Abstract
Diabetic nephropathy (DN) is an important diabetic complication, and podocyte apoptosis plays a critical role in the development of DN. In the present study, we examined the preventive effect of the total flavone glycosides of Flos Abelmoschus manihot (TFA) on urinary microalbumin and glomerular podocyte apoptosis in experimental DN rats. The preliminary oral administration of TFA (200 mg/kg/day) for 24 weeks significantly decreased the urinary microalbumin to creatinine ratio and 24-h urinary total protein in streptozotocin-induced DN rats. Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay indicated glomerular cell apoptosis in DN rats was significantly improved by pretreatment with TFA. Furthermore, fluorescence-activated cell sorting and Hoechst 33342 staining suggested preincubation with hyperoside (50 and 200 μg/mL), the major active constituent of TFA, could significantly mitigate cultured podocyte apoptosis induced by the advanced glycation end-products (AGEs). Western blot analysis showed that increased caspase-3 and caspase-8 expressions induced by AGEs were also inhibited by pretreatment with hyperoside at both doses. Our results demonstrate that TFA pretreatment can decrease urinary albumin excretion in early-stage DN, which might be accomplished by preventing renal damage and podocyte apoptosis.
Key Words: apoptosis, diabetic nephropathy, hyperoside, podocyte, total flavone glycosides of FlosAbelmoschus manihot
Introduction
Diabetic nephropathy (DN) is the major microvascular complication of diabetes worldwide.1 The clinical characteristics of DN are progressive albuminuria and gradual decline of renal function, which results from the multiple pathophysiologic dysfunctions of diabetes.2 The appearance of microalbuminuria is regarded as the hallmark of DN onset at its early stage, and the continuous existence of albuminuria plays a pivotal role in the damage of both renal structure and function.3 Although the treatment and understanding of DN are progressing, the level of albuminuria and renal damage in some DN patients continues advancing in a seemingly irreversible pattern.4 The novel underlying mechanisms and therapeutic measures remain to be explored.
The increased urinary albumin excretion is due to the dysfunction of the glomerular barrier, which is accepted to be a key target for prevention and treatment of DN. Among the elementary ingredients of glomeruli structure, the podocyte and its foot process play the pivotal role in maintenance of permselective function of the glomerular barrier.5 The loss of glomerular podocytes and decreased podocyte density are early pathological manifestations and predict the onset of DN.6,7 Podocyte loss precedes the development of comprehensive renal dysfunction and albuminuria in diabetic patients and animal models.8,9 Podocyte loss resulting from apoptosis has played an important role in the onset of albuminuria and the pathogenesis of DN.10 Recent studies demonstrate that preventing podocyte apoptosis could ameliorate renal injury and decreases proteinuria in DN.11,12
As a well-known traditional Chinese medicine, Flos Abelmoschus manihot L. Medic has been used as the neuroprotective drug for cerebral ischemic reperfusion injury in recent studies.13,14 Total flavone glycosides of Flos A. manihot (TFA) contain seven chemically identified flavone glycosides15 and have therapeutic effects on cerebral ischemic reperfusion and postmenopausal osteoporosis.13,16 Our previous clinical studies showed for the first time that TFA can improve proteinuria and renal function impairments in early-stage 2 DN,17 but the underlying mechanisms remain unknown. In this study, we aimed to investigate the preventive effect of TFA on microalbuminuria and apoptosis of glomerular cells in experimental early-stage DN rats. Furthermore, the protective effect of the major active constituent of TFA, hyperoside, on cultured podocyte apoptosis was explored to elucidate the underlying molecular pharmacologic mechanism.
Materials and Methods
Reagents
TFA (content of flavone glycosides over 99%) was extracted from Flos A. manihot L. Medic by the Department of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing, China. All of the seven flavone glycosides in TFA were characterized by high-performance liquid chromatography, and their chemical structures were identified. The profile compositions of TFA are hyperoside (43.2%), hibifolin (27.1%), isoquercetin (13.7%), quercetin-3′-O-glucoside (8.8%), quercetin-3-O-robinobioside (3.8%), myricetin (3.2%), and quercetin (0.2%). The hyperoside contents in different preparations were measured for quality control. TFA was suspended in 1% carboxymethyl cellulose solution at different concentrations for oral administration.
DN model
Male Sprague–Dawley rats 7–8 weeks old weighing 180–200 g were purchased from the Experimental Animal Center, Nanjing University of Chinese Medicine. Diabetes was induced in rats by a single intravenous injection of streptozotocin (STZ) (Sigma, St. Louis, MO, USA) (70 mg/kg of body weight) dissolved in 10 mmol/L sodium citrate (pH 5.5). The nondiabetic rats that received the same volume of citrate buffer served as the control group. Seven days after STZ injection, rats with high blood glucose levels in the range of 13.9–22.2 mmol/L were considered as rats with successfully established diabetes mellitus (DM). The urinary proteins were screened every 8 weeks, and a urinary microalbumin to creatinine (mAlb/Cr) ratio of >30–300 mg/g was taken as the screening standard for DN. According to previous studies,18,19 the DM rats could develop into early-stage DN models in about 16–24 weeks. The STZ-induced rats received continuous oral administration of TFA solution right after the establishment of DM models. The DM rats were randomly divided into three groups: DM rats treated with carboxymethyl cellulose solution (DN group) or a low or high dose of TFA (50 and 200 mg/kg/day TFA, respectively). In our preliminary experiments, oral administration of TFA (200 mg/kg/day) for 8 weeks could significantly decrease the urinary microalbumin excretion and glomerular hyperfiltration in STZ-induced DN mice. Each of the four groups had eight rats. All rats were fed a standard pellet laboratory diet and were provided with water ad libitum.
Experimental protocols
The experimental protocols were approved by the Animal Ethics Committee of Jiangsu Province (Nanjing). Blood glucose levels of DM rats were monitored every week using a Bayer glucose monitor (Bayer Co., Bergkamen, Germany). The DM rats were given supportive regular insulin treatment (Wanbang Biopharmaceuticals, Xuzhou, China) at a dose of 1 IU/kg of body weight (three times every week) when necessary to maintain blood glucose in the range of 13.9–22.2 mmol/L for preventing apparent exhaustion or ketosis during the experiment. The rats with blood glucose in the range of 13.9–22.2 mmol/L did not receive insulin treatment to avoid the possible synergistic effect or interaction of insulin and TFA. Every 8 weeks after drug administration, individual 24-h urine sample collections were performed using metabolic cages. All the animals were prevented from drinking for 24 h during the urine sample collection to avoid systemic error.
Urinary albumin concentrations were measured by Exocell (Philadelphia, PA, USA) kits using anti-rat albumin antibody. The urinary creatinine levels were measured by the enzymatic colorimetric method. Results of urinary albumin were normalized to creatinine levels and expressed as the urinary mAlb/Cr ratio. All animals were sacrificed after 24 weeks of treatment, and fresh kidney cortices were excised and stored at −80°C until further analysis.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay and Hoechst 33342 staining
To observe the glomerular apoptotic cells, apoptosis-related molecules were measured in glomeruli by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) using a commercially available kit (Chemicon International, Temecula, CA, USA) according to a previous study.20 The presence of TUNEL-positive glomerular cells in formalin-fixed renal tissue were identified by examining 30 glomeruli in each rat (×400 magnification). Moreover, the changes of apoptosis in podocytes cultured on coverslips were also investigated by Hoechst 33342 (Beyotime Institute of Biotechnology, Haimen, China) staining. Apoptosis was expressed by the percentage of podocytes with nuclear condensation nuclear cells at least 200 cells per condition.
Western blot
Cultured podocytes were harvested from the plates and lysed with a buffer containing 1% Nonidet P-40, a protease inhibitor cocktail. After determination of protein concentrations, cell lysates were subjected to 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis before transfer to polyvinylidene difluoride membranes. The membranes were blocked with 5% defatted milk powder in 1× Tris-buffered saline containing 0.1% Tween-20 for 30 min and incubated overnight with polyclonal rabbit anti-rat caspase-3 or caspase-8 primary antibody (Bioworld Technology, St. Louis Park, MN, USA) at 4°C. After phosphate-buffered saline (PBS) washing (10 min×3) and incubation with alkaline phosphatase–labeled secondary antibodies for 60 min, signals were detected with an enhanced 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium color development kit (Beyotime Institute of Biotechnology). Membranes were incubated with rabbit polyclonal anti-β-actin antibodies (Zhongshan Goldenbridge Biotechnology, Beijing, China) to serve as the control for equal loading. The density of each band was determined using NIH Image software and expressed as a relative value to the density of the corresponding band β-actin immunoblot.
Cell culture
Conditionally immortalized murine podocytes were cultivated in RPMI 1640 medium containing 10% fetal bovine serum, 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM l-glutamine. To permit immortalized growth, the culture medium was supplemented with 10 U/mL recombinant mouse γ-interferon to induce the expression of T antigen, and cells were cultured at 33°C (permissive conditions) as in a previous study.21 To induce differentiation, cells were cultured on type I or collagen IV at 37°C without γ-interferon for at least 7 days. We confirmed the podocytes differentiation under nonpermissive condition (37°C) by immunofluorescence staining for podocyte marker nephrin and WT-1. Only the differentiated podocytes were used in the study.
Determination of podocyte apoptosis by fluorescence-activated cell sorting
Podocytes were cultivated on six-well culture plates coated with collagen IV in RPMI 1640 medium with 10% fetal bovine serum for 12 h. Cells were washed with PBS and then exposed to 500 μg/mL PBS containing bovine serum albumin (BSA) (the control) or advanced glycation end-products (AGEs) with BSA for overnight in RPMI 1640 medium with 1% fetal bovine serum. In the hyperoside pretreatment group, a low or high concentration of hyperoside (50 or 200 μg/mL, respectively) was added to the RPMI 1640 medium 1 h before AGE-BSA exposure. Each experimental condition was performed in triplicate. After a 24-h incubation, cell culture medium was collected and stored. PBS used for washing (5 min×3) was combined with the saved culture medium. The concentration of detached cells was determined using a hemocytometer, and each sample was counted three times. The percentages of apoptosis and necrosis in the adherent-cell fraction were determined by fluorescence-activated cell sorting (FACS) after annexin V–fluorescein isothiocyanate and propidium iodide labeling. The numbers of apoptotic and necrotic cells were calculated by multiplying the percentages of apoptosis and necrosis as determined by FACS by the total number of adherent cells. The total number of cells was defined as the number of detached cells plus the number of adherent cells.
Statistical analysis
All results were expressed as mean±SD values, and differences were analyzed by SPSS version 16.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance with a one-tailed Student's t test was used to identify significant differences in multiple comparisons. The post hoc comparisons using the Student–Newman–Keuls test were used for intergroup comparisons of multiple variables. P<.05 was considered statistically significant.
Results
Effect of TFA on urinary albumin excretion of DN rats
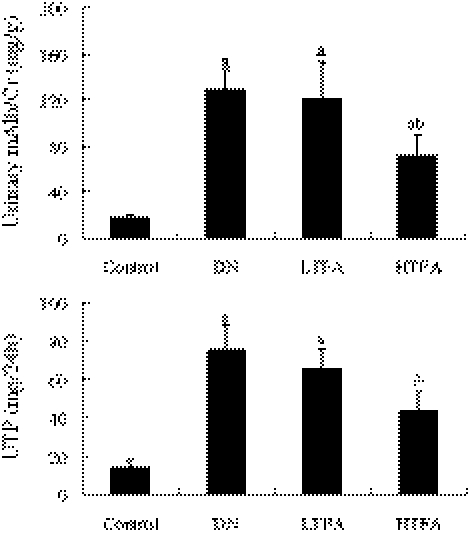
The effect of TFA on urinary albumin in early-stage DN rats was evaluated by measuring urinary mAlb/Cr and 24-h urinary total protein (UTP) (Fig. 1). DN rats induced by STZ for 24 weeks showed significant (P<.05) elevations of urinary mAlb/Cr and 24-h UTP (128.1±17.6 mg/g and 75.6±12.5 mg/24 h, respectively) compared with the control group (17.6±2.8 mg/g and 14.1±3.6 mg/24 h, respectively), demonstrating that we successfully established the early-stage DN animal model. Pretreatment with TFA (50 mg/kg/day) had no significant influence, whereas a higher dose of TFA (200 mg/kg/day) decreased urinary mAlb/Cr and 24-h UTP (71.6±17.9 mg/g and 43.9±9.8 mg/24 h, respectively; P<.05) in DN rats 24 weeks after the initiation. The albuminuria of the DN rats was significantly decreased by pretreatment with TFA at the dose of 200 mg/kg/day.
FIG. 1.
Effect of pretreatment with total flavone glycosides of Flos A. manihot (TFA) for 24 weeks on urinary microalbumin of rats with diabetic nephropathy (DN) induced by streptozotocin (n=8 per group): control group (nondiabetic normal rats), DN group (diabetes mellitus rats treated with vehicle carboxymethylcellulose solution), and LTFA and HTFA groups (diabetes mellitus rats treated with 50 [low-dose] or 200 [high-dose] mg/kg/day TFA, respectively). The urinary microalbumin to creatinine (mAlb/Cr) ratio, measured 24 weeks after treatment, was expressed in milligrams/gram, and the 24-h urinary total protein (UTP) was expressed in milligrams/24 h. Data are mean±SD values. P<.05 is statistically significant. aSignificantly different compared with the control group. bSignificantly different compared with the DN group.
Effect of TFA on glucose and lipid metabolic parameters of DN rats
DN rats also showed significant increases in glucose and glycosylated hemoglobin (HbA1c) levels (21.6±5.7 mmol/L and 10.8±3.2%, respectively) compared with the control group (5.2±1.8 mmol/L and 6.4±1.6%, respectively). DN rats also had higher serum carboxymethyllysine (CML) levels (76.5±12.4 ng/mL) in comparison with the nondiabetic rats (22.8±8.2 ng/mL). Pretreatment with TFA (50 and 200 mg/kg/day) for 24 weeks did not affect plasma glucose, HbA1c, and CML levels compared with DN rats treated with the vehicle (Table 1). There was no significant difference in glucose and lipid metabolic factors among the DN group and low- and high-dose TFA-treated groups at the end of the experiment.
Table 1.
Effect of Pretreatment with Total Flavone Glycosides of Flos A. manihot for 24 Weeks on Glucose and Lipid Metabolic Parameters of Streptozotocin-Induced Diabetic Nephropathy Rats
| |
Group (n=8) |
|||
|---|---|---|---|---|
| Control | DN | LTFA | HTFA | |
| Blood glucose (mmol/L) | 5.2±1.8 | 21.6±5.7a | 18.3±4.2a | 19.8±3.4a |
| HbA1c (%) | 6.4±1.6 | 10.8±3.2a | 10.1±2.5a | 9.6±2.8a |
| TC (mmol/L) | 4.6±1.1 | 5.1±1.2 | 4.8±0.8 | 4.6±0.9 |
| TG (mmol/L) | 1.4±0.6 | 2.3±0.9a | 1.9±0.4a | 2.1±0.6a |
| HDL (mmol/L) | 1.8±0.4 | 1.5±0.6 | 1.5±0.4 | 1.4±0.3 |
| LDL (mmol/L) | 2.8±0.9 | 3.6±0.8a | 3.4±0.8a | 3.2±0.6a |
| CML (ng/mL) | 22.8±8.2 | 76.5±12.4a | 72.9±10.8a | 65.4±11.5a |
Data are mean±SD values.
Significantly different compared with the control group, P<.05.
CML, carboxymethyllysine; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglycerides.
Effect of TFA on apoptosis of glomerular cells in DN rats
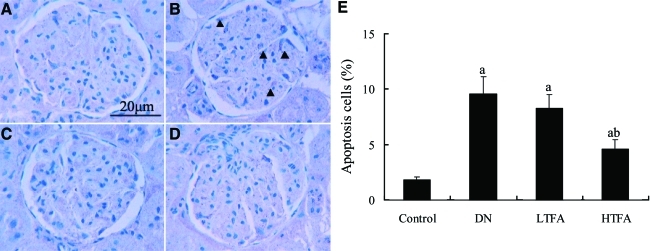
As shown in the TUNEL assay (Fig. 2), the number of glomerular apoptotic cells was significantly increased in DN rats (9.6±1.5%) compared with the normal control rats (1.8±0.3%, P<.05). The increased apoptotic cells within glomeruli were significantly abrogated by pretreatment with TFA. TFA at a dose of 50 mg/kg/day had a lesser and nonsignificant effect on glomerular cell apoptosis.
FIG. 2.
Effect of pretreatment with TFA for 24 weeks on glomerular cells apoptosis in DN rats by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay (n=6 per group): (A) control group, (B) DN group, (C) LTFA group, and (D) HTFA group. ×400 magnification. Positive glomerular cells in formalin-fixed renal slices were determined by examining at least 30 glomeruli in each rat. (E) The bar graph represents the mean percentages of apoptotic cells. Data are mean±SD values. P<.05 is statistically significant. aSignificantly different compared with the control group. bSignificantly different compared with the DN group. Color images available online at www.liebertonline.com/jmf
Effect of hyperoside on podocyte apotosis and necrosis induced by AGE-BSA
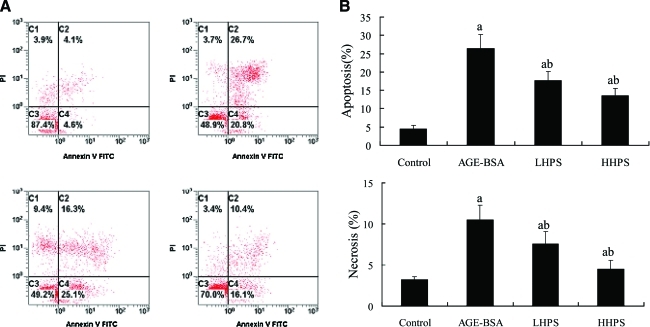
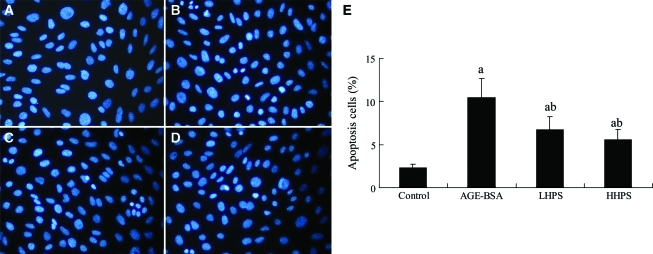
FACS was undertaken to test whether apoptosis and necrosis in cultured podocytes induced by AGEs could be alleviated by hyperoside (Fig. 3). Pretreatment with both doses of hyperoside (50 and 200 μg/mL) significantly dose-dependently decreased AGE-BSA-induced apoptosis and necrosis. In addition, the effect of hyperoside on cultured podocyte apoptosis induced by AGE-BSA was determined by Hoechst 33342 staining (Fig. 4). Apoptotic cells were significantly increased in podocytes stimulated by 500 μg/mL AGE-BSA (10.5±2.2%) compared with those incubated with equal concentration of PBS-BSA (2.3±0.4%) (P<.05). These increases were significantly attenuated by preincubation for 1 h with 50 or 200 μg/mL hyperoside.
FIG. 3.
Effect of hyperoside preincubation on podocyte apoptosis and necrosis induced by advanced glycation end-product (AGE)–bovine serum albumin (BSA) exposure: control group (500 μg/mL phosphate-buffered saline–BSA incubation for 24 h), AGE-BSA group (500 μg/mL AGE-BSA incubation for 24 h), and low-dose and high-dose hyperoside (LHPS and HHPS, respectively; 50 or 200 μg/mL, respectively, hyperoside preincubation for 1 h followed by AGE-BSA exposure) groups. (A) Apoptosis was quantified by fluorescence-activated cell sorting after annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) labeling. The abscissa and ordinate are representative fluorescence-activated cell sorting data of the fluorescence intensity of annexin V-FITC and propidium iodide (PI), respectively. (B) The bar graphs represent the mean percentages of apoptotic podocytes. Data are mean±SD values. P<.05 is statistically significant. aSignificantly different compared with the control group. bSignificantly different compared with the AGE-BSA group. Color images available online at www.liebertonline.com/jmf
FIG. 4.
Effect of hyperoside preincubation on cultured podocyte apoptosis after AGE-BSA exposure: (A) control group, (B) AGE-BSA group, and (C) LHPS and (D) HHPS groups. Apoptosis was assessed by Hoechst 33342 staining. (E) The percentage of podocytes with nuclear condensation was determined by examining at least 200 cells per condition ( ×400 magnifications), and representative data are shown in the four columns of the bar graph. Data are mean±SD values. P<.05 is statistically significant. aSignificantly different compared with the control group. bSignificantly different compared with the AGE-BSA group. Color images available online at www.liebertonline.com/jmf
Effect of hyperoside on activation of caspase pathways in podocytes induced by AGE-BSA
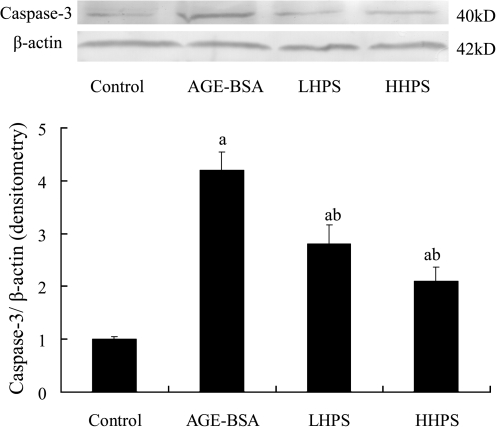
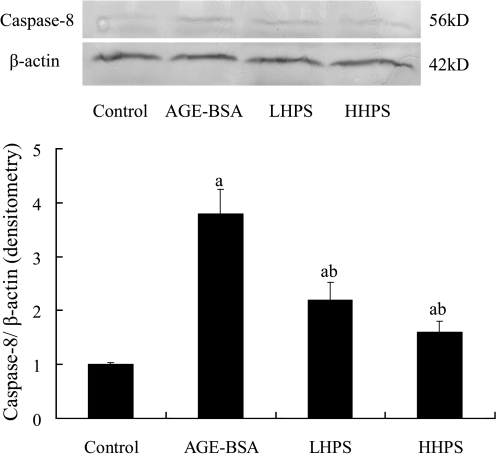
To verify that inactivation of the pro-apoptotic caspase pathway was responsible for the effect of hyperoside on podocyte apoptosis, we further measured the caspase-8 and caspase-3 protein expressions by western blot analysis during the observation. Pretreatment with 50 or 200 μg/mL hyperoside significantly decreased caspase-3 (Fig. 5) and caspase-8 (Fig. 6) overexpression in cultured podocytes induced by 500 μg/mL AGE-BSA in a dose-related manner.
FIG. 5.
Effect of hyperoside preincubation on caspase-3 expression in podocytes induced by AGE-BSA. (Top) Expression of activated caspase-3 protein was detected by western blot analysis, and each band was scanned and subjected to densitometry. (Bottom) The columns in the bar graph show the intensities of their protein relative to β-actin. Results are expressed relative to those of the control group, which were arbitrarily assigned a value of 1. Data are mean±SD values. P<.05 is statistically significant. aSignificantly different compared with the control group. bSignificantly different compared with the AGE-BSA group.
FIG. 6.
Effect of hyperoside preincubation on caspase-8 expression in podocytes induced by AGE-BSA. (Top) Expression of caspase-8 protein was detected by western blot analysis, and each band was scanned and subjected to densitometry. (Bottom) The columns in the bar graph show the intensities of their protein relative to β-actin. Results are expressed relative to those of the control group, which were arbitrarily assigned a value of 1. Data are mean±SD values. P<.05 is statistically significant. aSignificantly different compared with the control group. bSignificantly different compared with the AGE-BSA group.
Discussion
A. manihot L. Medic is recorded as Huangshu Kuihua in ancient literature of Chinese traditional herbs, and TFA is considered to be the major pharmacologically active constituent of A. manihot L. Medic.15,22 TFA has been found to have therapeutic effect on cerebral ischemic reperfusion injury and poststroke depression in the corresponding animal models.13,23 The chemical constituents of TFA have been isolated, and their structures have been identified by spectroscopic analysis. TFA contains seven flavone glycosides that were identified as quercetin-3-O-robinobioside, hyperoside, isoquercetin, hibifolin, myricetin, quercetin-3′-O-glucoside, and quercetin.15 Our previous clinical studies demonstrated that oral administrations of TFA for 8 weeks significantly reduced proteinuria and improved renal function in early stage DN of type 2 diabetes patients.17
The appearance of microalbuminuria is regarded as not only an early clinical hallmark of DN, but also an established independent risk factor for diabetic cardiovascular diseases.3 Our present study indicated that preliminary oral administration of TFA at the dose of 200 mg/kg/day for 24 weeks could significantly decrease the urinary albumin excretion (urinary mAlb/Cr and 24-h UTP) in STZ-induced DN rats. There were no significant changes among DN groups in glucose and lipid metabolic parameters such as the levels of blood glucose, total cholesterol, triglycerides, low-density lipoprotein, high-density lipoprotein, HbA1c, and CML. This protective effect of TFA was probably not due to the metabolic improvement during the investigation. Although our previous unpublished data also showed that oral administration of 200 mg/kg/day TFA for 8 weeks had no significant influence on mean arterial blood pressure of DN mice, we still could not completely exclude the possibility of changes in hemodynamic factors.
The loss of glomerular resident cells resulting from apoptosis has been regarded as a notable sign in STZ-induced diabetic rats, and it occurred mainly in 6 months after the induction.12,24 Our TUNEL assay showed that 200 mg/kg/day TFA pretreatment for 24 weeks could significantly prevent glomerular cell apoptosis in STZ-induced DN rats. Although it was not clear about the cellular type of apoptosis due to the methodical limitations, we could make sure that TFA had an anti-apoptotic effect on glomerular cells in STZ-induced DN rats. The results of previous studies showed that enhanced oxidative stress in conjunction with activated inflammatory markers plays the key role in pathogenesis and progression of apoptosis in chronic hyperglycemia in diabetic nephropathy.25,26 A recent study showed that NADPH oxidase-mediated superoxide production can contribute to glomerular podocyte damage and apoptosis in salt-induced hypertensive renal failure.27 Methylglyoxal-induced oxidative stress caused apoptosis of renal podocytes in Zucker diabetic fatty rats.28 Many flavones or flavonoids found in natural plants have antioxidant activity based on their chemical structure. The mechanism is like to due to direct scavenging or quenching of oxygen free radicals or excited oxygen species as well as inhibition of oxidative enzymes that generate these reactive oxygen species.29 Some of them are used as dietary foods for medical purposes.30,31 Our next studies will further elucidate the possible role of oxidative stress and inflammation in the anti-apoptotic effect of TFA.
A recent pharmacokinetic study simultaneously identified the components in blood and kidney dialysis fluid after oral administration of TFA.32 These results showed that unbound constituents of TFA in rat blood circulation and kidney are hyperoside and isoquercetin, which might be the potential active components of TFA. Hyperoside was identified as one of the most abundant ingredients chemically characterized in TFA in a previous study.15 To elucidate the protective effect of TFA on diabetes-related glomerular cell apoptosis, we further investigated the pharmacologic effect of pretreatment with hyperoside on cultured podocyte apoptosis.
Diabetes is characterized by AGE accumulation, which is regarded as the chief cause for diabetic complications, especially DN.33 A recent in vitro study on cultured podocytes demonstrated that AGEs lead to to podocyte apoptosis through the receptor for AGEs.34 Therefore, the apoptosis of cultured podocytes induced by AGEs could be adopted as a cellular model for screening of drugs. It is interesting that our in vitro study demonstrated that preincubation with hyperoside at a concentration of 50 or 200 μg/mL could significantly reduce AGE-BSA-induced apoptosis and necrosis in cultured podocytes in a dose-related manner. Pretreatment with 200 μg/mL hyperoside could also abate podocyte detachment (data not shown). This might be involved in the molecular mechanisms underlying the therapeutic effect of TFA.
The caspase family of cysteine proteases plays a key role in apoptosis.35 With inductions of some endogenous or exogenous signals, the activated caspase mediates apoptosis by proteolysis of specific substrates.36 There are two classes of caspases involved in apoptosis: initiators (activation by cellular receptor) and effectors (activation by mitochondrial permeability transition). Pro-apoptotic signals autocatalytically activate initiator caspases such as caspase-8, which in turn activate the process effector caspases such as caspase-3, leading to cell collapse.37 Caspase activation functions as the key intracellular pathway, the evoking caspase-like effect, resulting in triggering of cellular apoptosis.38 A recent in vitro study suggested that attenuating the deleterious crosstalk between AGEs and RAGE could inhibit apoptotic cell death and activated caspase-3 activity induced by AGEs in glomerular tubular cells.39
Our data further showed that hyperoside significantly decreased the elevation of activated caspase-3 and caspase-8 expression induced by AGE-BSA, suggesting that its alleviating effect on apoptosis was mediated by a caspase-dependent pathway. This is direct evidence for the protective effect of TFA on podocyte injury in diabetic status, and this mechanism might be a persuasive explanation for the antiproteinuric effect of TFA on DN rats. Because the decreased number induced by apoptosis is the chief manifestation of podocyte damage in DN,5,31 we could deduce that it is likely that TFA reduced urinary protein excretion in DN rats partly by ameliorating podocyte apoptosis. The point should be clarified in our in vivo experiment that the increased serum levels of the clearly identified AGE CML in DN rats were not improved by pretreatment of TFA. It is probable that TFA had an influence on the interaction between AGEs and the receptor for AGEs, and this hypothesis needs further supportive findings.
In summary, our data demonstrates that TFA pretreatment could decrease urinary albumin excretion and findings of renal damage in STZ-induced DN rats at the condition's early stage, which might be accomplished by ameliorating podocyte apoptosis. Our results provided evidence for the potential use of TFA as a novel therapeutic agent for albuminuria of DN at the early stage.
Acknowledgments
This work was supported by the Nature Scientific Foundation of Jiangsu Province (grant numbers BK2008490 and BK2010595). The authors gratefully acknowledge Ms. Jin-Hua Liu (Department of Internal Medicine, Nanjing University of Chinese Medicine) for her technical assistance in animal model establishment and drug administration.
Author Disclosure Statement
All the authors have no potential conflicts of interest to declare.
References
- 1.Gross JL. de Azevedo MJ. Silveiro SP. Canani LH. Caramori ML. Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association: Standards of medical care in diabetes—2010. Diabetes Care. 2010;33:11–61. [Google Scholar]
- 3.Klausen K. Borch-Johnsen K. Feldt-Rasmussen B. Jensen G. Clausen P. Scharling H. Appleyard M. Jensen JS. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 4.Mauer M. Zinman B. Gardiner R. Suissa S. Sinaiko A. Strand T. Drummond K. Donnelly S. Goodyer P. Gubler MC. Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf G. Chen S. Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 6.Wolf G. Chen S. Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 7.Jefferson JA. Shankland SJ. Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 8.Drummond K. Mauer M. The early natural history of nephropathy in type 1 diabetes II. Early renal structural changes in type 1 diabetes. Diabetes. 2002;51:1580–1587. doi: 10.2337/diabetes.51.5.1580. [DOI] [PubMed] [Google Scholar]
- 9.Susztak K. Raff AC. Schiffer M. Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 10.Menini S. Iacobini C. Oddi G. Ricci C. Simonelli P. Fallucca S. Grattarola M. Pugliese F. Pesce C. Pugliese G. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia. 2007;50:2591–2599. doi: 10.1007/s00125-007-0821-y. [DOI] [PubMed] [Google Scholar]
- 11.Sohn E. Kim J. Kim CS. Kim YS. Jang DS. Kim JS. Extract of the aerial parts of Aster koraiensis reduced development of diabetic nephropathy via anti-apoptosis of podocytes in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun. 2010;391:733–738. doi: 10.1016/j.bbrc.2009.11.129. [DOI] [PubMed] [Google Scholar]
- 12.Pesce C. Menini S. Pricci F. Favre A. Leto G. DiMario U. Pugliese G. Glomerular cell replication and cell loss through apoptosis in experimental diabetes mellitus. Nephron. 2002;90:484–488. doi: 10.1159/000054738. [DOI] [PubMed] [Google Scholar]
- 13.Wen JY. Chen ZW. Protective effect of pharmacological preconditioning of total flavones of abelmoschl manihot on cerebral ischemic reperfusion injury in rats. Am J Chin Med. 2007;35:653–661. doi: 10.1142/S0192415X07005144. [DOI] [PubMed] [Google Scholar]
- 14.Cheng XP. Qin S. Dong LY. Zhou JN. Inhibitory effect of total flavone of Abelmoschus manihot L. Medic on NMDA receptor-mediated current in cultured rat hippocampal neurons. Neurosci Res. 2006;55:142–145. doi: 10.1016/j.neures.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Lai X. Liang H. Zhao Y. Wang B. Simultaneous determination of seven active flavonols in the flowers of Abelmoschus manihot by HPLC. J Chromatogr Sci. 2009;47:206–210. doi: 10.1093/chromsci/47.3.206. [DOI] [PubMed] [Google Scholar]
- 16.Puel C. Mathey J. Kati-Coulibaly S. Davicco MJ. Lebecque P. Chanteranne B. Horcajada MN. Coxam V. Preventive effect of Abelmoschus manihot (L.) Medik. on bone loss in the ovariectomised rats. J Ethnopharmacol. 2005;99:55–60. doi: 10.1016/j.jep.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 17.Yu JY. Xiong NN. Guo HF. [Clinical observation on diabetic nephropathy treated with alcohol of Abelmoschus manihot] Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15:263–265. [PubMed] [Google Scholar]
- 18.Nagai K. Arai H. Yanagita M. Matsubara T. Kanamori H. Nakano T. Iehara N. Fukatsu A. Kita T. Doi T. Growth arrest-specific gene 6 is involved in glomerular hypertrophy in the early stage of diabetic nephropathy. J Biol Chem. 2003;278:18229–18234. doi: 10.1074/jbc.M213266200. [DOI] [PubMed] [Google Scholar]
- 19.van den Born J. Pisa B. Bakker MA. Celie JW. Straatman C. Thomas S. Viberti GC. Kjellen L. Berden JH. No change in glomerular heparan sulfate structure in early human and experimental diabetic nephropathy. J Biol Chem. 2006;281:29606–29613. doi: 10.1074/jbc.M601552200. [DOI] [PubMed] [Google Scholar]
- 20.Lee SC. Han SH. Li JJ. Lee SH. Jung DS. Kwak SJ. Kim SH. Kim DK. Yoo TH. Kim JH. Chang SH. Han DS. Kang SW. Induction of heme oxygenase-1 protects against podocyte apoptosis under diabetic conditions. Kidney Int. 2009;76:838–848. doi: 10.1038/ki.2009.286. [DOI] [PubMed] [Google Scholar]
- 21.Saleem MA. O'Hare MJ. Reiser J. Coward RJ. Inward CD. Farren T. Xing CY. Ni L. Mathieson PW. Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 22.Lai X. Zhao Y. Liang H. Bai Y. Wang B. Guo D. SPE-HPLC method for the determination of four flavonols in rat plasma and urine after oral administration of Abelmoschus manihot extract. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:108–114. doi: 10.1016/j.jchromb.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 23.Liu M. Jiang QH. Hao JL. Zhou LL. Protective effect of total flavones of Abelmoschus manihot L. Medic against poststroke depression injury in mice and its action mechanism. Anat Rec (Hoboken) 2009;292:412–422. doi: 10.1002/ar.20864. [DOI] [PubMed] [Google Scholar]
- 24.Brosius FC. Alpers CE. Bottinger EP. Breyer MD. Coffman TM. Gurley SB. Harris RC. Kakoki M. Kretzler M. Leiter EH. Levi M. McIndoe RA. Sharma K. Smithies O. Susztak K. Takahashi N. Takahashi T. Animal Models of Diabetic Complications Consortium: mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20:2503–2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J. Zhang R. Torreggiani M. Ting A. Xiong H. Striker GE. Vlassara H. Zheng F. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol. 2010;176:2163–2176. doi: 10.2353/ajpath.2010.090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh DK. Winocour P. Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol. 2011;7:176–184. doi: 10.1038/nrendo.2010.212. [DOI] [PubMed] [Google Scholar]
- 27.Cheng XW. Kuzuya M. Sasaki T. Inoue A. Hu L. Song H. Huang Z. Li P. Takeshita K. Hirashiki A. Sato K. Shi GP. Okumura K. Murohara T. Inhibition of mineralocorticoid receptor is a renoprotective effect of the 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor pitavastatin. J Hypertens. 2011;29:542–552. doi: 10.1097/HJH.0b013e328341cedf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J. Sohn E. Kim CS. Kim JS. Renal podocyte apoptosis in Zucker diabetic fatty rats: involvement of methylglyoxal-induced oxidative DNA damage. J Comp Pathol. 2011;144:41–47. doi: 10.1016/j.jcpa.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Terao J. Dietary flavonoids as antioxidants. Forum Nutr. 2009;61:87–94. doi: 10.1159/000212741. [DOI] [PubMed] [Google Scholar]
- 30.Seelinger G. Merfort I. Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008;74:1667–1677. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 31.Rahman I. Biswas SK. Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Xue C. Guo J. Qian D. Duan JA. Shang E. Shu Y. Lu Y. Identification of the potential active components of Abelmoschus manihot in rat blood and kidney tissue by microdialysis combined with ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:317–325. doi: 10.1016/j.jchromb.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Wendt T. Tanji N. Guo J. Hudson BI. Bierhaus A. Ramasamy R. Arnold B. Nawroth PP. Yan SF. D'Agati V. Schmidt AM. Glucose, glycation, and RAGE: implications for amplification of cellular dysfunction in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1383–1395. doi: 10.1097/01.asn.0000065100.17349.ca. [DOI] [PubMed] [Google Scholar]
- 34.Chuang PY. Yu Q. Fang W. Uribarri J. He JC. Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int. 2007;72:965–976. doi: 10.1038/sj.ki.5002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson MR. Apoptosis: unmasking the executioner. Cell Death Differ. 1998;5:646–652. doi: 10.1038/sj.cdd.4400394. [DOI] [PubMed] [Google Scholar]
- 36.Mishra R. Emancipator SN. Kern T. Simonson MS. High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int. 2005;67:82–93. doi: 10.1111/j.1523-1755.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 37.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Wang J. Lenardo MJ. Roles of caspases in apoptosis, development, and cytokine maturation revealed by homozygous gene deficiencies. J Cell Sci. 2000;113:753–757. doi: 10.1242/jcs.113.5.753. [DOI] [PubMed] [Google Scholar]
- 39.Matsui T. Yamagishi S. Takeuchi M. Ueda S. Fukami K. Okuda S. Irbesartan inhibits advanced glycation end product (AGE)-induced proximal tubular cell injury in vitro by suppressing receptor for AGEs (RAGE) expression. Pharmacol Res. 2010;61:34–39. doi: 10.1016/j.phrs.2009.07.004. [DOI] [PubMed] [Google Scholar]