Abstract
The activation of Ag-specific T cells locally in the CNS could potentially contribute to the development of immune-mediated brain diseases. We addressed whether Ag-specific T cells could be stimulated in the CNS in the absence of peripheral lymphoid tissues by analyzing Ag-specific T cell responses in organotypic brain slice cultures. Organotypic brain slice cultures were established 1 h after intracerebral OVA Ag microinjection. We showed that when OVA-specific CD8+ T cells were added to Ag-containing brain slices, these cells became activated and migrated into the brain to the sites of their specific Ags. This activation of OVA-specific T cells was abrogated by the deletion of CD11c+ cells from the brain slices of the donor mice. These data suggest that brain-resident CD11c+ cells stimulate Ag-specific naive CD8+ T cells locally in the CNS and may contribute to immune responses in the brain.
In situ stimulation of Ag-specific T cells at the sites of local injury or Ag expression might contribute to the development of immune-mediated diseases of the CNS. It is generally accepted that T cells traffic into the CNS when activated T cells cross the blood-brain barrier (1-3). However, the migration of nonactivated Ag-specific T cells into the CNS (4) and the circulation of naive lymphocytes in the cerebral spinal fluid (5) have also been demonstrated. Whether these cells contribute to the development of autoimmune diseases of the CNS was studied in the myelin basic protein (MBP)3 1–11 TCR transgenic (Tg) animal model (4). These studies showed that comparable numbers of T cells can be isolated from the CNS of both TCR Tg and wild-type mice but, surprisingly, in contrast to those found in wild-type animals, most of the MBP-specific T cells found in the CNS exhibited a naive phenotype. A similar observation was made using nonself Agspecific TCR Tg animals (4). Additionally, it was shown that CNS cells from MBP TCR Tg mice inhibited MBP-specific immune responses in vivo, indicating that CNS autoimmunity is partly regulated by in situ induction of tolerance in the brain (4). However, in all of these experiments T cell activation was analyzed in the absence of additional stimuli and in the noninflamed CNS.
In contrast to these studies, we demonstrated that following intracerebral (IC) microinjection of OVA (IC-OVA), OVA-specific CD8+ T cells expanded systemically and then migrated into the brain where they completed additional proliferation cycles (6). Ag-specific T cells isolated from the brain following antigenic microinjection were activated and responded to in vitro secondary Ag challenge. Additionally, CD8+ T cells accumulated and persisted at sites of Ag in the brain without replenishment from the periphery (6). We also demonstrated that although the microinjection caused little traumatic injury, it did not lead to the recruitment of CD8+ T cells into the brain but attracted activated Ag-specific CD8+ T cells from cognate Ag injection sites (6). These data brought up the possibility that small traumatic injuries or other insults to the CNS might influence the activation of naive Ag-specific T cells in the brain. Considering the importance of this possibility and its relevance to both understanding the basic mechanisms of immune-mediated brain diseases as well as therapeutic implications in defining whether peripheral immunosuppression is sufficient to treat autoimmune diseases of the CNS, we decided to analyze Ag-specific T cell activation in the brain in the absence of peripheral immune organs.
To study the activation of Ag-specific T cells in the brain independently of peripheral immunity, we generated brain slices from animals after an intracerebral microinjection of OVA Ag and cocultured these slices with OVA-specific CD8+ T cells. Ag-specific CD8+ T cells migrated into the organotypic brain slices where they further proliferated and colocalized with CD11c+ cells. The depletion of these CD11c+ cells abrogated the in situ activation of Ag-specific T cells in the brain slices. These data, combined with our previous observation that Ag-specific T cells preferentially migrate into the CNS in the presence of Ag (6), suggest that brain CD11c+ cells can stimulate naive Ag-specific T cells in situ in the inflamed brain.
Materials and Methods
Experimental animals
Male C57BL/6 (B6) mice and male diphtheria toxin receptor (DTR) Tg mice that carry a transgene encoding a simian fusion protein, DTR-GFP, were purchased from The Jackson Laboratory. Mice transgenic for a TCR recognizing the OVA257–264 (SIINFEKL) peptide bound to H2-Kb in a B6 background (OT-I) (7, 8) were provided by Dr. K. Hogquist (University of Minnesota, Minneapolis, MN). C57BL/6-Tg (OT-I)-RAG1tm1Mom (OTIRAG) mice were purchased from Taconic Farms. All mice were housed in a pathogen-free facility at the University of Wisconsin Medical School Animal Care Unit (Madison, WI). All experiments involving mice were performed in accordance with published National Institutes of Health guidelines and approved by the Committee on Animal Care, University of Wisconsin, Madison, WI.
Abs and protein Ag reagents
An Ab detecting CD8a (clone 53-6.7, conjugated to PE or allophycocyanin) was purchased from BD Pharmingen. Abs purified from hybridoma lines producing anti-CD11c (clone N418) and anti-Vα2 (clone B20.1) (American Type Culture Collection) were conjugated with biotin, FITC, PE-Cy5, or Cy5 as described previously (9).
OVA, pigeon cytochrome c (PCC), and diphtheria toxin (DT) were purchased from Sigma-Aldrich. OVA and PCC preparations were tested for endotoxin content and showed no B cell-activating capacity for naive cells isolated from B6 mice (data not shown). MIP-3β/CCL19 was purchased from R&D Systems.
Intracerebral immunization
For IC Ag delivery, 4-wk-old male mice were anesthetized by i.p. injection of a ketamine (90 mg per kg of body weight)-xylazine (10 mg per kg of body weight) mixture. OVA (60 μg per 20 μl of sterile PBS), PCC (60 μg per 20 μl of sterile PBS), or an equal volume of PBS was injected into the frontal lobe with an insulin syringe attached to a penetrating depth controller. The injection was restricted to the ventral-posterior region of the frontal lobe with a penetrating depth of 1.5 mm from the surface of the brain. The solution was injected slowly and the syringe was held in place for an additional minute to reduce the back filling of injected solution. Some DTR mice also received a single i.p. injection of DT (4 ng per g of body weight) or an equal volume of PBS 24 h before intracerebral immunization to deplete preexisting CD11c+ cells (10).
Lymphocyte isolation
Spleen tissue of OT-I mice was processed to single cell suspensions by standard methods (11). The isolated cells were further processed for positive enrichment of CD8+ T cells by magnetic sorting and were incubated in HBSS for 30 min to delete adhesive macrophages and dendritic cells (DCs). Cytofluorimetric analysis showed that ~95% of cells were CD8+Vα2+ T cells (OVA-specific) and <0.5% of them were CD11c+ after the depletion (data not shown).
Lymphocytes were also isolated from organotypic culture as described previously (12). Lymphocytes were isolated from the slices and/or the harvested culture medium. The isolated lymphocytes were washed with cold HBSS, resuspended in staining buffer (HBSS containing 5% FBS), stained, and processed for cytofluorimetric analysis. For each experiment, the total number of cells was calculated.
CFSE labeling
The succinimidyl ester group of CFSE reacts with intracellular amines, forming fluorescent conjugates that are retained in the cytoplasm. When cells divide, CFSE labeling is distributed equally between two daughter cells, which are half as fluorescent as the parent cell. As a result, each successive generation in a population of proliferating cells is marked by a halving of fluorescent intensity that is readily detected by a flow cytometer (13). Enriched and purified OT-I cells (2 × 106cells/ml) were incubated with 2.5 μM CFSE in HBSS for 10 min at 37°C. FBS was added to a concentration of 20% to arrest CFSE staining. Cells were washed in PBS or artificial cerebrospinal fluid (ACSF) and processed for cytofluorimetric analysis or for organotypic brain slice culture.
Organotypic brain slice preparation and culture
One hour after intracerebral injection, mice were decapitated during isoflurane anesthesia and brains were immediately extracted and immersed in ACSF (126 mM NaCl, 26 mM NaHCO3, 1.8 mM KCl, 2.1 mM CaCl2, 1.4 mM MgSO4, 1.2 mM KH2PO4, and 10 mM glucose) at 0–4°C. Three adjacent coronal brain slices (400 μm) were obtained, with the second, central slice containing the injection track. Each slice was cut into two hemi-slices at the midline, and hemi-slices from each side were kept in the same chamber for recovery. All slices were maintained in ACSF saturated with 95% O2-5% CO2 at 34°C for 1 h followed by 24°C for 1 h for recovery. Hemi-slices were transferred from each chamber onto one Falcon cell culture insert with pore size of 0.4 μm (BD Biosciences) in 6-well plates. Hemi-slices from DTR mice were incubated with DT (4 ng/ml) for 18 h to deplete newly derived CD11c+ cells in the brain, and the incubation was followed by a thorough wash. Enriched, purified CFSE+CD8+ T cells from OT-I mice were washed with ACSF and 1 × 105cells (in 10 μl) were added onto each hemi-slice. Slices were cultured in ACSF saturated with 95% O2-5% CO2 at 37°C in a humidified atmosphere with 5% CO2 for 48–72 h. In some experiments, MIP-3β (200 ng/ml) was added to ACSF to investigate the migration of brain-resident APCs.
Cytofluorimetric analysis
Cells isolated from each culture were incubated with mAbs, anti-Vα2, anti-CD11c, and anti-CD8a for 30–60 min on ice. An unlabeled anti-FcγR Ab (clone 2.4G2) was used to block nonspecific binding. Stained cells were analyzed by four-color flow cytometry (FACSCalibur instrument; BD Biosciences) for expression of cell surface markers. Cytofluorimetric data were analyzed using FlowJo (Tree Star). For each sample, cells were gated based on positive and negative controls, and the percentage and the absolute number of Ag-specific CD8+ T cells and/or CD11c+ cells were calculated.
Immunohistochemical analysis and confocal microscopy
Organotypic brain slices were rinsed with HBSS three times, incubated in 2% paraformaldehyde for 20 min, embedded in OCT compound (Sakura Finetek), frozen in liquid nitrogen, and sectioned at 5-μm intervals. Sections were rinsed and incubated with anti-CD11c Ab conjugated with PECy5. The distribution of CFSE+ (OVA-specific CD8+) cells and/or CD11c+ cells was examined and imaged using a confocal laser-scanning microscope (Bio-Rad MRC 1000; Bio-Rad Laboratories).
Statistical analysis
One-tailed Students’ t test was used for comparisons of two groups. In all cases, p < 0.05 was considered statistically significant.
Results
Ag-specific CD8+ T cells migrate into organotypic brain slices, colocalize with CD11c+ cells in situ, and proliferate in response to their specific Ag
We previously demonstrated that a single IC OVA injection (IC-OVA) induced a robust OVA-specific CD8+ T cell response and the recruitment of OVA-specific CD8+ T into the brain parenchyma where they colocalized with class II+ DEC205+CD11c+ DCs (6, 14, 15). To study whether brain-resident CD11c+ cells can stimulate Ag-specific T cells in situ, we used an organotypic brain slice culture system. We established organotypic brain slice cultures 1 h after IC-OVA injection to allow Ag uptake in the CNS but limit the recruitment of peripheral DCs into the brain (Fig. 1A). To determine the time limit on systemic DC recruitment into the brain, we relied on a recent study that showed there was no detectable increase of DC recruitment into the brain within 1 day following the adoptive transfer of lymphocytic choriomeningitis virus (LCMV)-specific cells in LCMV-infected mice (16). To find out whether APCs in the brain slices could induce the activation of Ag-specific T cells in the brain tissues, we cocultured the organotypic brain slices with CFSE-labeled, OVA-specific CD8+ T cells. Three days after coculture, we prepared 5-μm cryostat sections from brain slices and analyzed the presence and localization of CFSE+ OVA-specific CD8+ T cells. In the absence of Ag, CFSE+CD8+ T cells adhered to the surface of the brain slices (data not shown) but failed to migrate into the tissues (Fig. 1B, left hemisphere). In contrast, in the presence of Ag, CFSE+CD8+ T cells adhered to the surface and migrated into the tissues (Fig. 1B, right hemisphere). CD11c-expressing cells were seen occasionally scattered throughout the brain parenchyma of both IC-OVA-immunized and control hemi-slices (Fig. 1B, inset in left panel). More CD11c+ cells were observed in the area adjacent to the IC-OVA injection site and some of the CD11c+ cells localized in close proximity to the newly recruited CFSE+ OVA-specific CD8+ T cells (Fig. 1B, inset in right panel). These results indicate that OVA-specific CD8+ T cells are recruited into the brain slices and, more specifically, into the Ag injection site where they form clusters with CD11c+ cells.
FIGURE 1.

Ag-specific CD8+ T cells migrate and colocalize with CD11c+ cells harboring their cognate Ag (OVA) in organotypic brain slices. A, Diagram illustrates experimental outline. Brain hemi-slices were prepared from B6 mice 1 h after an IC-OVA injection and cocultured with OVA-specific CFSE-labeled CD8+ T cells. Three days after culture, lymph cells were isolated from cocultures and analyzed. B, Confocal images in top panels show the accumulation of OVA-specific CD8+ T cells (green) along the IC-OVA injection track in the brain (right panel). Brain sections prepared from the hemi-slice 3 days after coculture were stained with anti-CD11c Abs (insets only). The inset in each image shows CD11c+ cells (red) and OVA-specific CD8+ T cells (green) in the center of brain slices. A diagram at the bottom shows the anatomic location of images taken and depicts the distribution of OVA-specific CD8+ T cells (green dots) in the brain slices. Data represent analyses from two independent experiments. CC, Corpus callosum; AC, anterior commissure; LV, lateral ventricle; Scale, 250 μm (25 μm for insets).
To determine whether Ag-specific T cells were activated in cultures with injected OVA-containing brain slices, we measured OVA-specific CD8+ T cell proliferation 3 days after IC-OVA injection into organotypic brain slice cocultures. OVA-specific CD8+ T cells were detected by their expression of a specific TCR, Vα2. IC-OVA injection significantly increased the survival of Ag-specific CD8+ T cells in the brain slice cultures (Fig. 2A). Additionally, IC-OVA injection resulted in a significant increase in both the proliferation (Fig. 2, B and C) and the total number of OVA-specific CD8+ T cells (Fig. 2C) in brain slice cultures. To interpret these results, one has to consider that a low percentage of memory cells is also present in OT-1 Tg mice. OT-1 cell suspensions contain <3% memory/effector T cells based on low expression of LFA-1 and CD44 molecules (data not shown), suggesting that the majority of Vα2β5 CD8+ T cells before coculture display a naive phenotype. Additionally, the CFSE analysis conducted on the portion of proliferating cells indicates that >3% of total cells proliferated in the presence of organotypic brain slices (Fig. 2C). Altogether, these data suggest that naive Ag-specific T cells can proliferate in the presence of organotypic brain slices that contain their specific Ag.
FIGURE 2.
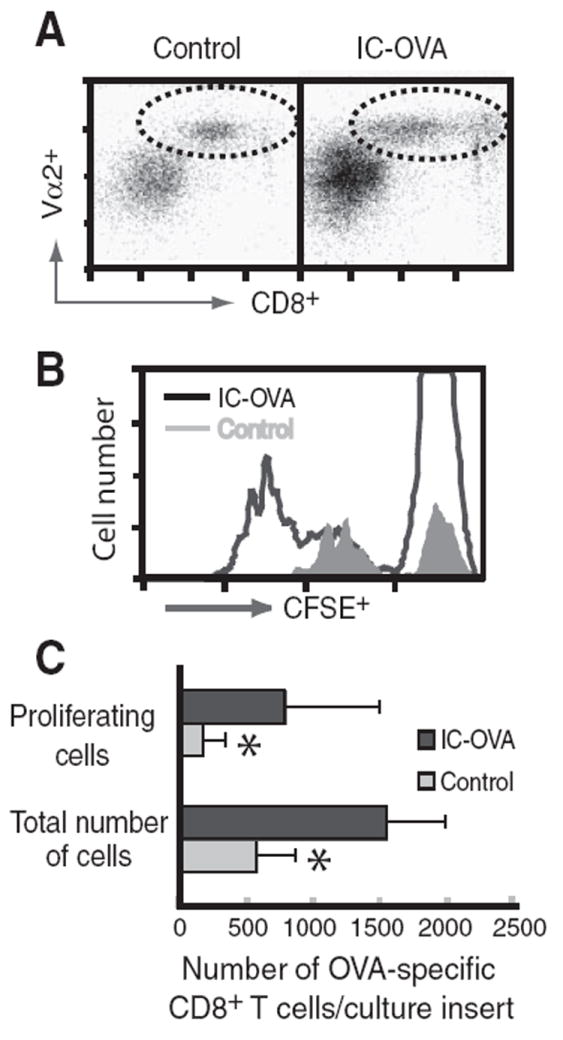
The number of OVA-specific (CFSE+Vα2+CD8+) T cells significantly increases in the culture containing brain slices prepared from the IC-OVA hemisphere. Brain hemi-slices with or without (control) an IC-OVA injection site were prepared from B6 mice 1 h after injection and cocultured with CFSE-labeled Vα2+CD8+ T cells. After three days, lymphocytes were isolated from cocultures and analyzed by cytofluorometry. A, Dot plots (gated on CFSE+ lymphocytes) show increased OVA-specific CD8+ T cell frequency (dotted circle) in the cultures with the IC-OVA injection site. B, Histograms show the proliferation of OVA-specific CD8+ T cells in the cultures as detected by cell cycle-dependent decrease of CFSE intensity. C, Quantitative analysis of OVA-specific CD8+ T cells in brain slice cultures. Bars represent the mean ± SE number of OVA-specific CD8+ T cells. *, p < 0.05, paired Student’s t test. Data are representative and were derived from seven independent experiments.
To dissect the effect of IC injection-induced trauma from the Ag-specific immune response and to determine whether the observed close proximity of CD11c+ cells and CD8+ T cells in the brain tissue reflects Ag presentation and T cell activation, we intracerebrally microinjected OVA Ag into one hemisphere and PCC, an irrelevant protein, into the contra-lateral hemisphere. Brain slices were prepared from each hemisphere and cocultured with OVA-specific CD8+ T cells. Three days after coculture, brain slices were lifted from these cultures and lymphocytes were isolated from the tissues to investigate the accumulation and proliferation of OVA-specific CD8+ T cells in situ in the brain. IC-OVA microinjection resulted in a significant increase in the number of OVA-specific CD8+ T cells within the brain slices, whereas PCC microinjection did not (Fig. 3). These data indicate that IC-delivered Ags are the major force to stimulate Ag presentation and activation of Ag-specific T cells in situ in the brain.
FIGURE 3.

Ag-specific CD8+ T cell accumulation in organotypic brain slices. Brain hemi-slices containing the IC injection site were prepared from B6 mice 1 h after IC-OVA and IC-PCC immunizations and cocultured with OVA-specific T cells. Three days after culture, the hemi-slices were separated from the culture and lymphocytes were isolated from each hemi-slice and analyzed by cytofluorometry. A, Dot plots (gated on lymphocytes) show the percentage of OVA-specific CD8+ T cells (Vα2+CD8+; dotted circle) per slice (percentage given in the upper right corner of each plot). B, Quantitative analysis of OVA-specific CD8+ T cells in each slice. Bars represent the mean ± SE number of CD8+ T cells. *, p < 0.05, paired Student’s t test. Data are representative and were derived from three independent experiments.
CD11c+ cells migrate from organotypic brain slices into the culture supernatant
We demonstrated that OVA-specific CD8+ T cells proliferated in cocultures with organotypic brain hemi-slices derived from IC-OVA-microinjected brains (Fig. 2). Some of the OVA-specific CD8+ T cells accumulated in the brain slices (Figs. 1B and 3), but many of them remained free floating in the culture supernatants (data not shown). These results suggest that brain-resident DCs migrate from the brain slices into the culture supernatants. To test this possibility, we analyzed the frequency of CFSE− and CD11c+ cells in the supernatants of brain slice cultures. CFSE negativity was applied as an additional marker for demonstrating the brain origin of CD11c+ cells in the supernatants of organotypic cultures. In these experiments, mice received an IC-OVA microinjection into one hemisphere and an IC-PBS injection in the contra-lateral hemisphere, and brain slices from each hemisphere were cocultured with OVA-specific CD8+ T cells. Three days after coculture, free-floating cells were isolated and processed for immunocytochemical and cytofluorimetric analysis. CD11c+ cells were detected in the supernatant of IC-OVA brain slice cultures (Fig. 4A), and the number of CFSE−CD11c+ cells was increased significantly compared with those in the supernatant of IC-PBS brain slice cultures (Fig. 4B). These results suggest that CNS-resident CD11c+ cells migrate out of these organotypic brain slices in the presence of Ag-specific T cells.
FIGURE 4.

Brain-resident CD11c+ cells accumulate in the culture supernatant of organotypic brain slices. Brain hemi-slices containing the IC injection sites were prepared from B6 mice 1 h after IC-OVA or IC-PBS injection and cocultured with OVA-specific CD8+ T cells. Three days after coculture, free-floating cells were harvested from the supernatant of each culture and processed for confocal images and cytofluorimetric analysis. A, Representative confocal image shows CFSE−CD11c+ (blue) and OVA-specific CD8+ T cells (CFSE+; green) in the culture supernatant of IC-OVA-injected hemi-slices. Scale bar, 20 μm. B, Histograms (gated on CFSE− cells) show an increased number of CD11c+ cells in the culture supernatant with brain slices containing IC-OVA injection sites. Data are representative of three independent experiments.
MIP-3β increases the migration of CD11c+ cells from brain slices
We showed that brain-resident CD11c+ cells migrated out of brain slices to OVA-specific CD8+ T cells in organotypic brain slice cultures (Fig. 4B). The results suggest that brain-resident CD11c+ cells are mature and mobile. To further address this issue, we microinjected OVA Ag into both hemispheres of the brain and prepared brain slices for coculture with OVA-specific CD8+ T cells in the presence or absence of MIP-3β (200 ng/ml), a chemokine known to attract mature DCs. Two days after the cultures, free-floating cells were harvested and analyzed by cytofluorometry. MIP-3β treatment significantly increased the total number of CFSE−CD11c+ cells in the supernatant of brain slice cultures (Fig. 5). These results indicate that brain-resident CD11c+ cells respond to MIP-3β chemokine and migrate into culture supernatants containing this chemokine.
FIGURE 5.

MIP-3β chemokine enhances the migration of brain-resident CD11c+ cells from organotypic brain slices. Brain hemi-slices were prepared from B6 mice 1 h after bilateral IC-OVA immunizations and cocultured with OVA-specific CD8+ T cells with or without MIP-3β (200 ng/ml). Two days after culture, cells were harvested from the supernatant of each culture and processed for cytofluorimetric analysis. A, Histograms (gated on CFSE− cells) show an increased number of CD11c+ cells in the culture with MIP-3β. B, Quantitative analysis of CFSE−CD11c+ cells in culture with or without MIP-3β. Bars represent the mean ± SE number of CD11c+ cells. *, p < 0.05, paired Student’s t test. Data are representative and were derived from three independent experiments.
In vitro depletion of brain-resident CD11c+ cells abrogates OVA-specific CD8+ T cell proliferation in organotypic brain slice cultures
To asses the role of endogenous brain CD11c+ cells in inducing Ag-specific T cell activation, we used a Tg strain of mice (CD11c-DTR) that expresses a transgene encoding a simian fusion protein, human DTR-GFP, under control of the murine CD11c promoter (10). Due to transgene expression, cells expressing CD11c can be selectively depleted in this Tg mouse strain. The human DTR has a significantly higher affinity to DT than its mouse counterpart, which results in significant toxicity only to cells expressing CD11c upon DT injection. For these experiments, CD11c+ cells were depleted from CD11c-DTR Tg mice by a single i.p. DT treatment (4 ng per g of body weight) or PBS as a control (Fig. 6A). Twenty-four hours later, OVA Ag was microinjected into the left hemisphere of these mice and the brains were processed for organotypic brain slice cultures 1 h after injection. Brain slices were cultured for an additional 18 h in the presence or absence of DT (4 ng/ml) to further deplete any recently recruited or derived CD11c+ cells in the brain. The DT treatment effectively depleted >95% of brain-resident CD11c+ cells (Fig. 6B). After DT treatment, the brain slices were cocultured with OVA-specific CD8+ T cells for 3 days. Brain slices that were generated from CD11c-DTR mice following DT treatments did not induce the clonal expansion of Ag-specific T cells in these cultures (Fig. 6C). These results indicate that CD11c+ DCs in the brains are required for Ag-specific T cell activation in organotypic brain slice cultures.
FIGURE 6.

The clonal expansion of OVA-specific CD8+ T cells is abolished by depletion of CD11c+ cells from DTR mice. A, Diagram illustrates experimental outline. Brain hemi-slices were prepared from DTR mice 1 h after IC-OVA immunizations with or without a primary DT injection and cocultured with OVA-specific CD8+ T cells from OTI-RAG mice with or without secondary DT treatment. Three days after co-culture, lymphocytes were isolated and processed for cytofluorimetric analysis. B, Histograms (gated on lymphocytes) show the depletion of CD11c+ cells from culture after DT treatments. C, Histograms (gated on CD8+ cells) show that the proliferation of OVA-specific CD8+ T cells in culture is abolished after DT treatments. Data are representative of three independent experiments.
Discussion
Previously it has been shown that CNS-resident APCs process Ag in the brain and promote immune responses (14, 15, 17, 18). Whether brain-resident APCs can locally process Ag and activate Ag-specific T cells in situ in the inflamed brain is a critical question in understanding the basic mechanisms of immune-mediated diseases of the CNS. In this study, we investigated the stimulatory function of brain-resident CD11c+ cells on Ag-specific T cells in situ in the brain using organotypic brain slices. This experimental approach allowed us to exclude the possibility of peripheral activation of Ag-specific T cells.
Organotypic brain slices have been used to investigate mechanisms and treatment strategies for neurodegenerative disorders such as cerebral ischemia, Alzheimer’s disease, epilepsy, neuro-toxicity, traumatic injury, and multiple sclerosis (19). In brain slice cultures the cytoarchitecture and the connectivity of different anatomical regions and different types of cells are preserved (20-23). Taking advantage of this model, we demonstrated that there are stimulatory resident CD11c+ cells in the mouse brain and that these cells cluster with Ag-specific CD8+ T cells in situ in the CNS and stimulate Ag-specific T cells in situ in the absence of a peripheral immune system in vitro in organotypic brain slices (Figs. 1-3).
Previously, it was suggested that naive T cells enter the inflamed CNS and become activated by local APCs in the brain tissue and that this process contributes to the initiation of epitope spreading in vivo in the CNS (24). Our study strengthens this observation and suggests that naive Ag-specific T cells can be stimulated in situ in the brain parenchyma. Naive Ag-specific T cell migration into the CNS has been demonstrated previously in response to autoimmunity (4) or trauma-induced inflammation (6). The local stimulation of naive Ag-specific T cells by APCs in the CNS in situ might contribute to the amplification of neuroinflammation. In support of this view, it was shown that ectopic lymphoid tissues form in the inflamed brain, where DCs and lymphocytes are in close contact with each other during experimental autoimmune encephalomyelitis (25), and in patients with progressive-relapsing multiple sclerosis (26). DC localization in ectopic lymphoid tissues supports their critical role in the maintenance of inflammatory responses in the CNS (27).
The activation and recruitment of CNS APCs (such as DCs, macrophages, and microglia) in inflammatory diseases and infections in the CNS have been shown previously (24, 28-32). These APCs are thought to be critical in the restimulation of effector T cells in the CNS. However, in an elegant study it was demonstrated that although there is a shift in the dynamics of microglia, macrophage, and DC activation profiles and recruitment in the CNS during chronic viral infection, CD11c+ DCs are the only potent stimulators of memory CTLs in the brain parenchyma during immunotherapeutic clearance of persistent viral infection (16). Additionally, an experimentally increased level of DCs in the brain accelerated experimental autoimmune encephalomyelitis clinical symptoms (33). In our studies we also focused on the CD11c-expressing APCs in the CNS. Because the majority of developed mouse DCs express CD11c (10, 16, 32, 34), we postulate that the bulk of brain-resident CD11c-expressing cells that can activate naive T cells belongs to the population of DCs. This is supported by our observation that when CD11c+ DCs were depleted using the CD11c-DTR mice, there was no activation of Ag-specific T cells in the brain slice cultures (Fig. 6). Although other cells were proposed to function as APCs in the CNS, these data confirm that of all the different CNS-APC populations, only CD11c+ DCs possess the ability to activate naive T cells.
It was previously proposed that DCs play a critical role in the initiation of autoimmunity and in promoting T cell activation in the CNS. It has been demonstrated that presentation of Ags by two distinct MHC class II molecules allows DCs to mediate cooperative interactions of T cells with different TCR specificities, leading to enhanced accumulation and IFN-γ production in the CNS (15). We previously detected a >10-fold amplification of T cells and an increased production of IFN-γ proinflammatory mediator as a response to DC Ag presentation in the brain. We proposed that this amplification might have an important role in the outcome of neuroinflammation. We also demonstrated that simultaneous DC-T cell clustering involves CD40-CD40 ligand interactions and high local IL-2 concentrations (6, 15). All of these previous data supported a novel role for DCs in locally enhancing T cell functions in the brain in vivo.
In the present work, by using a very sensitive functional approach, we demonstrate that CD11c+ cells can be found in the brain in the absence of the peripheral immune system and that these cells can stimulate naive T cells in vitro. Although DCs are the only APCs that can stimulate naive and memory T cells (35), we cannot exclude the possibility that other brain cells could contribute to naive T cell activation in organotypic brain cultures. Brain slices have been shown to induce glial cell activation (36), and microglia have been demonstrated to up-regulate CD11c expression upon inflammatory stimulus (16, 37, 38). However, we did not detect Ag-specific T cell proliferation in the absence of OVA Ag in brain slice cultures (Fig. 2B). Additionally, microglia can be easily distinguished from DCs (16), and these cells cannot efficiently stimulate naive APCs (32). In fact, it was shown that CNS-resident CD11b+CD11c−CD45low microglia purified from the inflamed CNS are largely incapable of activating either naive or effector T cells (32).
Both inhibitory protective and stimulatory functions were found to be associated with brain DCs (24, 30, 32, 33, 39-41). The changing function of DCs in the CNS might be associated with their high functional and phenotypic plasticity. DCs can be characterized according to their maturation status, their ability to migrate, capture, and present Ags to naive T cells, and their ability to induce either a productive immune response or self-tolerance (42). Understanding their function in the CNS is critical in light of numerous studies that demonstrate a substantial accumulation of DCs in the brain and spinal cord in response to local inflammation induced by autoimmunity, infection, or trauma (24, 28-32).
In an animal model of multiple sclerosis it was proposed that CD11c-expressing, brain-resident DCs at the blood-brain barrier and in the meninges are critical for the initial Ag presentation and the induction of T cell infiltration into the CNS (33) and for the activation of naive T cells in epitope spreading in vivo (24). The activation of naive T cells in the CNS might contribute to the amplification of neuroinflammation. In our study, we investigated this important issue using organotypic brain slice cultures. We showed that CD11c+ cells in situ in brain slices induce naive Ag-specific T cell accumulation and activation. These results, combined with our previous observation that Ag-specific T cells preferentially migrate into the CNS in the presence of Ag (6), strongly suggest that brain APCs can stimulate naive Ag-specific T cells in situ.
These data demonstrate that CD11c+ cells in the CNS contribute to the activation of naive T cells in the inflamed brain. Although the major pathway for the presentation of brain Ags is likely to be systemic, involving both antigenic drainage and DC migration from the inflamed CNS, our data suggest that local T cell activation by brain-residing CD11c+ APCs is also possible. The significance of this pathway needs to be further studied. The contribution of CD11c+ cells to the development and maintenance of CNS autoimmunity and inflammation indicate that these cells pose a novel target for the treatment of inflammatory diseases of the CNS.
Acknowledgments
We thank Khen Macvilay for careful cytofluorimetric data acquisition.
Footnotes
This work was supported by National Institutes of Health Grant R01-NS 37570-01A2 (to Z.F.).
Abbreviations used in this paper: MBP, myelin basic protein; ACSF, artificial cerebrospinal fluid; B6, C57BL/6; DC, dendritic cell; DT, diphtheria toxin; DTR, DT receptor; IC, intracerebral; PCC, pigeon cytochrome c; Tg, transgenic.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Wekerle H. T-cell autoimmunity in the central nervous system. Intervirology. 1993;35:95–100. doi: 10.1159/000150299. [DOI] [PubMed] [Google Scholar]
- 2.Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Semin Immunol. 1999;11:125–137. doi: 10.1006/smim.1999.0168. [DOI] [PubMed] [Google Scholar]
- 3.Hickey WF. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991;1:97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 4.Brabb T, von Dassow P, Ordonez N, Schnabel B, Duke B, Goverman J. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med. 2000;192:871–880. doi: 10.1084/jem.192.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seabrook T, Au B, Dickstein J, Zhang X, Ristevski B, Hay JB. The traffic of resting lymphocytes through delayed hypersensitivity and chronic inflammatory lesions: a dynamic equilibrium. Semin Immunol. 1999;11:115–123. doi: 10.1006/smim.1999.0167. [DOI] [PubMed] [Google Scholar]
- 6.Ling C, Sandor M, Suresh M, Fabry Z. Traumatic injury and the presence of antigen differentially contribute to T-cell recruitment in the CNS. J Neurosci. 2006;26:731–741. doi: 10.1523/JNEUROSCI.3502-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly JM, Sterry SJ, Cose S, Turner SJ, Fecondo J, Rodda S, Fink PJ, Carbone FR. Identification of conserved T cell receptor CDR3 residues contacting known exposed peptide side chains from a major histocompatibility complex class I-bound determinant. Eur J Immunol. 1993;23:3183–3326. doi: 10.1002/eji.1830231239. [DOI] [PubMed] [Google Scholar]
- 8.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 9.Fabry Z, Waldschmidt MM, Hendrickson D, Keiner J, Love-Homan L, Takei F, Hart MN. Adhesion molecules on murine brain microvascular endothelial cells: expression and regulation of ICAM-1 and Lgp 55. J Neuroimmunol. 1992;36:1–11. doi: 10.1016/0165-5728(92)90026-h. [DOI] [PubMed] [Google Scholar]
- 10.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qing Z, Sewell D, Sandor M, Fabry Z. Antigen-specific T cell trafficking into the central nervous system. J Neuroimmunol. 2000;105:169–178. doi: 10.1016/s0165-5728(99)00265-9. [DOI] [PubMed] [Google Scholar]
- 12.Sewell DL, Nacewicz B, Liu F, Macvilay S, Erdei A, Lambris JD, Sandor M, Fabry Z. Complement C3 and C5 play critical roles in traumatic brain cryoinjury: blocking effects on neutrophil extravasation by C5a receptor antagonist. J Neuroimmunol. 2004;155:55–63. doi: 10.1016/j.jneuroim.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyons AB. Divided we stand: tracking cell proliferation with carboxy-fluorescein diacetate succinimidyl ester. Immunol Cell Biol. 1999;77:509–515. doi: 10.1046/j.1440-1711.1999.00864.x. [DOI] [PubMed] [Google Scholar]
- 14.Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173:2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- 15.Karman J, Chu HH, Co DO, Seroogy CM, Sandor M, Fabry Z. Dendritic cells amplify T cell-mediated immune responses in the central nervous system. J Immunol. 2006;177:7750–7760. doi: 10.4049/jimmunol.177.11.7750. [DOI] [PubMed] [Google Scholar]
- 16.Lauterbach H, Zuniga EI, Truong P, Oldstone MB, McGavern DB. Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection. J Exp Med. 2006;203:1963–1975. doi: 10.1084/jem.20060039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling C, Sandor M, Fabry Z. In situ processing and distribution of intracerebrally injected OVA in the CNS. J Neuroimmunol. 2003;141:90–98. doi: 10.1016/s0165-5728(03)00249-2. [DOI] [PubMed] [Google Scholar]
- 18.Karman J, Ling C, Sandor M, Fabry Z. Dendritic cells in the initiation of immune responses against central nervous system-derived antigens. Immunol Lett. 2004;92:107–115. doi: 10.1016/j.imlet.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- 20.Wolf SA, Fisher J, Bechmann I, Steiner B, Kwidzinski E, Nitsch R. Neuroprotection by T-cells depends on their subtype and activation state. J Neuroimmunol. 2002;133:72–80. doi: 10.1016/s0165-5728(02)00367-3. [DOI] [PubMed] [Google Scholar]
- 21.Filipovic R, Zecevic N. Interaction between microglia and oligodendrocyte cell progenitors involves golli proteins. Ann NY Acad Sci. 2005;1048:166–174. doi: 10.1196/annals.1342.015. [DOI] [PubMed] [Google Scholar]
- 22.Huuskonen J, Suuronen T, Miettinen R, van Groen T, Salminen A. A refined in vitro model to study inflammatory responses in organotypic membrane culture of postnatal rat hippocampal slices. J Neuroinflammation. 2005;2:25. doi: 10.1186/1742-2094-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbny YI, Erdelyi F, Szabo G, Banks MI. Properties of a population of GABAergic cells in murine auditory cortex weakly excited by thalamic stimulation. J Neurophysiol. 2006;96:3194–3208. doi: 10.1152/jn.00484.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 25.Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:11–23. doi: 10.1016/j.jneuroim.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 26.Serafini B, Columba-Cabezas S, Di Rosa F, Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol. 2000;157:1991–2002. doi: 10.1016/S0002-9440(10)64838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Hanly A, Petito CK. HLA-DR-positive dendritic cells of the normal human choroid plexus: a potential reservoir of HIV in the central nervous system. Hum Pathol. 1998;29:88–93. doi: 10.1016/s0046-8177(98)90395-1. [DOI] [PubMed] [Google Scholar]
- 29.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- 30.Suter T, Biollaz G, Gatto D, Bernasconi L, Herren T, Reith W, Fontana A. The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activationl. Eur J Immunol. 2003;33:2998–3006. doi: 10.1002/eji.200323611. [DOI] [PubMed] [Google Scholar]
- 31.NewmanT A, Galea I, van Rooijen N, Perry VH. Blood-derived dendritic cells in an acute brain injury. J Neuroimmunol. 2005;166:167–172. doi: 10.1016/j.jneuroim.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ TH-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 33.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 34.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 35.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 36.Damiani CL, O’Callaghan JP. Recapitulation of cell signaling events associated with astrogliosis using the brain slice preparation. J Neurochem. 2007;100:720–726. doi: 10.1111/j.1471-4159.2006.04321.x. [DOI] [PubMed] [Google Scholar]
- 37.Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, Stern LJ, Strominger JL, Riese R. Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reichmann G, Schroeter M, Jander S, Fischer HG. Dendritic cells and dendritic-like microglia in focal cortical ischemia of the mouse brain. J Neuroimmunol. 2002;129:125–132. doi: 10.1016/s0165-5728(02)00184-4. [DOI] [PubMed] [Google Scholar]
- 39.Dittel BN, Visintin I, Merchant RM, Janeway CA., Jr Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J Immunol. 1999;163:32–39. [PubMed] [Google Scholar]
- 40.Weir CR, Nicolson K, Backstrom BT. Experimental autoimmune encephalomyelitis induction in naive mice by dendritic cells presenting a self-peptide. Immunol Cell Biol. 2002;80:14–20. doi: 10.1046/j.1440-1711.2002.01056.x. [DOI] [PubMed] [Google Scholar]
- 41.Kleindienst P, Brocker T. Concerted antigen presentation by dendritic cells and B cells is necessary for optimal CD4 T-cell immunity in vivo. Immunology. 2005;115:556–564. doi: 10.1111/j.1365-2567.2005.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]


