S), in ischemic group (I) and in quercetin (5.0 mg/Kg b.w.) treated group (Q3); (B) Diameter changes of order 2 arterioles, expressed as percent of baseline, under baseline conditions and during BCCAO and reperfusion in quercetin (5.0 mg/Kg b.w.) treated group (Q3), in chelerythrine + quercetin (5.0 mg/Kg b.w.) treated group (CQ) and in tyrphostin 47 + quercetin (5.0 mg/Kg b.w.) treated group (TQ); (C) Diameter changes of order 2 arterioles, expressed as percent of baseline, under baseline conditions, and during BCCAO and reperfusion in quercetin (5.0 mg/Kg b.w.) treated group (Q3), in L-NAME + chelerythrine + quercetin (5.0 mg/Kg b.w.) treated group (LCQ), in LTQ = L-NAME + tyrphostin 47 + quercetin (5.0 mg/Kg b.w.) group and chelerythrine + tyrphostin 47 + quercetin (5.0 mg/Kg b.w.) treated group (CTQ). °p < 0.01 vs. baseline; *p < 0.01 vs. I group; #p < 0.01 vs. Q3 group.
In each animal one order 4 arteriole, two order 3, and two order 2 arterioles were studied during each experiment. We chose to present only the data regarding order 2 vessels, the most responsive arterioles, as previously reported (Lapi et al., 2012).
Arteriolar diameters were measured with a computer-assisted method (MIP Image, CNR, frame by frame). The results of diameter measurements were comparable with those obtained by shearing method (±0.5 μm). To avoid bias due to single operator measurements, two independent “blinded” operators measured the vessel diameters. Their measurements overlapped in all cases.
The increase in permeability was calculated and reported as normalized gray levels (NGL): NGL = (I − Ir)/Ir, where Ir is the average baseline gray level at the end of vessel filling with fluorescence (average of five windows located outside the blood vessels with the same windows being used throughout the experimental procedure), and I is the same parameter at the end of BCCAO or RE.
Adherent leukocytes (i.e., cells on vessel walls that did not move over a 30-s observation period) were quantified in terms of number/100 μm of venular length (v.l.)/30 s using higher magnification (32×, microscope objectives). In each experimental group 45 venules were studied.
Perfused capillary length (PCL) was measured by MIP Image in an area of 150 μm × 150 μm. In this system the length of perfused capillaries is easily established by the automated process because it is outlined by dextran (Xu et al., 2002).
Moreover, for each experimental group 27 pial venules were studied to evaluate the single pial venule blood flow, Q, calculated according to the following equation: Q = α × VCL × A, where α was a constant related to the vessel diameter, VCL was the red blood cell centerline velocity and A was the cross-section area.
Mean arterial blood pressure (MABP; Viggo-Spectramed P10E2 transducer – Oxnard, CA, USA – connected to a catheter in the femoral artery) and heart rate were monitored with a Gould Windograf recorder (model 13-6615-10S, Gould, OH, USA). Data were recorded and stored in a computer. Blood gas measurements were carried out on arterial blood samples withdrawn from arterial catheter at 30 min time period intervals (ABL5; Radiometer, Copenhagen, Denmark). The hematocrit was measured under baseline conditions, at the end of BCCAO and at RE.
Western blot analysis
Protein concentrations were determined by the Bio-Rad protein assay (Bio-Rad). To detect the proteins of interest, specific antibodies: anti-eNOS (mouse monoclonal antibody, 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phosphorylated eNOS [Rabbit (polyclonal) Anti-eNOS (pS116) phosphospecific antibody unconjugated, Invitrogen] and anti-β-actin (mouse monoclonal, 1:1000, Sigma) were used. Immunoreaction was revealed using anti-mouse IgG conjugated to peroxidase, 1:2000 (GE Healthcare) by the ECL reagent (GE Healthcare). The optical density of the bands was determined by Chemi Doc Imaging System (Bio-Rad) and normalized to the optical density of β-actin. eNOS and phosphorylated eNOS were evaluated to define concentrations of the expressed and active proteins, respectively.
Statistical analysis
All reported values are means ± SD. Data were tested for normal distribution with the Kolmogorov–Smirnov test. Parametric (Student’s t-tests, Anova, and Bonferroni post hoc test) or non-parametric tests (Wilcoxon, Mann–Whitney, and Kruskal–Wallis tests) were used; non-parametric tests were applied to compare diameter and length data among experimental groups. The statistical analysis was carried out by SPSS 14.0 statistical package. Statistical significance was set at p < 0.05.
Results
Pial arterioles were classified in five orders according to diameter, length, and branching as previously reported (Lapi et al., 2012).
Sham-operated animals
In sham-operated animals there were neither changes in arteriolar diameter nor increase in leakage (NGL: 0.02 ± 0.01) nor adhesion of leukocytes (1.0 ± 0.5/100 μm v.l./30 s) and perfused capillary length was 100 ± 4% of baseline during the same period of observation as in the other experimental groups.
The animals treated with PKC inhibitor chelerythrine (3.0 mg/Kg b.w.) or TK inhibitor tyrphostin 47 (2.2 mg/Kg b.w.) did not show significant changes in all parameters when compared with baseline (Table 1).
Table 1.
Drug treatment (T): doses, route, and time of administration in the different experimental groups.
| Group | H/R plus T | NO H/R plus T | Time | N |
|---|---|---|---|---|
| I | Saline solution 1.5 ml i.v. | Prior to BCCAO, at Reperfusion beginning | 20 | |
| Q3 | Quercetin 5.0 mg/Kg b.w. i.v. | Prior to BCCAO, at R beginning | 9 | |
| CQ | Chelerythrine 3.0 mg/Kg b.w. i.v. | Prior to quercetin | 9 | |
| Quercetin 5.0 mg/Kg b.w. i.v | Prior to BCCAO, at R beginning | |||
| TQ | Tyrphostin 47 2.2 mg/Kg b.w. i.v. | Prior to quercetin | 9 | |
| Quercetin 5.0 mg/Kg b.w. i.v | Prior to BCCAO, at R beginning | |||
| LCQ | l-NAME 10.0 mg/Kg b.w. i.v. | Prior to quercetin | 9 | |
| Chelerythrine 3.0 mg/Kg b.w. i.v. | Prior to quercetin | |||
| Quercetin 5.0 mg/Kg b.w. i.v | Prior to BCCAO, at R beginning | |||
| LTQ | l-NAME 11.0 mg/Kg b.w. i.v. | Prior to quercetin | 9 | |
| Tyrphostin 47 2.2 mg/Kg b.w. i.v. | Prior to quercetin | |||
| Quercetin 5.0 mg/Kg b.w. i.v | Prior to BCCAO, at R beginning | |||
| CTQ | Chelerythrine 3.0 mg/Kg b.w. i.v. | Prior to quercetin | 9 | |
| Tyrphostin 47 2.2 mg/Kg b.w. i.v. | Prior to quercetin | |||
| Quercetin 5.0 mg/Kg b.w. i.v | Prior to BCCAO, at R beginning | |||
| GQ | Glibenclamide 1.0 mg/100 g b.w. i.v. | Prior to quercetin | 9 | |
| Quercetin 5.0 mg/Kg b.w. i.v | Prior to BCCAO, at R beginning | |||
| C | Chelerythrine 3.0 mg/Kg b.w. i.v | Prior to BCCAO, at R beginning | 9 | |
| T | Tyrphostin 47 2.2 mg/Kg b.w. i.v. | Prior to BCCAO, at R beginning | 9 | |
| G | Glibenclamide 1.0 mg/100 g b.w. i.v. | Prior to BCCAO, at R beginning | 9 | |
| S | Saline solution 1.5 ml i.v. | Twice at 50 min interval | 13 | |
| SC | Chelerythrine 3.0 mg/Kg b.w. i.v. | Twice at 50 min interval | 5 | |
| ST | Tyrphostin 47 2.2 mg/Kg b.w. i.v. | Twice at 50 min interval | 5 |
H/R: animals subjected to hypoperfusion and reperfusion and NO H/R: animals not subjected to hypoperfusion and reperfusion; N, number of rats utilized.
Pilot studies showed i.v. administration of chelerythrine (3.0 mg/Kg b.w.) abolished the arteriolar responses due to topical application of Phorbol-12,13-dibutyrate (PDBu), a PKC activator, at the dosage 100 μM (n = 7; Hudetz et al., 1995). Under control conditions, PDBu was effective in inducing transient arteriolar dilation by 12.0 ± 2.0% of baseline in sham-operated animals within 10 ± 1 min of administration, followed by a significant decrease in arteriolar diameter by 10.0 ± 1.5% of baseline within 15 ± 1 min (n = 7).
Tyrphostin 47 (2.2 mg/Kg b.w.) infusion completely suppressed order 2 arterioles dilation due to topical application of vascular endothelial growth factor (VEGF), a TK activator at the dosage 0.1 nM (n = 7; Morii et al., 1986). The diameter increased by 16.0 ± 2.5% of baseline in sham-operated animals treated with VEGF, n = 7. MABP did not undergo with any significant variation.
BCCAO-reperfusion
At the end of BCCAO, ischemic animals (group I) showed a decrease in diameter of order 2 vessels by 19.0 ± 3.0% of baseline (Figure 1A), with increased permeability (NGL: 0.21 ± 0.03, p < 0.01 compared with S group) as previously reported (Lapi et al., 2012).
At the end of reperfusion, order 2 arterioles constricted by 14.5 ± 3.3% of baseline (Figure 1A), venular permeability significantly increased (NGL: 0.47 ± 0.04, p < 0.01 vs. S group), with marked leukocyte adhesion (9.0 ± 2.0/100 μm v.l./30 s; p < 0.01 vs. S group) in venules and decreased capillary perfusion (PCL; 60 ± 7% of baseline, p < 0.01 vs. S group; Tables 1 and 2). In single pial venules (SPV, diameter 30–40 μm) the blood flow was reduced by 30 ± 1% of baseline (p < 0.01 vs. baseline: 247 ± 10 nl/s).
Table 2.
Variations of the main parameters in sham-operated (S) group, ischemic (I) group, quercetin (Q3) group (5 mg/Kg b.w.), chelerythrine plus quercetin (CQ) group, tyrphostin 47 plus quercetin (TQ) group, l-NAME and chelerythrine plus quercetin (LCQ) group, l-NAME and tyrphostin 47 plus quercetin (LTQ) group, chelerythrine and tyrphostin 47 plus quercetin (CTQ) group, glibenclamide plus quercetin (GQ) group at the end of reperfusion.
| Group | Arteriolar diameter (%) | Microvascular permeability (NGL) | Leukocyte adhesion (number of leukocytes/100 μm of venular length/30 s) | Capillary perfusion (%) |
|---|---|---|---|---|
| S | 100.0 ± 5.0 | 0.02 ± 0.01 | 1.0 ± 0.5 | 100 ± 4 |
| I | 85.5 ± 3.3° | 0.47 ± 0.04° | 9.0 ± 2.0° | 60 ± 7° |
| Q3 | 140.0 ± 4.0°* | 0.15 ± 0.02°* | 3.0 ± 1.0°* | 90 ± 4°* |
| CQ | 119.0 ± 2.4°*# | 0.40 ± 0.05°# | 7.0 ± 1.0°# | 62 ± 7°# |
| TQ | 120.7 ± 2.5°*# | 0.39 ± 0.04°# | 4.0 ± 1.0°*# | 81 ± 9°* |
| LCQ | 93.5 ± 3.0*# | 0.42 ± 0.03°# | 7.0 ± 1.0°# | 63 ± 5°# |
| LTQ | 91.0 ± 2.0°*# | 0.37 ± 0.03°*# | 5.0 ± 1.0°* | 80 ± 6°*# |
| CTQ | 89.5 ± 1.0°*# | 0.42 ± 0.03°# | 8.0 ± 2.0°# | 60 ± 4°# |
| GQ | 99.0 ± 4.0 *# | 0.22 ± 0.03°*# | 3.5 ± 1.0°* | 85 ± 4°* |
Arteriolar diameter and capillary perfusion are reported as percent changes of 100% baseline values. NGL, normalized gray levels. °p < 0.01 vs. S group, *p < 0.01 vs. I group, #p < 0.01 vs. Q3 group.
Quercetin
At the end of BCCAO quercetin highest dose caused (Q3 group) an increase in order 2 arteriole diameter by 13.4 ± 3.4% of baseline (p < 0.01 vs. ischemic animals; Figure 1A), while preventing microvascular permeability (NGL: 0.08 ± 0.02, p < 0.01 vs. S and I groups).
At RE order 2 arterioles dilated by 40 ± 4% of baseline (p < 0.01 vs. ischemic animals; Figure 1A), while leakage of FD70 was prevented (NGL: 0.15 ± 0.02, p < 0.01 vs. S and I groups) as well as leukocyte adhesion (3.0 ± 1.0/100 μm v.l./30 s; p < 0.01 vs. S and I groups). Capillary perfusion was protected (PCL: 90 ± 4% of baseline, p < 0.01 vs. S and I groups; Tables 1 and 2). SPV blood flow was significantly increased by 50.0 ± 0.9% of baseline: 235 ± 12 nl/s, p < 0.01 vs. baseline and I group.
PKC inhibition plus quercetin
Chelerythrine, PKC inhibitor, administered before quercetin (group CQ), blunted quercetin-induced effects after BCCAO, because there was reduction in diameter of all pial arterioles as observed in ischemic animals at the end of BCCAO (Figure 1B), while MABP did not significantly change (Table 3). Leakage significantly increased (NGL: 0.19 ± 0.02) compared to the animals treated with 5.0 mg/Kg b.w. of quercetin (p < 0.01 vs. Q3 group, p = NS vs. I group).
Table 3.
Mean arterial blood pressure (MABP) under baseline conditions, at the end of bilateral common carotid artery occlusion (BCCAO) and at the end of reperfusion (RE) in sham-operated (S) group, ischemic (I) group, quercetin (Q3) group (5 mg/Kg b.w.), chelerythrine plus quercetin (CQ) group, tyrphostin 47 plus quercetin (TQ) group, l-NAME and chelerythrine plus quercetin (LCQ) group, l-NAME and tyrphostin 47 plus quercetin (LTQ) group, chelerythrine and tyrphostin 47 plus quercetin (CTQ) group, glibenclamide plus quercetin (GQ) group.
| Group | Baseline (mmHg) | BCCAO (mmHg) | RE (mmHg) |
|---|---|---|---|
| I | 101.0 ± 3.5 | 99.0 ± 4.0 | 100.0 ± 3.5 |
| Q3 | 104.0 ± 5.5 | 102.0 ± 4.0 | 103.0 ± 5.0 |
| CQ | 99.0 ± 5.0 | 98.0 ± 4.0 | 100.0 ± 4.5 |
| TQ | 100.0 ± 4.0 | 99.0 ± 5.0 | 100.0 ± 5.0 |
| LCQ | 101.0 ± 5.5 | 99.0 ± 5.0 | 100.0 ± 5.0 |
| LTQ | 102.0 ± 3.5 | 100.0 ± 4.5 | 101.0 ± 4.5 |
| CTQ | 101.0 ± 5.0 | 100.0 ± 5.0 | 101.5 ± 4.0 |
| GQ | 98.0 ± 4.5 | 96.0 ± 4.0 | 99.0 ± 5.5 |
Values are means ± SD.
At RE, all arterioles dilated: by 19.0 ± 2.4% of baseline in order 2 (p < 0.01 compared with I and Q3 groups; Figure 1B) with no significant changes in MABP (Table 3). Leakage was marked (0.40 ± 0.05, p < 0.01 vs. Q3 groups); the adherent leukocytes were 7.0 ± 1.0/100 μm v.l./30 s, as observed in ischemic animals (p = NS vs. I group and p < 0.01 vs. Q3 group); PCL significantly diminished compared with Q3 group (62 ± 7% of baseline, p = NS vs. I group, p < 0.01 vs. Q3 group; Tables 1 and 2). SPV blood flow was significantly reduced by 25.7 ± 0.8% of baseline: 215 ± 11 nl/s, p < 0.01 vs. baseline, I and Q group.
TK inhibition plus quercetin
Tyrphostin 47, TK inhibitor administration, before quercetin (group TQ) did not affect quercetin-induced dilation of arterioles: by 5.0 ± 2.7% of baseline in order 2 (p < 0.01 vs. controls) at the end of BCCAO (Figure 1B) without significant change in MABP (Table 3). Leakage was marked (NGL: 0.18 ± 0.02) as observed in ischemic animals (p = NS compared with I group, p < 0.01 vs. Q3 group).
At RE tyrphostin 47 partially blunted only the dilation in smaller arterioles (increase in order 2 diameter by 20.7 ± 2.5% of baseline, p < 0.01 vs. I and Q3 groups; Figure 1B), while MABP did not significantly change (Table 3). There was increased leakage (NGL: 0.39 ± 0.04) compared to Q3 group (p < 0.01 vs. Q3 group), while leukocyte adhesion was partly prevented (4.0 ± 1.0/100 μm v.l./30 s, p < 0.01 vs. I and Q3 groups) and capillary perfusion was partially protected (PCL: 81 ± 9% of baseline, p < 0.01 vs. I group; Tables 1 and 2). In single pial venules blood flow decreased by 16.5 ± 0.8% of baseline: 293 ± 8 nl/s, p < 0.01 vs. baseline, I and Q group.
NO synthase and PKC inhibition plus quercetin
At the end of BCCAO, l-NAME, eNOS inhibitor, administered before PKC inhibitor, chelerythrine, and quercetin (group LCQ) caused a decrease in diameter of all arterioles: by 8.2 ± 2.0% of baseline in order 2 (p < 0.05 vs. ischemic animals, p < 0.01 vs. Q3 group; Figure 1C), without significant changes in MABP (Table 2). Permeability increased (NGL: 0.21 ± 0.03, p = NS vs. I group, p < 0.01 vs. Q3 group), as reported in ischemic animals.
At RE, all arterioles constricted: by 6.5 ± 3.0% of baseline in order 2 (p < 0.01 vs. Q3 group; Figure 1C), while no significant changes in MABP were detected (Table 3). Leakage was slightly different when compared with ischemic animals (NGL: 0.42 ± 0.03, p = NS vs. I group, p < 0.01 vs. Q3 group). The other parameters did not significantly change compared with CQ group: adherent leukocytes were 7.0 ± 1.0/100 μm v.l./30 s, p < 0.01 vs. Q3 group; p = NS vs. I group), while PCL was 63 ± 5% of baseline (p < 0.01 vs. Q3 group, p = NS vs. I group; Tables 1 and 2). SPV blood flow decreased by 28.1 ± 0.6% of baseline: 226.3 ± 10.5 nl/s, p < 0.01 vs. baseline and Q group.
NO synthase and TK inhibition plus quercetin
At the end of BCCAO, l-NAME, eNOS inhibitor, prior to tyrphostin 47, TK inhibitor, and quercetin (group LTQ) caused decrease in diameter of all arterioles: by 9.0 ± 3.0% of baseline in order 2 (p < 0.01 vs. Q3 group; Figure 1C). MABP did not significantly change (Table 3). Leakage was attenuated (NGL: 0.15 ± 0.02, p < 0.01 vs. I group) compared to ischemic animals.
At RE all arterioles constricted: by 9.0 ± 2.0% of baseline in order 2 (p < 0.01 vs. Q3 group; Figure 1C), without significant increase in MABP (Table 3). Leakage was not so high as in ischemic animals (NGL: 0.37 ± 0.03, p < 0.01 vs. I group); adhesion of leukocytes was attenuated (5.0 ± 1.0/100 μm v.l./30 s, p < 0.01 vs. I group) and capillary perfusion was higher than in ischemic animals (PCL: 80 ± 6% of baseline, p < 0.01 vs. I and Q3 groups; Tables 1 and 2). SPV blood flow was reduced by 23.5 ± 0.5% of baseline: 250 ± 9 nl/s, p < 0.01 vs. baseline, I and Q group.
PKC and TK inhibition plus quercetin
At the end of BCCAO, the inhibition of both PKC and TK prior to quercetin (group CTQ) caused a decrease in diameter of all arterioles: by 12.0 ± 1.5% of baseline in order 2 (p < 0.01 vs. Q3 group; Figure 1C); however, MABP did not significantly change (Table 3). Leakage was marked (NGL: 0.20 ± 0.02, p = NS vs. I group, p < 0.01 vs. Q3 group).
At RE the arterioles constricted: by 10.5 ± 1.0% of baseline in order 2 (p < 0.01 vs. Q3 group; Figure 1C), while MABP did not significantly change (Table 3). Leakage was pronounced (NGL: 0.42 ± 0.03, p < 0.01 vs. Q3 group); the adherent leukocytes increased in number (8.0 ± 2.0/100 μm v.l./30 s, p < 0.01 vs. Q3 group), PCL diminished (60 ± 4% of baseline, p = NS vs. I group, p < 0.01 vs. Q3 group; Tables 1 and 2). SPV blood flow was markedly decreased by 31.8 ± 0.7% of baseline: 237.3 ± 9.5 nl/s, p < 0.01 vs. baseline and Q group.
ATP-sensitive potassium (KATP) channel inhibition plus quercetin
At the end of BCCAO KATP channel inhibition prior to quercetin (group GQ) caused a slight decrease in diameter of all arterioles, by 5.4 ± 2.1% of baseline in order 2 (p < 0.01 vs. I and Q3 groups), while microvascular permeability was not affected (NGL: 0.10 ± 0.02, p < 0.01 vs. I group). MABP did not significantly change (Table 3).
At RE the arterioles did not significantly dilate compared with baseline (p < 0.01 vs. I and Q3 groups), while MABP did not significantly change (Table 3). FD70 leakage was moderately prevented compared with that observed in Q3 group (NGL: 0.22 ± 0.03, p < 0.01 vs. S, I, and Q3 groups). Adherent leukocytes were 3.5 ± 1.0/100 μm v.l./30 s (p < 0.01 vs. S and I groups). Capillary perfusion slightly decreased (PCL: 85 ± 4% of baseline, p < 0.01 vs. S and I groups; Tables 1 and 2). SPV blood flow was significantly reduced by 29.0 ± 0.4% of baseline: 274 ± 11 nl/s, p < 0.01 vs. baseline and Q group.
PKC, TK, and KATP channel inhibition prior to BCCAO and reperfusion
Protein kinase C inhibition by chelerythrine (C group) or TK inhibition by tyrphostin 47 (T group) or KATP channel inhibition by glibenclamide (G group) did not affect the microvascular changes observed in rats submitted to BCCAO and reperfusion (Table 1).
Finally, physiological parameters, such as hematocrit, MABP, heart rate, pH, PCO2, and PO2 did not change in the different experimental groups up to RE.
eNOS expression
Western blot analysis showed that at the end of reperfusion eNOS protein concentration significantly increased in animals treated with highest dose quercetin compared with I and S group. On the contrary, there was no significant increase in animals treated with chelerythrine and tyrphostin 47 before quercetin when compared with I group (Figure 2). Moreover, eNOS protein concentrations were not uniformly expressed in cortex, striatum, and hippocampus, because the higher were detected in hippocampus and cortex, while the lower in striatum of all animals. The same trend was observed for phosphorylated eNOS protein concentrations: no significant increase after combined PKC and TK inhibition.
Figure 2.
Western blotting of eNOS expression in three cerebral zones [cortex (A), striatum (B), hippocampus (C)] at the end of reperfusion in sham-operated group (S group), in quercetin-treated (5 mg/Kg b.w.) group (Q3 group), in ischemic group (I group) and in chelerythrine and tyrphostin 47 plus quercetin-treated group (CTQ group). Corresponding densitometric values (mean ± SD) are reported on the bottom. °p < 0.01 vs. S group, #p < 0.01 vs. Q3 group.
Discussion
In rats submitted to hypoperfusion by BCCAO and reperfusion there were peculiar microvascular responses, characterized by decrease in arteriolar diameter, increase in microvascular permeability and leukocyte adhesion, reduction in capillary perfusion, as previously reported (Lapi et al., 2012).
Quercetin, a plant derived compound with multiple proposed therapeutic effects (Rice-Evans, 2001), was effective in reducing pial microvascular alterations caused by BCCAO and reperfusion, inducing dilation of arterioles, prevention of leakage and leukocyte adhesion, facilitating capillary perfusion. These protective effects have been observed to be partly reduced by inhibition of nitric oxide release by l-NAME, eNOS inhibitor, able to decrease dilation of arterioles and capillary perfusion, to increase leakage, while there were no significant effect on leukocyte adhesion (Lapi et al., 2012).
Previous data indicate quercetin is able to activate or inhibit intracellular signaling pathways (Williams et al., 2004), such as those triggered by PKC or TK; in particular, quercetin increases PKC activity derived from rat brain (Chiwororo and Ojewole, 2010). Moreover, PKC has been suggested to regulate eNOS expression and activity in vascular cells, through different isoform activation (Davda et al., 1994; Fleming et al., 2001; Michell et al., 2001). PKC-β and PKC-ε appear to inhibit NO release, while PKC-α and PKC-δ are likely to increase NO formation in vessels (Partovian et al., 2005; Zhang et al., 2005; Motley et al., 2007; Fleming, 2008). Therefore, we hypothesized quercetin could activate different intracellular signaling pathways in our model. To define mechanisms involved in the quercetin-induced protective effects, PKC inhibition exerted by chelerythrine before quercetin was induced during BCCAO and reperfusion. The results indicate PKC inhibition significantly affected quercetin-triggered effects: the arterioles partially dilated at the end of reperfusion, while leakage and leukocyte adhesion were marked accompanied by capillary perfusion impairment. Therefore, our data suggest quercetin partially caused arteriolar dilation by activation of PKC intracellular signaling pathway promoting NO release. PKC appeared uniformly distributed in all pial arterioles, because there was homogenous response of all arteriolar orders after its inhibition. Furthermore, inhibition of both NO release and PKC signaling pathway abolished dilation induced by quercetin causing slight constriction of pial arterioles (by 6.5 ± 3.0% of baseline) at the end of reperfusion and marked decrease in single pial venule blood flow (by 25.7 ± 0.8% of baseline). Therefore, it is reasonable to suggest that the final result triggered by quercetin was mainly related to NO release, able to prevent blood–brain barrier disruption and to facilitate capillary perfusion.
Some in vitro studies indicate PKC activation can induce cerebral arteries constriction (Osol et al., 1991; Bonev et al., 1997). Our data show that PKC stimulation by PDBu induced dilation at first and then constriction of pial arterioles under baseline conditions. However, PKC inhibition by chelerythrine was not able to affect pial arteriolar diameter both in sham-operated animals and prior to BCCAO.
Quercetin is known to activate also the TK intracellular signaling pathway, as previously reported (Williams et al., 2004). In particular, TK has been reported to facilitate eNOS activation followed by increased NO release in arterioles under different conditions (Ayajiki et al., 1996; Corson et al., 1996; Fleming et al., 1998). Therefore, another hypothesis was TK inhibition could influence the quercetin-induced effects. Our data indicate TK inhibition by tyrphostin 47 administered prior to quercetin partly reduced dilation particularly in the smallest arterioles; this partial arteriolar dilation prevention might be due to decrease in NO release. The different arterioles responses to TK inhibition may be related to differentiated TK receptor distribution or involvement in dilation of pial arterioles. Consequently, the smaller ones were more responsive to tyrphostin 47; however, on the other end the decrease in single pial venule blood flow was by 16.5 ± 0.8% of baseline, corresponding to attenuated leukocyte adhesion and capillary failure. Microvascular permeability increased when compared with that observed in quercetin-treated groups. Therefore, TK inhibition appears to affect more vessel permeability (higher compared to animals treated by quercetin alone) than other vascular parameters. Combined NO release and TK signaling pathway inhibition abolished quercetin-induced arteriolar dilation causing moderate constriction (by 9 ± 2% of baseline) at the end of reperfusion corresponding to decrease in single pial venule blood flow by 23.5 ± 0.5% of baseline. Therefore, TK pathway may partly contribute to the overall effects exerted by quercetin on pial microvasculature.
It is important to note PKC and TK intracellular signaling pathways were mainly triggered by quercetin administration, because PKC or TK inhibition did not cause any protective effects on pial microcirculation in animals not treated by quercetin and submitted to hypoperfusion and reperfusion.
It is worth underlying combined inhibition of PKC and TK pathways completely reversed protection triggered by quercetin causing the strongest arteriolar constriction (by 10.5 ± 1.0% of baseline) at the end of reperfusion and marked decrease in single pial venule blood flow by 31.8 ± 0.7% of baseline. These effects indicate PKC and TK signaling pathways could mediate quercetin-induced pial vessel responses. In several previous studies quercetin has been observed to bind to the ATP-binding sites of a large number of proteins, such as PKC – modulating many cellular responses – such as inducible adhesion molecule ICAM-1 expression in human endothelial cells (Kobuchi et al., 1999; Williams et al., 2004). Furthermore, quercetin may activate the MAPK pathway (ERK 2, JNK1, and p38) leading to expression of survival and defensive genes (i.e., glutathione-S-transferase, etc.) resulting in survival and protective mechanisms (homeostasis response; Kong et al., 2000). The effects on pial arteriolar dilation exerted by quercetin might be related to activation of NO releasing mechanisms through stimulation of both PKC and TK intracellular signaling pathways. Finally, both PKC and TK activation have been suggested to facilitate vascular preconditioning, i.e., coronary vasculature protection from endothelial dysfunction by ischemic preconditioning (brief heart exposure to ischemia before prolonged ischemia). These effects have been related to NO release through PKC and TK stimulation, as linear or parallel pathways (Sakamoto et al., 2005), and to KATP channel (both mitochondrial and sarcoplasmatic) involvement in dilation mechanisms.
In our model, coupled inhibition of both PKC and TK pathways was able to abolish all effects exerted by quercetin, while inhibition of NO release did not completely suppress dilation. Western blot analysis, indeed, indicates that combined PKC and TK inhibition caused a decrease in eNOS expression and activation completely blunting quercetin’s effects. Therefore, these effects on pial arteriolar dilation are mostly due to eNOS activation, although other mechanisms may contribute, such as KATP channel involvement. To support this hypothesis we tested KATP channel blocking effects on arteriolar dilation triggered by quercetin and we observed suppression of dilation. Therefore, it is reasonable to suggest these channels contribute to arteriolar dilation and consequently to the overall effects induced by quercetin.
Furthermore, quercetin effects on leukocyte adhesion may be due to inhibition of adhesion molecule expression and quercetin anti-inflammatory properties. Therefore, quercetin’s action appears to be complex, depending on several mechanisms including NO release stimulation. However, further studies are required to clarify all involved molecular mechanisms, in particular, the PKC isoforms role in the quercetin-induced protection.
In conclusion, quercetin prevented microvascular leakage and leukocyte adhesion accompanied by increase in arteriolar diameter and protection of capillary perfusion facilitating adequate blood flow as indicated by enhanced pial venular blood flow. Our data indicate quercetin’s effects appear to be related to an increase in eNOS expression, NO release, and activity with the involvement, to a different extent, of PKC- and TK-dependent intracellular pathways. However, PKC and TK activation may also play a role in the prevention of blood–brain barrier disruption in association with quercetin scavenger activity, effective in preventing ROS formation and blood–brain barrier impairment (Fraser, 2011).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ayajiki K., Kindermann M., Hecker M., Fleming I., Busse R. (1996). Intracellular pH and tyrosine phosphorylation but not calcium determine shear stress-induced nitric oxide production in native endothelial cells. Circ. Res. 78, 750–758 [DOI] [PubMed] [Google Scholar]
- Bonev A. D., Jaggar J. H., Rubart M., Nelson M. T. (1997). Activators of protein kinase C decrease Ca 2+ spark frequency in smooth muscle cells from cerebral arteries. Am. J. Physiol. Cell Physiol. 273, C2090–C2095 [DOI] [PubMed] [Google Scholar]
- Chau C. H., Chen K. Y., Deng H. T., Kim K. J., Hosoya K., Terasaki T., Shih H. M., Ann D. K. (2002). Coordinating Etk/Bmx activation, and VEGF upregulation to promote cell survival, and proliferation. Oncogene 21, 8817–8829 10.1038/sj.onc.1206032 [DOI] [PubMed] [Google Scholar]
- Chiwororo W. D. H., Ojewole J. A. O. (2010). Dual effects of quercetin on rat isolated portal vein smooth muscle contractility. Cardiovasc. J. Afr. 21, 132–136 [PMC free article] [PubMed] [Google Scholar]
- Cho J. Y., Kim I. S., Jang Y. H., Kim A. R., Lee S. R. (2006). Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global cerebral ischemia. Neurosci. Lett. 404, 330–335 10.1016/j.neulet.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Cogolludo A., Frazziano G., Briones A. M., Cobeno L., Moreno L., Lodi F., Salaices M., Tamargo J., Perez-Vizcaino F. (2007). The dietary flavonoid quercetin activates BKCa currents in coronary arteries via production H2O2. Role in vasodilation. Cardiovasc. Res. 73, 424–431 10.1016/j.cardiores.2006.09.008 [DOI] [PubMed] [Google Scholar]
- Colantuoni A., Lapi D., Paterni M., Marchiafava P. L. (2005). Protective effects of insulin during ischemia-reperfusion injury in hamster cheek pouch microcirculation. J. Vasc. Res. 42, 55–66 10.1159/000083092 [DOI] [PubMed] [Google Scholar]
- Corson M. A., James N. L., Latta S. E., Nerem R. M., Berk B. C., Harrison D. G. (1996). Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ. Res. 79, 984–991 [DOI] [PubMed] [Google Scholar]
- Davda R. K., Chandler L. J., Guzman N. J. (1994). Protein kinase C modulates receptor-independent activation of endothelial nitric oxide synthase. Eur. J. Pharmacol. 266, 237–244 10.1016/0922-4106(94)90132-5 [DOI] [PubMed] [Google Scholar]
- Fleming I. (2008). “Biology of nitric oxide synthases,” in Handbook of Physiology Microcirculation, eds Tuma R. F., Duràn W. N., Ley K. (Amsterdam, NL: Academic Press; ), 56–80 [Google Scholar]
- Fleming I., Bauersachs J., Fisslthaler B., Busse R. (1998). Ca2+ -independent activation of the endothelial nitric oxide synthase in response to tyrosine phosphatase inhibitors and fluid shear stress. Circ. Res. 82, 686–695 [DOI] [PubMed] [Google Scholar]
- Fleming I., Fisslthaler B., Dimmeler S., Kemp B. E., Busse R. (2001). Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ. Res. 88, E68–E75 10.1161/hh1101.092677 [DOI] [PubMed] [Google Scholar]
- Fraser P. A. (2011). The role of free radical generation in increasing cerebrovascular permeability. Free Radic. Biol. Med. 51, 967–977 10.1016/j.freeradbiomed.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Fryer R. M., Schultz J. E., Hsu A. K., Gross G. J. (1999). Importance of PKC and tyrosine kinase in single or multiple cycles of preconditioning in rat hearts. Am. J. Physiol. 276, H1229–H1235 [DOI] [PubMed] [Google Scholar]
- Hudetz A. G., Fehér G., Weigle C. G., Knuese D. E., Kampine J. P. (1995). Video microscopy of cerebrocortical capillary flow: response to hypotension and intracranial hypertension. Am. J. Physiol. 268, H2202–H2210 [DOI] [PubMed] [Google Scholar]
- Huk I., Nanobash V. J., Weigel G., Neumayer C., Partyka L., Patton S., Malinski T. (1998). Bioflavonoid quercetin scavengers superoxide and increase nitric oxide concentration in ischemia-reperfusion injury: an experimental study. Br. J. Surg. 85, 1080–1085 10.1046/j.1365-2168.1998.00787.x [DOI] [PubMed] [Google Scholar]
- Kobuchi H., Roy S., Sen C. K., Nguyen H. G., Packer L. (1999). Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am. J. Physiol. 277, C403–C411 [DOI] [PubMed] [Google Scholar]
- Kong A. N., Yu R., Chen C., Mandlekar S., Primiano T. (2000). Signal transduction events elicited by natural products: role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch. Pharm. Res. 23, 1–16 10.1007/BF02976458 [DOI] [PubMed] [Google Scholar]
- Lapi D., Marchiafava P. L., Colantuoni A. (2008). Pial microvascular responses to transient bilateral common carotid artery occlusion: effects of hypertonic glycerol. J. Vasc. Res. 45, 89–102 10.1159/000109078 [DOI] [PubMed] [Google Scholar]
- Lapi D., Vagnani S., Pignataro G., Esposito E., Paterni M., Colantuoni A. (2012). A. Protective effects of quercetin on rat pial microvascular changes during transient bilateral common carotid artery occlusion and reperfusion. Front. Physiol. 3:32. 10.3389/fphys.2012.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell B. J., Chen Z. P., Tiganis T., Stapleton D., Katsis F., Power D. A., Sim A. T., Kemp B. E. (2001). Coordinated control of endothelial nitric-oxide synthase phosphorilation by protein kinase C and the cAMP-dependent protein kinase. J. Biol. Chem. 276, 17625–17628 10.1074/jbc.C100122200 [DOI] [PubMed] [Google Scholar]
- Moreau P., Takasa H., Küng C. F., van Rooijen M. M., Schaffner T., Lüscher T. F. (1995). Structure and function of the rat basilar artery during chronic nitric oxide synthase inhibition. Stroke 26, 1922–1928 10.1161/01.STR.26.10.1922 [DOI] [PubMed] [Google Scholar]
- Morii S., Ngai A. C., Winn R. (1986). Reactivity of rat pial arterioles and venules to adenosine and carbon dioxine: with detailed description of the closed cranial window technique in rats. J. Cereb. Blood Flow Metab. 6, 34–41 10.1038/jcbfm.1986.89 [DOI] [PubMed] [Google Scholar]
- Motley E. D., Eguchi K., Patterson M. M., Palmer P. D., Suzuki H., Eguchi S. (2007). Mechanism of endothelial nitric oxide synthase phosphorylation and activation by thrombin. Hypertension 49, 577–583 10.1161/01.HYP.0000255954.80025.34 [DOI] [PubMed] [Google Scholar]
- Negash S., Gao Y., Zhou W., Liu J., Chinta S., Raj J. U. (2007). Regulation of cGMP-dependent protein kinase-mediated vasodilation by hypoxia-induced reactive species in ovine fetal pulmonary veins. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L1012–L1020 10.1152/ajplung.00061.2007 [DOI] [PubMed] [Google Scholar]
- Ngai A. C., Ko K. R., Morii S., Winn H. R. (1988). Effect of sciatic nerve stimulation on pial arterioles in rats. Am. J. Physiol. 254, H133–H139 [DOI] [PubMed] [Google Scholar]
- Oriji G. K. (1999). Endothelin-induced prostacyclin production in rat aortic endothelial cells: role of calcium. Prostaglandins Leukot. Essent. Fatty Acid 61, 45–49 10.1054/plef.1999.0072 [DOI] [PubMed] [Google Scholar]
- Osol G., Laher I., Cipolla M. (1991). Protein kinase C modulates basal myogenic tone in resistance arteries from cerebral circulation. Circ. Res. 68, 359–367 [DOI] [PubMed] [Google Scholar]
- Pagliaro P., Gattullo D., Rastaldo R., Losano G. (2001). Involvement of nitric oxide in ischemic preconditioning. Ital. Heart J. 2, 660–668 [PubMed] [Google Scholar]
- Partovian C., Zhuang Z., Moodie K., Lin M., Ouchi N., Sessa W. C., Walsh K., Simons M. (2005). PKC alpha activates eNOS and increases arterial blood flow in vivo. Circ. Res. 97, 482–487 10.1161/01.RES.0000179775.04114.45 [DOI] [PubMed] [Google Scholar]
- Picq M., Dubois M., Munari-Silem Y., Prigent A. F., Pacheco H. (1989). Flavonoid modulation of protein kinase C activation. Life Sci. 44, 1563–1571 10.1016/0024-3205(89)90450-5 [DOI] [PubMed] [Google Scholar]
- Rice-Evans C. (2001). Flavonoid antioxidants. Cur. Med. Chem. 8, 797–807 [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Yonoki Y., Kubota Y., Kuwagata M., Saito M., Nakahara T., Ishii K. (2005). Inducible nitric oxide synthase inhibitors abolished histological protection by late ischemic preconditioning in rat retina. Exp. Eye Res. 82, 512–518 10.1016/j.exer.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Williams R. J., Spencer J. P. E., Rice-Evans C. (2004). Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med. 36, 838–849 10.1016/j.freeradbiomed.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Xu X. L., Feinstein D. L., Santiza R. A., Koenig H. M., Pellegrino D. A. (2002). Agonist-specific differences in mechanisms mediatine eNOS-dependent pial arteriolar dilation in rats. Am. J. Physiol. 282, H237–H243 [DOI] [PubMed] [Google Scholar]
- Zhang J., Baines C. P., Zong C., Cardwell E. M., Wang G., Vondriska T. M., Ping P. (2005). Functional proteomic analysis of a three-tier PKC – Akt-eNOS signalling module in cardiac protection. Am. J. Physiol. Heart Circ. Physiol. 288, H954–H961 10.1152/ajpheart.00987.2004 [DOI] [PubMed] [Google Scholar]