Abstract
Cognitive deficits, including impairments in working memory that have been linked to the prefrontal cortex, are among the most debilitating and difficult to treat features of schizophrenia. Consequently, the identification of potential targets informed by the pathophysiology of the illness is needed to develop novel pharmacological approaches for ameliorating these deficits. Postmortem studies of the prefrontal cortex in schizophrenia subjects have revealed disturbances restricted to a subpopulation of inhibitory neurons that includes chandelier neurons, whose axon terminals synapse on the axon initial segment of pyramidal neurons. Chandelier neurons play an important role in synchronizing pyramidal neuron activity and appear to be a critical component of the prefrontal cortical circuitry that subserves working memory function. Therefore, in this paper we review evidence suggesting that drugs which selectively enhance chandelier neuron-mediated inhibition of prefrontal pyramidal neurons may improve working memory dysfunction in schizophrenia. Potential novel targets for such agents include GABAA receptors that contain the α2 subunit. In addition, we discuss potential complementary mechanisms for enhancing inhibitory input to pyramidal cell bodies, including drugs with activity at the CB1 receptor of the endocannabinoid system. The development of pathophysiologically-based treatments that selectively remediate disturbances in specific neural circuits underlying working memory may provide an effective approach to improving cognitive deficits in schizophrenia.
Keywords: Prefrontal cortex, working memory, gamma oscillation, GABAA receptor α2 subunit, CB1, cannabinoid, parvalbumin, chandelier neuron
COGNITIVE DYSFUNCTION AND THE PRE-FRONTAL CORTEX IN SCHIZOPHRENIA
Disturbances in cognitive function are considered to be a core feature of schizophrenia, and include impairments in attention, memory, and executive functions, such as the abilities to plan, initiate, and regulate goal-directed behavior [33,58,81,113]. These cognitive deficits are present throughout the course of the illness, including the premorbid [27], first-onset [113], and later stages [14,58], and thus do not appear to be attributable to either treatment with antipsychotic medication or the chronic nature of the illness. In addition, cognitive dysfunction in schizophrenia is reported to be more debilitating and to have a greater detrimental effect on daily activities than psychotic symptoms [46], and is also associated with poorer long-term outcomes [46,55]. Furthermore, cognitive deficits are less responsive than the positive symptoms of schizophrenia to available psychotropic medications [7], although newer atypical antipsychotic medications may provide some benefit compared to older typical antipsychotic medications [13,50,135]. Consequently, the development of novel pathophysiologically-based treatments for the cognitive impairments in schizophrenia has recently become a primary focus for the National Institute of Mental Health [63].
An important type of cognitive deficit to pharmacologically target in schizophrenia is working memory, the process in which information is actively maintained for short periods of time and utilized to direct behavior [45] and which involves activation of the prefrontal cortex (PFC) [42,43,64]. For example, imaging studies have demonstrated that subjects with schizophrenia tend to perform poorly and have reduced PFC activation during various working memory tasks [18,21,100,136]. In addition, deficits in PFC activation during working memory tasks primarily occur at working memory loads that distinguish schizophrenia subjects from control subjects [21,103]. Furthermore, in subjects with schizophrenia who perform normally on working memory tasks, activity in the PFC may actually be increased, suggesting a reduced efficiency in PFC function [18,19].
A cellular mechanism that may subserve working memory function in the PFC involves the spatially and temporally synchronized firing of pyramidal neurons during the delay period of working memory tasks. For example, during working memory tasks, some PFC neurons selectively fire during the delay period between the temporary presentation of a stimulus and the later initiation of a response [42,45]. In addition, anatomical studies have demonstrated reciprocal interconnections between groups of layer 3 pyramidal neurons in monkey PFC, suggesting that neuronal activity during the delay period may be maintained, at least partially, through an excitatory feedback loop [88,107]. Furthermore, gamma band oscillations, which reflect the synchronized firing of neuronal networks at the 30–80 Hz frequency range, have also been associated with working memory tasks. For example, in working memory tasks, gamma oscillations are induced and sustained during the delay period [123,124], and the magnitude, or power, of gamma oscillations in the PFC appears to increase in proportion to memory load [62]. Thus, these data suggest that working memory is an emergent property of the gamma band activity of a distributed network of neurons, one node of which is localized to the PFC.
In addition, abnormalities in gamma band oscillations have been reported in schizophrenia (for review, see [74]). For example, in the frontal cortex of subjects with schizophrenia, impairments in phase-locking of gamma activity to the stimulus onset, including increased latency and reduced frequency, have been found [121]. In addition, abnormalities in the phase coherence of gamma oscillations between electrode sites, a measure of temporal synchronization in spatially-separated cortical areas, were also found, suggesting abnormalities in long-distance synchronization as well [121]. Furthermore, within the PFC in schizophrenia, reduced gamma band power has also been observed during the delay period of a working memory task1.
Inhibitory (GABA) neurons in the PFC appear to be a critical component of the neural circuitry that enables synchronized pyramidal neuron activity and working memory processes. For example, distinct subsets of inhibitory neurons are involved in the induction and maintenance of gamma oscillations in pyramidal neurons (for review, see [38,84]). In addition, during working memory tasks, fast-spiking GABA neurons in monkey PFC are active during the delay period [108,109,137] and can influence the time course of activation of pyramidal neurons at different stages of the task [24]. Finally, injection of GABA receptor antagonists into monkey PFC impairs performance on working memory tasks [112]. Interestingly, alterations in markers of GABA neurotransmission in the PFC of subjects with schizophrenia have been consistently reported in postmortem studies [3,11,12,48,90,128,129]. Therefore, the development of new treatments for working memory disturbances in schizophrenia first requires an understanding of the nature of PFC GABA neuron disturbances in the illness.
ALTERATIONS IN A SUBSET OF PREFRONTAL CORTICAL GABA NEURONS IN SCHIZOPHRENIA
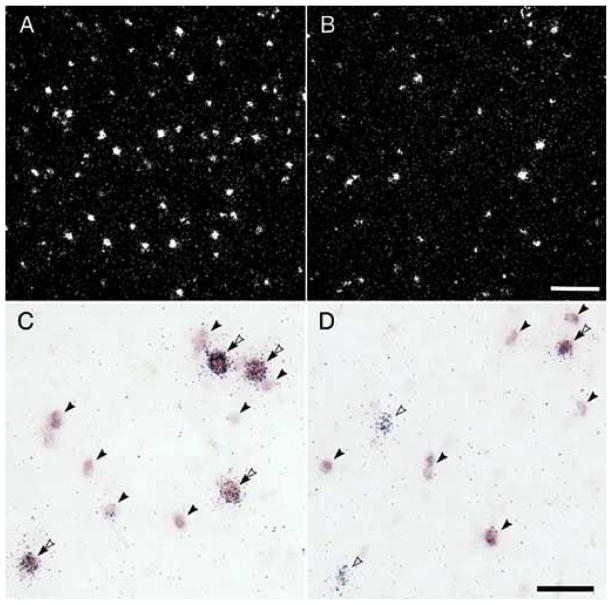
Convergent findings indicate that markers of GABA neurotransmission are altered in the PFC in subjects with schizophrenia. For example, several studies have found that the mRNA expression level of glutamate decarboxylase (GAD67), an enzyme responsible for the synthesis of GABA, is reduced in the PFC in schizophrenia [3,48,90,128,129]. With the exception of one study in elderly, chronically hospitalized, and demented individuals with schizophrenia [53], reduced GAD67 mRNA expression in the PFC may be one of the most consistent findings in postmortem studies of schizophrenia. Furthermore, the reduction in GAD67 mRNA expression appears to be restricted to a subset of GABA neurons. For example, while the density of neurons with detectable levels of GAD67 mRNA is significantly decreased in subjects with schizophrenia (Fig. 1A,B) [3,129], the relative cellular level of GAD67 mRNA expression per neuron is unchanged [129]. Together, these observations suggest that the majority of PFC GABA neurons express normal levels of GAD67 mRNA in schizophrenia, but a minority (approximately 25–35%) fail to express detectable levels of GAD 67 mRNA. Thus, the reduction of GAD67 mRNA levels to an undetectable level in this subset of neurons suggests that GABA synthesis in these neurons is greatly impaired.
Fig. 1.
Photomicrographs of PFC tissue sections processed by in situ hybridization for single label GAD67 mRNA (top) and for dual label GAD67 and PV mRNAs (bottom) in matched pairs of control (left) and schizophrenia (right) subjects. Top photomicrographs: Darkfield images from a matched pair of control (A) and schizophrenic (B) subjects processed by single label in situ hybridization for GAD67 mRNA using 35S-labeled probes [129]. Silver grains represent GAD67 mRNA label. Note the decreased density of grain clusters, representing GAD67 mRNA-labeled neurons, but similar grain cluster size, in the subject with schizophrenia. Scale bar = 150 μm. Bottom photomicrographs: Dual-label in situ hybridization showing simultaneous detection of PV and GAD67 mRNAs in PFC in a different pair of control (C) and schizophrenia (D) subjects [57]. GAD67 mRNA label was visualized as color reaction product by a digoxigenin-labeled probe, and silver grains represent parvalbumin mRNA label, which was detected by a 35S-labeled probe. GAD67 mRNA-labeled profiles (solid arrowhead), parvalbumin mRNA-labeled profiles (open arrowhead), and dual labeled profiles (double arrowhead) were detected. Similar to the top photomicrographs, the density of GAD67 mRNA-labeled profiles is reduced in the subject with schizophrenia compared to the control subject. Furthermore, in the control subject, GAD67 mRNA was detected in all parvalbumin mRNA-positive grain clusters, whereas in the schizophrenia subject, GAD 67 mRNA expression was undetectable in some of the parvalbumin mRNA-labeled profiles. Scale bar = 50 μm. Adapted from [57,129].
Furthermore, in the same set of subjects, mRNA levels for the GABA membrane transporter (GAT1), which is responsible for the re-uptake of released GABA, were also undetectable in a similar proportion of GABA neurons [130]. A reduction in the re-uptake of GABA at axon terminals may represent an attempt to raise synaptic levels of GABA in order to compensate for decreased GABA synthesis. However, as discussed above, the presence of working memory deficits in individuals with schizophrenia, which have been linked to reductions in GABA-mediated neuronal synchronization, suggests that this compensatory response to raise synaptic levels of GABA is not sufficient. In summary, these data suggest that in schizophrenia both the synthesis and re-uptake of GABA are greatly reduced in a subset of PFC inhibitory neurons.
The functional significance of disturbances restricted to a minority of GABA neurons in schizophrenia may be further informed by determining the identity of the affected GABA neurons. Indeed, distinct subpopulations of PFC GABA neurons, which exhibit unique anatomical, biochemical, and electro-physiological properties, appear to play specialized roles in regulating pyramidal neuron activity [30, 49,69]. For example, chandelier neurons, which are among the subpopulation of GABA neurons that express the calcium-binding protein parvalbumin, exhibit a fast-spiking, non-adapting firing pattern [23,69]. Chandelier neurons provide a linear array of axon terminals (termed cartridges) that exclusively synapse along the axon initial segment of pyramidal neurons [40,76,120]. In addition, the subpopulation of GABA neurons expressing the neuropeptide cholecystokinin exhibit a regular spiking, adapting firing pattern, and provide axon terminals that primarily target pyramidal cell bodies [70,98,102]. The proximity of their inhibitory synapses near or at the site of action potential generation in pyramidal neurons suggests that both parvalbumin and cholecystokinin neurons are positioned to powerfully regulate the output of pyramidal neurons. In contrast, GABA neurons that express the calcium-binding protein calretinin exhibit a regular-spiking, adaptive firing pattern [69] and provide axon terminals that target more distal dendritic sites on pyramidal cells or other GABA neurons [30,80].
Several lines of evidence suggest that the affected subset of PFC GABA neurons includes those that express parvalbumin. For example, parvalbumin mRNA expression was decreased in the PFC of subjects with schizophrenia, whereas calretinin mRNA levels were unchanged [57]. Interestingly, in direct contrast to the findings for GAD67 and GAT1 mRNAs, the density of neurons with detectable levels of parvalbumin mRNA was not changed in subjects with schizophrenia, whereas the average level of parvalbumin mRNA expression per neuron was significantly decreased. These findings suggest that 1) the majority of these neurons exhibit a disturbance in parvalbumin mRNA expression, and 2) the number of parvalbumin neurons is not altered in schizophrenia, an observation consistent with the results of studies of parvalbumin-immunoreactive neurons [10,139]. Interestingly, dual label studies revealed that almost all parvalbumin mRNA-labeled neurons in control subjects expressed GAD67 mRNA, whereas in subjects with schizophrenia, approximately half of parvalbumin mRNA-labeled neurons lacked detectable levels of GAD67 mRNA (Fig. 1C,D) [57]. Together, these findings suggest that the deficit in GAD67 mRNA expression is present predominately in the parvalbumin subpopulation of GABA neurons, and that these neurons are still present but are functionally impaired in schizophrenia.
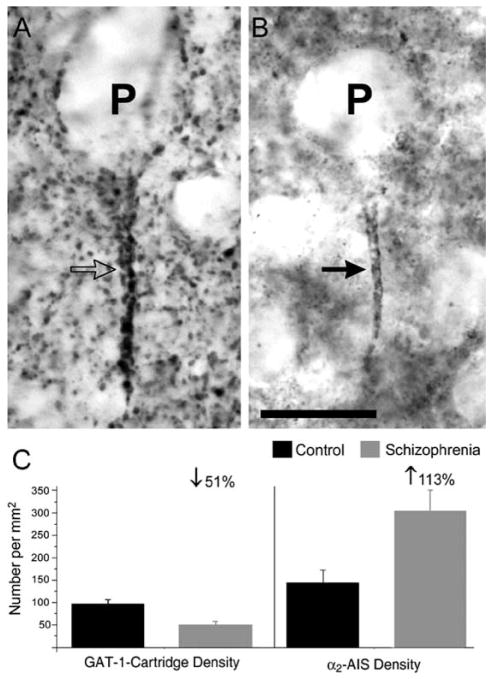
Within the parvalbumin neuron subpopulation, chandelier neurons appear to be particularly affected in schizophrenia. For example, the density of PFC chandelier neuron axon cartridges immunoreactive for GAT1 was significantly reduced in subjects with schizophrenia (Fig. 2) [104,140]. In concert with the mRNA studies, these findings suggest that in schizophrenia, many chandelier neurons express decreased levels of parvalbumin mRNA and undetectable levels of GAD67 and GAT1 mRNA, with the latter associated with decreased detectability of GAT1 protein in chandelier neuron axon cartridges. Furthermore, parallel studies in subjects with other psychiatric disorders, and in monkeys exposed chronically to antipsychotic medications in a fashion that mimics the treatment of schizophrenia, revealed that the above findings appear to be specific to the pathophysiology of schizophrenia and not a consequence of prolonged neurotropic medication [57,104,129,130].
Fig. 2. Pre- and postsynaptic inhibitory markers at the pyramidal neuron axon initial segment in schizophrenia.
A: Chandelier neuron axon cartridge immunoreactive for GAT1 (open arrow) is closely aligned to the axon initial segment of a pyramidal neuron (P). B: Axon initial segment of a pyramidal neuron immunoreactive for the GABAA receptor α2 subunit (solid arrow) (α2-AIS). Scale bar = 20 μm. C: Bar graphs showing the mean (± SD) number of GAT1-cartridges (left) and α2-AIS (right) per mm2 in the same schizophrenic subjects and matched control subjects. Adapted from [131].
Furthermore, these deficits appear to be relatively specific for parvalbumin neurons and are not present in the majority of other GABA neurons in the PFC in schizophrenia. For example, the majority of PFC GABA neurons express normal levels of both GAD67 and GAT1 mRNAs [129,130]. In addition, overall protein and mRNA expression levels of another synthesizing enzyme for GABA, GAD65, also appear to be unchanged in the PFC in schizophrenia [48]. Furthermore, the mRNA expression level of calretinin mRNA, which is found in approximately half of all PFC GABA neurons in monkey PFC [23], is also unchanged in schizophrenia [57]. Finally, the overall density of GAT1-immunoreactive boutons, presumably representing the majority of GABA axon terminals, is also unchanged in the PFC in schizophrenia [139]. Thus, the majority of other PFC GABA neurons appear to be relatively unaffected in schizophrenia.
The consequences of schizophrenia-related disturbances in chandelier neurons may be informed by an analysis of GABAA receptors at the postsynaptic targets, the axon initial segments of pyramidal neurons. GABAA receptors are composed of different subunits that have distinctive subcellular distributions and convey different physiological properties [41]. For example, the α2 subunit is prominently localized at pyramidal neuron axon initial segments in the superficial layers of human cerebral cortex [78]. Indeed, although associated with only ~ 15% of all GABAA receptors in the cortex [41], the α2 subunit is found at > 95% of inhibitory synapses onto pyramidal neuron axon initial segments, at least in rat hippocampus [97,98]. Furthermore, GABAA receptors containing the α2 subunit may have a higher affinity for GABA, and faster activation and slower de-activation times, compared to GABAA receptors containing the more commonly expressed α1 subunit [73,75]. Thus, GABAA receptors containing the α2 subunit appear to be anatomically positioned and functionally adapted to mediate a potent inhibitory influence on the output of pyramidal neurons, and thus may provide critical insight into the consequences of chandelier cell dysfunction on GABA activity at pyramidal neuron axon initial segments.
Interestingly, in schizophrenia, the density of PFC pyramidal neuron axon initial segments immuno-reactive for the α2 subunit (α2-AIS) is significantly increased by more than 100% compared to control subjects (Fig. 2) [132]. This increase in α2-AIS density appears to reflect higher detectable levels of α2 subunits at the AIS rather than an increase in pyramidal neuron density [115,116]. However, it is unclear whether the increase in α2-AIS density reflects an increase in total density of GABAA receptors or an increased proportion of GABAA receptors containing the α2 subunit at the AIS. Attempts to quantify the density of AIS labeled for other GABAA receptor subunits have been unsuccessful so far, possibly due to the much greater density of α1 and γ2 subunits at other locations [97] which prohibits the resolution of labeled AIS by light microscopy. Interestingly, α2-AIS density was inversely correlated with GAT1-cartridge density in the same subjects with schizophrenia (Fig. 2,3). These findings suggest that GABAA receptors are up-regulated at pyramidal neuron axon initial segments in response to deficient GABA activity in chandelier axon terminals in schizophrenia, and that a greater loss of GABA activity may be associated with greater compensatory increase in GABAA receptors.
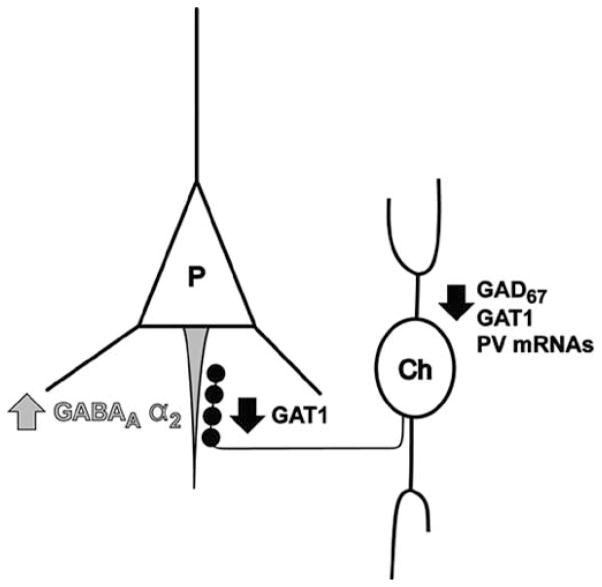
Fig. 3.
Circuit diagram of abnormalities in chandelier neuron synapses onto pyramidal neurons in the prefrontal cortex in schizophrenia. Chandelier neurons (Ch) provide linear arrays of axon terminals that synapse onto the axon initial segments (grey) of pyramidal neurons (P). In schizophrenia, a subpopulation of GABA neurons that includes chandelier neurons expresses undetectable levels of GAD67 and GAT1 mRNAs and lower levels of parvalbumin mRNA. Furthermore, chandelier axon cartridges (black) express lower levels of GAT1 protein. Postsynaptically at the axon initial segment of pyramidal neurons, the density of GABAA receptors containing the α2 subunit is increased. Together, these data suggest that in the PFC of subjects with schizophrenia, chandelier neurons provide deficient inhibition to pyramidal neuron axon initial segments.
In summary, postmortem studies in schizophrenia have discovered alterations in PFC GABA neurons, including the reduced synthesis and re-uptake of GABA, that are most prominent in the parvalbumin neuron subpopulation, which includes chandelier neurons (Fig. 3). These chandelier neurons appear to provide deficient inhibition to the axon initial segments of PFC pyramidal neurons, resulting in a compensatory postsynaptic up-regulation of GABAA receptors. These deficits appear to be relatively specific for parvalbumin neurons and are not present in the majority of other PFC GABA neurons. Finally, these disturbances do not appear to be attributable to chronic treatment with antipsychotic medications.
Interestingly, these schizophrenia-related disturbances in PFC parvalbumin neurons may contribute to the reported deficits in gamma oscillations, and consequently working memory function, in schizophrenia. For example, parvalbumin neurons, in particular chandelier neurons, are critically involved in initiating and maintaining the oscillatory, synchronous discharges of pyramidal neurons at gamma frequency. Indeed, a single chandelier neuron, whose axon terminals synapse on the axon initial segment of hundreds of pyramidal neurons [120] can synchronize the firing of multiple pyramidal neurons in rat hippocampus [22]. Furthermore, genetic manipulations of genes selectively expressed in parvalbumin neurons can have dramatic effects on gamma oscillations. For example, the knockout of the potassium channel Kv3.1, which is selectively expressed in parvalbumin neurons, results in a 2–4 fold increase in power in gamma frequency range [66]. The absence of these potassium channels appears to delay repolarization and prolong action potential waveforms in parvalbumin neurons, and thereby increase synaptic efficacy through increased GABA release. Finally, in parvalbumin-knockout mice, kainate-induced neuronal oscillations in the hippocampus exhibit much higher power at the gamma frequency range than in wild type mice [133]. During periods of repetitive firing, parvalbumin appears to normally buffer residual intra-terminal calcium levels and thereby limit the amount of GABA that is released. The absence of parvalbumin appears to facilitate GABA release during repetitive discharges, and thereby allows chandelier neurons, for example, to recruit and maintain more pyramidal neurons to fire synchronously, resulting in higher gamma power [133]. Perhaps in schizophrenia, the decrease in parvalbumin mRNA expression represents an unsuccessful compensatory attempt to improve chandelier neuron inhibitory regulation of gamma oscillations in PFC pyramidal neurons.
Thus, schizophrenia-related deficits in PFC parvalbumin neurons, which play a critical role in regulating gamma oscillations of pyramidal neurons, may be related to working memory dysfunction in schizophrenia. Therefore, we now begin a discussion of potential pharmacological targets that may selectively enhance parvalbumin neuron-mediated regulation of PFC pyramidal neuron firing, and thus help ameliorate working memory disturbances in individuals with schizophrenia.
POTENTIAL PHARMACOLOGICAL TARGETS TO ENHANCE THE FUNCTION OF PREFRONTAL PARVALBUMIN NEURONS IN SCHIZOPHRENIA
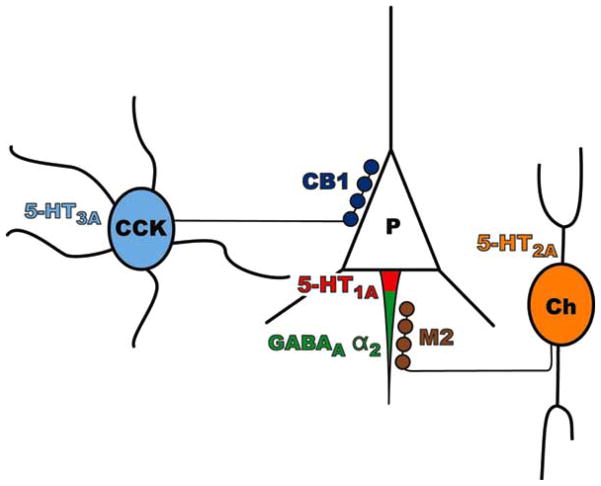
While some success has been reported in treating acute psychosis in schizophrenia using GABA-related agents, including benzodiazepines and valproic acid, as adjuncts to antipsychotic medications [20,134], most clinical trials have not assessed the utility of GABA-related agents in treating cognitive dysfunction in schizophrenia. Given the apparent subpopulation-specific abnormalities in PFC GABA neurons, the treatment of working memory dysfunction in schizophrenia may require drugs that selectively enhance parvalbumin neuron-mediated inhibitory regulation of pyramidal neurons without affecting signaling from other GABA neurons (Fig. 4).
Fig. 4.
Circuit diagram demonstrating potential pharmacological targets involving inhibitory neurons with proximal synapses onto prefrontal cortical pyramidal neurons in schizophrenia. The GABAA receptor α2 subunit (green) is heavily expressed at the pyramidal neuron axon initial segment, postsynaptic to chandelier neuron axon terminals. The M2 receptor (brown) is expressed presynaptically in parvalbumin neuron axon terminals. The CB1 receptor (dark blue) is expressed presynaptically in cholecystokinin neuron axon terminals that synapse onto pyramidal neuron cell bodies (P). The 5-HT1A receptor (red) is expressed postsynaptically in the pyramidal neuron axon initial segment, but is more proximal to the pyramidal neuron cell body that the chandelier cell axon terminals [31]. The 5-HT2A receptor (orange) is found in parvalbumin neurons which include chandelier neurons (Ch), while the 5-HT3A receptor (light blue) is found in cholecystokinin neurons (CCK).
The α2 Subunit of the GABAA Receptor
Drugs with selective agonist activity at GABAA receptors containing the α2 subunit may provide a direct and effective approach to enhancing chandelier neuron inhibition of PFC pyramidal neurons in schizophrenia. Indeed, the α2 subunit represents a highly selective target for enhancing inhibition at pyramidal neuron axon initial segments, since the α2 subunit is heavily expressed at inhibitory synapses onto pyramidal neuron axon initial segments [97,98,132] (Fig. 2). In addition, the density of α2-containing GABAA receptors appears to be increased at pyramidal neuron axon initial segments in schizophrenia (Fig. 2,3) [132]. Thus, it may even be possible to devise dosing strategies that would activate these receptors at pyramidal neuron axon initial segments without affecting synaptic sites in other brain regions that have normal levels of the GABAA receptor α2 subunit, thereby reducing potential side effects.
In particular, treatment with benzodiazepine-like agents selective for the α2 subunit would increase the frequency of GABAA receptor openings, enhancing chloride ion flow and thereby inhibitory postsynaptic potentials at pyramidal neuron axon initial segments. In addition, benzodiazepine-like agents would enhance inhibition at pyramidal neuron axon initial segments only in the presence of GABA and would not independently activate GABAA receptors. Thus, treatment with a α2-selective benzodiazepine would augment the postsynaptic inhibitory response at pyramidal neuron axon initial segments in a manner that incorporates the critical timing of chandelier neuron firing that is essential for synchronizing pyramidal neuron firing.
The principle of disynaptic inhibition is an example of the importance of maintaining normal regulatory mechanisms of chandelier neuron firing. Parvalbumin neurons, including chandelier cells, and pyramidal neurons in the PFC share excitatory input from various sources, including local axon collaterals from other PFC pyramidal neurons and axon terminals from the mediodorsal nucleus of the thalamus [85–87]. Thus, excitatory input from these sources stimulates both chandelier cells and pyramidal neurons simultaneously, resulting in a slightly delayed, secondary chandelier cell inhibitory input to pyramidal neuron axon initial segments. This disynaptic inhibitory input appears to limit the window of time, and thereby increase the temporal precision, for the summation of excitatory inputs needed to evoke pyramidal neuron firing, at least in rat hippocampus [106]. Thus, drugs that augment GABA release by chandelier cells during normal firing, or drugs that enhance the postsynaptic response to the release of GABA from chandelier cell axons such as α2-selective benzodiazepines, may be expected to improve disynaptic inhibition. However, drugs that generally increase the firing rate of chandelier cells, or drugs that activate postsynaptic GABAA receptors independent of the presence of GABA, may actually disrupt the timing of disynaptic inhibition.
Finally, recent studies suggest that the anxiolytic effects of benzodiazepines appear to be mediated by GABAA receptors that contain the α2 subunit, while the sedative effects are mediated by the α1 subunit [79]. Thus, α2-specific agents may both improve cognitive function and reduce stress responses that have been linked to psychosis exacerbations in schizophrenia [20], without inducing the sedation-related cognitive impairments associated with benzodiazepine use.
Serotonin Receptors
An alternative approach to enhancing inhibition of PFC pyramidal neurons in a circuit-specific manner could involve the use of the serotonin (5-HT) neuro-transmitter system as a pharmacological target. 5-HT receptors consist of a heterogeneous group of subtypes with distinct anatomical distributions and physiological functions. For example, the 5-HT1A receptor subtype is preferentially expressed postsynaptically at pyramidal neuron axon initial segments [26,31], the 5-HT2A subtype in parvalbumin neurons [65], and the 5-HT3A subtype in cholecystokinin neurons (Fig. 4) [35,93]. Furthermore, in monkey PFC, the majority of 5-HT-immunoreactive axons that form synapses appear to predominantly target inhibitory neurons rather than pyramidal neurons [119], suggesting that components of the 5-HT system may provide relatively selective targets for modulating GABA neuron function. Therefore, this section will discuss the role that these 5-HT receptor subtypes may play in PFC neural circuitry and will evaluate their possible use as drug targets for selectively improving inhibitory regulation of PFC pyramidal neurons in schizophrenia.
Targeting the 5-HT1A receptor, which mediates inhibition at pyramidal neuron axon initial segments, may provide another mechanism of compensating for schizophrenia-related deficits in inhibition to PFC pyramidal neurons (Fig. 4). The 5-HT1A receptor is a G protein coupled receptor involved in signaling pathways, including inhibiting adenylate cyclase and activating potassium channels (for review, see [110]). Activation of the 5-HT1A receptor results in inhibition of pyramidal neurons in rat and human neocortex, which can be reversed by 5-HT1A receptor antagonists [47,96]. Interestingly, 5-HT-immunoreactive axons do not appear in close proximity to 5-HT1A receptors at pyramidal neuron axon initial segments [31,119], suggesting that serotonin may activate 5-HT1A receptors and mediate an inhibitory effect at axon initial segments through volume transmission rather than through tightly coordinated synaptic inputs. Thus, a 5-HT1A receptor agonist may produce a tonic hyperpolarization of pyramidal neuron axon initial segments, which might help partially compensate for deficient GABA release from chandelier axon terminals.
However, the 5-HT1A receptor has also been reported to be located on other cell types, including GABA neurons [9], so 5-HT1A agonists may not selectively enhance inhibition at pyramidal neuron axon initial segments. Furthermore, while radiolabeled-ligand binding suggests that the overall level of 5-HT1A receptors is increased in the PFC of subjects with schizophrenia [17,56,118,122], the density of pyramidal neuron axon initial segment immunoreactive for the 5-HT1A receptor is unchanged in the PFC in schizophrenia [26]. The density of 5-HT transporter-immunoreactive axons is also unchanged in the PFC of subjects with schizophrenia [4]. Thus, in contrast to the higher levels of GABAA receptor α2 subunit at pyramidal neuron axon initial segments in schizophrenia, it may be more difficult to design a dose-dependent strategy whereby a 5-HT1A receptor agonist selectively affects pyramidal neuron axon initial segments.
The 5-HT2A receptor that induces excitation of PFC parvalbumin neurons may provide another potential target in schizophrenia (Fig. 4). 5-HT2A receptors are G protein-coupled, metabotropic receptors that facilitate neuronal excitability by reducing outward potassium currents and by stimulating phospholipase C (for review, see [110]). The 5-HT2A receptor subtype has been reported to be preferentially expressed in parvalbumin neurons in monkey PFC [65] (Fig. 4). Furthermore, activation of the 5-HT2A receptor appears to increase the activity of GABA neurons [117]. For example, 5-HT2A agonists increase extracellular GABA levels in rat frontal cortex and increase Fos protein expression in GAD67-immunoreactive neurons [1]. In addition, 5-HT2A receptor antagonists inhibit GABA release in rat frontal cortex [25].
However, several potential limitations to the use of the 5-HT2A receptor as a pharmacological target for improving cognition in schizophrenia must be considered. First, a 5-HT2A receptor agonist may increase parvalbumin neuron activity in a manner that exceeds the normal regulation of parvalbumin neuron firing, and may actually disrupt regulation of pyramidal neuron synchrony. Indeed, non-selective agonists of the 5-HT2A receptor, such as LSD, can induce psychosis. Furthermore, the atypical antipsychotic agents, which may improve some aspects of cognitive functioning in schizophrenia [13,50,135], actually antagonize the 5-HT2A receptor. Also, in monkey PFC, the 5-HT2A receptor is found in some inhibitory neurons that do not express parvalbumin, and also in the apical dendrites of pyramidal neurons [65], where 5-HT2A receptor agonists induce excitatory postsynaptic potentials [2]. Thus, drugs targeting the 5-HT2A receptor would not activate parvalbumin neurons in a cell-selective manner. Finally, several radiolabeled ligand binding studies have reported decreased 5-HT2A receptor levels in the PFC in schizophrenia [8,17,28,72,91], suggesting that the amount of 5-HT2A receptor available as substrate for pharmacological agents may be reduced in schizophrenia.
The 5-HT3A receptor is a cation-selective ligand-gated ion channel that may mediate fast synaptic neurotransmission (for review, see [110]). In rodent and monkey PFC, the 5-HT3A receptor is highly co-localized with markers of inhibitory neurons [35,65] and appears to be primarily restricted to cholecystokinin-containing GABA neurons, which provide inhibitory input to pyramidal neuron cell bodies (Fig. 4) [35,93]. Stimulation of the 5-HT3A receptor results in rapid depolarization of cholecystokinin neurons [35]. However, similar to concerns for the 5-HT2A receptor, it is unclear if increasing the firing rate of cholecystokinin neurons, rather than augmenting GABA release when cholecystokinin neurons fire normally, would improve or impair gamma synchronous pyramidal neuron activity. Furthermore, it is unclear whether the augmentation of cholecystokinin neuron-mediated inhibition can indeed partially compensate for a primary loss of parvalbumin neuron-mediated inhibition to PFC pyramidal neurons in schizophrenia.
Muscarinic Receptors of the Acetylcholine System
Another potential strategy for improving pyramidal cell synchronization involves targeting receptors that are selectively expressed presynaptically in parvalbumin neuron axon terminals and that augment the release of GABA during neuronal firing. For example, the M2 subtype of the muscarinic acetylcholine receptor is expressed presynaptically in the majority of parvalbumin-containing axon terminals that target pyramidal neuron axon initial segments and cell bodies (Fig. 4) [52]. In addition, carbachol, a cholinergic agonist, induces gamma oscillations in rat hippocampus, which can be blocked by muscarinic antagonists [15]. Furthermore, the anticholinergic side effects of a wide variety of drugs have been associated with greater cognitive impairment in schizophrenia [89]. Thus, the M2 receptor may represent a target for pharmacologically modulating parvalbumin neuron regulation of pyramidal neuron oscillations.
However, it is unclear whether the cholinergic enhancement of gamma oscillations is primarily due to the M2 receptor on parvalbumin axon terminals or through the other subtypes of muscarinic receptors. Second, the effect of stimulating M2 receptors on the perisomatic release of GABA and on pyramidal neuron activity in the PFC has not been directly demonstrated. Third, while an anticholinergic agent may have deleterious effects on cognitive functioning, it is unclear whether a cholinergic agonist would necessarily have the opposite effect. Fourth, presynaptic M2 receptors are also present in excitatory axon terminals, at least in cat visual cortex [34], and M2 immunoreactivity is also observed in dendrites of inhibitory neurons that are not parvalbumin-immunoreactive [34,52]. Thus, M2-selective agents may have more effects on other excitatory and inhibitory neurons than on parvalbumin-containing terminals. Consequently, studies are needed to identify other targets that are selectively expressed in parvalbumin neuron axon terminals and that selectively augment the release of GABA during parvalbumin neuron firing.
The CB1 Receptor of the Endocannabinoid System
The abuse of cannabis, or Δ9-tetrahydrocannibol (the psychoactive ingredient of hashish and marijuana), is prevalent in the schizophrenia population [111]. Interestingly, several lines of evidence suggest that cannabis abuse may influence the onset and clinical course of schizophrenia. For example, cannabis abusers have a relatively high prevalence of a comorbid schizophrenia diagnosis [111], and cannabis abuse prior to age 18 has been associated with increased risk of developing schizophrenia later in life [6,142]. In addition, the majority of patients with schizophrenia who abuse cannabis began using it at least one year prior to the onset of symptoms of schizophrenia [5,54], and the use of cannabis may be associated with an earlier onset of schizophrenia [77]. Furthermore, cannabis abuse in schizophrenia is not associated with differences in measures of anhedonia, suggesting that cannabis abuse may not be a form of self-medication [32]. Finally, cannabis abuse is also associated with worse outcomes in patients with schizophrenia, including earlier and more frequent relapses [77,83,95]. While other shared factors cannot be excluded (i.e. early cannabis abuse may be comorbid with subclinical prodromal symptoms of schizophrenia), these data suggest that cannabis abuse may help precipitate schizophrenia in vulnerable individuals and worsen the prognosis in individuals who continue to use cannabis.
An endogenous system of compounds with cannabis-mimicking activity, including anandamide and 2-arachido-noylglycerol, has recently been discovered (for review, see [39]). Endocannabinoids are lipid-based compounds that appear to be synthesized within the plasma membrane and are released extracellularly through passive diffusion or facilitated transport by a lipid-binding protein, and not through vesicular secretion. Endocannabinoids exert their effects through the family of cannabinoid (CB) receptors, which includes the CB1 receptor, the only CB receptor presently known to be located in the brain. CB receptors appear to act through a G-protein coupled signaling system which inhibits adenylate cyclase and voltage-gated calcium channels and activates potassium channels.
Interestingly, CB1 receptors appear to be primarily located presynaptically in GABA axon terminals that target pyramidal cell bodies in rat and human hippocampus [51,67,68,127]. Furthermore, the CB1 receptor is selectively expressed in cholecystokinin neurons, but not in parvalbumin neurons (Fig. 4) [67,82,127]. Cholecystokinin neurons provide inhibitory axon terminals that synapse onto pyramidal neuron soma [98,102] and may superimpose a “fine tuning” mechanism upon oscillating networks of synchronized pyramidal neurons that have been entrained by parvalbumin neurons [38].
Activation of CB1 receptors located on presynaptic GABA axon terminals appears to reduce inhibitory neurotransmission in hippocampal pyramidal neurons. For example, CB1 receptor agonists decrease the amplitude and frequency of GABAA receptor-mediated inhibitory postsynaptic currents in rat hippocampal pyramidal neurons [51,60,61,68], and also decrease GABA release in human hippocampal slices [67]. In addition, endocannabinoids appear to mediate depolarization-induced suppression of inhibition (DSI), a retrograde mechanism in which rapidly firing pyramidal neurons can down-regulate their own perisomatic inhibitory input. In DSI, prolonged firing of a pyramidal neuron, and subsequent elevations in the intracellular calcium levels, results in the retrograde release of endocannabinoids, which then act on presynaptic CB1 receptors located in cholecystokinin axon terminals to reduce proximal inhibitory input to that same pyramidal neuron [71,99,138]. For example, in rat hippocampus and cerebellum, DSI is blocked by CB1 receptor antagonists, and DSI is absent in CB1 receptor knockout mice [71,99,138,141].
These data suggest that CB1 receptors on cholecystokinin neuron axon terminals may play an important role in regulating proximal inhibitory input to pyramidal neurons during periods of repetitive firing, such as during gamma oscillations [51]. Indeed, the application of a CB1 receptor agonist reduces the magnitude of gamma oscillations in rat hippocampal slices [51], suggesting that the release of endocannabinoids disengages individual pyramidal neurons from synchronous, gamma oscillatory firing with other pyramidal neurons. Thus, endocannabinoids may play a role in regulating synchronous firing of pyramidal neurons and consequently working memory. Indeed, cannabis abuse has been associated with impairments in some cognitive tasks linked to the PFC, including working memory, attention, and other executive functions [37,105].
However, the anatomical distribution and physiological role of CB1 receptors in human PFC has not been carefully described yet. For example, it has not yet been shown that CB1 receptors in the PFC are also localized presynaptically in inhibitory terminals targeting pyramidal neuron bodies. In addition, it has not yet been demonstrated that activation of CB1 receptors mediates DSI in pyramidal neurons in monkey PFC. However, CB1 receptor agonists have been reported to decrease extracellular GABA levels in the frontal cortex of rats [36]. Thus, additional studies are necessary to further characterize the role of CB1 receptors in modulating inhibitory neurotransmission in monkey PFC.
Nonetheless, a potential pathophysiological process that may contribute to the increased susceptibility to schizophrenia in individuals who abuse cannabis may involve multiple impairments in proximal inhibitory input to PFC pyramidal neurons. For example, subjects with schizophrenia appear to have a reduction in inhibitory input to the axon initial segment of PFC pyramidal neurons, as described above (Fig. 3). The concomitant use of cannabis may cause an additional reduction in perisomatic inhibition to PFC pyramidal neurons from another population of GABA neurons, cholecystokinin neurons. Thus, two sources of proximal inhibitory input may be reduced in individuals who 1) have an underlying deficit in chandelier cell inhibition of pyramidal neuron axon initial segments, and 2) have superimposed cannibis-induced reduction of cholecystokinin neuron-mediated perisomatic inhibition to pyramidal neurons. Interestingly, peripubertal rats appear to be particularly susceptible to the deleterious effects of CB1 agonist administration on various short-term memory tasks, even after sustained drug-free intervals [114]. Thus, the combination of disease-specific and drug-induced reductions in perisomatic inhibitory input to PFC pyramidal neurons may precipitate or worsen cognitive dysfunction in schizophrenia.
These data suggest that in addition to the prevention and cessation of continued cannabis-abuse, perhaps the use of a CB1 receptor antagonist may improve proximal inhibitory input to pyramidal neurons, and possibly some aspects of working memory dysfunction, in schizophrenia (Fig. 4). Importantly, CB1 receptor antagonists would be predicted to selectively improve the maintenance of synchronous firing in pyramidal neurons, since prolonged firing of pyramidal neurons is required for endocannabinoids to be released and for DSI to occur [138,141]. Furthermore, the administration of a CB1 receptor antagonist appears to improve some aspects of short-term memory, at least in rodents [125]. Together, these data suggest that studies examining the effects of CB1 receptor antagonists on the synchronous firing of PFC pyramidal neurons and on performance of PFC-related cognitive tasks may be warranted.
Additionally, a pharmaceutical “cocktail” including a CB1 receptor antagonist and a GABAA receptor α2 agonist may provide a dual mechanism of improving inhibition to the cell body of PFC pyramidal neurons in a manner that incorporates the normal firing patterns of cholecystokinin neurons. CB1 receptors and α2-containing GABAA receptors are found pre- and postsynaptically, respectively, in cholecystokinin axon terminals that synapse at pyramidal cell bodies [67,98]. Thus, a CB1 receptor antagonist and an α2 agonist would enhance cholecystokinin neuron-mediated inhibitory input to PFC pyramidal neuron cell bodies by blocking endocannabinoid-mediated reductions in inhibition and enhancing GABA-mediated inhibitory postsynaptic potentials, respectively. Furthermore, this effect would be maximal during periods of gamma synchrony, since repetitive pyramidal neuron firing is necessary for endocannabinoids to be released. However, further studies are needed to determine whether the augmentation of cholecystokinin neuron inhibition can indeed partially compensate for a primary loss of parvalbumin neuron inhibition to pyramidal neurons in schizophrenia, and whether the effects of α2 agonists are synergistic at pyramidal soma and axon initial segments.
An additional potential limitation in utilizing the endocannabinoid system as a pharmacological target in schizophrenia is that CB1 receptors are also highly expressed in other brain regions, particularly in the hippocampus and certain nuclei of the amygdala [44,126]2. Furthermore, the CB1 receptor may be important for other processes including long-term potentiation in the hippocampus. While CB1 receptor agonists have been demonstrated to actually impair long-term potentiation in the hippocampus [101], the effects of a CB1 receptor antagonist on long-term potentiation are unclear and need to be more thoroughly studied. Interestingly, an initial radiolabeled-ligand binding study suggests that CB1 receptors may be preferentially expressed at higher levels in association cortices, including the PFC, in humans [44]. Furthermore, elevated levels of radiolabeled-ligand binding to the CB1 receptor in the PFC have been reported in subjects with schizophrenia compared to control subjects [29]. These initial studies at least suggest that the elevated levels of CB1 receptors in the PFC in subjects with schizophrenia may enable the use of a smaller dose of a CB1 receptor antagonist that would selectively improve inhibition in the PFC while minimizing detrimental effects in other brain regions. Therefore, additional studies are needed to characterize CB1 receptor expression in the PFC in both normal controls and subjects with schizophrenia.
CONCLUSIONS
In summary, cognitive impairments, including working memory deficits, are among the most debilitating and chronic aspects of schizophrenia, and new strategies informed by the pathophysiology of the illness are needed to develop novel therapies [63]. Designing drugs which selectively remediate deficiencies in parvalbumin neuron-mediated inhibition of PFC pyramidal neurons may provide a mechanism to improve synchronous firing of pyramidal neurons, and consequently, working memory dysfunction in schizophrenia. In particular, benzodiazepine-like agents which target the α2 subunit-containing GABAA receptors may provide a means to improve chandelier cell-mediated regulation of pyramidal neuron firing without potentially disrupting chandelier cell firing patterns. In addition, a CB1 receptor antagonist may provide an additional, comple-mechanism for improving inhibition to pyramidal neuron cell bodies, particularly during periods of repetitive firing such as in gamma synchrony. In the future, studies such as single cell population gene expression profiling techniques [16,59], are needed to identify other parvalbumin neuron-specific targets. Finally, developing a circuit-specific model of chandelier neuron dysfunction, such as a spatially-restricted reduction of GAD67 mRNA expression in parvalbumin neurons [92,94], may provide an important tool to determine whether the novel targets proposed could enhance parvalbumin neuron-mediated regulation of gamma synchronous pyramidal neuron firing in the PFC, and potentially alleviate working memory dysfunction in schizophrenia.
Acknowledgments
Studies conducted by the authors were supported by NIH grants MH 43784, MH 51234, MH 45156 and MH 64320.
Footnotes
Cho, R., Carter, C.S. Impaired task-set maintenance and frontal cortical gamma-band synchrony in schizophrenia. Cognitive Neurosci. Soc. Annual Meeting Abstr., 2004.
Eggan, S.M., Lewis, D.A. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate brain: A regional and laminar analysis. Soc. Neurosci. Abstr., 273, 1, 2004.
References
- 1.Abi-Saab WM, Bubser M, Roth RH, Deutch AY. 5-HT2 Receptor Regulation of Extracellular GABA Levels in the Prefrontal Cortex. Neuropsychopharm. 1999;20:92–6. doi: 10.1016/S0893-133X(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian GK, Marek GJ. Serotonin Induces Excitatory Postsynaptic Potentials in Apical Dendrites of Neocortical Pyramidal Cells. Neuro-pharmacology. 1997;36:589–99. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 3.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene Expression for Glutamic Acid Decarboxylase Is Reduced Without Loss of Neurons in Prefrontal Cortex of Schizophrenics. Arch Gen Psychiatry. 1995;52:258–66. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 4.Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-Specific Alterations in the Dopamine Innervation of the Prefrontal Cortex in Schizophrenic Subjects. Am J Psychiatry. 1999;156:1580–9. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- 5.Allebeck P, Adamsson C, Engstrom A, Rydberg U. Cannabis and Schizophrenia: A Longitudinal Study of Cases Treated in Stockholm County. Acta Psychiatr Scand. 1993;88:21–4. doi: 10.1111/j.1600-0447.1993.tb03408.x. [DOI] [PubMed] [Google Scholar]
- 6.Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and Schizophrenia. A Longitudinal Study of Swedish Conscripts. Lancet. 1987;2:1483–6. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- 7.Arndt S, Andreasen NC, Flaum M, Miller D, Nopoulos P. A Longitudinal Study of Symptom Dimensions in Schizophrenia: Prediction and Patterns of Change. Arch Gen Psychiatry. 1995;52:352–60. doi: 10.1001/archpsyc.1995.03950170026004. [DOI] [PubMed] [Google Scholar]
- 8.Arora RC, Meltzer HY. Serotonin2 (5-HT2) Receptor Binding in the Frontal Cortex of Schizophrenic Patients. J Neural Transm. 1991;85:19–29. doi: 10.1007/BF01244654. [DOI] [PubMed] [Google Scholar]
- 9.Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM. The 5-HT1A Serotonin Receptor Is Located on Calbindin-and Parvalbumin-Contining Neurons in the Rat Brain. Brain Res. 2003;959:58–67. doi: 10.1016/s0006-8993(02)03727-7. [DOI] [PubMed] [Google Scholar]
- 10.Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective Deficits in Prefrontal Cortical GABAergic Neurons in Schizophrenia Defined by the Presence of Calcium-Binding Proteins. Biol Psychiatry. 2002;52:708–15. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- 11.Benes FM, Berretta S. GABAergic Interneurons: Implications for Understanding Schizophrenia and Bipolar Disorder. Neuropsychopharm. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 12.Benes FM, Snyder-Marie A, Vincent S, Khan Y. Up-Regulation of GABA-A Receptor Binding on Neurons of the Prefrontal Cortex in Schizophrenic Subjects. Neuroscience. 1996;75:1021–31. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- 13.Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA. Neurocognitive Effects of Clozapine, Olanzapine, Risperdone, and Haloperidol in Patients With Chronic Schizophrenia or Schizoaffective Disorder. Am J Psychiatry. 2002;159:1018–28. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- 14.Breier A, Schreiber JL, Dyer J, Pickar D. National Institute of Mental Health Longitudinal Study of Chronic Schizophrenia. Arch Gen Psychiatry. 1991;48:239–46. doi: 10.1001/archpsyc.1991.01810270051007. [DOI] [PubMed] [Google Scholar]
- 15.Buhl EH, Tamas G, Fisahn A. Cholinergic Activation and Tonic Excitation Induce Persistent Gamma Oscillations in Mouse Somatosensory Cortex in vitro. J Physiol. 1998;513(Pt 1):117–26. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnet PW, Eastwood SL, Harrison PJ. Laser-Assisted Microdissection: Methods for the Molecular Analysis of Psychiatric Disorders at a Cellular Resolution. Biol Psychiatry. 2004;55:107–11. doi: 10.1016/s0006-3223(03)00642-5. [DOI] [PubMed] [Google Scholar]
- 17.Burnet PWJ, Eastwood SL, Harrison PJ. 5-HT1A and 5-HT2A Receptor MRNAs and Binding Site Densities Are Differentially Altered in Schizophrenia. Neuropsychopharm. 1996;15:442–55. doi: 10.1016/S0893-133X(96)00053-X. [DOI] [PubMed] [Google Scholar]
- 18.Callicot JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological Dysfunction of the Dorsolateral Prefrontal Cortex in Schizophrenia Revisited. Cereb Cortex. 2000;10:1078–92. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 19.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of Prefrontal Cortical Dysfunction in Schizophrenia: More Than Up or Down. Am J Psychiatry. 2003;160:2209–15. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter WT, Jr, Buchanan RW, Kirkpatrick B, Breier AF. Diazepam Treatment of Early Signs of Exacerbation in Schizophrenia. Am J Psychiatry. 1999;156:299–303. doi: 10.1176/ajp.156.2.299. [DOI] [PubMed] [Google Scholar]
- 21.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional Hypofrontality and Working Memory Dysfunction in Schizophrenia. Am J Psychiatry. 1998;155:1285–7. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 22.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of Neuronal Activity in Hippocampus by Individual GABAergic Interneurons. Nature. 1995;378:75–8. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 23.Condé F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local Circuit Neurons Immunoreactive for Calretinin, Calbindin D-28k, or Parvalbumin in Monkey Prefrontal Cortex: Distribution and Morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 24.Constantinidis C, Williams GV, Goldman-Rakic PS. A Role for Inhibition in Shaping the Temporal Flow of Information in Prefrontal Cortex. Nat Neurosci. 2002;5:175–80. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- 25.Cozzi NV, Nichols DE. 5-HT2A Receptor Antagonists Inhibit Potassium-Stimulated Gamma-Aminobutyric Acid Release in Rat Frontal Cortex. Eur J Pharmacol. 1996;309:25–31. doi: 10.1016/0014-2999(96)00325-1. [DOI] [PubMed] [Google Scholar]
- 26.Cruz DA, Eggan SM, Azmitia EC, Lewis DA. Serotonin1A Receptors at the Axon Initial Segment of Prefrontal Pyramidal Neurons in Schizophrenia. Am J Psychiatry. 2004;161:739–42. doi: 10.1176/appi.ajp.161.4.739. [DOI] [PubMed] [Google Scholar]
- 27.Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M. Behavioral and Intellectual Markers for Schizophrenia in Apparently Healthy Male Adolescents. Am J Psychiatry. 1999;156:1328–35. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- 28.Dean B, Hayes W. Decreased Frontal Cortical Serotonin2A Receptors in Schizophrenia. Schizophr Res. 1996;21:133–9. doi: 10.1016/0920-9964(96)00034-5. [DOI] [PubMed] [Google Scholar]
- 29.Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 Binding in the Human Central Nervous System: Regional Specific Changes in Density of Cannabinoid-1 Receptors Associated With Schizophrenia and Cannabis Use. Neuroscience. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- 30.DeFelipe J. Types of Neurons, Synaptic Connections and Chemical Characteristics of Cells Immunoreactive for Calbindin-D28K, Parvalbumin and Calretinin in the Neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- 31.DeFelipe J, Arellano JI, Gómez A, Azmitia EC, Muñoz A. Pyramidal Cell Axons Show a Local Specialization for GABA and 5-HT Inputs in Monkey and Human Cerebral Cortex. J Comp Neurol. 2001;433:148–55. doi: 10.1002/cne.1132. [DOI] [PubMed] [Google Scholar]
- 32.Dervaux A, Bayle FJ, Laqueille X, Bourdel MC, Le Borgne MH, Olie JP, Krebs MO. Is Substance Abuse in Schizophrenia Related to Impulsivity, Sensation Seeking, or Anhedonia? Am J Psychiatry. 2001;158:492–4. doi: 10.1176/appi.ajp.158.3.492. [DOI] [PubMed] [Google Scholar]
- 33.Elvevåg B, Goldberg TE. Cognitive Impairment in Schizophrenia Is the Core of the Disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- 34.Erisir A, Levey AI, Aoki C. Muscarinic Receptor M(2) in Cat Visual Cortex: Laminar Distribution, Relationship to Gamma-Aminobutyric Acidergic Neurons, and Effect of Cingulate Lesions. J Comp Neurol. 2001;441:168–85. doi: 10.1002/cne.1405. [DOI] [PubMed] [Google Scholar]
- 35.Ferezou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 Receptors Mediate Serotonergic Fast Synaptic Excitation of Neocortical Vasoactive Intestinal Peptide/Cholecystokinin Inter-neurons. J Neurosci. 2002;22:7389–97. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferraro L, Tomasini MC, Cassano T, Bebe BW, Siniscalchi A, O’Connor WT, Magee P, Tanganelli S, Cuomo V, Antonelli T. Cannabinoid Receptor Agonist WIN 55,212-2 Inhibits Rat Cortical Dialysate Gamma-Aminobutyric Acid Levels. J Neurosci Res. 2001;66:298–302. doi: 10.1002/jnr.1224. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, Morris R, Krauskopf D, Satz P. Cognitive Correlates of Long-Term Cannabis Use in Costa Rican Men. Arch Gen Psychiatry. 1996;53:1051–7. doi: 10.1001/archpsyc.1996.01830110089011. [DOI] [PubMed] [Google Scholar]
- 38.Freund TF. Interneuron Diversity Series: Rhythm and Mood in Perisomatic Inhibition. Trends Neurosci. 2003;26:489–95. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 39.Freund TF, Katona I, Piomelli D. Role of Endogenous Cannabinoids in Synaptic Signaling. Physiol Rev. 2003;83:1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 40.Freund TF, Martin KAC, Smith AD, Somogyi P. Glutamate Decarboxylase-Immunoreactive Terminals of Golgi-Impregnated Axoaxonic Cells and of Presumed Basket Cells in Synaptic Contact With Pyramidal Neurons of the Cat’s Visual Cortex. J Comp Neurol. 1983;221:263–78. doi: 10.1002/cne.902210303. [DOI] [PubMed] [Google Scholar]
- 41.Fritschy JM, Mohler H. GABAA-Receptor Heterogeneity in the Adult Rat Brain: Differential Regional and Cellular Distribution of Seven Major Subunits. J Comp Neurol. 1995;359:154–94. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 42.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic Coding of Visual Space in the Monkey’s Dorsolateral Prefrontal Cortex. J Neurophysiol. 1989;61:331–49. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 43.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal Neuronal Activity in Rhesus Monkeys Performing a Delayed Anti-Saccade Task. Nature. 1993;365:753–6. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 44.Glass M, Dragunow M, Faull RL. Cannabinoid Receptors in the Human Brain: A Detailed Anatomical and Quantitative Autoradiographic Study in the Fetal, Neonatal and Adult Human Brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 45.Goldman-Rakic PS. Cellular Basis of Working Memory. Neuron. 1995;14:477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 46.Green MF. What Are the Functional Consequences of Neurocognitive Deficits in Schizophrenia? Am J Psychiatry. 1996;153:321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 47.Grunschlag CR, Haas HL, Stevens DR. 5-HT Inhibits Lateral Entorhinal Cortical Neurons of the Rat In Vitro by Activation of Potassium Channel-Coupled 5-HT1A Receptors. Brain Res. 1997;770:10–7. doi: 10.1016/s0006-8993(97)00738-5. [DOI] [PubMed] [Google Scholar]
- 48.Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in Reelin and Glutamic Acid Decarboxylase67 (GAD67) Expression in Schizophrenia and Bipolar Disorder. Arch Gen Psychiatry. 2000;57:1061–9. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 49.Gupta A, Wang Y, Markram H. Organizing Principles for a Diversity of GABAergic Interneurons and Synapses in the Neocortex. Science. 2000;287:273–8. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 50.Gur RE, Kohler C, Ragland JD, Siegel SJ, Bilker WB, Loughead J, Phend N, Gur RC. Neurocognitive Performance and Clinical Changes in Olanzapine-Treated Patients With Schizophrenia. Neuropsychopharm. 2003;28:2029–36. doi: 10.1038/sj.npp.1300275. [DOI] [PubMed] [Google Scholar]
- 51.Hajos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, Freund TF. Cannabi-noids Inhibit Hippocampal GABAergic Transmission and Network Oscillations. Eur J Neurosci. 2000;12:3239–49. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 52.Hajos N, Papp EC, Acsady L, Levey AI, Freund TF. Distinct Interneuron Types Express M2 Muscarinic Receptor Immunoreactivity on Their Dendrites or Axon Terminals in the Hippocampus. Neuroscience. 1998;82:355–76. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- 53.Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-Wide Expression Analysis Reveals Dysregulation of Myelination-Related Genes in Chronic Schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–51. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hambrecht M, Hafner H. Cannabis, Vulnerability, and the Onset of Schizophrenia: an Epidemiological Perspective. Aust N Z J Psychiatry. 2000;34:468–75. doi: 10.1080/j.1440-1614.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- 55.Harvey PD, Howanitz E, Parrella M, White L, Davidson M, Mohs RC, Hoblyn J, Davis KL. Symptoms, Cognitive Functioning, and Adaptive Skills in Geriatric Patients With Lifelong Schizophrenia: A Comparison Across Treatment Sites. Am J Psychiatry. 1998;155:1080–6. doi: 10.1176/ajp.155.8.1080. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto T, Nishino N, Nakai H, Tanaka C. Increase in Serotonin 5-HT1A Receptors in Prefrontal and Temporal Cortices of Brains From Patients With Chronic Schizophrenia. Life Sci. 1991;48:355–63. doi: 10.1016/0024-3205(91)90556-q. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene Expression Deficits in a Subclass of GABA Neurons in the Prefrontal Cortex of Subjects With Schizophrenia. J Neurosci. 2003;23:6315–26. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heaton R, Paulsen JS, McAdams LA, Kuck J, Zisook S, Braff D, Harris J, Jeste DV. Neuropsychological Deficits in Schizophrenics: Relationship to Age, Chronicity, and Dementia. Arch Gen Psychiatry. 1994;51:469–76. doi: 10.1001/archpsyc.1994.03950060033003. [DOI] [PubMed] [Google Scholar]
- 59.Hemby SE, Ginsberg SD, Brunk B, Arnold SE, Trojanowski JQ, Eberwine JH. Gene Expression Profile for Schizophrenia: Discrete Neuron Transcription Patterns in the Entorhinal Cortex. Arch Gen Psychiatry. 2002;59:631–40. doi: 10.1001/archpsyc.59.7.631. [DOI] [PubMed] [Google Scholar]
- 60.Hoffman AF, Lupica CR. Mechanisms of Cannabinoid Inhibition of GABA(A) Synaptic Transmission in the Hippocampus. J Neurosci. 2000;20:2470–9. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffman AF, Riegel AC, Lupica CR. Functional Localization of Cannabinoid Receptors and Endogenous Cannabinoid Production in Distinct Neuron Populations of the Hippocampus. Eur J Neurosci. 2003;18:524–34. doi: 10.1046/j.1460-9568.2003.02773.x. [DOI] [PubMed] [Google Scholar]
- 62.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma Oscillations Correlate With Working Memory Load in Humans. Cereb Cortex. 2003;13:1369–74. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 63.Hyman SE, Fenton WS. Medicine. What Are the Right Targets for Psychopharmacology? Science. 2003;299:350–1. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
- 64.Inoue M, Mikami A, Ando I, Tsukada H. Functional Brain Mapping of the Macaque Related to Spatial Working Memory As Revealed by PET. Cereb Cortex. 2004;14:106–19. doi: 10.1093/cercor/bhg109. [DOI] [PubMed] [Google Scholar]
- 65.Jakab RL, Goldman-Rakic PS. Segregation of Serotonin 5-HT2A and 5-HT3 Receptors in Inhibitory Circuits of the Primate Cerebral Cortex. J Comp Neurol. 2000;417:337–48. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 66.Joho RH, Ho CS, Marks GA. Increased Gamma- and Decreased Delta-Oscillations in a Mouse Deficient for a Potassium Channel Expressed in Fast-Spiking Interneurons. J Neurophysiol. 1999;82:1855–64. doi: 10.1152/jn.1999.82.4.1855. [DOI] [PubMed] [Google Scholar]
- 67.Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, Mackie K, Vizi ES, Freund TF. GABAergic Interneurons Are the Targets of Cannabinoid Actions in the Human Hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- 68.Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically Located CB1 Cannabinoid Receptors Regulate GABA Release From Axon Terminals of Specific Hippocampal Interneurons. J Neurosci. 1999;19:4544–58. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawaguchi Y. Physiological Subgroups of Nonpyramidal Cells With Specific Morphological Characteristics in Layer II/III of Rat Frontal Cortex. J Neurosci. 1995;15:2638–55. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawaguchi Y, Kubota Y. Neurochemical Features and Synaptic Connections of Large Physiologically-Identified GABAergic Cells in the Rat Frontal Cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- 71.Kreitzer AC, Regehr WG. Cerebellar Depolarization-Induced Suppression of Inhibition Is Mediated by Endogenous Cannabinoids. J Neurosci. 2001;21RC174:1–5. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE. Selective Abnormalities of Prefrontal Serotonergic Receptors in Schizophrenia. A Postmortem Study. Arch Gen Psychiatry. 1993;50:810–8. doi: 10.1001/archpsyc.1993.01820220066007. [DOI] [PubMed] [Google Scholar]
- 73.Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and Deactivation Rates of Recombinant GABAA Receptor Channels Are Dependent on α-Subunit Isoform. Biophysical Journal. 1997;73:2518–26. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous Gamma Activity: A Review and Contribution to an Integrative Neuroscience Model of Schizophrenia. Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 75.Levitan ES, Schofield PR, Burt DR, Rhee LM, Wisden W, Kohler M, Fujita N, Rodriguez HF, Stephenson A, Darlison MG, Barnard EA, Seeburg PH. Structural and Functional Basis for GABAA Receptor Heterogeneity. Nature. 1988;335:76–9. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- 76.Lewis DA, Lund JS. Heterogeneity of Chandelier Neurons in Monkey Neocortex: Corticotropin-Releasing Factor and Parvalbumin Immunoreactive Populations. J Comp Neurol. 1990;293:599–615. doi: 10.1002/cne.902930406. [DOI] [PubMed] [Google Scholar]
- 77.Linszen DH, Dingemans PM, Lenior ME. Cannabis Abuse and the Course of Recent-Onset Schizophrenic Disorders. Arch Gen Psychiatry. 1994;51:273–9. doi: 10.1001/archpsyc.1994.03950040017002. [DOI] [PubMed] [Google Scholar]
- 78.Loup F, Weinmann O, Yonekawa Y, Aguzzi A, Wieser HG, Fritschy JM. A Highly Sensitive Immunoflourescence Procedure for Analyzing the Subcellular Distribution of GABAA Receptor Subunits in the Human Brain. J Histochem Cytochem. 1998;46:1129–39. doi: 10.1177/002215549804601005. [DOI] [PubMed] [Google Scholar]
- 79.Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and Neuronal Substrate for the Selective Attenuation of Anxiety. Science. 2000;290:131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 80.Lund JS, Lewis DA. Local Circuit Neurons of Developing and Mature Macaque Prefrontal Cortex: Golgi and Immunocytochemical Characteristics. J Comp Neurol. 1993;328:282–312. doi: 10.1002/cne.903280209. [DOI] [PubMed] [Google Scholar]
- 81.Mahurin RK, Velligan DI, Miller AL. Executive-Frontal Lobe Cognitive Dysfunction in Schizophrenia: A Symptom Subtype Analysis. Psychiatry Res. 1998;79:139–49. doi: 10.1016/s0165-1781(98)00031-6. [DOI] [PubMed] [Google Scholar]
- 82.Marsicano G, Lutz B. Expression of the Cannabinoid Receptor CB1 in Distinct Neuronal Subpopulations in the Adult Mouse Forebrain. Eur J Neurosci. 1999;11:4213–25. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 83.Martinez-Arevalo MJ, Calcedo-Ordonez A, Varo-Prieto JR. Cannabis Consumption As a Prognostic Factor in Schizophrenia. Br J Psychiatry. 1994;164:679–81. doi: 10.1192/bjp.164.5.679. [DOI] [PubMed] [Google Scholar]
- 84.McBain CJ, Fisahn A. Interneurons Unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 85.Melchitzky DS, González-Burgos G, Barrionuevo G, Lewis DA. Synaptic Targets of the Intrinsic Axon Collaterals of Supragranular Pyramidal Neurons in Monkey Prefrontal Cortex. J Comp Neurol. 2001;430:209–21. doi: 10.1002/1096-9861(20010205)430:2<209::aid-cne1026>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 86.Melchitzky DS, Lewis DA. Pyramidal Neuron Local Axon Terminals in Monkey Prefrontal Cortex: Differential Targeting of Subclasses of GABA Neurons. Cereb Cortex. 2003;13:452–60. doi: 10.1093/cercor/13.5.452. [DOI] [PubMed] [Google Scholar]
- 87.Melchitzky DS, Sesack SR, Lewis DA. Parvalbumin-Immunoreactive Axon Terminals in Macaque Monkey and Human Prefrontal Cortex: Laminar, Regional and Target Specificity of Type I and Type II Synapses. J Comp Neurol. 1999;408:11–22. [PubMed] [Google Scholar]
- 88.Melchitzky DS, Sesack SR, Pucak ML, Lewis DA. Synaptic Targets of Pyramidal Neurons Providing Intrinsic Horizontal Connections in Monkey Prefrontal Cortex. J Comp Neurol. 1998;390:211–24. doi: 10.1002/(sici)1096-9861(19980112)390:2<211::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 89.Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of Anticholinergic Load With Impairment of Complex Attention and Memory in Schizophrenia. Am J Psychiatry. 2004;161:116–24. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- 90.Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular Characterization of Schizophrenia Viewed by Microarray Analysis of Gene Expression in Prefrontal Cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 91.Mita T, Hanada S, Nishino N, Kuno T, Nakai H, Yamadori T, Mizoi Y, Tanaka C. Decreased Serotonin S2 and Increased Dopamine D2 Receptors in Chronic Schizophrenics. Biol Psychiatry. 1986;21:1407–14. doi: 10.1016/0006-3223(86)90332-x. [DOI] [PubMed] [Google Scholar]
- 92.Monyer H, Markram H. Interneuron Diversity Series: Molecular and Genetic Tools to Study GAGAergic Interneuron Diversity and Function. Trends Neurosci. 2004;27:90–7. doi: 10.1016/j.tins.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 93.Morales M, Bloom FE. The 5-HT3 Receptor Is Present in Different Subpopulations of GABAergic Neurons in the Rat Telencephalon. J Neurosci. 1997;17:3157–67. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nagy A. Cre Recombinase: The Universal Reagent for Genome Tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- 95.Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis Affects the Severity of Schizophrenic Symptoms: Results of a Clinical Survey. Psychol Med. 1986;16:515–20. doi: 10.1017/s0033291700010278. [DOI] [PubMed] [Google Scholar]
- 96.Newberry NR, Footitt DR, Papanastassiou V, Reynolds DJ. Actions of 5-HT on Human Neocortical Neurones in Vitro. Brain Res. 1999;833:93–100. doi: 10.1016/s0006-8993(99)01540-1. [DOI] [PubMed] [Google Scholar]
- 97.Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential Synaptic Localization of Two Major Γ-Aminobutyric Acid Type A Receptor α Subunits on Hippocampal Pyramidal Cells. Proc Natl Acad Sci USA. 1996;93:11939–44. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nyíri G, Freund TF, Somogyi P. Input-Dependent Synaptic Targeting of A2-Subunit-Containing GABAA Receptors in Synapses of Hippocampal Pyramidal Cells of the Rat. Eur J Neurosci. 2001;13:428–42. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- 99.Ohno-Shosaku T, Maejima T, Kano M. Endogenous Cannabinoids Mediate Retrograde Signals From Depolarized Postsynaptic Neurons to Presynaptic Terminals. Neuron. 2001;29:729–38. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 100.Park S, Holzman PS. Schizophrenics Show Spatial Working Memory Deficits. Arch Gen Psychiatry. 1992;49:975–82. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 101.Paton GS, Pertwee RG, Davies SN. Correlation Between Cannabinoid Mediated Effects on Paired Pulse Depression and Induction of Long Term Potentiation in the Rat Hippocampal Slice. Neuropharmacology. 1998;37:1123–30. doi: 10.1016/s0028-3908(98)00096-3. [DOI] [PubMed] [Google Scholar]
- 102.Pawelzik H, Hughes DI, Thomson AM. Physiological and Morphological Diversity of Immunocytochemically Defined Parvalbumin- and Cholecystokinin-Positive Interneurones in CA1 of the Adult Rat Hippocampus. J Comp Neurol. 2002;443:346–67. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- 103.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of Prefrontal Cortex Dysfunction to Working Memory and Symptoms in Schizophrenia. Am J Psychiatry. 2001;158:1105–13. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 104.Pierri JN, Chaudry AS, Woo TUW, Lewis DA. Alterations in Chandelier Neuron Axon Terminals in the Prefrontal Cortex of Schizophrenic Subjects. Am J Psychiatry. 1999;156:1709–19. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- 105.Pope HG, Jr, Yurgelun-Todd D. The Residual Cognitive Effects of Heavy Marijuana Use in College Students. JAMA. 1996;275:521–7. [PubMed] [Google Scholar]
- 106.Pouille F, Scanziani M. Enforcement of Temporal Fidelity in Pyramidal Cells by Somatic Feed-Forward Inhibition. Science. 2001;293:1159–63. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 107.Pucak ML, Levitt JB, Lund JS, Lewis DA. Patterns of Intrinsic and Associational Circuitry in Monkey Prefrontal Cortex. J Comp Neurol. 1996;376:614–30. doi: 10.1002/(SICI)1096-9861(19961223)376:4<614::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 108.Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional Tuning of Adjacent Interneurons and Pyramidal Cells During Working Memory: Evidence for Microcolumnar Organization in PFC. J Neurophysiol. 1999;81:1903–16. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- 109.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and Creation of Spatial Tuning by Disinhibition: GABAA Blockade of Prefrontal Cortical Neurons Engaged by Working Memory. J Neurosci. 2000;20:485–94. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of Mechanisms of Serotonin Receptor Signal Transduction. Pharm & Therapeutics. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 111.Reiger DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goddwin FK. Comorbidity of Mental Disorders With Alcohol and Other Drug Abuse. JAMA. 1990;264:2511–8. [PubMed] [Google Scholar]
- 112.Sawaguchi T, Matsumura M, Kubota K. Delayed Response Deficits Produced by Local Injection of Bicuculline into the Dorsolateral Prefrontal Cortex in Japanese Macaque Monkeys. Exp Brain Res. 1989;75:457–69. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
- 113.Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological Deficits in Neuroleptic Naive Patients With First-Episode Schizophrenia. Arch Gen Psychiatry. 1994;51:124–31. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 114.Schneider M, Koch M. Chronic Pubertal, but Not Adult Chronic Cannabinoid Treatment Impairs Sensorimotor Gating, Recognition Memory, and the Performance in a Progressive Ratio Task in Adult Rats. Neuropsychopharm. 2003;28:1760–9. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- 115.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally High Neuronal Density in the Schizophrenic Cortex: A Morphometric Analysis of Prefrontal Area 9 and Occipital Area 17. Arch Gen Psychiatry. 1995;52:805–18. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 116.Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated Neuronal Density in Prefrontal Area 46 in Brains From Schizophrenic Patients: Application of a Three-Dimensional, Stereologic Counting Method. J Comp Neurol. 1998;392:402–12. [PubMed] [Google Scholar]
- 117.Shen RY, Andrade R. 5-Hydroxy-tryptamine2 Receptor Facilitates GABAergic Neuro-transmission in Rat Hippocampus. J Pharmacol Exp Ther. 1998;285:805–12. [PubMed] [Google Scholar]
- 118.Simpson MDC, Lubman DI, Slater P, Deakin JFW. Autoradiography With [3H]8-OH-DPAT Reveals Increases in 5-HT1A Receptors in Ventral Prefrontal Cortex in Schizophrenia. Biol Psychiatry. 1996;39:919–28. doi: 10.1016/0006-3223(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 119.Smiley JF, Goldman-Rakic PS. Serotonergic Axons in Monkey Prefrontal Cerebral Cortex Synapse Predominantly on Interneurons As Demonstrated by Serial Section Electron Microscopy. J Comp Neurol. 1996;367:431–43. doi: 10.1002/(SICI)1096-9861(19960408)367:3<431::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 120.Somogyi P. A Specific Axo-Axonal Interneuron in the Visual Cortex of the Rat. Brain Res. 1977;136:345–50. doi: 10.1016/0006-8993(77)90808-3. [DOI] [PubMed] [Google Scholar]
- 121.Spencer KM, Nestor PG, Salisbury DF, Shenton ME, McCarley RW. Abnormal Neural Synchrony in Schizophrenia. J Neurosci. 2003;23:7407–11. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sumiyoshi T, Stockmeier CA, Overholser JC, Dilley GE, Meltzer HY. Serotonin1A Receptors Are Increased in Postmortem Prefrontal Cortex in Schizophrenia. Brain Res. 1996;708:209–14. doi: 10.1016/0006-8993(95)01361-x. [DOI] [PubMed] [Google Scholar]
- 123.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced Gamma-Band Activity During the Delay of a Visual Short-Term Memory Task in Humans. J Neurosci. 1998;18:4244–54. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tallon-Baudry C, Kreiter A, Bertrand O. Sustained and Transient Oscillatory Responses in the Gamma and Beta Bands in a Visual Short-Term Memory Task in Humans. Vis Neurosci. 1999;16:449–59. doi: 10.1017/s0952523899163065. [DOI] [PubMed] [Google Scholar]
- 125.Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, Soubrie P. Improvement of Memory in Rodents by the Selective CB1 Cannabinoid Receptor Antagonist, SR 141716. Psychopharmacology (Berl) 1996;126:165–72. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- 126.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical Distribution of Cannabinoid CB1 Receptors in the Rat Central Nervous System. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 127.Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 Receptors Are Localized Primarily on Cholecystokinin-Containing GABAergic Interneurons in the Rat Hippocampal Formation. Neuroscience. 1999;93:969–75. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- 128.Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, Freed WJ. Microarray Analysis of Gene Expression in the Prefrontal Cortex in Schizophrenia: A Preliminary Study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- 129.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased GAD67 MRNA Expression in a Subset of Prefrontal Cortical GABA Neurons in Subjects With Schizophrenia. Arch Gen Psychiatry. 2000;57:237–45. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 130.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. GABA Transporter-1 MRNA in the Prefrontal Cortex in Schizophrenia: Decreased Expression in a Subset of Neurons. Am J Psychiatry. 2001;158:256–65. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- 131.Volk DW, Lewis DA. Impaired Prefrontal Inhibition in Schizophrenia: Relevance for Cognitive Dysfunction. Physiol Behav. 2002;77:501–5. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- 132.Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal Alterations in Pre- and Postsynaptic Inhibitory Markers at Chandelier Cell Inputs to Pyramidal Neurons in Schizophrenia. Cereb Cortex. 2002;12:1063–70. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- 133.Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-Deficiency Facilitates Repetitive IPSCs and Gamma Oscillations in the Hippocampus. J Neurophysiol. 2003;89:1414–22. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- 134.Wassef A, Baker J, Kochan LD. GABA and Schizophrenia: a Review of Basic Science and Clinical Studies. J Clin Psychopharmacol. 2003;23:601–40. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- 135.Weickert TW, Goldberg TE, Marenco S, Bigelow LB, Egan MF, Weinberger DR. Comparison of Cognitive Peformances During a Placebo Period and an Atypical Antipsychotic Treatment Period in Schizophrenia: Critical Examination of Confounds. Neuropsychopharm. 2003;28:2217. doi: 10.1038/sj.npp.1300216. [DOI] [PubMed] [Google Scholar]
- 136.Weinberger DR, Berman KF, Zec RF. Physiologic Dysfunction of Dorsolateral Prefrontal Cortex in Schizophrenia. I Regional Cerebral Blood Flow Evidence. Arch Gen Psychiatry. 1986;43:114–24. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 137.Wilson FA, O Scalaidhe SP, Goldman-Rakic PS. Functional Synergism Between Putative Gamma-Aminobutyrate-Containing Neurons and Pyramidal Neurons in Prefrontal Cortex. Proc Natl Acad Sci USA. 1994;91:4009–13. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wilson RI, Nicoll RA. Endogenous Cannabinoids Mediate Retrograde Signalling at Hippocampal Synapses. Nature. 2001;410:588–92. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 139.Woo TU, Miller JL, Lewis DA. Shizophrenia and the Parvalbumin-Containing Class of Cortical Local Circuit Neurons. Am J Psychiatry. 1997;154:1013–5. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- 140.Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A Subclass of Prefrontal Gamma-Aminobutyric Acid Axon Terminals Are Selectively Altered in Schizophrenia. Proc Natl Acad Sci USA. 1998;95:5341–6. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The Cannabinoid CB1 Receptor Mediates Retrograde Signals for Depolarization-Induced Suppression of Inhibition in Cerebellar Purkinje Cells. J Neurosci. 2002;22:1690–7. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self Reported Cannabis Use As a Risk Factor for Schizophrenia in Swedish Conscripts of 1969: Historical Cohort Study. BMJ. 2002;325:1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]