Abstract
Garlic protects against degenerative diseases such as hyperlipidemia and cardiovascular diseases. However, raw garlic has a strong pungency, which is unpleasant. In this study, we examined the effect of high temperature/high pressure-processed garlic on plasma lipid profiles in rats. Sprague–Dawley rats were fed a normal control diet, a high cholesterol (0.5% cholesterol) diet (HCD) only, or a high cholesterol diet supplemented with 0.5% high temperature/high pressure-processed garlic (HCP) or raw garlic (HCR) for 10 weeks. The body weights of the rats fed the garlic-supplemented diets decreased, mostly because of reduced fat pad weights. Plasma levels of total cholesterol (TC), low-density lipoprotein cholesterol, and triglyceride (TG) in the HCP and HCR groups decreased significantly compared with those in the HCD group. Additionally, fecal TC and TG increased significantly in the HCP and HCR groups. It is notable that no significant differences in plasma or fecal lipid profiles were observed between the HCP and HCR groups. High temperature/high pressure-processed garlic contained a higher amount of S-allyl cysteine than raw garlic (P<.05). The results suggest that high temperature/high pressure-processed garlic may be useful as a functional food to improve lipid profiles.
Key Words: S-allyl cysteine, high temperature/high pressure-processed garlic, plasma lipid profiles
Introduction
Dyslipidemia associated with elevated cholesterol and triglycerides (TGs) is a leading cause of cardiovascular disease.1 Accordingly, attempts have been made to identify natural products that reduce blood lipid levels.2
Garlic (Allium sativum L.) has been consumed as a medicinal plant and as a food seasoning for millennia.3 Consuming garlic is very helpful for regulating plasma lipid levels and plasma anticoagulant activity as well as for preventing atherosclerosis.4–6 Additionally, hepatoprotective, anticancer, immune-enhancing, antioxidant, and chemopreventive activities of garlic have been reported,3,7 but the cardioprotective effects of garlic may be the most studied health-promoting effects.5,6 There is no doubt that garlic and garlic preparations possess anticoagulant ability.8 However, Kerckhoffs et al.9 reported that it is still uncertain whether garlic or garlic preparations can be used as lipid-lowering agents. Controversy exists regarding the plasma lipid-regulating and antioxidant properties of garlic.
The health-promoting effects of garlic are derived from many bioactive compounds.3 Most bioactive substances in garlic are affected by cooking or other processing methods. In particular, the main bioactive sulfur-containing compound in fresh garlic is allicin, which is very unstable under heat and is unpleasant to eat.10 Accordingly, the optimal conditions for preparing processed garlic are very important. However, knowledge about the influence of processing methods on bioactive properties of garlic is limited.11,12 In a preliminary experiment, the pungency of high temperature/high pressure-processed garlic was much weaker than that of raw garlic. However, as pungency still remains in processed garlic, green tea leaves were added to the garlic during high pressure cooking and removed after processing. The aim of this investigation was to determine the effect of high temperature/high pressure-processed garlic on the major bioactive sulfur-containing compounds in garlic as well as their bioactivity, particularly on lipid profiles of rats fed a high-cholesterol diet (HCD).
Materials and Methods
Garlic processing
Fresh garlic samples were obtained from a local market in Daejeon, Korea. The garlic was peeled, washed in water, and processed by two different methods. Raw garlic was prepared by crushing the garlic samples in a blender followed by freeze-drying. The high temperature/high pressure-processed garlic was processed at 120°C and high pressure of 1.5 kgf/m2 with 1% green tea leaves for 20 min in an autoclave (model HB-506-6, Hanbaek Scientific Co., Seoul, Korea). The green tea leaves were removed, and the garlic was freeze-dried and powdered in a mill (particle size, <25 μm).
All garlic samples were stored at −70°C until the experiment.
Hypercholesterolemic rat model and diets
Male Sprague–Dawley rats (weight, 120–140 g; 4–5 weeks of age) were obtained from Animal Husbandry of Damul Science (Daejeon) and housed in polycarbonate cages with controlled temperature (23±3°C) and humidity (55±10%) under a 12-h light:dark cycle. The rats were fed a pelleted commercial diet (Samyang Co., Seoul) for the first 2 weeks. After acclimation, the rats were randomly divided into four treatment groups of 10 rats each and allowed free access to water and the assigned diets for 10 weeks. The normal control (NC) diet was the AIN 93 diet. The HCD was 97.5% AIN 93 G diet with 0.5% cholesterol and 2% soybean oil. The crushed raw garlic (HCR) diet was the HCD plus 0.5% crushed garlic, and the HCP diet was the HCD diet with 0.5% high temperature/pressure-processed garlic. The control and experimental diets were isoenergenic. Diet composition was as follows: NC diet contained fat at 16.0% of kcal, protein at 20.3% of kcal, and carbohydrate at 63.8% of kcal; HCD diet contained fat at 20.1% of kcal, protein at 20.3% of kcal, and carbohydrate at 60.0% of kcal; HCR (raw garlic) diet contained fat at 20.3% of kcal, protein at 20.0% of kcal, and carbohydrate at 59.8% of kcal; and HCP (high temperature/high pressure-processed garlic) diet contained fat at 20.2% of kcal, protein at 20.0% of kcal, and carbohydrate at 59.8% of kcal. Energy content was calculated using 4 kcal/g for protein and carbohydrate and 9 kcal/g for fat.
The body weights of the rats, feces, and food were measured daily, and the feed efficiency ratio (FER) was calculated throughout the experiment. Animal experiments were conducted in compliance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.13 After 10 weeks, rats were fasted for 12 h and sacrificed by cervical decapitation, and fasting blood samples were collected in heparinized tubes. Heart, kidneys, spleen, and liver were immediately removed, rapidly washed in saline buffer, collected into cryovials, weighed, and immediately stored in liquid nitrogen for lipid peroxidation and antioxidant marker assays.
Plasma lipid profile measurements
Plasma TG, total cholesterol (TC), and high-density lipoprotein cholesterol concentrations were determined enzymatically using immunoassay kits (Asan Pharmaceuticals, Seoul) and an enzyme-lined immunosorbent assay reader (Pharmacia Biotech, Cambridge, United Kingdom) according to the manufacturer's protocol. Plasma low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation.14
Measurements of fecal lipid profile (TC and TG)
Feces powder was homogenized with chloroform:methanol (2:1) to a final volume 20 times the volume of the feces sample (0.2 g in 4 mL of solvent mixture). After dispersion, the whole mixture was agitated for 15–20 min on a shaker at room temperature. The solvent was washed with water (1 mL). After vortex-mixing, the mixture was centrifuged at low speed (1,500 g) to separate the two phases. After centrifugation, the upper phase was siphoned off, and the lower chloroform phase containing lipids was evaporated under vacuum in a rotary evaporator. Then, the residue was dissolved in ethanol and analyzed at 500 and 550 nm using TC and TG kits purchased from Asan Pharmaceuticals.15
Histopathological examination of liver
Livers were excised, fixed in formalin solution, stained with hematoxylin–eosin, and examined under a light microscope to assess fatty changes.
Determination of alliin and S-allyl cysteine by high-performance liquid chromatography
Dry garlic powder extraction under alliinase-inhibiting conditions
Dry garlic powder (1 g) was extracted at room temperature using 10 mL of methanol:water (80:20, vol/vol) and 0.05% formic acid (pH 3). An aliquot was diluted five times and filtered (pore size, 0.2 μm). Then, 10 μL was injected into the high-performance liquid chromatography (HPLC) apparatus.
Dry garlic powder extraction under alliinase-activating conditions
Dry garlic powder (1 g) was extracted at room temperature using 10 mL of water (pH 6–8). An aliquot was diluted five times and filtered (pore size, 0.2 μm), and 10 μL was injected.
HPLC instrumentation and method.16
Garlic extracts were subjected to HPLC using a Varian Prostar 210 pump and a Prostar 325 ultraviolet-visible detector (Varian Inc., Santa Clara, CA, USA). Compounds were separated on a 150-×3-mm (i.d.) (3 μm particle size) Thermo Quest Fortis C18 column at 20°C (Fortis Technologies Ltd., Neston, Cheshire, United Kingdom) and an ultraviolet detector operated at 208 nm. The column flow rate was 0.4 mL/min. The mobile phase consisted of (A) 20 mM sodium dihydrogen phosphate and 10 mM heptanesulfonic acid, pH 2.1 (adjusted with 85% orthophosphoric acid) and (B) acetonitrile:20 mM sodium dihydrogen phosphate and 10 mM heptanesulfonic acid, pH 2.1 (50:50, vol/vol). The gradient program used is listed in Table 1. Data were acquired using Galaxie software from Varian. Alliin, allicin, and S-allyl cysteine standards were purchased from LKT Labs (St. Paul, MN, USA). Quantitative HPLC determinations were performed based on standard curves.
Table 1.
Gradient Elution Program for High-Performance Liquid Chromatography Analysis
| |
Time (min) |
||||||
|---|---|---|---|---|---|---|---|
| Eluant | 0 | 5 | 25 | 26 | 28 | 30 | 40 |
| A | 100 | 70 | 46 | 0 | 0 | 100 | 100 |
| B | 0 | 30 | 54 | 100 | 100 | 0 | 0 |
Elution Phase A contained 20 mM sodium dihydrogen phosphate plus 10 mM heptane sulfonic acid (pH 2.1, adjusted with 85% orthophosphoric acid); elution Phase B contained acetonitrile:20 mM sodium dihydrogen phosphate plus 10 mM heptane sulfonic acid (pH 2.1, adjusted with 85% orthophosphoric acid) (50:50 vol/vol).
Statistical analysis
All data are presented as mean±SD values. An analysis of variance followed by Duncan's multiple range test was performed to evaluate differences among the groups, using SAS version 6.0 (SAS Institute, Cary, NC, USA). Statistical significance was defined as P<.05.
Results
Body and organ weights
The effect of processed garlic on growth characteristics is shown in Table 2. After 10 weeks of treatment, rat body weights increased significantly in the HCD group (554.7±29.0 g), compared with that in the NC group (525.0±24.9 g) group (P<.05). Treatment with HCR (511.4±26.1 g) or HCP (521.2±26.2 g) restored body weights to control levels. The FER in both the HCP and HCR groups decreased significantly, whereas the fecal weight in the HCP and HCR groups increased significantly, compared with those of the HCD group. The weights of liver, kidney, spleen, and heart increased in all groups, but the liver and heart weights in the HCP group were significantly lower than those in the HCD group (P<.05) (Table 3). Visible fat deposition in the retroperitoneal, mesenteric, epididymal, spleen, and total fat pad areas of rats decreased significantly in the HCR and HCP groups (P<.05) (Table 3). The amount of visible fat, except inguinal fat, was not significantly different in the HCR and HCP groups from that of the NC group.
Table 2.
Initial and Final Body Weight, Weight Gain, Food Intake, Food Efficiency Ratio, and Fecal Weight of Sprague–Dawley Rats After 10 Weeks of Feeding Experimental Diets
| |
Body weight (g) |
|
|
|
|
|
|---|---|---|---|---|---|---|
| Group | Initial | Final | Weight gain (g) | Food intake (g/day) | FER (%) | Fecal weight (g) |
| NC | 254.9±10.4NS | 525.0±24.9b | 271.6±12.1b | 20.3±1.3c | 20.3±1.0ab | 2.2±0.4b |
| HCD | 259.4±11.8 | 554.7±29.0a | 292.9±28.8a | 28.1±0.2a | 21.1±1.7a | 2.2±0.2b |
| HCR | 254.9±7.7 | 511.4±26.1b | 256.5±21.4b | 27.6±0.3b | 19.6±1.1b | 2.4±0.3ab |
| HCP | 252.8±10.2 | 523.9±20.8b | 271.4±14.2b | 27.8±0.2b | 20.1±1.2ab | 2.7±0.8a |
Data are mean±SD values (n=10) for each of the experimental groups.
Values with different letters within a column are significantly different by Duncan's multiple range test (P<.05).
Not significant at P>.05.
FER, food efficiency ratio; NC, normal control group; HCD, high cholesterol diet group; HCR, high cholesterol diet+0.5% raw garlic supplementation group; HCP, high cholesterol diet+0.5% high temperature/high pressure-processed garlic supplementation group.
Table 3.
Weight of Organs and Visible Fat in Sprague–Dawley Rats After 10 Weeks of Feeding Experimental Diets
| |
Weight (g) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Liver | Kidney | Spleen | Heart | Mesenteric | Retroperitoneal | Spleen | Epididymal | Inguinal | Total fat pad |
| NC | 13.21±1.12c | 3.11±0.04NS | 0.86±0.15c | 1.56±0.06b | 6.3±1.3b | 6.1±0.9b | 1.8±1.4b | 6.3±1.1b | 6.2±2.3a | 25.9±1.9bc |
| HCD | 28.14±3.96a | 3.56±0.32 | 1.40±0.26b | 1.64±0.16a | 7.5±0.6a | 16.7±2.5a | 4.0±1.4a | 8.8±0.8a | 5.9±1.2a | 39.9±1.8a |
| HCR | 25.08±2.36a | 3.31±0.15 | 1.20±0.20a | 1.47±0.16ab | 4.7±1.7c | 6.3±2.1b | 2.0±1.3b | 7.6±2.4b | 6.1±1.3bcd | 25.4±4.4bc |
| HCP | 25.96±1.59b | 3.44±3.76ab | 1.36±0.28ab | 1.52±0.11ab | 4.56±1.24c | 6.96±1.96b | 2.36±1.11b | 7.48±1.43b | 6.93±1.13b | 27.59±3.67b |
Data are mean±SD values (n=10) for each of the experimental groups.
Values with different letters within a column are significantly different by Duncan's multiple range test (P<.05).
Not significant at P>.05.
Plasma and fecal lipid profiles
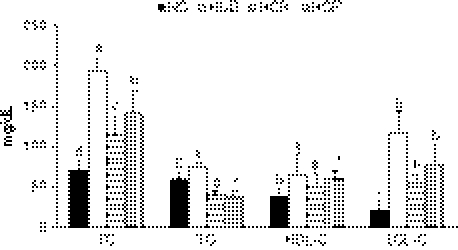
As shown in Figure 1, rats fed a diet enriched with high cholesterol for 10 weeks developed hypercholesterolemia; plasma TC was 192.8 mg/dL, versus 70.8 mg/dL in the NC group. The levels of TC, TG, and LDL-C in plasma decreased significantly in groups fed the processed garlic-supplemented diet compared with the HCD group (P<.05), demonstrating that dietary supplementation with processed garlic improved lipid profiles.
FIG. 1.
Plasma lipid levels in rats (10 animals per group) fed the normal or high cholesterol diet supplemented with raw or processed garlic: total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). NC, normal control group; HCD, high cholesterol diet group; HCR, high cholesterol diet +0.5% raw garlic supplementation group; HCP, high cholesterol diet +0.5% high temperature/high pressure-processed garlic supplementation group. aColumns with different letters within each lipid are significantly different by Duncan's multiple range test (P<.05).
In contrast, fecal levels of TC and TG (in μmol/day) increased significantly in the HCP group compared with those in the HCD group, demonstrating that high temperature/high pressure-processed garlic supplementation results in cholesterol excretion via the feces (Table 4).
Table 4.
Fecal Total Cholesterol and Triglyceride Levels in Sprague–Dawley Rats After 10 Weeks of Feeding Experimental Diets
| |
Level (μmol/day) |
|
|---|---|---|
| Group | TC | TG |
| NC | 1.6±0.2d | 0.5±0.1c |
| HCD | 2.4±0.5c | 0.9±0.1b |
| HCR | 3.5±0.7b | 1.3±0.3a |
| HCP | 3.3±0.6b | 1.3±0.3a |
Data are mean±SD values (n=10) for each of the experimental groups.
Values with different letters within a column are significantly different by Duncan's multiple range test (P<.05).
Serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and lactate dehydrogenase
The levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and lactate dehydrogenase in serum were significantly higher in the HCD group than in the NC group (P<.05), whereas these values decreased in the processed garlic-supplemented diet group. Aspartate and alanine aminotransferase levels decreased remarkably in the HCR and HCP groups compared with those in the HCD group (Table 5). Furthermore, serum glucose increased significantly in groups treated with garlic compared with the HCD group (P<.05), although albumin and protein levels were not significantly different (Table 5).
Table 5.
Blood Biochemical Parameters of Sprague–Dawley Rats After 10 Weeks of Feeding Experimental Diets
| Group | ALT (U/L) | AST (U/L) | ALP (U/L) | LDH (U/L) | Albumin (g/dL) | Protein (g/dL) | BUN (mg/dL) | Glucose (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| NC | 22.4±1.5d | 126.5±19.8b | 32.0±6.8b | 1,007.0±309.7b | 5.9±1.3NS | 7.1±0.7b | 13.8±2.7c | 155.9±15.9d |
| HCD | 154.1±15.0a | 240.0±33.0a | 50.4±4.4a | 1,325.6±515.9a | 5.8±0.5 | 7.5±0.6ab | 18.9±2.0a | 231.4±21.8a |
| HCR | 77.4±26.6c | 126.7±32.6b | 30.9±5.6b | 699.3±199.1b | 6.3±0.6 | 8.1±1.0a | 16.0±1.8bc | 183.8±27.2b |
| HCP | 101.7±13.1c | 148.1±21.3b | 41.6±16.5b | 872.0±341.0b | 6.37±1.16a | 8.29±1.02a | 16.43±2.72b | 191.5±46.2b |
Data are mean±SD values (n=10) for each of the experimental groups.
Values with different letters within a column are significantly different by Duncan's multiple range test (P<.05).
Not significant at P>.05.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; LDH, lactate dehydrogenase.
Histological changes in liver
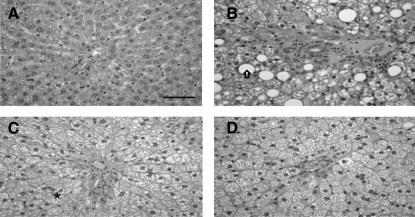
All rats in the NC group had normal liver histological findings (Fig. 2). However, the HCD group had prominent microvesicular steatosis and fatty changes around the portal triad in the liver. Livers from the processed garlic-treated group showed only microvesicular steatosis, which was less extensive than that in livers from rats receiving HCD alone.
FIG. 2.
Histopathological changes in liver of the different groups. (A) NCl rat. (B) HCD treatment induced prominent microvesicular steatosis (*) and fatty changes (arrow) around the portal triad in the liver. (C) HCR group. (D) HCP group. Livers from the HCP group showed only microvesicular steatosis, which was less extensive than that in livers from rats receiving HCD alone. P, portal vein. Hematoxylin–eosin stain. Bar=500 μm.
Major sulfur compounds in high temperature/high pressure-processed garlic by HPLC
Major sulfur-containing compounds such as allicin, alliin, and S-allyl cysteine in raw, high temperature, and high pressure-processed garlic were determined by HPLC. As shown in Table 6, allicin content in raw garlic was 2.6 mg/g, whereas that in high temperature/high pressure-processed garlic was very low at 0.09 mg/g. Additionally, raw garlic contained 4.29 mg/g alliin, whereas high temperature/high pressure-processed garlic contained 1.76 mg/g alliin. However, S-allyl cysteine content was twofold higher in the high temperature/high pressure-processed garlic than in raw garlic.
Table 6.
Allicin, Alliin, and S-Allyl Cysteine Content in Raw and High Temperature/High Pressure-Processed Garlic by High-Performance Liquid Chromatography
| Compound (mg/g) | Raw garlic | High temperature/high pressure-processed garlic |
|---|---|---|
| S-Allyl cysteine | 114.89±0.05 | 25.49±0.04* |
| Alliin | 4.29±0.03 | 1.76±0.06* |
| Allicin | 2.60±0.06 | 0.09±0.02* |
Data are mean±SD values.
Values in the same row are significantly different by Student's t test (P<.05).
Discussion
Bioactive compounds in garlic are affected by cooking or other processing methods. Accordingly, the optimal conditions for preparing processed garlic are very important. However, knowledge about the influence of processing methods on bioactive properties of garlic is limited.9,10 Therefore, the aims of this investigation were to determine the effects of high temperature and high pressure processing on the major bioactive sulfur-containing compounds of garlic as well as their bioactivity, particularly as they affected the lipid profiles of rats fed HCD.
We demonstrated that plasma TC and LDL-C levels in rats fed HCD (0.5% cholesterol) for only 10 weeks increased by 2.7 and 5.7 times, respectively, compared with those in rats fed NC diet. HCD supplemented with processed garlic or high temperature/high pressure-processed garlic significantly reduced plasma TC, TG, and LDL-C levels in rats but significantly increased fecal TC and TG levels, compared with those in rats fed HCD only. This is consistent with previous findings that plasma lipid levels decreased significantly in rats fed a processed garlic product and HCD compared with those fed HCD only.17,18 Previous studies have shown that raw garlic has profoundly decreases TC and TG levels without changing high-density lipoprotein cholesterol levels in rats fed HCD, whereas boiled garlic has little effect on these parameters.19,20
In a human study,21 supplementation with raw garlic, powdered garlic, or aged garlic extract (4 g/day) for 6 months significantly lowered LDL-C and other plasma lipid levels in adults with moderate hypercholesterolemia. Our findings in rats provide further support for the antihyperlipidemic, antihypercholesterolemic, and anti-atherosclerotic actions of processed garlic products because both crushed raw garlic and high temperature/high pressure-processed garlic exhibited remarkable antihyperlipidemic action by decreasing the levels of TG, LDL-C, and TC in plasma and increasing the levels of TG and TC in feces.
Very low amounts of allicin and alliin were detected in the high temperature/high pressure-processed garlic, whereas the S-allyl cysteine level was approximately twofold greater in the high temperature/high pressure-processed garlic than in raw garlic. S-Allyl cysteine is the main component of aged garlic extract, which decreases TG and TC.22 In particular, S-allyl cysteine, a water-soluble organosulfur compound, is a potent inhibitor of cholesterol synthesis and hence may be the major component of garlic responsible for reducing plasma cholesterol level.
The data from our study indicate that high temperature/high pressure-processed garlic improved lipid profiles, particularly decreases in TC and TG. Our studies further revealed that the lipid-lowering action of high temperature/high pressure-processed garlic originates, in part, from the organosulfur compounds, especially S-allyl cysteine. Improved lipid profiles following ingestion of high temperature/high pressure-processed garlic might be derived from the sulfur-containing compounds such as S-allyl cysteine, but not allicin. High temperature/high pressure-processed garlic is easily consumed by humans because of the lack of pungency without a loss in bioactivity.
In conclusion, high temperature/high pressure-processed garlic contained bioactive sulfur compounds in comparable amounts to those in raw garlic, particularly S-allyl cysteine. High temperature/high pressure-processed garlic improved plasma lipid profiles in rats fed a high cholesterol-containing diet, possibly by interrupting the enterohepatic circulation of cholesterol and cholesterol metabolites. These results suggest that high temperature/high pressure-processed garlic may have efficacy as a functional food for improving blood lipid profiles.
Acknowledgment
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (grant NRF 2009-0077171).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kritchevsky D. Diet and atherosclerosis. Am J Pathol. 1976;84:615–632. [PMC free article] [PubMed] [Google Scholar]
- 2.Hasani-Ranjbar S. Nayebi N. Moradi L. Mehri A. Larijani B. Abdollahi M. The efficacy and safety of herbal medicines used in the treatment of hyperlipidemia; a systematic review. Curr Pharm Des. 2010;16:2935–2947. doi: 10.2174/138161210793176464. [DOI] [PubMed] [Google Scholar]
- 3.Lawson LD. Garlic, a review of its medicinal effects and indicated active compounds. In: Lawson LD, editor; Bauer R, editor. Chemistry and Biological Activity, Series 691: Phytomedicines of Europe. American Chemical Society; Washington, DC: 1998. pp. 176–209. [Google Scholar]
- 4.Amagase H. Petesch BL. Matsuura H. Kasuga S. Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee SK. Maulik SK. Effect of garlic on cardiovascular disorders: a review. Nutr J. 2002;1:1–14. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman K. Lowe GM. Garlic and cardiovascular disease: a critical review. J Nutr. 2006;136:736S–740S. doi: 10.1093/jn/136.3.736S. [DOI] [PubMed] [Google Scholar]
- 7.Kim MH. Kim MJ. Lee JH. Han JI. Kim JH. Sok D-E. Kim MR. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. J Med Food. 2011;14:732–738. doi: 10.1089/jmf.2010.1454. [DOI] [PubMed] [Google Scholar]
- 8.Lawson LD. Ransom DK. Hughes BG. Inhibition of whole blood platelet aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb Res. 1992;65:141–156. doi: 10.1016/0049-3848(92)90234-2. [DOI] [PubMed] [Google Scholar]
- 9.Kerckhoffs DAJM. Brouns F. Hornstra G. Mensink RP. Effects on the human serum lipoprotein profile of beta-glucan, soy protein and isoflavones, plant sterols and stanols, garlic and tocotrienols. J Nutr. 2002;132:2494S–2505S. doi: 10.1093/jn/132.9.2494. [DOI] [PubMed] [Google Scholar]
- 10.Touloupakis E. Ghanotakis DF. Nutraceutical use of garlic sulfur-containing compounds. Adv Exp Med Biol. 2011;698:110–121. doi: 10.1007/978-1-4419-7347-4_9. [DOI] [PubMed] [Google Scholar]
- 11.Constenla DT. Lozano JE. Effect of pretreatments and processing conditions on the chemical, physical, microbiological and sensory characteristics of garlic paste. J Food Process Eng. 2005;28:313–329. [Google Scholar]
- 12.Haciseferogullari H. Ozcan M. Demir F. Calisir S. Some nutritional and technological properties of garlic. J Food Process Eng. 2005;68:463–469. [Google Scholar]
- 13.National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- 14.Bairaktarit ET. Tzallas C. Kalientzidou M. Tselepis AD. Siamopoulos KC. Seferiadis KI. Elisaf MS. Evaluation of alternative calculation methods for determining low-density lipoprotein cholesterol in hemodialysis patients. Clin Biochem. 2004;37:937–940. doi: 10.1016/j.clinbiochem.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Folch J. Lees M. Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissures. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 16.Arnault I. Christides JP. Mandon N. Haffner T. Kahane R. Auger J. High-performance ion-pair chromatography method for simultaneous analysis of alliin, deoxyalliin, allicin and dipeptide precursors in garlic products using multiple mass spectrometry and UV detection. J Chromatogr A. 2003;991:69–75. doi: 10.1016/s0021-9673(03)00214-0. [DOI] [PubMed] [Google Scholar]
- 17.Rahman K. Historical perspective on garlic and cardiovasular disease. J Nutr. 2001;131:977S–979S. doi: 10.1093/jn/131.3.977S. [DOI] [PubMed] [Google Scholar]
- 18.Yeh YY. Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr. 2001;131(3 Suppl):989S–993S. doi: 10.1093/jn/131.3.989S. [DOI] [PubMed] [Google Scholar]
- 19.Gorinstein S. Jastrzebski Z. Namiesnik J. Leontowicz H. Leontowicz M. Trakhtenberg S. The atherosclerotic heart disease and protecting properties of garlic: contemporary data. Mol Nutr Food Res. 2007;51:1365–1381. doi: 10.1002/mnfr.200700064. [DOI] [PubMed] [Google Scholar]
- 20.Gorinstein S. Leontowicz H. Leontowicz M. Drzewiecki J. Raw and boiled garlic enhances plasma antioxidant activity and improves plasma lipid metaboilsm in cholesterol-fed rats. Life Sci. 2006;78:655–663. doi: 10.1016/j.lfs.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 21.Gardner CD. Lawson LD. Block E. Chatterjee LM. Kiazand A. Balise RR. Kraemer HC. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: a randomized clinical trial. Arch Intern Med. 2007;167:346–353. doi: 10.1001/archinte.167.4.346. [DOI] [PubMed] [Google Scholar]
- 22.Yeh YY. Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr. 2001;131(3 Suppl):989S–993S. doi: 10.1093/jn/131.3.989S. [DOI] [PubMed] [Google Scholar]