Abstract
Studies have shown that cancer care near the end of life is more aggressive than many patients prefer. Using a cohort of deceased Medicare beneficiaries with poor-prognosis cancer, meaning that they were likely to die within a year, we examined the association between hospital characteristics and eleven end-of-life care measures, such as hospice use and hospitalization. Our study revealed a relatively high intensity of care in the last weeks of life. At the same time, there was more than a twofold variation within hospital groups with common features, such as cancer center designation and for-profit status. We found that these hospital characteristics explained little of the observed variation in intensity of end-of-life cancer care and that none reliably predicted a specific pattern of care. These findings raise questions about what factors may be contributing to this variation. They also suggest that best practices in end-of-life cancer care can be found in many settings and that efforts to improve the quality of end-of-life care should include every hospital category.
Previous studies have shown that patients nearing the end of life often do not receive the care they prefer.1–7 The observed gap between the typical preferences of patients and their families and the care received has stimulated efforts to offer better supportive care for the growing numbers of patients with poor-prognosis cancer—that is, patients who are likely to die in less than a year.8,9 When confronted with such poor survival chances in the face of cancer and other illness, the average patient prefers to spend as much time as possible in a home-like setting with good control of pain and other symptoms.3,4,6,10
In some regions of the United States, and in some hospitals, patients with short life expectancies receive relatively high levels of comfort-focused, palliative services and are less likely to die in a hospital or in a hospital’s intensive care unit. In other places, such patients are more likely to spend their last days in the hospital, often in intensive care units, receiving uncomfortable treatments—such as using a breathing tube connected to a ventilator—that are unlikely to prolong or enhance the quality of life. In some cases intense care may be driven by patient preferences, but commonly it is not.11–18
To identify hospital characteristics associated with higher levels of palliative and community-based care, such as hospice care or dying outside the hospital, we examined care received at the end of life by Medicare beneficiaries who died with poor-prognosis cancer. We also studied the extent of care variation within and across hospital groups, defined by common characteristics. In particular, we expected to find that hospitals with a specific focus on cancer care—including members of the National Comprehensive Cancer Network and hospitals designated as cancer centers by the National Cancer Institute—would be highly attentive to National Quality Forum metrics, such as hospice care, that are important to patients with poor-prognosis cancer.
We also examined the association between care delivered to Medicare beneficiaries with poor-prognosis cancer and other hospital characteristics, such as for-profit status. We hypothesized that for-profit status could be associated with more aggressive care under a fee-for-service payment structure or, conversely, associated with higher care quality because of greater access to capital that could be used for quality improvement efforts.
We found that hospital characteristics, such as a focus on cancer care and for-profit status, were very weakly associated with the nature of end-of-life care received by patients. At the same time, patterns of care varied markedly within groups of hospitals with common characteristics. A complex set of factors contributes to the decisions that are made about end-of-life care. However, these results suggest that, in the context of national average preferences, best practices in end-of-life cancer care can be found in many settings and are not consistently associated with any hospital features we studied.
Study Data And Methods
COHORT DEFINITION
From the Medicare Denominator files for 2003–07, we identified a 20 percent sample of fee-for-service Medicare beneficiaries who died at ages 66–99 and had continuous inpatient and outpatient Medicare insurance (Parts A and B) in the last six months of life (N = 215, 311). Decedents were included in the study if they met two conditions: First, they had at least one hospital discharge or at least two clinician visits in the last six months of life with International Classification of Diseases, Ninth Revision (ICD-9), cancer diagnosis codes associated with a high risk of near-term death; and second, they had at least one hospital admission for cancer care in the last six months of life.19,20
These criteria excluded patients with many common cancers not associated with a high likelihood of dying in the near term. For example, only 6 percent of the study population had prostate cancer, which is generally not a poor-prognosis condition. However, those patients with prostate cancer included in our cohort had metastatic disease—cancer that had spread beyond the area of the prostate—which is associated with near-term death.
HOSPITAL ASSIGNMENT
We attributed each cohort member’s medical care to the hospital providing the patient with the largest number of hospitalizations for cancer care in the last six months of life. Cancer care hospitalizations were defined as those with a primary diagnosis of cancer or a secondary diagnosis of poor-prognosis cancer.19 We obtained hospital bed count and for-profit status from the 2007 Medicare Provider of Service File.
We categorized hospitals into the following four mutually exclusive types: members of the National Comprehensive Cancer Network (n = 21); hospitals outside the network that were designated comprehensive cancer centers by the National Cancer Institute (n 22); hospitals that were not in the network or=designated comprehensive cancer centers but that were academic medical centers (n = 161); and community hospitals, those institutions not in the above groups (n = 4, 240).21–23
The National Comprehensive Cancer Network describes itself this way: “As the arbiter of high-quality cancer care, [the network] promotes the importance of continuous quality improvement and recognizes the significance of creating clinical practice guidelines appropriate for use by patients, clinicians, and other health care decision-makers.”23 Hospitals that are in the network have developed and promote the use of clinical cancer care guidelines, including those for palliative care.23–25
According to the National Cancer Institute, the designation of Comprehensive Cancer Center “requires more than state-of-the-art care and services and includes a strong research base interactive with a wide spectrum of prevention, care, education, information and dissemination activities that broadly serve communities, regions of the country and often the Nation.”21
OUTCOMES
For each patient, we studied care measures in the six months preceding death, such as hospitalization, hospice use, intensive care unit use, and number of unique physicians providing care. We used the hospital discharge status of “expired” to identify patients who died in the hospital. Using Medicare hospice files, we measured the number of days of hospice use for each patient.We also determined whether initiation of hospice use was late, defined as within three or fewer days of death. Late initiation of hospice care is considered an indicator of poor quality care because patients receive little palliative benefit. Such late hospice use has been aptly described as “using hospice to manage death rather than palliate disease.”26(p319)
We used billing codes to determine the receipt of chemotherapy in the last fourteen days of life—a stage of illness where it is less likely to provide benefit and more likely to cause discomfort due to toxicity.16–18 We also used the codes to indicate the receipt of three uncomfortable procedures—feeding tube placement, insertion of a breathing tube for assisted ventilation, and cardiopulmonary resuscitation—that are unlikely to prolong life or improve its quality in patients with debilitating disease.12–15
Five of our measures are quality indicators that have been endorsed by the National Quality Forum for end-of-life cancer patients.27 Higher rates of intensive care unit use in the last month of life, receiving chemotherapy in the last four-teen days of life, dying in a hospital, and receiving hospice care for less than three days are considered undesirable (that is, they are indicators of low-quality care), while hospice use in the last month of life is desirable (an indicator of high-quality care).
STATISTICAL ANALYSIS
We tabulated decedent characteristics and medical care events by hospital type, size, and for-profit status. We then developed multilevel models with the individual decedent as the unit of analysis to examine the association between hospital characteristics (type, size, for-profit status) and cancer care events. Our final models adjusted for individual patients’ age; sex; race (black or nonblack); cancer type;28 comorbidity count;19 income estimate based on ZIP code;29,30 hospital type, using National Comprehensive Cancer Center members as the reference; hospital bed count, using less than 150 beds as the reference; and hospital for-profit status, using not-for-profit status as the reference.
We tested the validity of our care attribution to assigned hospitals by calculating a loyalty measure for each patient in the last six months of life. This measure was defined as hospital days at assigned hospital divided by all hospital days. We also assessed how close to the time of death the hospitalization occurred at the assigned hospital.
LIMITATIONS
We identified our cohort using cancer diagnoses demonstrated to be highly predictive of short-term death. But because our administrative claims data did not include the cause of death, we cannot be certain that our subjects died as a result of cancer. However, each died with the diagnosis of poor-prognosis cancer. This means that the care they received immediately preceding death reflects treatment of patients known to have a high disease burden of cancer, whatever the final cause of death.20
Our objective was to compare care experienced by patients at hospitals with distinct characteristics. This required us to assign each cohort member to a hospital and resulted in the exclusion of patients not hospitalized for cancer care in the last six months of life. The exclusion of these patients is an important limitation of our study. For a comparison of included patients with those excluded because of a lack of a hospitalization, see the online Appendix.31
We selected care measures based on the literature on patient preferences at the end of life. However, we have no information on individual patient and family care preferences for cohort members, or on their satisfaction with the care the patient received. It is possible that patients desiring more aggressive end-of-life care are more likely to turn to academic hospitals, while those preferring less aggressive care tend to use community hospitals. Nonetheless, we found no literature supporting such a trend.
Additional details about our methods and data can be found in the online Appendix.31
Study Results
DESCRIPTIVE STATISTICS OF PATIENTS AND HOSPITALS
We identified 237,098 patients dying with poor-prognosis cancer between 2003 and 2007. Of these, we excluded 21,787 (9.1 percent) because they were not hospitalized for cancer care in the last six months of life. Among the final cohort of 215,311 patients, the mean age at death was seventy-eight years (Exhibit 1). The five most common cancer types were lung or bronchus (31.0 percent), unspecified primary (9.3 percent), hematologic (9.2 percent), colon or rectum (8.5 percent), and pancreatic (6.2 percent).
Exhibit 1.
Characteristics Of Medicare Patients Who Died With Poor-Prognosis Cancer, 2003–07
| Hospital type |
|||||
|---|---|---|---|---|---|
| Characteristic | All | NCCN cancer centers |
Non-NCCN NCI cancer centers |
Academic hospitals |
Community hospitals |
| Number of hospitals | 4,444 | 21 | 22 | 161 | 4,240 |
| Number of decedents | 215,311 | 4,921 | 4,075 | 19,590 | 186,725 |
| Percent of cohort | 100.0 | 2.3 | 1.9 | 9.1 | 86.7 |
| Age at death (%) | |||||
| 66–74 | 35.4 | 51.5 | 43.9 | 38.3 | 34.5 |
| 85–99 | 19.5 | 9.6 | 14.1 | 18.0 | 20.0 |
| Female (%) | 48.6 | 46.1 | 48.5 | 49.0 | 48.6 |
| Black (%) | 9.3 | 11.9 | 14.2 | 17.3 | 8.3 |
| Most common types of cancer (%) | |||||
| Lung/bronchus | 31.0 | 20.1 | 20.2 | 26.0 | 32.0 |
| Unspecified | 9.3 | 8.1 | 8.9 | 9.0 | 9.4 |
| Hematologic | 9.2 | 12.5 | 11.8 | 9.5 | 9.0 |
| Number of other chronic conditionsa (%) | |||||
| 0 | 42.4 | 52.2 | 47.3 | 44.1 | 41.8 |
| 1 | 28.6 | 28.0 | 28.0 | 28.2 | 28.7 |
| 2 | 16.6 | 12.7 | 14.3 | 15.6 | 16.8 |
| 3 or more | 12.4 | 7.2 | 10.4 | 12.1 | 12.7 |
| Estimated household incomeb (mean $) | 53,679 | 57,286 | 57,285 | 53,847 | 53,685 |
SOURCE Authors’ analysis of Medicare data. NOTES NCCN is National Comprehensive Cancer Network. Non-NCCN NCI is National Cancer Institute centers, excluding those in the NCCN. Academic hospital is defined according to Note 22 in text.
Other chronic conditions in the last two to six months of life are congestive heart failure, chronic pulmonary disease, dementia, diabetes with end organ damage, peripheral vascular disease, chronic renal failure, chronic liver disease, and coronary artery disease.
Estimated income is derived from 2006 estimates based on the 2000 US census from the Dartmouth Primary Care Service Area Project (Note 29 in text).
In the last six months of life, 4,444 hospitals provided the preponderance of inpatient cancer care to this cohort (Exhibit 1). Of these hospitals, 85 percent were not-for-profit (data not shown). In terms of bed numbers, 63 percent were small (less than 150 beds), 20 percent were medium (150–300 beds), and 17 percent were large (more than 300 beds) (data not shown).
The patients at hospitals in the National Comprehensive Cancer Network were slightly younger and had fewer noncancer morbidities than patients in the National Cancer Institute–designated facilities and academic and community hospitals (Exhibit 1). Compared to academic and community hospital patients, National Comprehensive Cancer Network and National Cancer Institute center patients were less likely to have lung or bronchus cancer and more likely to have hematologic malignancies.
Patients at National Cancer Institute centers and academic hospitals were more likely to be black than patients at National Comprehensive Cancer Network and community hospitals (Exhibit 1). With the exception of race, patient characteristics differed little by hospital size and for-profit status. All differences described here were significant (p < 0.05). Further details are available in Appendix Exhibit 1S.31
UNADJUSTED CARE MEASURES
Overall, considering unadjusted care measures by hospital type, size, and for-profit status, we found that in the last month of life patients received high levels of inpatient care (Exhibit 2). In the last month of life, only 54 percent of the cohort received some hospice care. More details are available in Appendix Exhibit 2S.31
Exhibit 2.
Unadjusted Cancer Care Measures, By Hospital Characteristics
| Type |
Size |
For-profit status |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | All | NCCN cancer centers |
Non- NCCN NCI cancer centers |
Academic hospitals |
Community hospitals |
<150 beds |
150– 300 beds |
>300 beds |
Not-for- profit |
For- profit |
| No. of decedents | 215,.311 | 4,921 | 4,075 | 19,590 | 186,725 | 51,494 | 62,956 | 100,861 | 189,629 | 25,682 |
| Death in hospital (%) | 30.2 | 32.6 | 32.4 | 33.8 | 29.7 | 28.4 | 30.2 | 31.2 | 30.2 | 30.3 |
| HOSPICE | ||||||||||
| Hospice use, last month of life (%) |
53.8 | 53.4 | 52.4 | 50.3 | 54.2 | 50.9 | 54.3 | 54.9 | 53.6 | 55.4 |
| Days in hospice, last month of life (per decedent) |
8.4 | 8.6 | 8.1 | 7.6 | 8.5 | 8.2 | 8.3 | 8.5 | 8.4 | 8.5 |
| Hospice initiated, last 3 days of life (%) |
8.5 | 7.1 | 7.9 | 8.3 | 8.6 | 7.5 | 9.0 | 8.8 | 8.4 | 9.2 |
| HOSPITAL | ||||||||||
| Hospitalized, last month of life (%) |
64.9 | 60.2 | 61.7 | 64.4 | 65.1 | 66.3 | 65.4 | 63.8 | 64.7 | 66.3 |
| Days in hospital, last month of life (per decedent) |
5.3 | 5.6 | 5.6 | 5.9 | 5.3 | 4.7 | 5.4 | 5.6 | 5.3 | 5.4 |
| INTENSIVE CARE UNIT | ||||||||||
| ICU use, last month of life (%) | 24.7 | 23.3 | 26.3 | 26.0 | 24.6 | 21.6 | 25.6 | 25.7 | 24.1 | 28.9 |
| Days in ICU, last month of life (per decedent) |
1.4 | 1.4 | 1.5 | 1.5 | 1.3 | 1.0 | 1.4 | 1.5 | 1.3 | 1.7 |
| OTHER MEASURES | ||||||||||
| Chemotherapy, last 14 days of life (%) |
6.2 | 6.0 | 5.4 | 5.3 | 6.3 | 5.4 | 6.5 | 6.5 | 6.1 | 6.6 |
| Potentially life-prolonging procedure,a last month of life (%) |
9.3 | 10.3 | 13.1 | 12.6 | 8.9 | 7.0 | 9.4 | 10.5 | 9.2 | 10.7 |
| Saw 10 or more physicians, last six months of life (%) |
48.1 | 58.2 | 54.3 | 57.6 | 46.7 | 34.2 | 49.1 | 54.5 | 48.1 | 47.5 |
SOURCE Authors’ analysis of Medicare data. NOTES NCCN is National Comprehensive Cancer Network. Non-NCCN NCI is National Cancer Institute centers, excluding those in the NCCN. Academic hospital is defined according to Note 22 in text. ICU is intensive care unit.
Potentially life-prolonging procedures are feeding tube placement, insertion of a breathing tube for assisted ventilation, and cardiopulmonary resuscitation.
ADJUSTED CARE MEASURES
We present our findings as rate ratios: the rate of a given care measure for one hospital group, such as National Comprehensive Cancer Network centers, divided by the rate of the same measure in a reference hospital group, such as community hospitals. After adjusting for patient and hospital characteristics, we observed relative differences of 15 percent or greater across the four hospital types for only four of the eleven measures (Exhibit 3). The first was the percentage of patients initiating hospice care within three days of death. The stepwise higher relative rates across the groups can be interpreted to mean that— compared to National Comprehensive Cancer Network hospitals, the reference group—very late hospice initiation was 13 percent higher in National Cancer Institute hospitals, 19 percent higher in academic hospitals, and 29 percent higher in community hospitals.
Exhibit 3.
Adjusted Relative Rates Of Cancer Care Measures, By Hospital Type
| Hospital type |
|||
|---|---|---|---|
| Measure | Non-NCCN NCI cancer centers |
Academic hospitals |
Community hospitals |
| Death in hospital | 1.02 | 1.08** | 0.98 |
| HOSPICE | |||
| Hospice use, last month of life | 0.97 | 0.93** | 1.04 |
| Days in hospice, last month of life | 0.92** | 0.86** | 0.98 |
| Hospice initiated, last 3 days of life | 1.13 | 1.19** | 1.29** |
| HOSPITAL | |||
| Hospitalized, last month of life | 1.04 | 1.09** | 1.09** |
| Days in hospital, last month of life | 1.02 | 1.07** | 1.01 |
| INTENSIVE CARE UNIT | |||
| ICU use, last month of life | 1.15** | 1.13** | 1.11** |
| Days in ICU, last month of life | 1.08** | 1.10** | 1.05 |
| OTHER MEASURES | |||
| Chemotherapy, last 14 days of life | 1.00 | 1.05 | 1.36** |
| Potentially life-prolonging procedure,a last month of life | 1.29** | 1.25** | 0.99 |
| Saw 10 or more physicians, last 6 months of life | 0.91** | 0.98 | 0.90** |
SOURCE Authors’ analysis of Medicare data. NOTES Adjusted for patients’ age, sex, race, estimated 2006 median household income of ZIP code, chronic comorbidity count category (0, 1, and more than 1), and cancer category (lung, unspecified, hematologic, and other); and hospitals’ bed count and for-profit status. The reference group is hospitals in the National Comprehensive Cancer Network (NCCN). Non-NCCN NCI is National Cancer Institute centers, excluding those in the NCCN. ICU is intensive care unit. Academic hospital is defined according to Note 22 in text. Community hospitals are those not in any of the other hospital categories.
Potentially lifeprolonging procedures are feeding tube placement, insertion of a breathing tube for assisted ventilation, and cardiopulmonary resuscitation.
p < 0:05
Second, compared to National Comprehensive Cancer Network hospitals, patients in the other three hospital types experienced more care in the intensive care unit in the last month of life (Exhibit 3). Third, patients cared for in community hospitals were much more likely to receive chemotherapy in the last fourteen days of life than patients in other hospitals. And fourth, potentially life-prolonging procedures in the last month of life were more common in National Cancer Institute centers and academic hospitals, compared to the other two types of hospitals. Additional details, including confidence intervals, are presented in Appendix Exhibit 3S.31
Patients cared for in medium-size and large hospitals, as opposed to small ones, received more care by almost every measure (Exhibit 4). The effects were slight for days in hospice care compared to days in the hospital, intensive care unit use, late chemotherapy, and potentially life-prolonging procedures. Patients cared for at for-profit compared to not-for-profit hospitals were more likely to receive aggressive hospital-based care, although the two types of hospitals varied little in terms of hospice services use. Additional details, including confidence intervals, are presented in Appendix Exhibit 4S.31
Exhibit 4.
Adjusted Relative Rates Of Cancer Care Measures, By Hospital Size And For-Profit Status
| Hospital size |
|||
|---|---|---|---|
| Measure | 150–300 beds | >300 beds | For-profit |
| Death in hospital | 1.06** | 1.06** | 1.02 |
| HOSPICE | |||
| Hospice use, last month of life | 1.07** | 1.10** | 1.04** |
| Days in hospice, last month of life | 1.02** | 1.07** | 1.02** |
| Hospice initiated, last 3 days of life | 1.19** | 1.21** | 1.11** |
| HOSPITAL | |||
| Hospitalized, last month of life | 0.99 | 0.97** | 1.02** |
| Days in hospital, last month of life | 1.14** | 1.16** | 1.05** |
| INTENSIVE CARE UNIT | |||
| ICU use, last month of life | 1.18** | 1.20** | 1.22** |
| Days in ICU, last month of life | 1.37** | 1.41** | 1.33** |
| OTHER MEASURES | |||
| Chemotherapy, last 14 days of life | 1.18** | 1.20** | 1.09** |
| Potentially life-prolonging procedure,a last month of life | 1.30** | 1.37** | 1.24** |
| Saw 10 or more physicians, last 6 months of life | 1.37** | 1.49** | 1.04** |
SOURCE Authors’ analysis of Medicare data. NOTES Adjusted for patients’ age, sex, race, estimated 2006 median household income of ZIP code, chronic comorbidity count category (0, 1, and more than 1), and cancer category (lung, unspecified, hematologic, and other); and hospitals’ bed count and for-profit status. The reference groups are, for hospital size, hospitals with fewer than 150 beds and, for for-profit status, not for profit. ICU is intensive care unit.
Potentially life-prolonging procedures are feeding tube placement, insertion of a breathing tube for assisted ventilation, and cardiopulmonary resuscitation.
p < 0:05
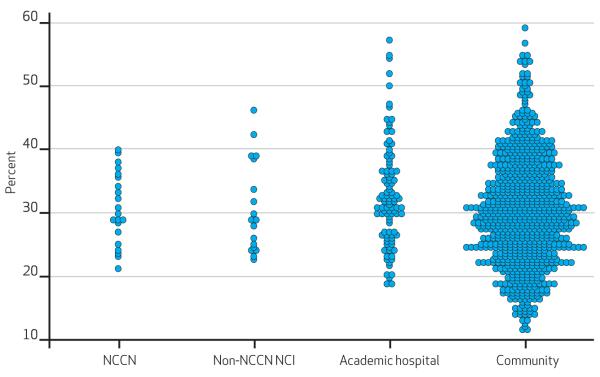
There were slight or modest differences across the four hospital types, across hospital sizes, and across hospital profit status categories. However, marked variation was noted within all of those categories. Generally, more than a twofold variation was noted within the hospital groups with common features (Exhibit 5). No hospital characteristic reliably predicted a specific pattern of care. Appendix Exhibit 5S portrays the range of variation on each measure within each hospital group.31
Exhibit 5. Percentage Of Patients Dying In The Hospital, By Hospital Type.
SOURCE Authors’ analysis of Medicare data. NOTES Each circle represents one hospital and its rate of in-hospital death among Medicare beneficiaries dying with poor-prognosis cancer. NCCN is National Comprehensive Cancer Network. Non-NCCN NCI is National Cancer Institute centers, excluding those in the NCCN. Academic hospital is defined according to Note 22 in text. Community hospitals are those not in any of the other hospital categories. Adjusted for patients’ age, sex, race, estimated 2006 median household income of ZIP code, chronic comorbidity count category (0, 1, and more than 1), and cancer category (lung, unspecified, hematologic, and other). Plots of other care measures are presented in Appendix Exhibit 5S (see Note 31 in text).
Hospital loyalty measures revealed that patients received the overwhelming majority of all inpatient days—for cancer and noncancer care—at their assigned hospital (mean: 92 percent; median: 100 percent; data not shown). The mean period between the last hospitalization at the assigned center and death was twenty-seven days; the median was nine days. In sensitivity analyses that included hospitalizations for cancer and noncancer care, a change of hospital assignment occurred for less than 2 percent of the cohort.
Discussion
OVERALL CARE INTENSITY
Our study of Medicare patients dying with poor-prognosis cancer revealed a relatively high intensity of care in the last weeks of life. Some experts, including oncologists, have labeled this pattern of care aggressive or overaggressive.26,32–34 Although we noted trends across hospital types and characteristics, these differences were dwarfed by the variation within hospital groups defined by common features such as hospital type, size, or for-profit status. These findings highlight the range of care experienced by cancer patients at the end of life and raise questions about the sources of this variation.
THE LITERATURE ON PATIENTS’ PREFERENCES
The fundamental question is whether the care received by these patients is the care that they and their families wanted. Patients, including those with cancer, vary in their preferences for more or less aggressive end-of-life care. However, variation in regional intensity of care for chronically ill patients in the last six months of life does not generally reflect Medicare beneficiaries’ preferences.3,4,34,35 The majority of patients prefer comfort over curative care and would rather die at home than in the hospital.4,6,7,34
Other studies have shown that health care intensity corresponds closely with overall local practice patterns and local medical service capacity, such as hospital bed supply or physician supply.6,36–38 Our data did not permit a study of patients’ preferences. However, our findings did not support the possibility that the observed wide variation in aggressiveness of care could be explained by patients with distinctive preferences selecting specific care settings. Such an explanation would require that patients know in advance the intensity of care common at each hospital and choose their care setting based on that knowledge.
There is no hospital-specific, publicly available information about end-of-life care intensity to guide patients’ hospital selection. Our findings showed that hospitals with a specific clinical focus on cancer care, such as the National Comprehensive Cancer Network and National Cancer Institute centers, differ only modestly in their end-of-life care patterns from hospitals that do not have such a focus.
An alternative explanation for the observed variation is that hospitals unknowingly provided widely differing care practices that strongly shaped the care patients received.26,33,39 As noted, we found that hospice use was low for all hospital types and regardless of size and for-profit status—only 54 percent in the last of month of life. We also found that no hospital group excelled on other measures of end-of-life care, such as in-hospital death rate or days in the intensive care unit during the last month of life. These results indicate a need for a broad reexamination of end-of-life cancer care and whether it meets the needs and wants of patients.
CHEMOTHERAPY CLOSE TO DEATH
No hospital group clearly excelled on the end-of-life care outcomes we examined. However, one measure on which all types of hospitals may be making progress warrants particular consideration. Overall, late use of chemotherapy in this cohort appeared lower than the rates of up to 12 percent that were reported in studies of patients known to be dying of cancer in the late 1990s.26,33,39,40 This lower rate may result from differences in the populations included in these studies and in the distinct data sources used—clinical records compared to claims—although Medicare claims have been found to be a valid way to ascertain the use of clinician-administered chemotherapy.41
The difference between our rates and those published previously could reflect diffusion of care standards promoted by oncologists recently, including quality standards that explicitly label late chemotherapy as overly aggressive care.17,33,39 Recent analyses have confirmed that end-of-life care for patients with chronic illness is changing quickly.11,42
COULD MORE INTENSE CARE BE BENEFICIAL?
Some doctors, patients, caregivers, and policy makers might question whether overall cancer survival is higher for patients cared for at hospitals with greater intensity of end-of-life treatment. Our study cannot directly answer this question. Other researchers have failed to find a survival benefit for elderly patients cared for in hospitals and regions with higher intensity of care. And although a recent study described a short-term survival benefit when high intensity was compared to low intensity, there appeared to be no difference between high and average intensity.36,37,43,44
These studies examining the potential benefit of higher-intensity care were not limited to patients with poor-prognosis cancer—patients for whom we expect that any survival benefit, if one exists at all, would be small. Indeed, recent studies show that the introduction of palliative care to poor-prognosis cancer patients can prolong life while improving its quality.45–48 A corollary to this conclusion is that relative to palliative care, aggressive care, in these populations, shortens life while reducing its quality.
Conclusion
Decisions about end-of-life care may be among the most complex made by clinicians and patients. The sources of this complexity are diverse and include the imperfect nature of predictions of life expectancy, varying and often insufficiently explored patient and family preferences, the strong current of local practice patterns, financial incentives, and local capacity for inpatient and palliative care.
Our study of a national sample of older patients who died with poor-prognosis cancers reveals that, in general, a high volume of inpatient services is delivered at the end of life. Furthermore, we found that no type of hospital stands apart in providing care more consistent with measures endorsed by the National Quality Forum, such as lower rates of intensive care unit use in the last month of life, chemotherapy in the last fourteen days of life, death in a hospital, and receiving hospice care for less than three days; and higher rates of hospice use in the last month of life. Each hospital needs to examine the care it provides to patients believed to be nearing death, and question its alignment with patient preferences—whether they be for early supportive care or aggressive treatment in the last days of life.
Acknowledgments
This research was supported, in part, by the Robert Wood Johnson Foundation and the National Institute on Aging (Grant Nos. P01 AG19783 and Beeson K23AG028947 to Julie Bynum). The authors acknowledge Kristen K. Bronner, managing editor of the Dartmouth Atlas of Health Care, for development of graphic data displays.
Biography
 Nancy E. Morden is an assistant professor at Dartmouth Medical School.
Nancy E. Morden is an assistant professor at Dartmouth Medical School.
In this month’s Health Affairs, Nancy Morden and coauthors report on their study examining the intensity of health care delivered to Medicare enrollees who are terminally ill with cancer. They found that the overall intensity of care is high, and probably far more so than many patients would prefer—but also highly variable, even among hospitals of similar size, tax status, and designation as a cancer center. Because best practices in end-of-life cancer care can be found in many settings, however, they recommend that efforts to improve the quality of end-of-life care extend to every hospital category.
Morden is an assistant professor in the Department of Community and Family Medicine at Dartmouth Medical School; a researcher at the Dartmouth Institute for Health Policy and Clinical Practice; and an investigator in the Cancer Control Research Program at the Dartmouth-Hitchcock Norris Cotton Cancer Center. She received her medical degree from Harvard University and her master’sdegree in public health from the University of Washington, Seattle.
 Chiang-Hua Chang is a research instructor at the Dartmouth Institute.
Chiang-Hua Chang is a research instructor at the Dartmouth Institute.
Chiang-Hua Chang is a research instructor at the Dartmouth Institute for Health Policy and Clinical Practice. Involved in research collaborations that include the Primary Care Service Project and the Dartmouth Atlas of Health Care, Chang holds a master’s degree in biostatistics from the University of Michigan and a doctoral degree in health policy and clinical practice from Dartmouth College.
 Joseph O. Jacobson is the chief quality officer at the Dana Farber Cancer Institute.
Joseph O. Jacobson is the chief quality officer at the Dana Farber Cancer Institute.
Joseph Jacobson is the chief quality officer at the Dana Farber Cancer Institute and an associate clinical professor at Harvard Medical School. Board certified in internal medicine, medical oncology, and hematology, he was a founding member of the American Society of Clinical Oncology’s Quality Oncology Practice Initiative. Jacobson received a master’sdegreein clinical effectiveness from Harvard University and a medical degree from Boston University.
 Ethan M. Berke is an associate professor at Dartmouth Medical School.
Ethan M. Berke is an associate professor at Dartmouth Medical School.
Ethan Berke is an associate professor in community and family medicine at Dartmouth Medical School, a researcher at the Dartmouth Institute for Health Policy and Clinical Practice, and an investigator in the Cancer Control Research Program at the Dartmouth-Hitchcock Norris Cotton Cancer Center. He obtained his master’s degree in public health with a concentration in epidemiology from the University of Washington and his medical degree from Albany Medical College.
 Julie P.W. Bynum is an associate professor at the Dartmouth Institute.
Julie P.W. Bynum is an associate professor at the Dartmouth Institute.
Julie Bynum is an associate professor of medicine and associate director of the Center for Health Policy Research at the Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical School. A practicing geriatrician, Bynum received her medical degree from the Johns Hopkins University School of Medicine and holds a master’s degree in public health from the Johns Hopkins Bloomberg School of Public Health.
 Kimberly M. Murray is a research associate at the Maine Medical Center Research Institute.
Kimberly M. Murray is a research associate at the Maine Medical Center Research Institute.
Kimberly Murray is a research associate at the Center for Outcomes Research and Evaluation at the Maine Medical Center Research Institute. She received her master’s degree in public policy from the University of Southern Maine.
 David C. Goodman is a professor at Dartmouth Medical School.
David C. Goodman is a professor at Dartmouth Medical School.
David Goodman is a professor of pediatrics and of health policy at Dartmouth Medical School. He also is director of the Center for Health Policy Research at the Dartmouth Institute for Health Policy and Clinical Practice. A co–principal investigator of the Dartmouth Atlas, Goodman holds a master’sdegree in evaluative clinical sciences from Dartmouth College and a medical degree from the State University of New York Upstate Medical Center, Syracuse.
Contributor Information
Nancy E. Morden, the Dartmouth Medical School, a researcher at the Dartmouth Institute for Health Policy and Clinical Practice, and an investigator in the Cancer Control Research Program at the Dartmouth-Hitchcock Norris Cotton Cancer Center, in Lebanon, New Hampshire..
Chiang-Hua Chang, the Dartmouth Institute for Health Policy and Clinical Practice..
Joseph O. Jacobson, the Dana Farber Cancer Institute, in Boston, Massachusetts, and an associate clinical professor at Harvard Medical School..
Ethan M. Berke, Dartmouth Medical School, a researcher at the Dartmouth Institute for Health Policy and Clinical Practice, and an investigator in the Cancer Control Research Program at the Dartmouth-Hitchcock Norris Cotton Cancer Center..
Julie P.W. Bynum, the Dartmouth Institute for Health Policy and Clinical Practice and associate director of the institute’s Center for Health Policy Research..
Kimberly M. Murray, the Maine Medical Center Research Institute’s Center for Outcomes Research and Evaluation, in Portland..
David C. Goodman, Dartmouth Medical School and director of the Center for Health Policy Research at the Dartmouth Institute..
NOTES
- 1.Brooksbank M. Palliative care: where have we come from and where are we going? Pain. 2009;144(3):233–5. doi: 10.1016/j.pain.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Clark D. From margins to centre: a review of the history of palliative care in cancer. Lancet Oncol. 2007;8(5):430–8. doi: 10.1016/S1470-2045(07)70138-9. [DOI] [PubMed] [Google Scholar]
- 3.Barnato AE, Anthony DL, Skinner J, Gallagher PM, Fisher ES. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med. 2009;24(6):695–701. doi: 10.1007/s11606-009-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnato AE, Herndon MB, Anthony DL, Gallagher PM, Skinner JS, Bynum JP, et al. Are regional variations in end-of-life care intensity explained by patient preferences? A study of the US Medicare population. Med Care. 2007;45(5):386–93. doi: 10.1097/01.mlr.0000255248.79308.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui D, Elsayem A, De la Cruz M, Berger A, Zhukovsky DS, Palla S, et al. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303(11):1054–61. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pritchard RS, Fisher ES, Teno JM, Sharp SM, Reding DJ, Knaus WA, et al. Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment. J Am Geriatr Soc. 1998;46(10):1242–50. doi: 10.1111/j.1532-5415.1998.tb04540.x. [DOI] [PubMed] [Google Scholar]
- 7.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA. 1995;274(20):1591–8. [PubMed] [Google Scholar]
- 8.Miniño AM, Xu J, Kochanek KD, Tejada-Vera B. Death in the United States, 2007 [Internet] National Center for Health Statistics; Hyattsville (MD): [cited 2012 Mar 7]. Dec, 2009. (NCHS Data Brief No. 26). Available from: http://www.cdc.gov/ nchs/data/databriefs/db26.pdf. [Google Scholar]
- 9.Foley KM, Gelband H, editors. Improving palliative care for cancer. National Academies Press; Washington (DC): 2001. [PubMed] [Google Scholar]
- 10.Gruneir A, Mor V, Weitzen S, Truchil R, Teno J, Roy J. Where people die: a multilevel approach to understanding influences on site of death in America. Med Care Res Rev. 2007;64(4):351–78. doi: 10.1177/1077558707301810. [DOI] [PubMed] [Google Scholar]
- 11.Goodman DC, Fisher ES, Chang C-H, Morden NE, Jacobson JO, Murray K, et al. Quality of end-of-life cancer care for Medicare beneficiaries: regional and hospital-specific analyses. Dartmouth Institute for Health Policy and Clinical Practice; Lebanon (NH): [cited 2012 Mar 7]. Nov 16, 2010. Available from: http://www.dartmouthatlas.org/ downloads/reports/Cancer_report_11_16_10.pdf. [PubMed] [Google Scholar]
- 12.Oyogoa S, Schein M, Gardezi S, Wise L. Surgical feeding gastrostomy: are we overdoing it? J Gastrointest Surg. 1999;3(2):152–5. doi: 10.1016/s1091-255x(99)80025-0. [DOI] [PubMed] [Google Scholar]
- 13.Ehlenbach WJ, Barnato AE, Curtis JR, Kreuter W, Koepsell TD, Deyo RA, et al. Epidemiologic study of inhospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361(1):22–31. doi: 10.1056/NEJMoa0810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Astrow AB, Sood JR, Nolan MT, Terry PB, Clawson L, Kub J, et al. Decision-making in patients with advanced cancer compared with amyotrophic lateral sclerosis. J Med Ethics. 2008;34(9):664–8. doi: 10.1136/jme.2007.022731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Wright AA, Huskamp HA, Nilsson ME, Maciejewski ML, Earle CC, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med. 2009;169(5):480–8. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao S, Shafiq J, Vardy J, Adams D. Use of chemotherapy at end of life in oncology patients. Ann Oncol. 2009;20(9):1555–9. doi: 10.1093/annonc/mdp027. [DOI] [PubMed] [Google Scholar]
- 17.Neuss MN, Desch CE, McNiff KK, Eisenberg PD, Gesme DH, Jacobson JO, et al. A process for measuring the quality of cancer care: the Quality Oncology Practice Initiative. J Clin Oncol. 2005;23(25):6233–9. doi: 10.1200/JCO.2005.05.948. [DOI] [PubMed] [Google Scholar]
- 18.Harrington SE, Smith TJ. The role of chemotherapy at the end of life: “when is enough, enough”? JAMA. 2008;299(22):2667–78. doi: 10.1001/jama.299.22.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iezzoni LI, Heeren T, Foley SM, Daley J, Hughes J, Coffman GA. Chronic conditions and risk of inhospital death. Health Serv Res. 1994;29(4):435–60. [PMC free article] [PubMed] [Google Scholar]
- 20.Berke EM, Smith T, Song Y, Halpern MT, Goodman DC. Cancer care in the United States: identifying end-of-life cohorts. J Palliat Med. 2009;12(2):128–32. doi: 10.1089/jpm.2008.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Office of Cancer Centers . Guidelines for comprehensive designation [Internet] NCI; Bethesda (MD): [cited 2012 Mar 7]. Available from: http://cancercenters.cancer.gov/grants_funding/comprehensiveness.html. [Google Scholar]
- 22.Association of American Medical Colleges . Council of Teaching Hospitals and Health Systems (COTH) [Internet] AAMC; Washington (DC): [cited 2012 Mar 7]. Available from: http://www.aamc.org/members/coth/ [Google Scholar]
- 23.National Comprehensive Cancer Network . NCCN annual report— 2010 [Internet] NCCN; Fort Washington (PA): [cited 2012 Mar 7]. 2010. Available from: http://www.nccn.org/about/pdf/annual_report.pdf. [Google Scholar]
- 24.Levy MH, Back A, Benedetti C, Billings JA, Block S, Boston B, et al. NCCN clinical practice guidelines in oncology: palliative care. J Natl Compr Canc Netw. 2009;7(4):436–73. doi: 10.6004/jnccn.2009.0031. [DOI] [PubMed] [Google Scholar]
- 25.National Comprehensive Cancer Network . NCCN guidelines for supportive care: palliative care [Internet] NCCN; Fort Washington (PA): [cited 2012 Mar 7]. 2011. Available for download from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. [Google Scholar]
- 26.Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315–21. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 27.National Quality Forum . NQF-endorsed palliative care and end-of-life care: endorsement maintenance standards [Internet] NQF; Washington (DC): [cited 2012 Mar 8]. Available from: http://www.quality forum.org/Projects/n-r/Palliative_Care_and_End-of-Life_Care/Table_of_Measures.aspx. [Google Scholar]
- 28.Health Care Costs and Utilization Project . Clinical Classifications Software (CCS) for ICD-9-CM [Internet] Agency for Healthcare Research and Quality; Rockville (MD): [last modified 2012 Mar 2; cited 2012 Mar 8]. Available from: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. [Google Scholar]
- 29.Primary Care Service Area Team of Dartmouth Medical School . The Primary Care Service Area Project [Internet] Dartmouth Institute for Health Policy and Clinical Practice; Lebanon (NH): [cited 2012 Mar 8]. Available from: http://pcsa.dartmouth.edu/index.html. [Google Scholar]
- 30.Census Bureau . American FactFinder [Internet] Census Bureau; Washington (DC): [cited 2012 Mar 8]. Available from: http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml. [Google Scholar]
- 31.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 32.Cintron A, Hamel MB, Davis RB, Burns RB, Phillips RS, McCarthy EP. Hospitalization of hospice patients with cancer. J Palliat Med. 2003;6(5):757–68. doi: 10.1089/109662103322515266. [DOI] [PubMed] [Google Scholar]
- 33.Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–6. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose JH, O’Toole EE, Dawson NV, Lawrence R, Gurley D, Thomas C, et al. Perspectives, preferences, care practices, and outcomes among older and middle-aged patients with late-stage cancer. J Clin Oncol. 2004;22(24):4907–17. doi: 10.1200/JCO.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 35.Voogt E, van der Heide A, Rietjens JA, van Leeuwen AF, Visser AP, van der Rijt CC. Attitudes of patients with incurable cancer toward medical treatment in the last phase of life. J Clin Oncol. 2005;23(9):2012–9. doi: 10.1200/JCO.2005.07.104. [DOI] [PubMed] [Google Scholar]
- 36.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–98. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 37.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138(4):273–87. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wennberg JE, Fisher ES, Stukel TA, Skinner JS, Sharp SM, Bronner KK. Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States. BMJ. 2004;328(7440):607. doi: 10.1136/bmj.328.7440.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Earle CC, Neville BA, Landrum MB, Souza JM, Weeks JC, Block SD, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care. 2005;17(6):505–9. doi: 10.1093/intqhc/mzi061. [DOI] [PubMed] [Google Scholar]
- 40.Emanuel EJ, Young-Xu Y, Levinsky NG, Gazelle G, Saynina O, Ash AS. Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med. 2003;138(8):639–43. doi: 10.7326/0003-4819-138-8-200304150-00011. [DOI] [PubMed] [Google Scholar]
- 41.Du XL, Key CR, Dickie L, Darling R, Geraci JM, Zhang D. External validation of medicare claims for breast cancer chemotherapy compared with medical chart reviews. Med Care. 2006;44(2):124–31. doi: 10.1097/01.mlr.0000196978.34283.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodman DC, Esty AR, Fisher ES, Chang C-H. Dartmouth Institute for Health Policy; Lebanon (NH): [cited 2012 Mar 8]. Apr 12, 2011. Trends and variation in end-of-life care for Medicare beneficiaries with severe chronic illness [Internet] Available from: http://www.dartmouthatlas.org/downloads/reports/EOL_Trend_Report_0411.pdf. [PubMed] [Google Scholar]
- 43.Skinner J, Staiger D, Fisher ES. Looking back, moving forward. N Engl J Med. 2010;362(7):569–74. doi: 10.1056/NEJMp1000448. discussion 74. [DOI] [PubMed] [Google Scholar]
- 44.Barnato AE, Chang CC, Farrell MH, Lave JR, Roberts MS, Angus DC. Is survival better at hospitals with higher “end-of-life” treatment intensity? Med Care. 2010;48(2):125–32. doi: 10.1097/MLR.0b013e3181c161e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–9. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 47.Connor SR, Pyenson B, Fitch K, Spence C, Iwasaki K. Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage. 2007;33(3):238–46. doi: 10.1016/j.jpainsymman.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Saito AM, Landrum MB, Neville BA, Ayanian JZ, Weeks JC, Earle CC. Hospice care and survival among elderly patients with lung cancer. J Palliat Med. 2011;14(8):929–39. doi: 10.1089/jpm.2010.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]