Abstract
The protective effect of raspberry ketone against nonalcoholic steatohepatitis (NASH) was tested by using a high-fat diet-induced NASH model, and its mechanism was explored. Forty Sprague–Dawley rats with a 1:1 male to female ratio were randomly divided into five groups: the normal control (NC) group (n=8) fed normal diet for 8 weeks, the model control (MC) group (n=8) fed high-fat diet (82% standard diet, 8.3% yolk powder, 9.0% lard, 0.5% cholesterol, and 0.2% sodium taurocholate), and the raspberry ketone low-dose (0.5%) (RKL) group (n=8), the raspberry ketone middle-dose (1%) (RKM) group (n=8), and the raspberry ketone high-dose (2%) (RKH) group (n=8) fed high-fat diet for 4 weeks. After 8 weeks of experiment, all the rats were sacrificed, and blood lipid parameters (total cholesterol [TC], triglycerides [TG], high-density lipoprotein cholesterol [HDL-C], and low-density lipoprotein cholesterol [LDL-C]), liver function parameters (serum alanine aminotransferase [ALT], aspartate aminotransferase [AST], and alkaline phosphatase [ALP]), leptin (LEP), free fatty acid (FFA), tumor necrosis factor α (TNF-α), blood glucose (GLU), and insulin (INS) with calculated INS resistance index (IRI) and INS-sensitive index (ISI) were measured in rats. Therefore, we determined the peroxisome proliferator-activated receptor (PPAR)-α activity in liver homogenate and the levels of low-density lipoprotein receptor (LDLR), high-sensitivity C-reactive protein (hs-CRP), adiponection (APN), superoxide dismutase, and malondialdehyde (MDA). The liver tissues of rats in each group were imaged by electron microscopy with hematoxylin–eosin as the staining agent. The levels of TG, TC, LDL-C, ALT, AST, ALP, GLU, INS, IRI, FFA, LEP, TNF-α, MDA, and hs-CRP of MC rats were significantly increased (P<.05, P<.01). Therefore, the levels of HDL-C, ISI, PPAR-α, LDLR, and APN were significantly decreased (P<.05, P<.01). Compared with the MC group, each parameter in the RKL, RKM, and RKH groups was significantly improved (P<.05, P<.01). Thus raspberry ketone was an effective intervention for NASH in rats. It was believed that raspberry ketone had a dual effect of liver protection and fat reduction, and the mechanism was probably mediated by alleviation of fatty degeneration of liver cells, decreased liver inflammation, correction of dyslipidemia, reversal of LEP and INS resistance, and improved antioxidant capacity.
Key Words: nonalcoholic steatohepatitis, raspberry ketone
Introduction
Raspberry (Rubus rosaceae L.) fruits contain various vitamins, amino acids, minerals, sugars, organic acids, and other nutrients as well as bioactive compounds such as flavonoids, ellagic acid, and anthocyanins, which could inhibit cancer cells and resist cardiovascular disease.1,2
Raspberry ketone (RK) [4-(4-hydroxyphenyl) butan-2-one], one of the major aromatic compounds of raspberry,3 is widely used as a fragrance in cosmetics and as a flavoring agent in foodstuffs.4 In one study investigating the intragastric administration of RK (1 mmol/kg), about 90% of the dose was excreted as metabolites via the urine within 24 h in rats, guinea pigs, and rabbits.5 However, there have been no reports on the biological effects of RK. RK was discovered in blackberries by Japanese researchers.6 Morimoto et al.7 reported that a dose of 1% RK was sufficient to prevent high-fat diet-induced increases in body and tissue weights; it was also shown that 1% RK remedially affected the increases in body weight, visceral adipose tissues weights, and hepatic triacylglycerol content in mice fed with a high-fat diet. These results indicate that RK prevents the obesity and the fatty liver induced by feeding a high-fat diet. Meng et al.8 showed that RK could reduce the weight of high-fat diet-induced obese rats, suggesting that RK exerts anti-obesity functions by mediating lipid disorders, improving insulin (INS) resistance (IR), and reversing leptin (LEP) resistance. On the other hand, the study of Zhou et al.9 illustrated that RK can also treat nonalcoholic steatohepatitis (NASH) by affecting lipid disorders, improving LEP resistance, inhibiting lipid peroxidation, and modulating inflammatory responses, perhaps through enhancement of peroxisome proliferator-activated receptor (PPAR)-γ expression.
The incidence of high-fat diet-induced fatty liver is increasing,10 making it the subject of intense research because current drug treatments are not highly effective.11 NASH is also an intermediate stage of nonalcoholic fatty liver disease (NAFLD). NAFLD encompasses the metabolic response to liver damage, including IR. The pathological changes in NAFLD are similar to those in alcoholic liver disease, but patients with NAFLD have no history of excessive alcohol consumption. In recent years, studies have shown that approximately 15% of patients with nonalcoholic fatty liver can develop NASH from simple fatty liver.12 Furthermore, it can progress to fibrosis and cirrhosis, and 3% of patients may develop liver failure.13 A 20-year follow-up study showed that fatty degeneration of liver cells alone was not progressive, whereas 20% patients with NASH can developed cirrhosis in 5–10 years.14 Moreover, up to 28% of patients with cirrhosis related to NASH developed cirrhosis in 10 years with some liver-related deaths and hepatocellular carcinoma.15 Therefore, NAFLD research has become a major focus liver disease research.16
Currently, treatments of NAFLD include various dietary, lifestyle, and drug therapies that act as hypoglycemic and hepatoprotective agents.17–19 It has now been confirmed that NASH is closely related with obesity, diabetes type 2, hyperlipidemia, and other metabolic syndrome.20,21 Therefore, lipid-lowering drugs are commonly used in clinical treatment of fatty liver.22,23 However, some lipid-lowering drugs have potential liver toxicity, and some fail to decrease liver fatty deposits and may exacerbate liver injury.24,25 Therefore, drugs with the dual role of lipid-lowering and liver-protecting effects will be more appropriate for the prevention and treatment of fatty liver.26
The “two hits” theory of NASH, raised by Day and James,27 holds that the first hit is mainly from IR, causing the fatty degeneration of liver cells; the second hit is mainly that IR is intensified under the functions of cytokines and oxidative stress, and the function of liver cells is damaged, causing fat-induced hepatitis. In the two hits, IR plays an important role, and the impaired lipid metabolism in liver is the basis of various forms of fatty liver.
Many epidemiological data and laboratory observations have also confirmed that RK is effective in weight loss,28 but only a few have reported that RK also has effects on NASH.8
In this study, RK's effects on the ultrastructure of mouse liver tissues were used to evaluate the effect of RK on NASH. Furthermore, biochemical, enzyme histochemistry, immunohistochemistry, and enzyme-linked immunosorbent assay analyses were applied in this study. In addition, not only were the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglycerides (TG), glucose (GLU), INS, INS-sensitive index (ISI), LEP, and tumor necrosis factor α (TNF-α) of blood samples and malondialdehyde (MDA) contents, superoxide dismutase (SOD) activity, and PPAR-γ of liver tissues detected, but also for the first time blood lipid parameters (high-density lipoprotein cholesterol [HDL-C] and low-density lipoprotein cholesterol [LDL-C]), liver function parameters (ALT, AST, and alkaline phosphatase [ALP]) of serum, and free fatty acid (FFA) with calculated IR index (IRI). PPAR-α in liver homogenate and low-density lipoprotein receptor (LDLR), high-sensitivity C-reactive protein (hs-CRP), and adiponectin (APN) were detected as well.29–31 Also, the liver tissues of rats in each group were imaged by electron microscopy with hematoxylin–eosin as the staining agent.
We used rats as a model for high-fat diet-induced NASH, determining the main variables related with NASH. We also investigated the main target of RK in NASH.32,33 The results assist in the development of a safe and effective natural intervention for NASH.
Materials and Methods
Primary compound
Natural RK (purity ≥99%) was purchased from Wuhan Hezhong Chemical Manufacture Co. Ltd. RK was diluted with salad oil to concentrations of 0.5%, 1%, and 2% for use.
Experimental animals
Forty male and female Sprague–Dawley specific pathogen-free grade rats weighing 80–100 g (animal license number SCXK [Liaoning Province] 2008-0005) were purchased from the Animal Experiment Center of China Medical University.
Standard diet containing 25% maize, 15% soybean flour, 15% barley meal, 12% bran, 10% cabbage, 10% fish protein concentrate, 5% bone meal, 2% salt, and 1% yeast was purchased from the Animal Experiment Center of China Medical University.
High-fat diet containing 82% standard diet, 8.3% yolk powder, 9.0% lard, 0.5% cholesterol, and 0.2% sodium taurocholate was purchased from Shenyang Yuhong Qianmin Animal Feed Factory, with cobalt-60 irradiation before use.
Primary reagents
The following reagents were obtained: INS, LEP, FFA, TNF-α, enzyme-linked immunosorbent assay kit (GBD Co., lot 21010512); PPAR-α, LDLR, hs-CRP, APN, enzyme-linked immunosorbent assay kit (R&D Co.); SOD, MDA, Coomassie Brilliant Blue staining kit (Nanjing Jiancheng Bioengineering Institute, lot 20100112); cholesterol (analytically pure, Sinopharm Chemical Reagent Co. Ltd., lot F20091029, AR); and other reagents including formaldehyde, ceresin wax, absolute ethyl alcohol, chloral hydrate, and glutaraldehyde (domestic analytically pure).
Primary instruments
Instruments were obtained as follows: the TDL-5A centrifuge from ShangHai Anting Scientific Instrument Factory, the model 550 microplates from Bio-Rad, the model DY89 electric glass homogenizer from Ningbo Xinzhi Biotechnology Co., Ltd., the BL610 type electronic balances from Beijing Sartorius-Mechatronics Co., Ltd., the model OLS200 constant temperature water bath from Grant Co., the model CL-2000 full-automatic biochemical analyzer from Shimazu Corp., the electronic scale from Zhongshan Hengxin Electronic Co., Ltd., the paraffin slice machine from Leica Co., the optical microscope from Olympus Co., the model H-7650 transmission electron microscope from Hitachi Ltd., the model LKBV5 ultrathin slicer from LKB Instruments, and the trace liquid removal device from Eppendorf Co.
Establishment of the experimental animal model
The animals were raised in a specific pathogen-free barrier system animal laboratory of the Animal Experiment Center of China Medical University. During the experimental period, the animal room held four rats per cage, with free access to water and food, under conditions of temperature controlled at 20–26°C, humidity at 40–70%, and a 12/12-h day–night light cycle. Rats were fed with normal diet for 1 week and then randomly divided into five groups: normal control (NC) group (n=8) fed normal diet for 8 weeks, the model control (MC) group (n=8) fed high-fat diet (82% standard diet, 8.3% yolk powder, 9.0% lard, 0.5% cholesterol, and 0.2% sodium taurocholate), the RK low-dose (RKL) group (n=8), the RK middle-dose (RKM) group (n=8), and the RK high-dose (RKH) group (n=8). Rats were first fed with high-fat diet for 4 weeks, and then these rats were given intragastrically 0.5%, 1%, or 2% RK. The first two groups of rats were intragastrically administered salad oil at the same dose (2 mL/day per rat) once a day at 10:00 a.m., lasting for 4 weeks.
Sample collection and preparation
On the weekend night after 8 weeks, rats were fasted except for water for 12 h and weighed the next morning, followed by intraperitoneal injection of 10% chloral hydrate at a concentration of 0.35 mL/100 g, and blood samples were collected from the abdominal aorta and held at 4°C. After centrifugation using the TDL-5A at 3000 rpm for 10 min, the final sera were collected and stored at −20°C. In addition, part of the right hepatic lobe was taken, fixed with 10% formaldehyde, paraffin-embedded, and sliced, with hematoxylin–eosin staining, for histologic examination of the liver.
Observation of changes of liver tissue ultramicrostructure by transmission electron microscopy
After sampling, 2.5% of glutaral (prepared with dipotassium sodium arsenate) was used to fix the samples for 2 h. Samples were washed with phosphate-buffered saline three times. After fixing with 1% osmic acid (CsO4), samples were washed three times with phosphate-buffered saline. Samples were then sequentially dehydrated with 50% and 70% alcohol followed by 80%, 90%, and 100% acetone. After immersion overnight, samples were polymerized at 35°C, 45°C, and 60°C for 24 h (total 72 h). Samples were sliced with an ultramicrotome (model LKBV5), treated with uranyl acetate–lead citrate dye, and then observed with a transmission electron microscope (model H-7650). The slice thickness was 50–70 nm.
Data analysis
Data analysis was performed using SPSS version 13.0 statistical software. The experimental data were expressed as mean±SD values. Comparison of mean values in groups was conducted with single-factor analysis of variance (one-way analysis of variance), and pairwise comparison was performed with the least significant difference method. If P<.05, differences between groups were considered statistically significant.
Results
Effects of RK on serum biochemical indicators in rats
As shown in Table 1, compared with NC rats, the blood lipid level of MC rats was significantly increased (P<.01), and the HDL-C level was significantly decreased (P<.01). Furthermore, compared with MC, the TG, TC, and LDL-C levels in RKL, RKM, and RKH rats were significantly increased (P<.01).
Table 1.
Effect of Raspberry Ketone on Total Cholesterol, Triglycerides, High-Density Lipoprotein Cholesterol, and Low-Density Lipoprotein Cholesterol Contents in Rat Serum
| |
|
Level (mmol/L) |
|||
|---|---|---|---|---|---|
| Group | Number of rats | TG | TC | HDL-C | LDL-C |
| NC | 8 | 0.3800±0.0833 | 1.2450±0.2661 | 1.0200±0.1523 | 0.1888±0.0314 |
| MC | 8 | 0.6525±0.2768** | 1.9938±0.2611** | 0.7250±0.1161** | 1.3288±0.1660** |
| RKL | 8 | 0.4263±0.0721†† | 1.1188±0.2230†† | 1.0025±0.1766†† | 0.2263±0.0969†† |
| RKM | 8 | 0.4975±0.0913† | 1.4663±0.2929†† | 1.0688±0.2015†† | 0.2650±0.1090†† |
| RKH | 8 | 0.4888±0.0706† | 1.3463±0.2253†† | 1.0313±0.2183†† | 0.3213±0.0633*†† |
Compared with the normal control (NC) group, *P<.05, **P<.01; compared with the model control (MC) group, †P<.05, ††P<.01.
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; RKH, RKM, and RKL, high-, middle-, and low-dose raspberry ketone, respectively; TC, total cholesterol; TG, triglycerides.
As shown in Table 2, the serum levels of AST, ALT, and ALP in MC rats were significantly higher than those in NC rats (P<.01), which indicated that this was a successful model of liver injury. The serum levels of AST, ALT, and ALP in RKL, RKM, and RKH rats were significantly decreased in comparison with MC rats (P<.05, P<.01) except for the serum level of AST in RKH rats (P>.05).
Table 2.
Effect of Raspberry Ketone on Aspartate Aminotransferase, Alanine Aminotransferase, and Alkaline Phosphatase Activities in Rat Serum
| |
|
Level (U/L) |
||
|---|---|---|---|---|
| Group | Number of rats | AST | ALT | ALP |
| NC | 8 | 132.75±11.02 | 45.00±20.10 | 50.00±31.52 |
| MC | 8 | 181.25±17.27** | 163.25±19.14** | 86.13±15.84** |
| RKL | 8 | 161.88±17.23**† | 91.25±49.17**†† | 44.25±15.52†† |
| RKM | 8 | 161.63±21.22**† | 60.25±24.69†† | 59.38±21.65† |
| RKH | 8 | 165.23±14.13** | 61.25±24.76†† | 58.13±31.44† |
Compared with the NC group, **P<.01; compared with the MC group, †P<.05, ††P<.01.
ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase.
Effect of RK on GLU, INS, IRI, and ISI indicators in rats
The levels of blood GLU and INS in MC rats were increased compared with NC rats (P<.01) (Table 3). Therefore, RK had a tendency to decrease blood GLU. The levels of blood GLU in all the RK dose groups were lower than in MC rats (P<.01). All RK dose groups had significantly decreased levels of INS compared with MC rats. As shown in Table 3, we computed the IRI and ISI based on fasting GLU and fasting INS. The results showed that the IRI of MC rats was elevated compared with that in NC rats, but the IRI values of all RK groups were significantly lower than that of MC rats. In contrast, ISI values of all the RK groups were significantly higher than in the MC group.
Table 3.
Effect of Raspberry Ketone on Glucose, Insulin, Insulin Resistance Index, and Insulin-Sensitive Index in Rat Serum
| Group | Number of rats | GLU (mmol/L) | INS (μIU/mL) | IRI | ISI |
|---|---|---|---|---|---|
| NC | 8 | 5.80±1.44 | 3.4934±0.1255 | 0.9015±0.2269 | 0.0527±0.0158 |
| MC | 8 | 9.80±2.54** | 5.9342±0.8046** | 2.6081±0.8319** | 0.0188±0.0068** |
| RKL | 8 | 6.48±1.08†† | 4.0342±0.2023*†† | 1.1648±0.2344†† | 0.0394±0.0069**†† |
| RKM | 8 | 6.31±1.10†† | 3.9936±0.1209*†† | 1.1251±0.2345†† | 0.0407±0.0067*†† |
| RKH | 8 | 6.24±1.36†† | 3.9609±0.2476*†† | 1.0970±0.2440†† | 0.0422±0.0087*†† |
Compared with the NC group, *P<.05, **P<.01; compared with the MC group, ††P<.01.
GLU, glucose; INS, insulin; IRI, insulin resistance index; ISI, insulin-sensitivr index.
Effect of RK on serum FFA, LEP, and TNF-α indicators in rats
Table 4 shows that the levels of serum FFA, LEP, and TNT-α in MC rats were significantly higher than in the NC group. Compared with MC rats, the levels of serum FFA, LEP, and TNF-α in RKL rats were significantly lower (P<.01). In addition, FFA, LEP, and TNF-α in RKM rats were also significantly decreased (P<.05, P<.01). However, the level of serum FFA in RKH rats was not significantly different compared with MC rats (P>.05), but LEP and TNF-α were significantly lower compared with MC rats (P<.01).
Table 4.
Effect of Raspberry Ketone on Free Fatty Acids, Leptin, and Tumor Necrosis Factor-α in Rat Serum
| Group | Number of rats | FFA (μg/L) | LEP (ng/mL) | TNF-α (pg/mL) |
|---|---|---|---|---|
| NC | 8 | 83.6295±18.2886 | 2.4088±0.5742 | 166.7349±36.8859 |
| MC | 8 | 158.8191±46.2329** | 7.5511±2.2619** | 524.4951±86.4024** |
| RKL | 8 | 87.3506±19.4831†† | 2.8314±0.7684†† | 177.8109±42.6634† |
| RKM | 8 | 119.7349±24.9286*† | 3.0096±0.8342†† | 192.2714±49.4108†† |
| RKH | 8 | 132.0080±52.6466* | 4.7654±1.6085**†† | 277.0231±88.0478**†† |
Compared with the NC group, *P<.05, **P<.01; compared with the MC group, †P<.05, ††P<.01.
FFA, free fatty acid; LEP, leptin; TNF-α, tumor necrosis factor-α.
Effect of RK on antioxidant capacity of liver tissue indicators in rats
MDA directly reflects the rate of spread and the strength of tissue cell lipid peroxidation, and it indirectly reflect the degree of cellular damage. We found that the higher the content of MDA, the more serious the biofilm damage (Table 5). Our statistical analysis indicates that the MDA content of liver homogenate of MC rats was significantly higher (P<.01) than that of NC rats, with no significant difference compared with RKM and RKL rats (P>.05). In conclusion, with respect to decreased MDA activation in liver, homogenate of RKH was most effective.
Table 5.
Effect of Raspberry Ketone on Antioxidant Capacity Index in Rat Liver Tissue
| Group | Number of rats | MDA (nmol/mg of protein) | SOD (U/mg of protein) |
|---|---|---|---|
| NC | 8 | 35.0875±8.5332 | 183.6705±42.5496 |
| MC | 8 | 47.9707±3.1872** | 142.5405±33.1428 |
| RKL | 8 | 41.8731±7.1281 | 167.9278±48.5945 |
| RKM | 8 | 47.4445±12.3921** | 205.3188±56.6528†† |
| RKH | 8 | 36.2798±6.4538†† | 189.1805±43.9669† |
Compared with the NC group, **P<.01; compared with the MC group, †P<.05, ††P<.01.
MDA, malondialdehyde; SOD, superoxide dismutase.
Determination of SOD activity reflects the antioxidant capacity in vivo, as well as measuring tissue cell antioxidant levels. Compared with the NC group, the SOD activity in liver homogenate of MC rats was decreased (Table 5), but the difference was not significant. The SOD activities in all RK groups were increased, but only RKM and RKH were significantly higher than in the MC group (P<.05, P<.01).
Effect of RK on PPAR-α and LDLR indicators in liver homogenate of rats
From Table 6, we concluded that levels of PPAR-α and LDLR in liver homogenates were both lower than in NC rats (P<.01). Levels of PPAR-α and LDLR of RKH, RKM, and RKL rats were significantly higher than those of MC rats (P<.01).
Table 6.
Effect of Raspberry Ketone on Peroxisome Proliferator-Activated Receptor-α and Low-Density Lipoprotein Receptor in Rat Liver Tissue
| Group | Number of rats | PPAR-α (ng/L) | LDLR (ng/L) |
|---|---|---|---|
| NC | 8 | 57.5005±1.5341 | 21.1895±0.6209 |
| MC | 8 | 45.1443±6.1367** | 17.0636±0.4566** |
| RKL | 8 | 53.7886±1.9742*†† | 18.6343±0.6088**†† |
| RKM | 8 | 51.3928±3.1948**†† | 19.9965±0.9695**†† |
| RKH | 8 | 53.1225±1.7253*†† | 18.6864±0.5751**†† |
Compared with the NC group, *P<.05, **P<.01; compared with the MC group, ††P<.01.
LDLR, low-density lipoprotein receptor; PPAR-α, peroxisome proliferator-activated receptor-α.
Effect of RK on hs-CRP and APN indicators in liver homogenate of rats
The hs-CRP of MC rats was significantly higher compared with NC rats (P<.01), and the APN level was significantly lower (P<.01) (Table 7). There were significant differences between all RK groups and MC rats (P<.01).
Table 7.
Effect of Raspberry Ketone on High-Sensitivity C-Reactive Protein and Adiponectin in Rat Liver Tissue
| Group | Number of rats | hs-CRP (ng/L) | APN (ng/L) |
|---|---|---|---|
| NC | 8 | 13.4823±0.3927 | 30.6324±1.7886 |
| MC | 8 | 17.8985±0.9411** | 18.5323±2.4704** |
| RKL | 8 | 15.5305±0.5521**†† | 26.3950±1.7864**†† |
| RKM | 8 | 16.4698±0.5705**†† | 21.6254±2.5946**†† |
| RKH | 8 | 15.7399±0.9179**†† | 25.1076±1.5932**†† |
Compared with the NC group, **P<.01; compared with the MC group, ††P<.01.
APN, adiponectin; hs-CRP, high-sensitivity C-reactive protein.
Effect of RK on histomorphology of liver tissue in rats
Anatomical observation of liver
The general appearance of the liver was observed carefully when rat liver was dissected. Livers of the NC group showed a sharp fringe, soft texture, good elasticity, and a smooth surface with a wine color. Livers of the MC group showed diffuse intumescence, a dull and thick fringe, doughy texture, no elasticity, introcession when pressed, yellow surface color, greasy cross-section, and adhesion with surrounding tissue, which indicates that high-fat feed induces severe adiposis hepatica in rats. The liver appearances of the RKL, RKM, and RKH rat groups were between those of the NC and MC groups. The liver color of the high-dose group was close to that of the NC group, which indicates that RK reduces fat and lessens the symptom of adiposis hepatica.
Pathological observation of liver
Hematoxylin–eosin staining was applied to liver tissue and evaluated under a light microscope. The effect of RK on experimental rat liver under pathological conditions was observed.
In the NC group (Fig. 1A), the structure of rat liver was clear. The acini hepatis, portal area, and other structures were complete and clear. The liver cells aligned regularly with central veins as the center showed a radial pattern. The size of liver cells was normal, the cell nucleus was in the center, and the cytoplasm was uniform. No dropsy, apomorphosis, necrosis, and inflammatory cell infiltration were observed. Also, the sinus hepaticus demonstrated no distension and engorgement.
FIG. 1.
Histological examination of liver tissue sections stained with hematoxylin–eosin dye: (A) NC group, (B) MC group, (C) RKL group, (D) RKM group, and (E) RKH group. ×400.
In the MC group (Fig. 1B), diffuse pimelosis of different extents appeared in the liver, which showed that the volume of pimelosis cells increased, fat vacuoles of different quantities and sizes were in the cytoplasm, the cell nucleus was pushed to the fringe, the structure of the acini hepatis was damaged seriously, the sinus hepaticus was narrowed and even disappeared with compression, and the hepatic cord arrayed irregularly. Dropsy, apomorphosis, necrosis, and inflammatory cell infiltration of liver cells were observed. Because of fat deposition in the liver, vacuoles, dropsy, spotty and flaky necrosis, and inflammatory cell infiltration appeared under the optical microscope after slicing, which demonstrates that the rat model of nonalcoholic fatty hepatitis was established successfully.
In the RKL group (Fig. 1C), the liver structure of experimental animals recovered inconspicuously. The demarcation of acini hepatis was not clear. The liver cells showed different extents of adipose degeneration. The liver cell cord disordered, and the sinus hepaticus disappeared. Inflammatory cell infiltration and punctiform necrosis were present in the portal area.
In the RKM group (Fig. 1D), the liver structure recovered to some extent. Also, the liver cell surrounding the central veins exhibited some recovery, showing a radial arrangement, and the sinus hepaticus recovered. The liver cells around acini hepatis showed different extents of adipose degeneration. The liver cell cord was disordered, and the sinus hepaticus disappeared. There was still inflammatory cell infiltration in the portal area.
In the RKH group (Fig. 1E), most liver structures recovered to near normal. The demarcation of acini hepatis was clear. The liver cell cord showed a radial arrangement, and the sinus hepaticus recovered. There were a few lipid droplets in the liver cell cytoplasm, and a little inflammatory cell infiltration was also found in the portal area.
These results showed that RK can reduce liver lipidoses of nonalcoholic fatty hepatitis rats and prevent the generation of adiposis hepatica. It also indicates that RK can to a large extent reduce the symptom of nonalcoholic fatty hepatitis induced by high-fat diet.
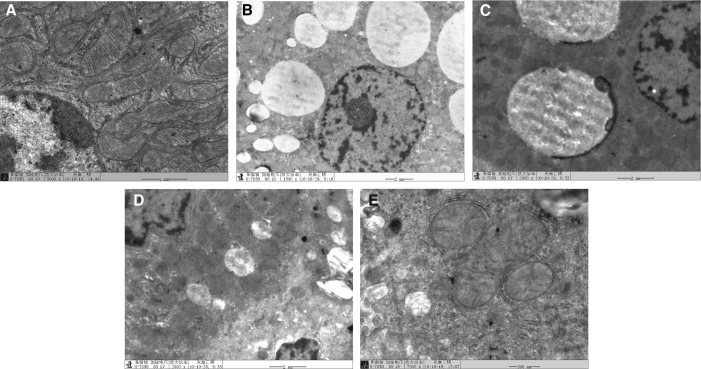
Electron microscopy
In the NC group (Fig. 2A), the ultramicrostructure of rat liver cells was normal. The liver cell nucleus was round or close to round. The rough endoplasmic reticulum was abundant and clear and held together by amylon. The apparato reticulare structure was clear, the cytochondriome was abundant, and the crista was complete and clear.
FIG. 2.
The appearance of liver cells in each group by transmission electron microscopy: (A) NC group, (B) MC group, (C) RKL group, (D) RKM group, and (E) RKH group.
In the MC group (Fig. 2B), the ultramicrostructures of rat liver cells were obviously abnormal. The liver cell matrix was thin, with a high degree of lipidation, filled with large amounts of fat vacuoles; even the cell nucleus was pressed by lipid droplets. The rough endoplasmic reticulum in the cytoplasm had obvious distention and degranulation. The crista and glycogen granules were floating free from the cytochondriome. Meanwhile, caryotheca crenation and nuclear chromatic agglutination were observed in some liver cells and Kupffer cells.
In the RKL group (Fig. 2C), the lipid droplets in cells were decreased with little engorgement in the cytochondriome. All structures of the liver cells were damaged to different extents. The cytochondriome and rough endoplasmic reticulum in the cytoplasm had light engorgement or broken crista, but the pathological changes were obviously improved compared with the MC group.
In the RKM group (Fig. 2D), a few fat vacuoles were observed in the liver cells. The cytoplasm had slight lipidation, the rough endoplasmic reticulum was abundant and held together with amylon, and the cytochondriome showed engorgement, but the cristae were clear on observation.
In the RKH group (Fig. 2E), the cell nuclei of liver cells were generally normal. The cytochondriome was abundant, and a few cytochondriomes were varicose. The rough endoplasmic reticulum was abundant and held together with amylon, and small lipid droplets were found occasionally.
Discussion
This study indicates that RK has conspicuous preventive and therapeutic effects on nonalcoholic fatty hepatitis due to the following mechanisms:
Decrease in adipose degeneration in liver cells
Serum transaminases are sensitive to liver cell damage. ALT mainly resides in liver cell endochylema, whereas AST mainly resides in cytochondriomes, with a small amount in the endochylema.34 Therefore, ALT is more sensitive to liver cell damage than AST, whereas AST can reflect the extent of damage to liver cells more precisely than ALT. ALP in liver is located in liver cell membranes and microvilli of the cholangiole; thus the combined detection of ALT, AST, and ALP reflects the extent of damage to liver cells. The activities of ALT, AST, and ALP in serum from the MC group of rats were higher than those in the NC group, which indicates that the NASH rat had severe liver cell damage. After treatment with RK, the activities of ALT, AST, and ALP in MC group rat serum decreased significantly. The combination of macroscopic observation of liver and histopathological observation of hematoxylin–eosin staining under the electron microscope showed that RK can reduce fatty deposition of liver cells and promote cytostasis to decrease the degeneration and extent of necrosis in liver cells.
Reduction of inflammatory cell infiltration of hepatic tissue
When liver cells have adipose degeneration, NASH induces conspicuous inflammation in hepatic acini accompanied with portal area inflammation and hepatic fibrosis. Liver tissues with hematoxylin–eosin staining under the optical microscope demonstrated different inflammatory cell infiltration in acini hepatis.35,36 After treatment with RK, inflammatory cell infiltration in the liver was obviously decreased. The aforementioned phenomenon indicated that the inflammatory extent of liver tissue had been diminished because of both a decrease of the inflammatory cell population and functional degradation.
APN is a cell factor that is excreted by adipose tissue and closely related to metabolism. RK can increase the expression of liver APN and sensitivity to INS. Its mechanism may be related to restraint of nonenzymatic conversion, increase of APN externalization, and reduction of the effect of IR.37,38 RK also prevents oxidation of fat to protect the stability of liver cell membranes, reduce externalization of the inflammatory factor TNF-α, and lessen inflammation of liver cells.
Correct blood lipid metabolic disorder
The increase of FFA is an important initiation factor of NASH. Changes in any aspect of englobement, synthesis, and metabolism of FFA may result in more deposition of triacylglycerol in liver and finally result in adiposis hepatica. In this study, rats consumed fatty acid from the high-fat diet, which causes increases in FFA and triacylglycerol content in blood, and thus triacylglycerol was deposited much more thickly than in the control group. After treatment with RK, the lipochondria on the liver surface decreased upon macroscopic observation, the liver wet weight decreased, and the FFA and triacylglycerol contents in blood serum dropped significantly, which indicates that cleaning the liver and adjusting the fat diet can correct the blood lipid metabolism of the NASH rat and reduce fatty deposition in the liver by reducing the extent of adipose degeneration of liver cells.
Improve IR
The first hit of the “two hits” theory of NASH pathogenesis mainly expresses IR. IR has significant correlation with distribution of body fat. Accompanying the increase in adipose cell population, the sensitivity of the population degrades gradually, which is an obstacle in the normal pancreatic island–adipose cell axis feedback mechanism to generate hyperinsulinemia, while hyperinsulinemia can further aggravate the blood lipid metabolic disorder. In this experiment, INS serum content and the IRI of the NASH rat model greatly increased, whereas the ISI decreased significantly. After treatment with RK, INS serum content, blood GLU level, and IRI of the MC group decreased significantly, whereas the ISI increased, indicating that IR is drastically improved by RK.
Improve LEP resistance
LEP is a hormone secreted by adipocytes that can control food intake, adjust energy metabolism, participate in sugar and fat metabolism in liver, stimulate generation of liver GLU, decrease the synthesis of TG, and elevate the sensitivity of liver and peripheral tissue to INS. Previous research discovered that the LEP content of nonalcoholic fatty liver patients has a positive correlation with INS content. In this study, serum LEP levels of the NASH rat model increased greatly. After treatment with RK, LEP levels decreased significantly, which indicates that RK can improve LEP resistance.
Reduce the release of TNF-α
TNF-α, an inflammatory cytokine released by liver macrophages, can affect the cytochrome P450 enzyme system to cause cytochondriome damage and further result in a “cascade” of inflammatory reactions and aggravate adipose degeneration and hepatocellular damage. Our study found that the serum TNF-α content increased significantly in the NASH rat model, which confirmed that TNF-α has an important effect in NASH morbidity. After treatment with RK, serum TNF-α content decreased significantly, which indicates that RK can reduce the release of TNF-α.
Elevate oxidation resistance
Reduced glutathione and erythrocuprein (SOD) are major antioxidants in liver cells. When the balance between oxidant and antioxidant is disrupted, oxidative stress is generated. In this study, the liver homogenate SOD activity in the NASH rat model is significantly lower, the antioxidation capability of liver cells is weakened, and the MDA content increases greatly. Nevertheless, in each dose group of RK, the liver homogenate SOD activity was elevated significantly, and MDA content decreased notably, which indicates that RK could protect against oxidative stress.
Adjust lipid metabolism
PPAR-α is a key factor for adjusting an organism's energy metabolism and the fatty acid oxidation of the cytochondriome, peroxisome, and corpusculus. LDLR is a glucoprotein on the cell surface. Both PPAR-α and LDLR have a great regulatory effect on in vivo lipid metabolism. Our research found that PPAR-α and LDLR levels of rat liver in the NASH model groups are lower than those of the NC group (P<.01). The decrease of PPAR-α and LDLR results in fatty acid metabolism disequilibrium and lipidoses in liver, which is one of the reasons for NASH morbidity.
Conclusions
In this study, a nonalcoholic fatty hepatitis model was established successfully, and RK was used for adiposis hepatica interference with a high-fat diet. The results show that RK can decrease lipid levels in blood serum and hepatic tissue, reduce the generation of FFA, and finally protect liver cells. RK can prevent damage of liver cells and reduce the effect of liver inflammatory reaction by improving IR and LEP resistance. RK can further restrain oxidative stress and the lipid peroxidation reaction, maintain the balance between oxidation and antioxidation, and prevent damage in liver cells. RK can also diminish the active damage of liver cells induced by high-fat diet to protect liver cells. RK can reduce the inflammatory damage and adipose degeneration extent of liver to recover the structure and function of liver cells gradually. Thus it is considered that RK has both liver-protective and lipid-adjusting effects. The mechanism of action may be to lessen adipose degeneration of liver cells, reduce inflammatory cell infiltration of hepatic tissue, correct blood lipid metabolic disorders, improve IR and LEP resistance, reduce the release of TNF-α, and elevate the oxidation resistance.
Acknowledgment
We acknowledge financial support from the Special Fund for Agro-scientific Research in the Public Interest (grant 201103037).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lu LT. A study on the genus Rubus of China. J System Evol. 1983;21:13–25. [Google Scholar]
- 2.Gu Y. Li WL. Wang CY. Yu H. Shi ZM. Peng LJ. Investigation on wild Rubus resources in Yunnan province. Plant Sci J. 2000;18:49–55. [Google Scholar]
- 3.Gallois A. Quantitative evaluation of raspberry ketone using thin-layer chromatography. Sci Aliments. 1982;2:99–106. [Google Scholar]
- 4.Guichard E. Identification of the flavoured volatile components of the raspberry cultivar Lloyd George. Sci Aliments. 1982;2:173–185. [Google Scholar]
- 5.Sporstol S. Scheline RR. The metabolism of 4-(4-hydroxyphenyl) butan-2-one (raspberry ketone) in rats, guinea-pigs and rabbits. Xenobiotica. 1982;12:249–257. doi: 10.3109/00498258209052463. [DOI] [PubMed] [Google Scholar]
- 6.Beekwilder J. van der Meer IM. Sibbesen O. Broekgaarden M. Qvist I. Mikkelsen JD. Hall RD. Microbial production of natural raspberry ketone. Biotechnol J. 2007;10:1270–1279. doi: 10.1002/biot.200700076. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto C. Satoh Y. Hara M. Inoue S. Tsujita T. Okuda H. Anti-obese action of raspberry ketone. Life Sci. 2005;77:194–204. doi: 10.1016/j.lfs.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Meng XJ. Zhou Y. Liu X. Zheng F. Experimental study on the anti-obesity action of simple obesity in rats by raspberry ketone. Food Industry. 2008;1:1–3. [Google Scholar]
- 9.Zhou Y. Meng XJ. Liu X. Li B. Experimental study on the intervention of nonalcoholic steatohepatitis in rats by raspberry ketone. Food Industry. 2008;2:6–9. [Google Scholar]
- 10.Nonalcoholic steatohepatitis clinical research network. Hepatology. 2003;37:224. doi: 10.1002/hep.510370203. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 12.Pulzi FBU. Cisternas R. Melo MR. Ribeiro CMF. Malheiros CA. Salles JE. New clinical score to diagnose nonalcoholic steatohepatitis in obese patients. Diabetol Metab Syndr. 2011;3:3. doi: 10.1186/1758-5996-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth SG. Gordon FD. Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;126:137–145. doi: 10.7326/0003-4819-126-2-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 14.Falck-Ytter Y. Younossi ZM. Marchesini G. McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21:17–26. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- 15.Younossi ZM. Diehl AM. Ong JP. Nonalcoholic fatty liver disease: an agenda for clinical research. Hepatology. 2002;35:746–752. doi: 10.1053/jhep.2002.32483. [DOI] [PubMed] [Google Scholar]
- 16.Hojo M. Watanabe S. Pharmacological therapy of nonalcoholic steatohepatitis. Hepatol Res. 2011;41:209–216. doi: 10.1111/j.1872-034X.2011.00780.x. [DOI] [PubMed] [Google Scholar]
- 17.Fan JG. Ding XD. Zeng Y. Nonalcoholic steatohepatitis: Society of American symposium on liver disease [in Chinese] Liver. 2003;8:59–61. [Google Scholar]
- 18.Ni YJ. Liu HY. Zhang SC, et al. Hepatic lipase and lipoprotein lipase in the pathogenesis of fatty liver [in Chinese] Chin J Digest. 1999;19:324–327. [Google Scholar]
- 19.Rouach H. Fataccioli V. Gentil M. French SW. Morimoto M. Nordmann R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–355. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Aguilar FF. Etiopathogenesis of non-alcoholic steatohepatitis [in Spanish] Gastroenterol Hepatol. 2005;28:396–406. doi: 10.1157/13077761. [DOI] [PubMed] [Google Scholar]
- 21.Marchesini G. Marzocchi R. Agostini F. Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005;16:421–427. doi: 10.1097/01.mol.0000174153.53683.f2. [DOI] [PubMed] [Google Scholar]
- 22.Ni YJ. Liu HY. Pathogenesis of fatty liver and the diagnosis and treatment [in Chinese] Int J Dig Dis. 1997;17:158–161. [Google Scholar]
- 23.Li YM. Lipid-lowering drugs used in the treatment of fatty liver in the conflict [in Chinese] Modern Med Health. 2003;19:127–128. [Google Scholar]
- 24.Yan M. Lu R. Jia X. Meng F. Zhao X. Different serum lipid adjustment drugs for the treatment of hyperlipidemic fatty liver [in Chinese] Chin J Hepatol. 2001;9:355–357. [PubMed] [Google Scholar]
- 25.Zhao XW. Diagnosis and treatment of fatty liver in progress [in Chinese] J Trad Chin Med. 2002;43:943–945. [Google Scholar]
- 26.Yang HY. Tzeng YH. Chai CY. Hsieh AT. Chen JR. Chang LS. Yang SS. Soy protein retards the progression of non-alcoholic steatohepatitis via improvement of insulin resistance and steatosis. Nutrition. 2011;2:16. doi: 10.1016/j.nut.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Day CP. James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 28.Park KS. Raspberry ketone increases both lipolysis and fatty acid oxidation in 3T3-L1 adipocytes. Planta Med. 2010;76:1654–1658. doi: 10.1055/s-0030-1249860. [DOI] [PubMed] [Google Scholar]
- 29.Abdelmegeed MA. Yoo SH. Henderson LE. Gonzalez FJ. Woodcroft KJ. Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr. 2011;141:603–610. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukumitsu S. Aida K. Shimizu H. Toyoda K. Flaxseed lignan attenuates high-fat diet-induced fat accumulation and induces adiponectin expression in mice. Br J Nutr. 2008;100:669–676. doi: 10.1017/S0007114508911570. [DOI] [PubMed] [Google Scholar]
- 31.Sturzeneker MC. Ioshii SO. Villela Baroncini LA. Précoma DB. Olmesartan severely weakened the development of NASH in an animal model of hypercholesterolemia. Atherosclerosis. 2011;2:19. doi: 10.1016/j.atherosclerosis.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 32.Xu C. Wang G. Hao Y. Zhi J. Zhang L. Chang C. Correlation analysis between gene expression profile of rat liver tissues and high-fat emulsion-induced nonalcoholic fatty liver. Dig Dis Sci. 2011;56:2299–2308. doi: 10.1007/s10620-011-1599-9. [DOI] [PubMed] [Google Scholar]
- 33.Ji G. Yang Q. Hao J. Guo L. Xhen X. Hu J. Leng L. Jiang Z. Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int Immunopharmacol. 2011;11:762–768. doi: 10.1016/j.intimp.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 34.Adams LA. Angulo P. Lindor KD. Nonalcoholic fatty liver disease. Can Med Assoc J. 2005;173:734–745. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siebler J. Galle PR. Treatment of nonalcoholic fatty liver disease. World Gastroenterol. 2006;12:2161–2167. doi: 10.3748/wjg.v12.i14.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams LA. Angulo P. Treatment of non-alcoholic fatty liver disease. J Postgrad Med. 2006;82:315–322. doi: 10.1136/pgmj.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang YX. Li ZK. The recent study on fatty liver [in Chinese] Inner Mongolia Med J. 2006;38:459–461. [Google Scholar]
- 38.Liu HC. Yan JR. Xu N. Comparative study of effects and safety of atorvastatin and fluvastatin on aged patients with hyperlipidemia [in Chinese] Chin J Modern Med. 2006;14:133–l37. [Google Scholar]