Abstract
Under appropriate culture conditions, bone marrow (BM)-derived mesenchymal stem cells are capable of differentiating into diverse cell types unrelated to their phenotypical embryonic origin, including neural cells. Here, we report the successful generation of neural stem cell (NSC)-like cells from BM-derived human mesenchymal stem cells (hMSCs). Initially, hMSCs were cultivated in a conditioned medium of human neural stem cells. In this culture system, hMSCs were induced to become NSC-like cells, which proliferate in neurosphere-like structures and express early NSC markers. Like central nervous system-derived NSCs, these BM-derived NSC-like cells were able to differentiate into cells expressing neural markers for neurons, astrocytes, and oligodendrocytes. Whole-cell patch clamp recording revealed that neuron-like cells, differentiated from NSC-like cells, exhibited electrophysiological properties of neurons, including action potentials. Transplantation of NSC-like cells into mouse brain confirmed that these NSC-like cells retained their capability to differentiate into neuronal and glial cells in vivo. Our data show that multipotent NSC-like cells can be efficiently produced from BM-derived hMSCs in culture and that these cells may serve as a useful alternative to human neural stem cells for potential clinical applications such as autologous neuroreplacement therapies.
Keywords: Electrophysiology, Immunochemistry, Neuroreplacement
Introduction
Human neural stem cells (hNSCs) derived from embryos or fetal brains are self-renewing cells capable of differentiating into all major neural lineages of the brain, including neurons, astrocytes, and oligodendrocytes both in vitro and in vivo.1–4 These hNSCs hold great promise for neuroreplacement therapies in many central nervous system (CNS) diseases caused by cell loss and degeneration.5–7 However, several issues such as the need for concurrent immune suppression in hNSC transplantation, the difficulties related to sourcing hNSCs, as well as multiple ethical concerns, limit their clinical application. Thus, the ideal source for cell replacement therapies would be autologous stem cells derived from the patient’s own tissues.
Human mesenchymal stem cells (hMSCs) derived from adult bone marrow (BM) are a subset of self-renewable multipotent stem cells capable of differentiating into various mesenchymal lineages, including osteogenic, chondrogenic, adipogenic, and fibroblastic cells.8–10 Although adult stem cells are considered to be lineage-restricted, it has been demonstrated by a number of studies that hMSCs can differentiate or transdifferentiate into a diverse family of cell types that are unrelated to their phenotypical embryonic origin. For example, recent studies, including our own,11 have shown that hMSCs can differentiate into cells that express properties of neural lineages both in vitro under cultured conditions12–20 and in vivo after transplantation into the CNS.21–23 This suggests that certain populations of hMSCs possess the potential for neural generation. hMSCs are relatively easy to isolate from BM tissues and to expand in culture. Using neural cells produced from autologous hMSCs for transplantation in neuroreplacement therapies may eliminate the risk of immune rejection and avoid the controversial ethical issues associated with embryonic/fetal hNSCs.
Previous studies have induced hMSCs to produce neural-like cells in various culture systems with growth factors,17,19 chemical agents,20,24 or combinations of both.11,13,16 The conclusion that the hMSCs have differentiated into neuronal cells was based on the appearance of neuron-like morphology and an increase in the expression of neural gene markers.13,19 However, some culture conditions used for neural induction in these studies can cause non-neural cells to extend long thin neurites with aberrant neural gene marker expression.25–27 Additionally, in a normal state without neural induction, MSCs already expressed low levels of certain neural gene markers commonly used to characterize neural differentiation, such as nestin, Nurr1, Enolase2, glial fibrillary acidic protein (GFAP), and beta-III-tubulin.13,25,28,29 Therefore, the demonstration of neuronal morphology and expression of a limited set of neuronal marker genes may not be sufficient to prove neuronal differentiation and/or transdifferentiation of MSCs. Further functional evaluation, electrophysiology in particular, of those neuronal-like cells induced from MSCs is necessary.26,27,30 Two studies reported that hMSCs could be initially converted into multi-potent neural stem cell (NSC)-like cells and then induced to differentiate into neuronal cell types under appropriate culture conditions.16,17 However, one study failed to demonstrate the electrophysiological property of neuronal cells differentiated from these NSC-like cells.16 Similarly, the other study did not test the electrophysiological characteristics of resultant neuronal cells.17 The electrophysiological characteristics of neuronal cells produced from MSCs were only achieved by co-cultures with primary astrocytes31 or transfected with gene Notch intracellular domain followed by the administration of a certain combination of neurtrophins.32
In this report, we showed that NSC-like cells can be induced from hMSCs in a conditioned medium of hNSCs (CM-hNSCs) without using a co-culture system or gene transfection. These NSC-like cells are morphologically and phenotypically similar to brain-derived NSCs, forming neurosphere-like structures and expressing markers associated with NSCs, including octamer-binding protein 4 (Oct4), paired box gene 6 (Pax6), Sox1, Foxg1 (Bf1), and nestin. Subsequent differentiation of NSC-like cells yielded neural-like cells expressing strong and long-lasting neural markers for neuronal cells, including doublecortin (DCX), beta-III-tubulin, neurofilament 200 (NF-200), gamma aminobutyric acid (GABA), glutamate, and microtubule-associated protein 2 (MAP2), and for glial cells including GFAP, nerve/glial antigen 2 (NG2), and galactosylceramidase (GalC). Importantly, electrophysiological analyses demonstrated that neuronal cells differentiated from these NSC-like cells exhibited typical characteristics of excitable neurons, including evoked action potentials. Transplantation of these NSC-like cells into mouse brains revealed that these cells migrated and differentiated into neuronal cells expressing beta-III-tubulin and neuronal nuclei (NeuN) and glial cells expressing GFAP, respectively, suggesting that these NSC-like cells retained the ability for neural differentiation in vivo. Parts of this work have been reported previously as abstracts.33,34
Materials and Methods
Collection of a CM-hNSCs
hNSCs were obtained from Biowhittaker (CC-2599; Walkersville, MD, USA). We have reported the methods for the growth and maintenance of undifferentiated hNSCs previously.6,35–37 Briefly, hNSCs were cultured and expanded in T-75 flask containing 25 ml serum-free neurobasal medium (Gibco; Invitrogen, Carlsbad, CA, USA), human recombinant epidermal growth factor (EGF; 20 ng/ml) and basic fibroblast growth factor (bFGF; 20 ng/ml) (R&D Systems, Minneapolis, MN, USA), B27 (serum-free medium supplements formulated to provide optimal growth condition for NSC expansion, 1 : 50; Invitrogen), heparin (5 μg/ml; Sigma, St Louis, MO, USA), 2 mM L-glutamine, and an antibiotic–antimycotic mixture (1 : 100; Invitrogen). The cells were incubated at 37°C in a 5% CO2 humidified incubation chamber (Fisher, Pittsburgh, PA, USA). Under these culture conditions, hNSCs formed floating neuro-spheroids. To facilitate optimal growth, hNSC spheroids were sectioned into quarters every 2 weeks and fed by replacing 50% of the culture medium every 3 days. The CM-hNSCs were collected at the time of each medium change and filtered through a membrane with a pore size of 0.22 μm in diameter (Millipore Corporation, Billerica, MA, USA). The filtered CM-hNSCs were centrifuged at 800×g for 30 minutes at 18°C. The spun fraction of CM-hNSCs was examined by microscopic observation to be cell-free and further confirmed that there was no cell contamination after culturing of CM-hNSCs for 1 week.
Cultivation of MSCs
The hMSCs from four donors were obtained from AllCells LLC (Emeryville, CA, USA) and Cambrex Bioscience (Walkersville, MD, USA). These hMSCs are negative for surface markers associated with hematopoietic cells (e.g. CD11b, CD33, CD34, and CD133) and retain their ability to differentiate along various mesenchymal cell lineages under appropriate culture conditions, including osteogenic, chondrogenic, and adipogenic cells, according to the companies. These hMSCs were cultured in tissue culture-treated T-75 flasks at a concentration of 1×105 cells/cm2 in 20 ml of growth medium consisting of Dulbecco’s modified Eagle medium (Gibco; Invitrogen) supplemented with 5% fetal bovine serum (Stem Cell Technologies, Vancouver, Canada), and an antibiotic–antimycotic mixture (1 : 100; Invitrogen). The cells were incubated at 37°C in a 5% CO2 humidified incubation chamber (Fisher) and fed by replacing half the culture medium twice per week. Cells were passaged by incubating with 0.05% trypsin-ethylenediaminetetraacetic acid (EDTA; Gibco; Invitrogen) for ~5 minutes at room temperature to gently release the cells from the surface of the culture flask after reaching ~80% confluency. Culture medium was added to stop the trypsinization and the cells were centrifuged at 1500 rev/minute for 5 minutes at room temperature, resuspended, and transferred into new culture flasks following the protocol developed in our laboratory.11,38,39
NSC induction of hMSCs
Following 3–5 passages, hMSCs (1×105 cells/cm2) were transferred into T-75 tissue culture flask containing 20 ml CM-hNSCs at 37°C in a 5% CO2 humidified incubation chamber (Fisher). The CM-hNSCs were changed every 3 days. At the time for the first medium change, most of the cultivated cells attached to the plastic surface of the flask (~80%). Those plastic–non-adherent and floating cells (~20%) were removed by medium changes and discarded. Following 2–3 times of medium changes, all of the cells stayed attached to the culture flask. After reaching 80% of confluency, these attached cells were passaged with 0.05% trypsin-EDTA (Gibco; Invitrogen) for ~2 minutes at room temperature. Fresh CM-hNSC medium was added to stop the trypsinization and the cells were centrifuged at 1500 rev/minute for 3 minutes at room temperature, resuspended, and then divided among new culture flasks, which were preconditioned with CM-hNSCs overnight, at a concentration of 1×105 cells/cm2. About 3 weeks after the initial cultivation in CM-hNSCs, aggregate formation of hMSCs was observed. These cellular aggregates became floating neurosphere-like structures when expanded for an additional several days (3–7 days) in CM-hNSCs. The cells in the neurosphere-like structures were termed as NSC-like cells33,34 and used for our in vitro (immunocytochemistry and electrophysiology) and in vivo (transplantation) experiments.
Immunocytochemistry
Induction of NSC-like cells
The expression of stem cell and NSC markers was examined in cellular aggregates of hMSCs and NSC-like cells that were seeded onto poly-L-lysine (Sigma) coated glass coverslips and cultured in CM-hNSCs. The cell samples were rinsed with 0.01M phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS for 20 minutes. Following washing in PBS, the cells were blocked by 0.1% Triton X-100 (Sigma) and 3% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in PBS for additional 30 minutes. Single or double fluorescent immunocytochemical staining for stem cell and neural stem cell markers was performed by using mouse anti-Oct4 (1 : 600; Chemicon, Temecula, CA, USA), goat anti-Bf1 (1 : 500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-nestin (1 : 1000; Chemicon), rabbit anti-nestin (1 : 1000; Chemicon), rabbit anti-Sox1 (1 : 300; Chemicon), and goat anti-Pax6 (1 : 600; Santa Cruz Biotechnology) primary antibodies overnight at 4°C. Cells were then washed in PBS and incubated with corresponding secondary antibodies conjugated with fluorescent tetramethylrhodamine isothiocyanate (TRITC) and/or fluorescein isothiocyanate (FITC) for 2 hours at room temperature in the dark. Finally, cells were washed in PBS, counterstained with mounting medium containing 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA), and viewed by fluorescence microscopy (Zeiss, Jena, Germany).
Proliferation potential of NSC-like cells
To determine the proliferative potential of NSC-like cells, 1 μM bromodeoxyuridine (BrdU; Sigma) was added to the culture medium for 24–48 hours. The cells were then rinsed with 0.01M PBS and fixed with 4% paraformaldehyde in PBS for 20 minutes. For BrdU staining, cells were treated with 2 N HCl for 10 minutes at room temperature, washed in PBS, and blocked by 0.1% Triton X-100 and 3% donkey serum in PBS for additional 30 minutes. Double fluorescent immunocytochemical staining was performed by using sheep anti-BrdU (1 : 600; Fitzgerald Industries International, Acton, MA, USA) and other primary antibodies for NSC markers overnight at 4°C. Cells were then washed in PBS and incubated with corresponding secondary antibodies conjugated with fluorescent TRITC and FITC for 2 hours at room temperature in the dark. After PBS washing, cells were counterstained with mounting medium containing DAPI (Vector Laboratories), and viewed by fluorescence microscopy (Zeiss).
Terminal differentiation of NSC-like cells
The terminal neural differentiation was examined in NSC-like cell aggregates plated onto poly-L-lysine coated coverslips after differentiating for 3–10 days in neurobasal medium (Gibco; Invitrogen) containing B27 (1 : 50; Invitrogen) or N2 supplements (chemically defined supplements formulated to provide optimal growth condition for NSC expansion, 1 : 100; Invitrogen) and penicillin (100 U/ml)/streptomycine (1 mg/ml) without adding b-FGF and EGF. The cells were rinsed with 0.01M PBS and fixed with 4% paraformaldehyde in PBS for 20 minutes. Cell samples were then washed in PBS and blocked by 0.1% Triton X-100 and 3% donkey serum in PBS for additional 30 minutes. Single or double or triple fluorescent immunocytochemical staining was performed by using mouse anti-beta-III tubulin (1 : 600; Sigma), rabbit anti-beta-III tubulin (1 : 500; Covance, Richmond, CA, USA), goat anti-DCX (1 : 500; Santa Cruz Biotechnology), mouse anti-MAP2 (1 : 500; Chemicon), mouse anti-NeuN (1 : 1000; Chemicon), rabbit anti-NF-200 (1 : 600; Chemicon), mouse anti-fibronectin (1 : 1000; Sigma), mouse anti-GABA (1 : 500; Sigma), rabbit anti-glutamate (1 : 500; Sigma), rabbit anti-NG2 (1 : 500; Chemicon), rabbit anti-GalC (1 : 300; Chemicon), rabbit anti-GFAP (1 : 1000; Abcam, Cambridge, MA, USA), and mouse anti-GFAP (1 : 3000; Chemicon) primary antibodies overnight at 4°C. Cells were then washed in PBS and incubated with corresponding secondary antibodies conjugated with fluorescent TRITC, FITC, and amino-methyl-coumarin-acetate (AMCA; 1 : 300; Jackson Immuno Research Laboratories) for 2 hours at room temperature in the dark. Cells were counterstained with mounting medium containing DAPI (Vector Laboratories) after PBS washing and viewed by fluorescence microscopy (Zeiss). Cells immunostained by specific antibodies were counted and recorded as a percent of the total number of cells. For negative controls, primary antibodies were omitted and the same staining procedure was followed. Digitally-captured images from fluorescent microscopy of stained cells were analyzed by NIH Image software (NIH, Bethesda, MD, USA) with Cell Scoring, Particle Analysis, and Cell Analysis macros.
Electrophysiological recording
NSC-like cells and their parent hMSCs were plated on poly-L-lysine coated coverslips (Sigma) and cultured in neurobasal medium (Gibco; Invitrogen) containing B27 (1 : 50; Invitrogen) and penicillin (100 U/ml)/streptomycine (1 mg/ml), L-glutamine (2 mM), and laminin (1 μg/ml; Invitrogen) without adding mitogens. For some cell samples, 5 μM cytosine arabinofuranoside (Ara-C; Sigma), a selective inhibitor of DNA synthesis, was added in culture for 2 days to eliminate dividing glial cells. All experiments were performed at room temperature. After culturing for 2–10 days in this differentiation media, the coverslips were placed into a recording chamber and superfused with oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid. The composition of artificial cerebrospinal fluid is as follows: 122 mM NaCl, 3 mM KCl, 0.81 mM MgSO4, 2.5 mM CaCl2, 1 mM NaH2PO4, 24 mM NaHCO3, and 10 mM glucose (pH 7.4). Patch pipettes (5–15 MΩ) were fabricated from borosilicate glass capillary tubing (OD 1.5 mm; A-M Systems, Carlsborg, WA, USA) using a P-97 micropipette puller (Sutter Instrument, Novato, CA, USA) and filled with 140 mM K-gluconate, 11 mM EDTA, 1 mM CaCl2, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 3.5 mM KOH. Cells differentiated from NSC-like cells with neuron-like morphology (a relatively rounded cell body with extended processes) were chosen for whole-cell patch clamp recording as we previously reported.40,41 Cells with glia-like morphologies (flat, polygonal, and star-like large cell bodies and thicker processes) were excluded from electophysiological examination. Physiological signals were amplified and low-pass filtered using a MultiClamp 700B (Axon Instruments, Union City, CA, USA). Data were digitized at a rate of 10 000 Hz (Digidata 1440A; Axon Instruments) and later analyzed (pClamp 10; Axon Instruments).
NSC-like cell transplantation
NSC-like cells were incubated with 1 μM BrdU (Sigma) for 24–48 hours in CM-hNSCs to label nuclei, so these cells could be identified after transplantation, as we performed previously.6,11,35,37 In total, 10 mice (C57BL, 4-month-old) were used for NSC-like cell transplantation. Six mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and 1×105 NSC-like cells were injected stereotaxically into the cortex and striatum. The remaining four mice were injected with the same amount of hMSCs at the same regions as controls. Animal experiments were conducted in accordance with the guidelines of the University Animal Care Committee at the University of Illinois at Chicago (UIC). No drugs were used to suppress the immune system. Four weeks after transplantation, all mice were anesthetized with sodium pentobarbital (75 mg/kg, i.p.) and perfused by 4% paraformaldehyde in normal saline. The brains were removed and coronal sections of 30 μm in thickness were sliced. After the initial PBS washes, brain sections were treated with 2 N HCl for 30 minutes at room temperature. Then the sections were washed in PBS, blocked in PBS containing 0.25% Triton X-100 (Sigma) and 3% donkey serum (Jackson ImmunoResearch Laboratories) for 30 minutes, and followed by incubation with sheep anti-BrdU (1 : 500; Fitzgerald Industries International) and mouse anti-NeuN (1 : 1000; Chemicon) or mouse anti-beta-III-tubulin (1 : 600; Sigma) or mouse anti-GFAP (1 : 1000; Sigma) antibodies overnight at 4°C. The sections were then washed in PBS and incubated with corresponding secondary antibodies including TRITC-conjugated donkey anti-sheep IgG and FITC-conjugated donkey anti-mouse IgG (1 : 200; Jackson Immunoresearch Laboratories) for 2 hours at room temperature in the dark. Finally, sections were washed three times with PBS, counterstained with DAPI (Vector Laboratories), and viewed by confocal and/or fluorescence microscopy (Zeiss). Negative controls for immunohistochemistry consisted of secondary antibody application alone. The immunohistochemical results obtained from the group with NSC-like cell transplantation were compared to those of control group receiving hMSC injection.
Results
Immunocytochemistry
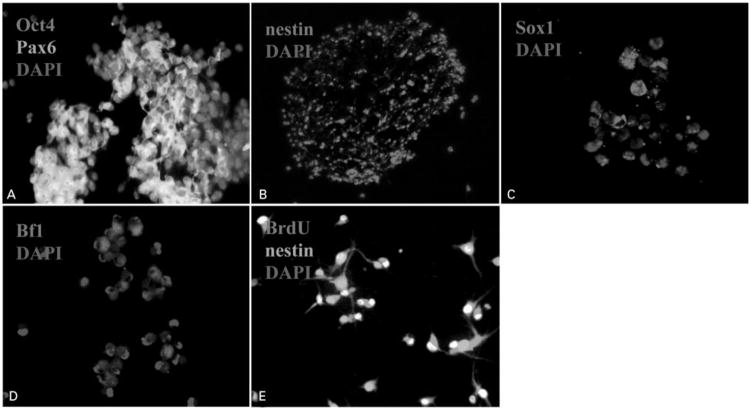
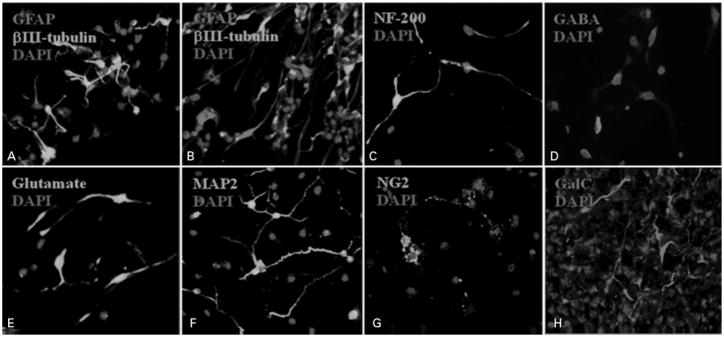
All hMSCs used for the neural induction displayed a fibroblast-like morphology in our culture system. After culturing hMSCs in CM-hNSCs for 3 weeks, cellular aggregates of hMSCs were formed. The percentage of hMSCs that formed cellular aggregates is estimated as 80% in the parent population of hMSCs. These aggregates grew quickly and had a doubling time of about 24–48 hours. When expanded in CM-hNSCs for additional 3–7 days, these cellular aggregates continued to increase in number and form neurosphere-like structures similar to hNSC-generated neurospheres in morphology. Immunocytochemical examination revealed that Oct4 and Pax6, proliferative stem cell markers, were coexpressed in the cellular aggregates of hMSCs (Fig. 1A). Nestin, a common NSC marker, was found in the vast majority of cells in the neurosphere-like structures (Fig. 1B). Sox1 and Bf1-immunoreactive cells were also observed in the NSC-like cells (Fig. 1C and D). These nestin-immunoreactive cells were proliferative cells, as evidenced by positive BrdU immunostaining in their nuclei (Fig. 1E), suggesting that these cells stay in an undifferentiated state in the presence of CM-hNSCs. During the neural development of hMSCs, the expression of Oct4 and Pax6 in these cells progressively decreased accompanied by a concomitant increase in early NSC markers. In addition, the resultant NSC-like cells lost fibroblast-like morphology and the expression of fibronectin (data not shown), the typical characteristics of their parent hMSCs. When exposed to neurobasal medium containing B27 or N2 for 5–10 days without adding any mitogens, NSC-like cells migrated out from the neurosphere-like structures and extended neurite-like processes. Subsequent immunocytochemical analysis demonstrated that the expression of neural progenitor cell markers such as nestin significantly decreased upon differentiation. NSC-like cells expressed astrocyte markers (GFAP, 60% of total cells) and neuron markers (beta-III-tubulin, 40% of total cells), respectively when examined at 3 days following differentiation (Fig. 2A and B). Further examination with different neural antibodies revealed that the neuronal cells differentiated from the NSC-like cells expressed NF-200, GABA, glutamate, and MAP2, respectively, at 7 days following differentiation (Fig. 2C–F). NG2 and GalC (markers for oligodendrocytes)-immunoreactive cells were also observed in a subset of NSC-like differentiated cells (Fig. 2G and H). Triple immunostaining with anti-DCX (a marker for immature migrating neurons), beta-III-tubulin (a marker for young neurons), and MAP2 (a marker for mature neurons) antibodies after 5–10 days differentiation revealed that some beta-III-tubulin-positive cells coexpressed DCX or MAP2 (Fig. 3A–D). This overlapping expression of markers for immature and mature neurons indicates that the cells were at different developmental stages, ranging from early to late, or that the NSC-like cells were capable of generating distinct neuronal subtypes. The number of cells expressing MAP2 increased with time in the differentiation medium, suggesting a gradual process for the maturation of neuronal cells. The NSC-like cells generated from hMSCs in our culture system are stable and enduring. The percentage of spheroid cells that form determined neural lineages is estimated >95% and the daughter cells of NSC-like cells retain capacity to differentiate into neuronal and glial cells for over 30 generations.
Figure 1.
Micrographs showing the production of neural stem cell (NSC)-like cells from bone marrow (BM)-derived human mesenchymal stem cells (hMSCs). (A) Octamer-binding protein 4 (Oct4) (red) and paired box gene 6 (Pax6) (green)-immunoreactive cells in the cellular aggregates of hMSCs (×20). Note that some Oct4-immunoreactive cells colocalized with Pax6-immunoreactive cells. (B) Nestin-immunoreactive cells (red) in the neurosphere-like structures (×10). (C) Bf1 (red)-immunoreactive cells in the NSC-like cells (×20). (D) Sox1 (red)-immunoreactive cells in the NSC-like cells (×20). (E) Bromodeoxyuridine (BrdU) (red) and nestin (green)-immunopositive cells (×20). Note that most of the nuclei of nestin-immunopositive cells were labeled by BrdU, suggesting that these cells are dividing cells. Cell nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue).
Figure 2.
Micrographs (×20) showing the differentiation of neural stem cell (NSC)-like cells in culture medium containing neurobasal and B27 without basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF). NSC-like cells differentiated into cells expressing beta-III-tubulin (a and b, green) and glial fibrillary acidic protein (GFAP) (A and B, red), respectively. A subpopulation of differentiated neuronal cells expressed neurofilament 200 (NF-200) (C, green), gamma aminobutyric acid (GABA) (D, red), glutamate (E, green), and microtubule-associated protein 2 (MAP2) (F, green), respectively. Some NSC-like cells differentiated into cells expressing nerve/glial antigen 2 (NG2) (G, green), a marker for oligodendrocyte progenitors. Some NSC-like cells differentiated into cells expressing galactosylceramidase (GalC) (H, red), a marker for oligodendrocytes. Cell nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue).
Figure 3.
A representative picture (×20) with triple fluorescent immunocytochemical staining for neuronal differentiation of the neural stem cell (NSC)-like cells in the culture medium consisting of neurobasal and B27 without basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF). NSC-like cells differentiated into neuronal cells expressing doublecortin (DCX) (A, blue), beta-III-tubulin (B, red), and microtubule-associated protein 2 (MAP2) (C, green). Note that some cells double labeled with different markers may represent the neuronal cells at different stages of differentiation (D, yellow). Counterstaining for cell nuclei was not performed in these cells.
Electrophysiology recording
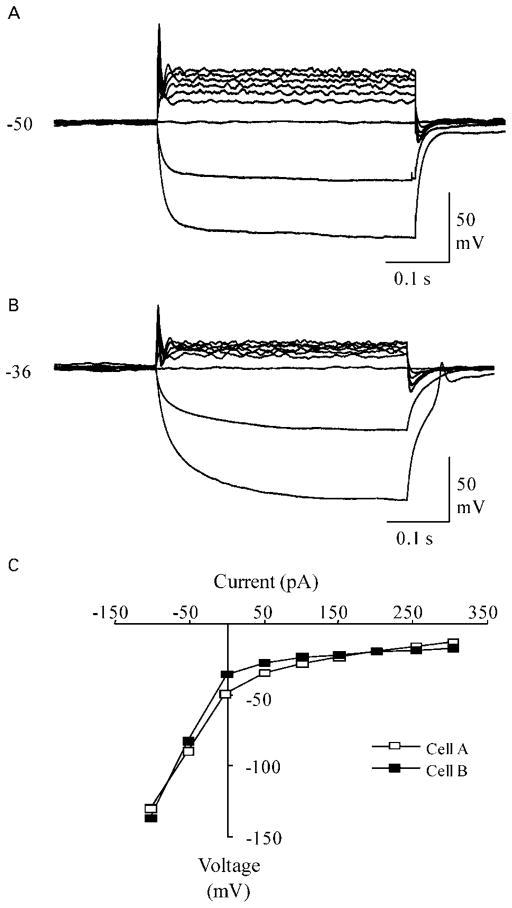
The electrophysiological characteristics of neuron-like cells differentiated from BM-derived NSC-like cells were accessed using whole-cell patch clamp recordings after culturing in differentiation media for 3–10 days, as previously described.40 The rest potentials for cells ranged from −25 to −58 mV with an average potential of −37.6±1.3 mV (average±SEM, n=60). When subjected to a series of current pulses, it was found that nearly all (~98%) of the cells with neuron-like morphology exhibited active membrane properties in response to the depolarizing current pulses. The most common response observed for 70% of the neuron-like cells was a single spike-like event early in the depolarizing current step (Fig. 4A and B). Cells that did not generate overshooting spike-like events usually had smaller bumps without overshoot in the beginning of the depolarizing current steps and rectifying current–voltage relationships. These ‘bumps’, which resemble immature action potentials, were detected in 27% of the neuron-like cells. Among recorded cells, only one cell completely lacked active membrane properties in response to stimulation and was presumably an undifferentiated cell. In contrast, hMSCs did not express any action potentials (data not shown). Taken together, our studies demonstrated that neuron-like cells differentiated from NSC-like cells have key features of functional neurons with active membrane properties and the ability to generate action potentials.
Figure 4.
Neuron-like membrane properties of cells differentiated from human bone marrow (BM)-derived neural stem cell (NSC)-like cells. (A, B) Whole-cell patch clamp recordings showing the responses of two cells to a series of current pulses. The cells in both panel A and panel B had spike-like events with overshoot and after-hyperpolarizations at the beginning of the current steps. The amplitude of the spike-like structures was 65 mV and the half-width was 4.8 ms for the cell in panel A. The amplitude was lower (50 mV) and the half-width was shorter (4.0 ms) for the cell in panel B. Cells were stimulated with 400 ms current steps beginning at −100 pA and increasing to 300 pA in 50 pA intervals. Resting membrane potential (mV) is indicated to the left of the voltage recordings. (C) Plot of the steady-state current–voltage relationship for the cells in panels A and B. Notice that the plots were rectifying (non-linear) for the cells in panels A and B, indicating an active neuron membrane response.
NSC-like cell transplantation
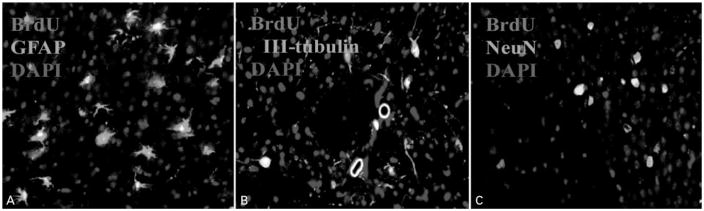
We further examined the survival and differentiation of NSC-like cells when transplanted into adult mouse brains. Brain sections were analyzed by double-fluorescent immunohistochemistry at 4 weeks following cell transplantation. Similar to our previous findings with hNSC transplantation in rodent brains, we identified that the majority of NSC-like cells (70%) differentiated into astrocytes with BrdU-labled nuclei, suggesting their donor origin (Fig. 5A). In contrast, ~20% BrdU-labeled cells coexpressed the early neuronal marker beta-III-tubulin (Fig. 5B), indicating that these cells had differentiated into neuronal cells. These beta-III-tubulin-immunopositive cells lack long processes and may be immature neurons. Since the observation for neural differentiation is performed at 4 weeks following transplantation, it is likely that the maturation of these neuronal cells will continue with time and longer observation periods may be necessary, as suggested by our previous transplantation study with hMSCs-derived neural cells. However, cells double-labeled with BrdU and NeuN, a marker for mature neurons, were also found in the areas near the injection site, showing that a subpopulation of cells had migrated and differentiated into mature neurons in the host brains (Fig. 5C). A small portion of transplanted cells labeled by BrdU only was also observed in the areas close to the injection site (data not shown), suggesting that these cells were not differentiated. There was no tumor formation observed in the mouse brains. We did not see any evidence of neural differentiation from the transplanted naïve hMSCs in the brains of the control mice. Our results demonstrated that NSC-like cells, which were generated in vitro from BM-derived hMSCs through CM-hNSCs-based cell reprogramming, can survive transplantation and retain their capacity for neural differentiation in vivo, giving rise to cells expressing neuronal and glial markers in the transplanted brains.
Figure 5.
Typical fluorescent immunohistochemical pictures (×20) in the mouse brain 4 weeks after neural stem cell (NSC)-like cell transplantation. NSC-like cells survived, migrated, and differentiated into astrocytes expressing glial fibrillary acidic protein (GFAP) (A), neuronal cells expressing beta-III-tubulin (B), and neuronal nuclei (NeuN) (C). Colocalization of bromodeoxyuridine (BrdU) (red) with GFAP or beta-III-tubulin or NeuN (green) immunoreactivity indicated that these cells originated from transplanted NSC-like cells. The yellow color in the pictures is produced by an overlap of BrdU (red) and beta-III-tubulin or NeuN (green) staining. Cell nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue).
Discussion
Many studies have shown that hMSCs can be induced to differentiate into neuronal and glial cells directly in vitro using a variety of protocols.13–20 Here we demonstrated that hMSCs can be converted into NSC-like cells initially when cultivated in CM-hNSCs. These resultant NSC-like cells can form neurosphere-like structures, express early neural stem cell markers, and differentiate into neurons and glial cells. Our results suggest that these NSC-like cells obtained in our protocol have been committed to neural cell lineages and are multipotent NSCs with similar characteristic to those NSCs derived from the CNS.
Oct4 is a key transcription factor essential for pluripotency and self-renewal of undifferentiated stem cells.42–44 Pax6 is a paired box-containing transcription factor crucial for the development of the embryonic CNS, eye, nose, and pancreas, and is expressed in the dividing neuroepithelial cells.45 The coexpression of Oct4 and Pax6 was observed in the cellular aggregates of hMSCs. Recently, Osumi and colleagues46 suggested that Pax6 may also be important for postnatal neurogenesis, promoting neuronal differentiation in context-dependent manners. The coexpression pattern of Oct4 and Pax6 in the cellular aggregates of hMSCs suggests that these cells may have obtained the potential for a specific neural differentiation although, to some extent, they may still retain the capacity to differentiate into a wide range of cells at this stage. Consistent with previous reports,16 the expression of Oct4 was reduced sharply with the onset of expression of multiple early neural stem/progenitor markers such as Sox1, Bf1, and nestin in NSC-like cells, suggesting that the conversion from hMSCs to NSC-like cells may undergo a legitimate program of neural lineage specification. The expression of the intermediate filament protein nestin, a marker for neural stem/progenitor cells,47,48 which was also found in reactive glial cells,49 was observed in the majority of cells in the neurosophere-like structures. Sox1 is a 39-kDa transcription factor that belongs to the Soxb1 subgroup. The onset of expression of Sox1 appears to coincide with the induction of ectodermal cells committed to the neural fate. Within the developing CNS, Sox1 maintains neural stem cells in an undifferentiated state and has been used as a marker for NSCs.50–52 Bf1 is a marker of neural progenitor cells specific to anterior CNS fate. The expression of Bf1 in the generated NSC-like cells may suggest that by default these NSC-like cells have adopted certain forebrain characteristics.53,54 In addition, these NSC-like cells can be further characterized by double immunostaining of nestin and BrdU, indicating that they were undifferentiated and undergoing mitosis in cultures.
The capacity of these NSC-like cells to differentiate into neural lineages in vitro in the differentiation medium seems similar to that of the real multipotent hNSCs derived from fetal and adult brains.2,4,47 In other words, these NSC-like cells can give rise to its three major types of neural cells, respectively, expressing neuronal (beta-III-tubulin, NF-200, GABA, glutamate, and MAP2), astrocytic (GFAP), and oligodendrocytic markers (NG2 and GalC). MAP2, a marker exclusively expressed in mature postmitotic neurons, was expressed in terminally differentiated neuronal cells and the proportion of MAP2-immunor-eactive cells increased after culturing for longer periods in the differentiation medium.
Although multiple key markers for NSCs and neural cells were expressed in NSC-like cells and differentiated neural cells, all these markers may also be expressed in other putative stem cell populations or in cultured cells. For example, it has been shown that cells treated with conditions in which they become sick will undergo profound changes in morphology and gene and protein expression profile that resemble neural cells.13,25–30 Thus, the expression of neural markers is not incontrovertible evidence that hMSCs have transdifferentiated to neural lineages. Electrophysiological determination of action potential existence in NSC-like cell-derived neurons would be more confirmative of neuronal generation and indicative of neuronal function. Previous studies have reported that cells with the functional properties of neurons can be generated from BM-derived MSCs by transfection of the gene Notch intra-cellular domain32 or co-culturing with astrocytes24,31 or neurons.55 More recently, there were reports demonstrating that neuron-like cells derived from hMSCs exhibited functional synaptic transmission,56 and were able to fire tetrodotoxin-sensitive action potentials when co-cultured with primary astrocytes.24 In contrast, we have generated neuron-like cells with active membrane properties from hMSCs by a simple culture system without gene transfection or co-cultures. These neuron-like cells displayed spike-like events with overshoot and brief after-hyperpolarization in whole-cell patch clamp recordings. These spike-like events can be blocked by the voltage-gated sodium channel inhibitor lidocaine and the inhibition can be reversed by saline washing,40 suggesting that the action potentials of the neuron-like cells are sodium-dependent. Our previous calcium imaging experiments also demonstrated that these neuron-like cells can respond to potassium chloride depolarization and L-glutamate application with an increased level of cytoplasmic calcium, suggesting that these cells may express voltage-gated calcium channels and functional L-glutamate receptors.40 Although glial cells express many of the voltage-gated ion channels found in neurons and can also produce action potentials, the channel density is typically not high enough to support fast action potentials.57,58 In our experiments, the frequency of the spike-like events with overshoot and after-hyperpolarization found in these BM-derived neuron-like cells is higher than would be expected for glial cells. We did not detect repetitive spikes in these neuron-like cells, implying that these neurons may not yet be fully developed.40,41 This observation was expected because the cells studied in our electrophysiology recording experiment were in culture for <2 weeks (3–10 days) and it normally takes two or more weeks in vitro for mature action potentials to develop in neurons differentiated from multipotent neural progenitor cells (NPCs) isolated from adult human brain.3,59 It may take even longer for these BM-derived neuronal cells to develop into real mature neurons in culture. Since hMSCs do not express sodium channels or action potentials,16,26,60,61 our results strongly suggest that the BM-derived NSC-like cells have differentiated into electrophysiologically functional neuronal cells.
In our studies, whether NSC-like cells could differentiate into neural cells in vivo was investigated in the brain of normal mouse after transplantation. When transplanted into mouse brain, NSC-like cells survived and differentiated into neuronal and glial cells expressing beta-III-tubulin, NeuN, and GFAP, respectively, at 4 weeks following transplantation. Our results demonstrated that BM-derived NSC-like cells were not only capable of differentiating into neural cells in vitro but also retained the capability of neural differentiation in vivo. Compared to differentiated mature neuronal cells, NSC-like cells produced by our protocol may be more suitable candidates for transplantation in clinical practice because immature stem cells can survive transplantation better and have greater potential to migrate and integrate into host brains,1,6,7 one of the significant advantages over other already existing protocols describing the generation of neuronal cells from MSCs. The results obtained from the present studies warrant further evaluation of the therapeutic potential of these BM-derived NSC-like cells in neurologically ‘diseased’ models.
The mechanism controlling stem cell fate decision remains unclear even though it has been extensively studied. Previous transplantation studies demonstrated that the acquisition of neural phenotypes by BM stem cells in the brains could be caused by the fusion of grafted cells and host neurons.21,23 However, findings of many other studies provide strong evidence, suggesting that the neural fate of the BM stem cells transplanted in vivo is a consequence of neural differentiation.11,22,62–64 Although cell fusion events may not be completely ruled out in transplanted brains and in some co-culture systems,55,65,66 the neural conversion of hMSCs cannot be attributed to cell fusion in our present studies. Since the CM-hNSCs used in our studies for neural induction have been confirmed to be a cell-free medium and no hNSCs contamination, the possibility of cell fusion between hNSCs and hMeSCs can be excluded in our culture system. It is well-known that the fate of adult stem cells is greatly influenced by contextual cues. For example, stem cells can respond and differentiate into specific cell types according to the cues existed in the environment to which they are exposed.11,21–23,62–67 Our experimental results showed that hMSCs cultured in CM-hNSCs can be efficiently converted into NSC-like cells, providing evidence that some soluble factors released from cultured hNSCs are important for the induction of NSC-like cells from BM-derived hMSCs. These extracellular factors may have the potential to epigenetically modify and/or alter the transcriptional state of hMSCs and increase their potential to develop into NSC-like cells. Identification of these soluble factors by global proteomic analysis of CM-hNSC contents and investigation of their effects on cell fate decision may elucidate the molecular mechanisms responsible for neural development of hMSCs and can lead to alternative methods for producing functional NSC-like cells of human origin from readily available sources of autologous adult stem cells.
Acknowledgments
This study was supported by The Charles H. and Bertha L. Boothroyd Foundation, The American Health Assistance Foundation (AHAF) grants to T. Qu, and NIH grant (NIH/NINDS 1 R01 NS052372-03) to J. Cheng.
References
- 1.Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–40. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 2.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–5. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westerlund U, Moe MC, Varghese M, Berg-Johnsen J, Ohlsson M, Langmoen IA, et al. Stem cells from the adult human brain develop into functional neurons in culture. Exp Cell Res. 2003;289:378–83. doi: 10.1016/s0014-4827(03)00291-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang SC, Werning M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–33. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 5.Ourednik V, Ourednik J, Park KI, Teng YD, Aboody KA, Auguste KI, et al. Neural stem cells are uniquely suited for cell replacement and gene therapy in the CNS. Novartis Found Symp. 2000;231:242–62. doi: 10.1002/0470870834.ch15. [DOI] [PubMed] [Google Scholar]
- 6.Qu T, Brannen CL, Kim HM, Sugaya K. Human neural stem cells improve cognitive function of aged brain. Neuroreport. 2001;12:1127–32. doi: 10.1097/00001756-200105080-00016. [DOI] [PubMed] [Google Scholar]
- 7.Storch A, Schwarz J. Neural stem cells and neurodegeneration. Curr Opin Investig Drugs. 2001;3:774–81. [PubMed] [Google Scholar]
- 8.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 9.Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–25. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 10.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–41. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 11.Qu T, Dong X, Sugaya I, Vaghani A, Pulido J, Sugaya K. Bromodeoxyuridine increases multipotency of human bone marrow-derived stem cells. Restor Neurol Neurosci. 2004;22:459–68. [PubMed] [Google Scholar]
- 12.Black IB, Woodbury D. Adult rat and human bone marrow stromal stem cells differentiate into neurons. Blood Cells Mol Dis. 2001;27:632–6. doi: 10.1006/bcmd.2001.0423. [DOI] [PubMed] [Google Scholar]
- 13.Bossolasco P, Cova L, Calzarossa C, Rimoldi SG, Borsotti C, Deliliers GL, et al. Neuroglial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005;193:312–25. doi: 10.1016/j.expneurol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282:148–52. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- 15.D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–81. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 16.Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117:4411–22. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Elkahloun AG, Messina SA, Ferrari N, Xi D, Smith CL, et al. Cellular and genetic characterization of human adult bone marrow-derived neural stem-like cells: a potential antiglioma cellular vector. Cancer Res. 2003;63:8877–89. [PubMed] [Google Scholar]
- 18.Mareschi K, Novara M, Rustichelli D, Ferrero I, Guido D, Carbone E, et al. Neural differentiation of human mesenchymal stem cells: evidence for expression of neural markers and eag K+ channel types. Exp Hematol. 2006;34:1563–72. doi: 10.1016/j.exphem.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–56. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 20.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 22.Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci U S A. 2003;100:1364–9. doi: 10.1073/pnas.0336479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weimann JM, Charlton CA, Brazelton TR, Hackman RC, Blau HM. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc Natl Acad Sci U S A. 2003;100:2088–93. doi: 10.1073/pnas.0337659100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu L, Zhu L, Huang Y, Lee TD, Forman SJ, Shih CC. Derivation of neural stem cells from mesenchymal stem cells: evidence for a bipotential stem cell population. Stem Cells Dev. 2008;17:1109–21. doi: 10.1089/scd.2008.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertani N, Malatesta P, Volpi G, Sonego P, Perris R. Neurogenic potential of human mesenchymal stem cells revisited: analysis by immunostaining, time-lapse video and microarray. J Cell Sci. 2005;118:3925–36. doi: 10.1242/jcs.02511. [DOI] [PubMed] [Google Scholar]
- 26.Lu P, Tuszynski MH. Can bone marrow-derived stem cells differentiate into functional neurons? Exp Neurol. 2005;193:273–8. doi: 10.1016/j.expneurol.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fischer I. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J Neurosci Res. 2004;77:192–204. doi: 10.1002/jnr.20147. [DOI] [PubMed] [Google Scholar]
- 28.Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A, et al. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72:319–26. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- 29.Woodbury D, Reynolds K, Black IB. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res. 2002;69:908–17. doi: 10.1002/jnr.10365. [DOI] [PubMed] [Google Scholar]
- 30.Jin K, Mao XO, Batteur S, Sun Y, Greenberg DA. Induction of neuronal markers in bone marrow cells: differential effects of growth factors and patterns of intracellular expression. Exp Neurol. 2003;184:78–89. doi: 10.1016/s0014-4886(03)00133-x. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y, Henderson D, Blackstad M, Chen A, Miller RF, Verfaillie CM. Neuroectodermal differentiation from mouse multipotent adult progenitor cells. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11854–60. doi: 10.1073/pnas.1834196100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–10. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma K, Shi G, Pappas GD, Qu T. Neural generation of human mesenchymal stem cells cultivated in a conditioned cell-free culture medium of human neural stem cells. Proceedings of the 36th Annual Meeting of Society for Neuroscience (SfN); 2006 Oct; Atlanta, GA, USA. [Google Scholar]
- 34.Ma K, Liu Q, Shi G, et al. Production of functional neuronal cells from human bone marrow tissues. Proceedings of the 37th Annual Meeting of Society for Neuroscience (SfN); 2007 Nov 3–7; San Diego, CA, USA. [Google Scholar]
- 35.Kim HM, Qu T, Kriho V, Lacor P, Smalheiser N, Pappas GD, et al. Reelin function in neural stem cell biology. Proc Natl Acad Sci U S A. 2002;99:4020–5. doi: 10.1073/pnas.062698299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwak YD, Brannen CL, Qu T, Kim HM, Dong X, Soba P, et al. Amyloid precursor protein regulates differentiation of human neural stem cells. Stem Cells Dev. 2006;15:381–9. doi: 10.1089/scd.2006.15.381. [DOI] [PubMed] [Google Scholar]
- 37.Dong X, Pulido JS, Qu T, Sugaya K. Differentiation of human neural stem cells into retinal cells. Neuroreport. 2003;14:143–6. doi: 10.1097/00001756-200301200-00026. [DOI] [PubMed] [Google Scholar]
- 38.Shi G, Ma K, Pappas GD, Qu T. Phenotypic characteristics of hybrid cells produced by cell fusion of porcine adrenal chromaffin cells with human mesenchymal stem cells: A preliminary study. Neurol Res. 2008;30:217–22. doi: 10.1179/016164107X241674. [DOI] [PubMed] [Google Scholar]
- 39.Sugaya I, Qu T, Sugaya K, Pappas GD. Genetically engineered human mesenchymal stem cells produce met-enkephalin at augmented higher levels in vitro. Cell Transplant. 2006;15:225–30. doi: 10.3727/000000006783981981. [DOI] [PubMed] [Google Scholar]
- 40.Fox L, Shen J, Ma K, Liu Q, Shi G, Pappas GD, et al. Membrane properties of neuron-like cells generated from adult human bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:1831–41. doi: 10.1089/scd.2010.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng J, Nath A, Knudsen B, Hochman S, Geiger JD, Ma M, et al. Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neuroscience. 1998;82:97–106. doi: 10.1016/s0306-4522(97)00174-7. [DOI] [PubMed] [Google Scholar]
- 42.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 43.Pochampally RR, Smith JR, Ylostalo J, Prockop DJ. Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood. 2004;103:1647–52. doi: 10.1182/blood-2003-06-1967. [DOI] [PubMed] [Google Scholar]
- 44.Pesce M, Scholer HR. Oct-4: control of totipotency and germline determination. Mol Reprod Dev. 2000;55:452–7. doi: 10.1002/(SICI)1098-2795(200004)55:4<452::AID-MRD14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 45.Tyas DA, Pearson H, Rashbass P, Price DJ. Pax6 regulates cell adhesion during cortical development. Cereb Cortex. 2003;13:612–9. doi: 10.1093/cercor/13.6.612. [DOI] [PubMed] [Google Scholar]
- 46.Osumi N, Shinohara H, Numayama-Tsuruta K, Maekawa M. Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells. 2008;26:1663–72. doi: 10.1634/stemcells.2007-0884. [DOI] [PubMed] [Google Scholar]
- 47.Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–5. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- 48.Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84:109–29. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- 49.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 50.Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–78. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- 51.Miyagi S, Kato H, Okuda A. Role of SoxB1 transcription factors in development. Cell Mol Life Sci. 2009;66:3675–84. doi: 10.1007/s00018-009-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci. 2005;28:583–8. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Tao W, Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992;8:957–66. doi: 10.1016/0896-6273(92)90210-5. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–96. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 55.Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392–402. doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- 56.Cho KJ, Trzaska KA, Greco SJ, McArdle J, Wang FS, Ye JH, et al. Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin-1{alpha} Stem Cells. 2005;23:383–91. doi: 10.1634/stemcells.2004-0251. [DOI] [PubMed] [Google Scholar]
- 57.Oh Y. Ion channels in neuroglial cells. Kaohsiung J Med Sci. 1997;13:1–9. [PubMed] [Google Scholar]
- 58.Sontheimer H. Voltage-dependent ion channels in glial cells. Glia. 1994;11:156–72. doi: 10.1002/glia.440110210. [DOI] [PubMed] [Google Scholar]
- 59.Moe MC, Varghese M, Danilov AI, Westerlund U, Ramm-Pettersen J, Brundin L, et al. Multipotent progenitor cells from the adult human brain: neurophysiological differentiation to mature neurons. Brain. 2005;128:2189–99. doi: 10.1093/brain/awh574. [DOI] [PubMed] [Google Scholar]
- 60.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99:2199–204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hung SC, Cheng H, Pan CY, Tsai MJ, Kao LS, Ma HL. In vitro differentiation of size sieved stem cells into electrically active neural cells. Stem Cells. 2002;20:522–9. doi: 10.1634/stemcells.20-6-522. [DOI] [PubMed] [Google Scholar]
- 62.Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, et al. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet. 2004;363:1432–7. doi: 10.1016/S0140-6736(04)16102-3. [DOI] [PubMed] [Google Scholar]
- 63.Crain BJ, Tran SD, Mezey E. Transplanted human bone marrow cells generate new brain cells. J Neurol Sci. 2005;233:121–3. doi: 10.1016/j.jns.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Munoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004;24:4585–95. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–5. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 66.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–8. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 67.Alexanian AR, Maiman DJ, Kurpad SN, Gennarelli TA. In vitro and in vivo characterization of neurally modified mesenchymal stem cells induced by epigenetic modifiers and neural stem cell environment. Stem Cells Dev. 2008;17:1123–30. doi: 10.1089/scd.2007.0212. [DOI] [PubMed] [Google Scholar]