Abstract
In this project, we strived to develop a decellularized human cornea to use as a scaffold for reconstructing the corneal epithelium and anterior stroma. Human cadaver corneas were decellularized by five different methods, including detergent- and nondetergent-based approaches. The success of each method on the removal of cells from the cornea was investigated. The structural integrity of decellularized corneas was compared with the native cornea by electron microscopy. The integrity of the basement membrane of the epithelium was analyzed by histology and by the expression of collagen type IV, laminin, and fibronectin. Finally, the ability of the decellularized corneas to support the growth of human corneal epithelial cells and fibroblasts was assessed in vitro. Corneas processed using Triton X-100, liquid nitrogen, and poly(ethylene glycol) resulted in incomplete removal of cellular material. Corneas processed with the use of sodium dodecyl sulfate (SDS) or with sodium chloride (NaCl) plus nucleases successfully removed all cellular material; however, only the NaCl plus nuclease treatment kept the epithelial basement membrane completely intact. Corneas processed with NaCl plus nuclease supported both fibroblast and epithelial cell growth in vitro, while corneas treated with SDS supported the growth of only fibroblasts and not epithelial cells. Decellularized human corneas provide a scaffold that can support the growth of corneal epithelial cells and stromal fibroblasts. This approach may be useful for reconstructing the anterior cornea and limbus using autologous cells.
Introduction
The optical clarity of the cornea is highly dependent on the integrity of its primary layers, including the outermost epithelial, the middle stromal, and the innermost endothelial layer. A number of tissue engineering approaches have been investigated for the reconstruction of the whole cornea or its individual layers as an alternative to using cadaver corneal tissue for transplants.1–5
Reconstruction of the corneal epithelium has been extensively studied with tissue-engineered corneal epithelium now in clinical use in selected academic centers worldwide.6–9 In most clinical applications, a small population of corneal epithelial stem/progenitor cells from the limbus are expanded in culture on a substrate, typically a basement membrane such as human amniotic membrane.10 The corneal epithelial sheet, with or without a carrier, is then transplanted to the diseased corneal surface. The main clinical indication for the use of tissue-engineered corneal epithelium is the treatment of limbal stem cell deficiency, a condition where the epithelial stem cells responsible for renewing the corneal epithelium have been lost. The early clinical results appear promising, especially when autologous-engineered epithelium is used and immune-mediated rejection is avoided.11 However, success with tissue-engineered corneal epithelium is not always attained and in some cases, despite a successful initial transplant, a normal corneal epithelium is only temporarily restored.12 Although there are many factors that contribute to the clinical failure of the tissue engineered epithelium, it seems that one of the major issues is the lack of an appropriate in vivo environment that can support the long-term survival and function of the corneal epithelium and its stem cells.
Besides factors essential for the health of the corneal epithelium such as a healthy tear film, proper eyelid closure, and the absence of destructive inflammation, the function and survival of the epithelium is also highly dependent on the structural and biochemical support from the underlying stroma. Previous studies in the cornea as well as other epithelial tissues have clearly demonstrated the importance of the interactions between the epithelium and its underlying layers.13 In the cornea, epithelial-stromal interactions have been found to play a critical role in regulating epithelial proliferation and differentiation during both normal and wound healing states.13–17
Based on this information, we hypothesized that tissue-engineering application of the corneal epithelium would be optimized and success would be achieved with the establishment of the proper stromal microenvironment to provide the necessary support to the transplanted epithelial cells. Therefore, we aimed at developing a construct that can be used for both epithelial and anterior stromal reconstruction.
Both synthetic and biological matrices have previously been tested for corneal tissue engineering.18–20 In recent years, decellularized organ matrices have also drawn attention, as they provide a more natural environment for the growth and differentiation of cells when compared with synthetic scaffolds.21 In the cornea, decellularized xenograft matrices have been studied primarily for stromal replacement.22–28 In this study, we undertook a tissue-engineering approach to evaluate methods for removing cells from human cadaver corneas while maintaining the integrity of the basement membrane and the stromal matrix. The optimized protocol resulted in a decellularized cornea fully capable of supporting the growth and differentiation of human corneal epithelial and stromal cells in vitro.
Materials and Methods
Decellularization of human cadaver corneas
Human donor corneoscleral buttons that had been stored in Optisol media and deemed unsuitable for transplantation were obtained from the Illinois Eye Bank, Chicago, IL. Most corneas had either passed their expiration date or had low cell counts, which made them undesirable for clinical use. The mean age of the donors was 50±25 years. The following five different methods were employed to remove cells from the human cadaver corneas. The sample size for all experiments was three, except as noted.
Method 1 (detergents)
Corneas were placed in 50 mL centrifuge tubes with sodium dodecyl sulfate (SDS) (Bio-Rad) or Triton X-100 (Sigma-Aldrich) in concentrations ranging from 0.1% to 1% at room temperature for 24 h under shaking. To remove the detergents, the corneas were washed thrice with phosphate-buffered saline (PBS), each for 24 h at 4°C on a rotating shaker.
A slight variation of the detergent-based method was also adapted from Daniel et al., where corneas were treated with 1% SDS for 12 h, after which they were rinsed with PBS 3–5 times for 2 h, in 75% ethanol for 12 h, and in PBS for 3–5 times for 1 h all at room temperature under shaking.29,30
Method 2 (liquid nitrogen)
Corneas were placed in 50 mL centrifuge tubes, were snap frozen with liquid nitrogen, and were left in the tube with the cap closed tight to create a hypoxic environment. The corneas were then incubated in the same tubes for 7 days at room temperature.31
Method 3 [poly(ethylene glycol)]
Corneas were treated with poly(ethylene glycol) (PEG) MW 1000 and MW 8000 (Sigma-Aldrich). PEG is an amphiphilic polymer that damages the cell membranes. The rationale for using PEG is to avoid using detergents. For our purpose, the decellularization protocol adapted from Uchimura et al. was modified to 45 min PEG exposure at room temperature under shaking.32 To remove PEG, corneas were washed thrice with PBS as just described.
Method 4 (osmotic gradient plus detergents)
Tissues were subjected to a stepwise treatment in which a combination of hypotonic and hypertonic buffers along with Triton X-100 and SDS was used. This procedure, adapted from a study on carotid arteries by Roy et al., was modified to preserve the extracellular matrix and remove cells from the cornea.33 In the first step, the cornea was treated with hypotonic buffer containing 10.0 mM Tris (pH 8.0) for 24 h at 4°C under continuous shaking. After 24 h, the cornea was placed in hypertonic buffer containing 1% Triton X-100, 1.5 M KCl, and 10.0 mM Tris (pH 8.0) under the same conditions. In the third step, hypertonic buffer was replaced by an extraction buffer containing 1% SDS and 10.0 mM Tris (pH 9.0) for 24 h. To remove SDS, the cornea was incubated in 1% Triton X-100 at 37°C for 30 min under shaking. Finally, the cornea was extensively washed with PBS with PBS changed every 24 h for up to 72 h under the same temperature and shaking conditions.
Method 5 (NaCl plus nucleases)
Corneas were subjected to nonsurfactant treatment involving incubation in 1.5 M sodium chloride (NaCl) solution for 48 h with NaCl change after 24 h.34 This method was modified in the second step by treating the corneas with DNAse 5 U/mL and RNAse 5 U/mL (Sigma-Aldrich) for 48 h. Corneas were then washed with PBS for 72 h with PBS changed every 24 h. The decellularization procedure was carried out at room temperature under continuous agitation.
Histologic evaluation of decellularized corneas
The removal of cellular components was investigated by routine histology and using the nuclear stains 4′,6-diamidino-2-phenylindole (DAPI) and/or propidium iodide (PI). Decellularized corneas were embedded in optimal cutting temperature (OCT) medium, sectioned with a cryostat, and fixed in 4% paraformaldehyde (PFA) solution in PBS for 20 min at room temperature. After fixation, the tissue sections were permeabilized with 0.5% Triton-X-100 in PBS for 30 min at room temperature and stained with PI or DAPI. Fluorescent images were captured with a Zeiss Axioscope2 upright phase-contrast and epifluorescence microscope. The integrity of the extracellular matrix was investigated with hematoxylin and eosin (H and E) staining and scanning electron microscopy. H and E stain was used to observe the corneal structure as well as the degree of decellularization. The presence of residual cellular/membranous material was assessed by immunostaining for human major histocompatibility complex (MHC) class I antigen (human leukocyte antigen [HLA]-A,B,C).
For scanning electron microscopy studies, the corneas were fixed with 2.5% glutaraldehyde and 2% PFA in PBS, pH 7.4, at room temperature for 2 h, then washed thrice in PBS buffer PH 7.4 for 5 min each. For secondary fixation, samples were fixed in 2% osmium tetraoxide in PBS for 1 h at room temperature; dehydrated in graded ethanol series: 25%, 50%, 75%, and 95% each for 15 min; and finally, in 100% ethanol with 3 changes of 5 min each. Samples were critical-point dried using a CPD 030- Balzers Critical Point Dryer (BAL-TEC). Samples were mounted on an aluminum stub and coated with gold/palladium with a thickness of ∼275 Å using a sputter coater (Denton Desk IV Sputter Coater). Scanning electron microscope JEOL (Japan Electron Optics Laboratory) 5600LV SEM was used to visualize and compare the anterior surface morphology and the arrangement of the collagen fibers network of control and decellularized corneas.
For transmission electron microscopy, samples were primary fixed in 2% PFA and 2.5% glutaraldehyde in sodium cacodylate, then secondary fixed with 1% osmium tetroxide at 4°C. Dehydration with ethanol was performed as described for SEM followed by infiltration with one part Spurr's resin to one part 100% ethanol over molecular sieves followed by embedding with fresh resin and polymerization. Polymerized blocks were sectioned at 90 nm on a Reichert-Jung Ultracut E ultramicrotome, then placed on 200 hex mesh parlodion/carbon coated nickel grids, dried, and stained with 4% uranyl acetate followed by Reynold's lead citrate. Stained grids were imaged with a JEOL 1200EX transmission electron microscope. The size and distance between collagen fibers was measured for a minimum of 50 fibers for each TEM section.
For periodic acid Schiff (PAS) staining, frozen sections were incubated in 0.5% periodic acid (MP Biomedicals) for 10 min at room temperature. The sections were then washed in running water for 5 min and then treated with hydrochloric acid Schiff's reagent for 7 min warmed at room temperature. This is followed by 5 min wash with running water.
Immunostaining
For immunostaining, human corneas in OCT were cut into 10 μm sections, fixed in 4% PFA for 20 min followed by permeabilization in 0.3% Triton-X in Tris Buffered Saline (TBS) for 10 min at room temperature. The sections were blocked with 10% donkey serum with 1% bovine serum albumin in TBS for 1 h at room temperature. The primary antibodies included the goat polyclonal anti-collagen type IV (1:100; Southern Biotech), mouse monoclonal anti-collagen type IV alpha 1 (1:100; DSHB), goat anti-keratin 12 (1:100; Santa Cruz Biotechnology), mouse anti-ABCG2 (1:100; Santa Cruz Biotechnology), mouse anti-p63 (1:100; Santa Cruz Biotechnology), rabbit anti-Ki67 (1:50; Epitomics), mouse anti-human HLA Class I (1:50; Biolegend), and goat anti-aldehyde dehydrogenase 1 (ALDH1) (1:50; EMD Chemicals). The primary antibody was applied either overnight at 4°C or for 2 h at room temperature. A fluoroscein isothiocyanate-conjugated donkey anti-goat, anti-rabbit or anti-mouse IgG (1:300-400; Jackson ImmunoResearch), or rhodamine-conjugated anti-goat IgG was used for 1 h at room temperature in the dark. For negative control, the primary antibody incubation step was omitted or instead isotype control was used. Slides were cover slipped with mounting medium with or without DAPI, and images were visualized using a Zeiss Axiovert fluorescence microscope and photographed with an AxioCam (Carl Zeiss) camera.
Cell culture
Human corneal epithelial and fibroblast cultures were initiated from fresh cadaver eyes obtained from the Illinois Eye Bank as described earlier.35 To isolate limbal epithelial cells, the limbus was separated from the central cornea with an 8 mm trephine. The limbal ring was incubated in 2 mg/mL Dispase II (Invitrogen) in PBS for 1 h at 37°C. The epithelial sheets were peeled off and digested in 0.25% trypsin-EDTA at 37°C for 30 min. Cells were washed, re-suspended in keratinocyte serum-free medium (KSFM; Invitrogen), and plated on the basement membrane side of the decellularized corneas at a density of 1×106 cells/cornea. To induce stratification, after 2 weeks, the media was changed to one part KSFM and three parts DMEM/F12 plus 2% serum, and the media was reduced to allow growth at the air-liquid interface.
To isolate corneal fibroblasts, the epithelium and Descemet's membrane were first removed after incubation in Dispase as described earlier. The remaining stroma was then cut into 1×1 mm pieces and incubated in 0.1% collagenase (Sigma) in DMEM at 37°C for 1.5 h on a shaker. The isolated keratocytes were expanded and differentiated into fibroblasts by culturing in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. After 1–2 passages, the cells were trypsinized and re-suspended in DMEM and 10% FBS. A total of 1×106 fibroblasts cells was resuspended in 70 μL and injected into the stroma of each decellularized cornea. This was done using a 25g needle on a 1cc syringe. The needle was inserted into the stroma to about 50% depth, and cells+media (volume about 20–25 μL) was forcefully injected into the stroma. A successful injection into the stroma was evident based on the immediate opacification of the stroma due to temporary increased fluid and stromal swelling. After achieving opacification in one spot, the needle was either advanced to a different spot or withdrawn and then to re-inserted into another spot. This process was repeated with 4–5 injections throughout the cornea (total volume about 100 μL) until the entire corneal stroma showed edematous opacification. It should be noted that the opacification is temporary and the fluid gets redistributed with time.
Cell viability and proliferation
LIVE/DEAD Viability/Cytotoxicity kit (Molecular Probes) was used to visualize live and dead cells in the cornea. The cornea was rinsed once with PBS and then incubated for 30 min at room temperature in calcein AM (2 μM) and ethidium homodimer (4 μM) solution. After incubation, the cornea was rinsed thrice in PBS to remove the excess solution. The tissue was then placed on a glass bottom dish and imaged using a Leica SP2 confocal microscope at excitaion/emmssion 495 nm/515 nm for live cells and excitation/emmision 495 nm/635 nm for dead cells.
Proliferation in the epithelium was assessed by immunostaining for Ki67. Fibroblast proliferation within the stroma was measured using an MTS assay. Briefly, equal number of fibroblasts were injected into decellularized corneal stromal pieces, then incubated in culture up to 4 weeks. At various time points, the corneal pieces were removed from culture and incubated in an MTS solution for 3 h. The calorimetric changes were measured by a spectrophotometer at 492 nm.
Results
Decellularization of human corneas
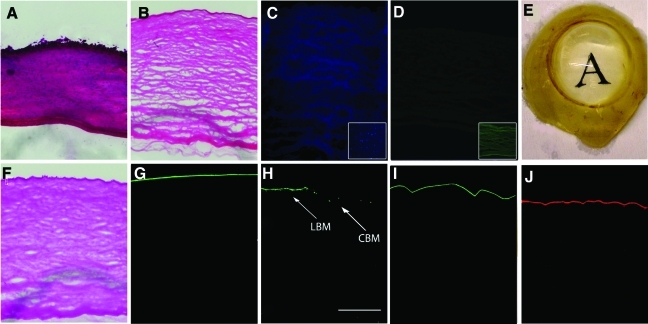
Human corneas decellularized with different methods were initially screened for the removal of cells with H and E staining, as well as the nuclear stain DAPI (Fig. 1A–D). Complete removal of cells was observed in corneas treated with the NaCl plus nuclease treatment (Fig. 1B–D) method as well as the osmotic gradient and 1% SDS processing methods (not shown). The use of liquid nitrogen (Fig. 1C inset), and PEG (1000 or 8000) did not result in complete removal of cells and cellular debris (Table 1).
FIG. 1.
Histological analysis of corneas exposed to the NaCl plus nuclease decellularization method. Hematoxylin and eosin staining in (A) control cornea and (B) decellularized cornea. (C) DAPI staining of NaCl decellularized cornea demonstrating absence of nuclei (inset showing incomplete decellularization with liguid nitrogen method), (D) HLA staining of NaCl decellularized cornea demonstrating no HLA (inset showing positive HLA staining with liquid nitrogen method), (E) Optically clear NaCl plus nuclease decellularized cornea after deturgescence with glycerol. The presence of the epithelial basement membrane in decellularized cornea was observed by (F) PAS staining and (G) by immunostaining against collagen type IV, (H) the alpha1 subtype of collagen type IV which is specific for the limbal basement membrane (LBM) (arrow), central corneal basement membrane (CBM) (arrow), (I) fibronectin, and (J) laminin staining. (H) Scale Bar=50 μm. NaCl, sodium chloride; DAPI, 4′,6-diamidino-2-phenylindole; HLA, human leukocyte antigen; PAS, periodic acid Schiff. Color images available online at www.liebertonline.com/tec
Table 1.
Results of Corneal Decellularization Using the Tested Methods
| Decellularization method | DAPI | Basement membrane | HLA staining |
|---|---|---|---|
| SDS | − | +/− | − |
| Triton-X | + | + | + |
| Liquid nitrogen | + | + | + |
| PEG (1000) | + | + | + |
| PEG (8000) | + | + | − |
| Osmotic gradient+SDS | − | +/− | − |
| NaCl plus nucleases | − | + | − |
DAPI: “+”=presence of nuclei/DNA, “−”=absence of nuclei/DNA; Basement Membrane: “+”=completely intact basement membrane, “+/−”=areas of basement membrane disruption; HLA: “+”=presence of membranous/cellular material by HLA staining, “−”=absence of HLA staining.SDS, sodium dodecyl sulfate; PEG, poly(ethylene glycol); NaCl, sodium chloride; DAPI, 4′,6-diamidino-2-phenylindole; HLA, human leukocyte antigen.
Evaluation of the extracellular matrix
The epithelium basement membrane was evaluated with PAS staining and immunostaining for collagen type IV. PAS staining stains carbohydrate in tissues and is routinely used in histologic studies to identify basement membranes that contain high proportions of glycoproteins/carbohydrates.
On PAS staining, the epithelial basement membrane of corneas treated with NaCl plus nucleases appeared intact and comparable to the untreated corneas (Fig. 1F). Likewise, on immunofluorescence, there was no difference in the staining for collagen type IV, laminin, and fibronectin between the NaCl plus nuclease-treated and fresh cadaver corneas (Fig. 1G, I, K). The basement membrane in the limbal region was evaluated more specifically using a monoclonal antibody against α1 chain of collagen type IV. There was also no difference compared with the control, suggesting that the treatment did not have a deleterious effect on the basement membrane of the limbal region (Fig. 1H). The detergent-based methods, in particular, SDS, was found to induce damage to the basement membrane by PAS staining in certain areas (data not shown) (Table 1).
The decellularization process generally caused significant swelling of the corneal tissue; however, after deturgescence with glycerol, the stroma was optically clear, indicating no gross change to the lamellar organization (Fig. 1E).
The effect of the NaCl plus nuclease treatment on the integrity of the matrix was further evaluated by scanning and transmission electron microscopy. Epithelial cells seen in control corneas (Fig. 2A, G) were absent in decellularized corneas with preservation of the limbal matrix (Fig. 2B, D) and epithelial basement membrane (Fig. 2H) after decellularization. No gross changes in the collagen fibers were noted in decellularized corneas except that the collagen fibers were slightly larger due to swelling as just noted (Fig. 2E, F). The mean diameter of the collagen fibers was 377±42 Å in normal corneas compared with 499±26 Å in decellularized corneas (p<0.001, n=50). There was no difference in the distances between the fibers (276±33 Å vs. 297±70 Å, n=50). Overall, the NaCl plus nuclease decellularization procedure did not appear to alter the ultrastructure of the cornea (Fig. 2).
FIG. 2.
Scanning electron micrographs (A–D). Anterior surface of the limbus in (A) control and (B) NaCl plus nuclease decellularized corneas. No significant difference is observed in the anterior surface of the central cornea (CC), and limbal (L) region. On cross-section of cornea, the collagen fibrils appear similar between (C) control and (D) NaCl plus nuclease treated corneas. Transmission electron micrographs (E–H). Collagen fibers in (E) control and (F) NaCl plus nuclease decellularized cornea. Epithelial basement membrane (BM), (arrow) is shown in (G) control and (H) NaCl plus nuclease decellularized cornea.
Ability to support cell growth
The ability of the decellularized corneas to support corneal epithelial and fibroblast cell growth was further evaluated in culture (Fig. 3). Limbal epithelial cells were plated on decellularized corneas. In NaCl plus nuclease decellularized corneas, the epithelial cells readily attached and by the end of week 2, became nearly confluent as revealed by the Live/Dead Assay (Fig. 3A). Stratification of the epithelium was successfully induced by raising the tissues to the air-liquid interface (Fig. 3D). Corneas decellularized using detergent-based methods, in particular, SDS (Methods 1 and4), did not support epithelial growth, due to poor attachment of the cells.
FIG. 3.
Cell viability and cytotoxicity assay and DAPI staining in NaCl plus nuclease treated corneas. The tissue was stained with calcein AM and imaged en face with confocal microscopy: (A) Corneal epithelial cells cultured for 2 weeks over decellularized cornea, demonstrating viability and differentiation of the superficial layer. (B) Corneal fibroblasts injected into the stroma of decellularized corneas and cultured for 5 days demonstrating viability, and (C) A cross-sectional image showing viable fibroblasts in the stroma and stratified epithelium on the anterior of cornea. DAPI staining of the cross sections demonstrating (D) stratified epithelium and (E) Fibroblasts after 2 weeks in culture, and (F) corneal fibroblasts 5 weeks after injection of fibroblasts and 3 weeks after plating epithelial cells. Scale Bar=300 μm for A, B, C and 100 μm for D, E, and F. Color images available online at www.liebertonline.com/tec
Corneal fibroblasts were injected into NaCl plus nuclease decellularized corneas. The cells attached and exhibited native, spread morphology. The viability assay performed up to 3 weeks revealed that the cells remained alive with evidence of proliferation (based on cell density) for the duration of the study (Fig. 3B, C). The fibroblasts appeared to migrate into the more anterior layers (closer to the epithelium) of the stroma (Fig. 3E, F). Corneas decellularized by detergent based methods likewise supported the growth of fibroblasts (data not shown).
Phenotypic evaluation of the corneal epithelial cells grown on decellularized corneas revealed that they can differentiate and express the corneal specific keratin 12 similar to that seen in human corneas (Fig. 4A–C). To examine the level of differentiation, the expression of markers typically associated with limbal stem and progenitor cells was also evaluated by immunofluorescence. The basal epithelium was found to express DeltaNp63and ABCG2, indicating that the construct supported a “limbal” (undifferentiated) phenotype in the basal layer (Fig. 4D–F, J–L). Proliferation was noted in the basal epithelial layer as evident by Ki67 staining (Fig. 4G–I)
FIG. 4.
Corneal epithelial cell were grown over NaCl plus nuclease decellularized corneas for 2 weeks and then raised to the air-liquid interface to induce differentiation (A–L). (A–C) Corneal specific cytokertain 12 expression is noted in all the superficial epithelial cells. The basal epithelium expresses stem/progenitor cell markers including (D–F) DeltaNp63 (J–L) ABCG2 and proliferation marker (G–I) Ki-67. Corneal fibroblasts grown in decellularized cornea after 1 week, demonstrating (M–O) ALDH1 staining. Scale Bar=50 μm. ALDH1, aldehyde dehydrogenase 1. Color images available online at www.liebertonline.com/tec
The fibroblasts grown in the decellularized corneal stroma were likewise evaluated for the expression of ALDH1, a marker of corneal keratocytes. This was performed in corneas ranging from 1 to 4 weeks after injection of fibroblasts. As early as 1 week, 64%±12% of the cells were found to be expressing ALDH1, suggesting that the majority had partially reverted to a keratocyte phenotype (Fig. 4M–O) (n=3 corneas, minimum 20 cells per sample). The percentage of ALDH1 staining did not differ significantly at subsequent time points examined (range 59%–87%), indicating no correlation with time in culture (p=NS by chi-square). Proliferation and migration of the fibroblast from the injection site toward the anterior and posterior stroma was noted by histology and MTS assay (Fig. 5).
FIG. 5.
Corneal fibroblasts Proliferation and Migration Assay. Corneal fibroblasts grown in a pocket made in stroma for (A) 1 day, (B) 7 days, (C) 14 days, (D) 21 days, and (E) 28 days, stained with DAPI demonstrate proliferation and migration of cells over time. (F) An MTS Assay showing proliferation of cells over a period of 4 weeks. Scale Bar=100 μm. Color images available online at www.liebertonline.com/tec
Discussion
The purpose of this study was to develop a decellularized human cornea as a suitable scaffold for tissue engineering of the corneal epithelium and its underlying stroma. The rationale behind this approach is that a tissue construct that best recreates the corneal and limbal microenvironment will likely offer the most optimal substrate for survival and function of the tissue engineered corneal epithelium. This may be particularly important, as in many clinical situations, the pathology or damage is extended beyond the epithelium and often includes the extracellular matrix and cells within the anterior stroma. A decellularized human cornea could potentially provide a matrix that is nearly identical to the native cornea on which appropriate cells can be repopulated to reconstruct the corneal epithelium with the underlying stromal support.
A total of five methods were investigated in this study for their effectiveness in cellular removal, preservation of the extracellular matrix, and the ability of the resulting decellularized cornea to support the growth of corneal cells. Among the various detergent and nondetergent based methods, a process using agitation, NaCl, DNAse, and RNAase was found to provide the most superior results with complete decelullarization and minimal disturbance of the basement membrane and the ultrastructure of the cornea. The use of liquid nitrogen and PEG (1000, 8000) resulted in incomplete removal of cells from corneas. The use of detergents, while successful in removing cellular material, rendered the corneas unsuitable for epithelial cell attachment afterward. This is consistent with a previous study on decellularized human cornea using 2% Triton X-100 and 0.1% NH4OH, after which laminin was no longer detectable in the epithelial basement membrane.36
There are several aspects of this study that are worth highlighting. In this study, stromal cells were included as supporting cells for the epithelial cells. Most tissue-engineering applications of the corneal epithelium have primarily included the underlying matrix for supporting the epithelium. Several recent studies have considered the importance of the stromal cells in corneal epithelial tissue engineering and have developed experimental approaches that incorporate stromal cells in the tissue construct.37–39 Our study is the first to use a decellularized human cadaver cornea instead of a xenograft cornea. Decellularized human corneas, while eliminating the theoretical risk of zoonotic infections, are also less likely to induce immunologic responses in a human host that may potentially be seen with xenogenic grafts.40 Corneal tissues that are structurally intact but are unsuitable for transplantation are good candidates for such applications.
Previous studies have highlighted the importance of corneal and limbal fibroblasts in corneal epithelial proliferation, differentiation, and wound healing.41 In this study, stromal fibroblasts were chosen given their well-known interaction with the corneal epithelium, including their production of epitheliotrophic factors.13,41 It should be noted that complete reconstruction of the stromal niche will likely require more than just fibroblasts and epithelial cells and perhaps may require other cell types such as neuronal cells. The purpose of this study, however, was not to identify all the necessary cells at this point, but rather to develop a construct that could be used to further study the various cell types that may be necessary.
The differentiation state of the cells that are used to repopulate the underlying stroma may also be important.42 In these experiments, keratocytes were differentiated in vitro into fibroblasts mainly because fibroblasts are easier to expand in culture. However, when exposed to corneal stroma, these fibroblasts appeared to revert back into a more keratocyte-like phenotype that may be important in maintaining transparency of the cornea. While we envision the final construct to only include a relatively thin anterior stroma, the choice of cell type may still affect its optical clarity. This may not be a critical factor for the limbal area but more important in the central cornea. Future in vivo experiments will help determine whether it is necessary to use keratocytes instead of fibroblasts in the construct.
This study, in addition, examined to what extent the decellularized human corneas can maintain the epithelial cells in an undifferentiated phenotype based on the expression of stem cell associated markers. Previous studies on human amniotic membrane have shown that the extracellular matrix helps to preserve cells in a less differentiated state.43–45 The decellularization process used in this study appears to preserve the corneal basement membrane and matrix proteins. Notably, the epithelial cells grown over the NaCl plus nuclease decellularized corneas appear to maintain a “limbal stem cell phenotype” as evident by the expression of ABCG2 and DeltaNp63. While neither of these markers are very specific markers of corneal epithelial stem cells, nonetheless, they confirm previous observations of the corneal/limbal basement membrane and its ability to keep cells in a less differentiated state.46
In summary, this is the first study on decellularization of the human cornea that has examined recellularization of the cornea with corneal epithelial cells and fibroblasts. The novel method for decellularization has potential applications in corneal and limbal tissue engineering, where a biopsy may be used to grow both epithelial cells and stromal cells to reconstruct the corneal epithelium with a stromal niche.
Acknowledgments
This research was supported by a Fight for Sight Grant-in-Aid, Illinois Society for the Prevention of Blindness, unrestricted grant from Research to Prevent Blindness, a career development grant K08EY017561-A1 to ARD, and a core grant EY01792 both from the National Institutes of Health, Bethesda, Maryland. ARD is also the recipient of a Career Development Award from Research to Prevent Blindness. This is also supported by a grant from the Eye Bank Association of America to MAS. The authors thank Ruth Zelkha, MS, for technical assistance in preparation of the images and the Illinois Eye Bank for generously providing human corneal tissue.
Disclosure Statement
No competing financial interests exist.
References
- 1.De Miguel M.P. Alio J.L. Arnalich-Montiel F. Fuentes-Julian S. de Benito-Llopis L. Amparo F. Bataille L. Cornea and ocular surface treatment. Curr Stem Cell Res Ther. 2010;5:195. doi: 10.2174/157488810791268663. [DOI] [PubMed] [Google Scholar]
- 2.Han B. Schwab I.R. Madsen T.K. Isseroff R.R. A fibrin-based bioengineered ocular surface with human corneal epithelial stem cells. Cornea. 2002;21:505. doi: 10.1097/00003226-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Griffith M. Osborne R. Munger R. Xiong X. Doillon C.J. Laycock N.L. Hakim M. Song Y. Watsky M.A. Functional human corneal equivalents from cell lines. Science. 1999;286:2169. doi: 10.1126/science.286.5447.2169. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson D.J. Li F. Shimmura S. Griffith M. Bioengineered corneas: how close are we? Curr Opin Ophthalmol. 2003;14:192. doi: 10.1097/00055735-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Nishida K. Yamato M. Hayashida Y. Watanabe K. Maeda N. Watanabe H. Yamamoto K. Nagai S. Kikuchi A. Tano Y. Okano T. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77:379. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- 6.Meller D. Pauklin M. Westekemper H. Steuhl K.P. Autologous transplantation of cultivated limbal epithelium. Ophthalmologe. 2010;107:1133. doi: 10.1007/s00347-010-2205-9. [DOI] [PubMed] [Google Scholar]
- 7.Pauklin M. Fuchsluger T.A. Westekemper H. Steuhl K.P. Meller D. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57. doi: 10.1159/000315020. [DOI] [PubMed] [Google Scholar]
- 8.Colabelli Gisoldi R.A. Pocobelli A. Villani C.M. Amato D. Pellegrini G. Evaluation of molecular markers in corneal regeneration by means of autologous cultures of limbal cells and keratoplasty. Cornea. 2010;29:715. doi: 10.1097/ICO.0b013e3181c91ac4. [DOI] [PubMed] [Google Scholar]
- 9.Thanos M. Pauklin M. Steuhl K.P. Meller D. Ocular surface reconstruction with cultivated limbal epithelium in a patient with unilateral stem cell deficiency caused by Epidermolysis bullosa dystrophica hallopeau-Siemens. Cornea. 2010;29:462. doi: 10.1097/ICO.0b013e3181b442ea. [DOI] [PubMed] [Google Scholar]
- 10.Tsai R.J.F. Li L.M. Chen J.K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe J.R. Daya S.M. Dimitriadi M. Martin R. James S.E. Survival of cultured allogeneic limbal epithelial cells following corneal repair. Tissue Eng. 2007;13:123. doi: 10.1089/ten.2006.0108. [DOI] [PubMed] [Google Scholar]
- 12.Kenyon K.R. Tseng S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 13.Li D.Q. Tseng S.C. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 14.Liu J. Wilson S. Mohan R. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999;18:293. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang L.Q. Huang Y.F. Huang J.X. Yang B.J. Chen B. The interactions of stromal-epithelial in a model of co-culture in the rabbit cornea. Zhonghua Yan Ke Za Zhi. 2007;43:251. [PubMed] [Google Scholar]
- 16.Melles G.R. Binder P.S. Moore M.N. Anderson J.A. Epithelial-stromal interactions in human keratotomy wound healing. Arch Ophthalmol. 1995;113:1124. doi: 10.1001/archopht.1995.01100090050022. [DOI] [PubMed] [Google Scholar]
- 17.Wilson S.E. Mohan R.R. Ambrosio R., Jr. Hong J. Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 18.Li F. Carlsson D. Lohmann C. Suuronen E. Vascotto S. Kobuch K. Sheardown H. Munger R. Nakamura M. Griffith M. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc Natl Acad Sci U S A. 2003;100:15346. doi: 10.1073/pnas.2536767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimmura S. Doillon C.J. Griffith M. Nakamura M. Gagnon E. Usui A. Shinozaki N. Tsubota K. Collagen-poly(N-isopropylacrylamide)-based membranes for corneal stroma scaffolds. Cornea. 2003;22:S81. doi: 10.1097/00003226-200310001-00012. [DOI] [PubMed] [Google Scholar]
- 20.Chirila T.V. An overview of the development of artificial corneas with porous skirts and the use of PHEMA for such an application. Biomaterials. 2001;22:3311. doi: 10.1016/s0142-9612(01)00168-5. [DOI] [PubMed] [Google Scholar]
- 21.Ott H.C. Matthiesen T.S. Goh S.K. Black L.D. Kren S.M. Netoff T.I. Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 22.Fu Y. Fan X. Chen P. Shao C. Lu W. Reconstruction of a tissue-engineered cornea with porcine corneal acellular matrix as the scaffold. Cells Tissues Organs. 2010;191:193. doi: 10.1159/000235680. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z. Zhou Y. Li N. Huang M. Duan H. Ge J. Xiang P. Wang Z. The use of phospholipase A(2) to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials. 2009;30:3513. doi: 10.1016/j.biomaterials.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y.G. Xu Y.S. Huang C. Feng Y. Li Y. Wang W. Development of a rabbit corneal equivalent using an acellular corneal matrix of a porcine substrate. Mol Vis. 2008;14:2180. [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto Y. Funamoto S. Sasaki S. Honda T. Hattori S. Nam K. Kimura T. Mochizuki M. Fujisato T. Kobayashi H. Kishida A. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials. 2010;31:3941. doi: 10.1016/j.biomaterials.2010.01.122. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y. Wu Z. Ge J. Wan P. Li N. Xiang P. Gao Q. Wang Z. Development and characterization of acellular porcine corneal matrix using sodium dodecylsulfate. Cornea. 2010;30:73. doi: 10.1097/ICO.0b013e3181dc8184. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki S. Funamoto S. Hashimoto Y. Kimura T. Honda T. Hattori S. Kobayashi H. Kishida A. Mochizuki M. In vivo evaluation of a novel scaffold for artificial corneas prepared by using ultrahigh hydrostatic pressure to decellularize porcine corneas. Mol Vis. 2009;15:2022. [PMC free article] [PubMed] [Google Scholar]
- 28.Oh J.Y. Kim M.K. Lee H.J. Ko J.H. Wee W.R. Lee J.H. Processing porcine cornea for biomedical applications. Tissue Eng Part C Methods. 2009;15:635. doi: 10.1089/ten.TEC.2009.0022. [DOI] [PubMed] [Google Scholar]
- 29.Daniel J. Abe K. McFetridge P.S. Development of the human umbilical vein scaffold for cardiovascular tissue engineering applications. ASAIO J. 2005;51:252. doi: 10.1097/01.mat.0000160872.41871.7e. [DOI] [PubMed] [Google Scholar]
- 30.McFetridge P.S. Daniel J.W. Bodamyali T. Horrocks M. Chaudhuri J.B. Preparation of porcine carotid arteries for vascular tissue engineering applications. J Biomed Mater Res Part A. 2004;70A:224. doi: 10.1002/jbm.a.30060. [DOI] [PubMed] [Google Scholar]
- 31.Amano S. Shimomura N. Yokoo S. Araki-Sasaki K. Yamagami S. Decellularizing corneal stroma using N2 gas. Mol Vis. 2008;14:878. [PMC free article] [PubMed] [Google Scholar]
- 32.Uchimura E. Sawa Y. Taketani S. Yamanaka Y. Hara M. Matsuda H. Miyake J. Novel method of preparing acellular cardiovascular grafts by decellularization with poly(ethylene glycol) J Biomed Mater Res Part A. 2003;67A:834. doi: 10.1002/jbm.a.10097. [DOI] [PubMed] [Google Scholar]
- 33.Roy S. Silacci P. Stergiopulos N. Biomechanical properties of decellularized porcine common carotid arteries. Am J Physiol Heart Circ Physiol. 2005;289:567. doi: 10.1152/ajpheart.00564.2004. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Andrades M. Cardona J.D. Ionescu A.M. Campos A. Perez M.D. Alaminos M. Generation of bioengineered corneas with decellularized xenografts and human keratocytes. Invest Ophthalmol Vis Sci. 2010;52:215. doi: 10.1167/iovs.09-4773. [DOI] [PubMed] [Google Scholar]
- 35.Djalilian A.R. Namavari A. Ito A. Balali S. Afshar A. Lavker R.M. Yue B.Y.J.T. Down-regulation of Notch signaling during corneal epithelial proliferation. Mol Vis. 2008;14:1041. [PMC free article] [PubMed] [Google Scholar]
- 36.Choi J.S. Williams J.K. Greven M. Walter K.A. Laber P.W. Khang G. Soker S. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials. 2010;31:6738. doi: 10.1016/j.biomaterials.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Barbaro V. Ferrari S. Fasolo A. Ponzin D. Di Iorio E. Reconstruction of a human hemicornea through natural scaffolds compatible with the growth of corneal epithelial stem cells and stromal keratocytes. Mol Vis. 2009;14:2084. [PMC free article] [PubMed] [Google Scholar]
- 38.Pang K. Du L. Wu X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials. 2010;31:7257. doi: 10.1016/j.biomaterials.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 39.Phu D. Wray L.S. Warren R.V. Haskell R.C. Orwin E.J. Effect of substrate composition and alignment on corneal cell phenotype. Tissue Eng Part A. 2010 doi: 10.1089/ten.tea.2009.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasimir M.T. Rieder E. Seebacher G. Nigisch A. Dekan B. Wolner E. Weigel G. Simon P. Decellularization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves. J Heart Valve Dis. 2006;15:278. [PubMed] [Google Scholar]
- 41.Espana E.M. Kawakita T. Romano A. Di Pascuale M. Smiddy R. Liu C.Y. Tseng S.C. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci. 2003;44:5130. doi: 10.1167/iovs.03-0584. [DOI] [PubMed] [Google Scholar]
- 42.Carrier P. Deschambeault A. Audet C. Talbot M. Gauvin R. Giasson C.J. Auger F.A. Guérin S.L. Germain L. Impact of cell source on human cornea reconstructed by tissue engineering. Invest Ophthalmol Vis Sci. 2009;50:2645. doi: 10.1167/iovs.08-2001. [DOI] [PubMed] [Google Scholar]
- 43.Mariappan I. Maddileti S. Savy S. Tiwari S. Gaddipati S. Fatima A. Sangwan V.S. Balasubramanian D. Vemuganti G.K. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc. 2010;5:1470. doi: 10.1038/nprot.2010.115. [DOI] [PubMed] [Google Scholar]
- 44.Tsai R.J. Tsai R.Y. Ex vivo expansion of corneal stem cells on amniotic membrane and their outcome. Eye Contact Lens. 2010;36:305. doi: 10.1097/ICL.0b013e3181efff40. [DOI] [PubMed] [Google Scholar]
- 45.Grueterich M. Espana E.M. Tseng S.C.G. Ex vivo expansion of limbal stem cells: Amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Chen S.Y. Hayashida Y. Chen M.Y. Xie H.T. Tseng S.C. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537. doi: 10.1089/ten.tec.2010.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]