Abstract
The field of skeletal muscle tissue engineering is currently hampered by the lack of methods to form large muscle constructs composed of dense, aligned, and mature myofibers and limited understanding of structure-function relationships in developing muscle tissues. In our previous studies, engineered muscle sheets with elliptical pores (“muscle networks”) were fabricated by casting cells and fibrin gel inside elastomeric tissue molds with staggered hexagonal posts. In these networks, alignment of cells around the elliptical pores followed the local distribution of tissue strains that were generated by cell-mediated compaction of fibrin gel against the hexagonal posts. The goal of this study was to assess how systematic variations in pore elongation affect the morphology and contractile function of muscle networks. We found that in muscle networks with more elongated pores the force production of individual myofibers was not altered, but the myofiber alignment and efficiency of myofiber formation were significantly increased yielding an increase in the total contractile force despite a decrease in the total tissue volume. Beyond a certain pore length, increase in generated contractile force was mainly contributed by more efficient myofiber formation rather than enhanced myofiber alignment. Collectively, these studies show that changes in local tissue geometry can exert both direct structural and indirect myogenic effects on the functional output of engineered muscle. Different hydrogel formulations and pore geometries will be explored in the future to further augment contractile function of engineered muscle networks and promote their use for basic structure-function studies in vitro and, eventually, for efficient muscle repair in vivo.
Introduction
Tissue engineering of skeletal muscle constructs holds promise for the treatment of significant loss in muscle function (due to trauma, ablation, or disease) that cannot be fully restored via the intrinsic ability of muscle to self-repair.1,2 Engineered muscle constructs can also be used as three-dimensional (3D) tissue models that complement the conventional two-dimensional (2D) cell cultures and animal models in studying the processes of muscle development, regeneration, and disease.3,4 While several important studies.5–7 have focused on developing methods to improve survival, neovascularization, and functional integration of engineered muscle tissues in vivo, the force generating capacity of skeletal muscle constructs still remains significantly inferior to that of the native muscle.8,9 Thus, methods to enhance the contractile function of engineered muscle constructs are expected to promote the design of efficient muscle tissue engineering therapies and better 3D tissue models for basic studies of muscle biology and function. Currently, the successful in vitro engineering of highly functional muscle tissues is hampered by: (1) the lack of techniques to reproducibly induce uniform 3D alignment of densely-packed, viable muscle cells within a relatively large tissue volume, and (2) the limited understanding of the structure-function relationships and mechanisms of myogenic differentiation in developing muscle tissues.
Native skeletal muscle is composed of highly aligned, densely packed, and cross-striated myofibers, with each myofiber containing force-generating myofibrils that act as units of muscle contraction.10 Muscle architecture, that is, the spatial arrangement of muscle fibers relative to the axis of force generation, is a critical determinant of muscle contractile function.11 Consequently, changes in muscle tissue architecture in physiological (e.g., fiber rotation during shortening of pennate muscle12) or pathological (e.g., muscle disarray and fibrosis in dystrophic muscle13) conditions can significantly affect generation of muscle contractile force. To recreate native muscle architecture in vitro, researchers have previously applied different topographical,14 chemical,15–17 or physical18 cues to align cells in 2D culture, or utilized cylindrically shaped cell-laden hydrogels or self-assembled organoids formed under static uniaxial tension to align muscle cells in 3D culture.4,8,9,19 However, no current tissue engineering methodologies allow for precise and reproducible control of local 3D myofiber alignment that would enable systematic studies of structure-function relationship encountered in healthy, diseased, or developing skeletal muscle.
To address some of these challenges, we have previously established a hydrogel molding approach to fabricate “engineered skeletal muscle networks” with controllable size and geometry.20,21 The use of computer-assisted design to systematically alter tissue mold dimensions and introduce tissue pores with defined geometry, distribution, and direction in this system allowed us to precisely control engineered tissue thickness, shape, and local and global alignment of differentiated muscle fibers. Specifically, by fabricating elastomeric tissue molds with parallel hexagonal posts arranged in staggered fashion, we were able to create fibrin gel-based muscle networks containing: (1) elliptical pores with length that was directly determined by the post length (PL) and width that was determined by both the post width and degree of cell-mediated gel compaction, and (2) dense, cross-striated myofibers that were in average aligned along the long axis of the elliptical pores.20 Although tissue pores are necessary to improve the metabolic supply to the embedded muscle cells and generate thicker viable tissues (up to 400 μm), increased number and size of the pores will increase the volume fraction of the void space in engineered muscle (i.e., tissue porosity) and, consequently, may exert negative effect on the generated contractile force. On the other hand, selective increase in the pore length, although expected to increase the porosity of the tissue, could also improve force generation by simultaneously increasing the degree of myofiber alignment. We therefore hypothesized that muscle networks with more elongated pores created by the longer posts would generate larger contractile forces despite increased tissue porosity.
To test this hypothesis, tissue molds with three different PLs and otherwise identical dimensions were used to fabricate skeletal muscle networks. After 2 weeks of culture, the network pore dimensions, tissue volume, myonuclear number, myofiber alignment, and contractile force generation (including twitch and tetanus amplitudes, twitch force kinetics, and tetanus-to-twitch ratio) were systematically assessed. We found that different pore lengths in engineered muscle networks affected their force generation capacity by altering both the alignment and total number of myofibers. On the other hand, resulting alterations in network geometry did not affect “force per myonucleus,” an approximate measure of the single myofiber force production and transmission. Overall, our results show that the degree of local cellular alignment during 3D muscle assembly is a direct determinant of myogenic differentiation and contractile function in engineered muscle.
Materials and Methods
Isolation of neonatal rat skeletal myoblasts
As previously described,20 muscle tissue from the lower hind limbs of 2–3-day-old Sprague-Dawley rats was digested with 1 mg/mL collagenase (Worthington) in Wyles solution (137 mM NaCl, 5 mM KCl, 21 mM HEPES, 0.7 mM Na2HPO4, 100 mM glucose, and 0.1 mg/mL BSA) for 2 h at 37°C. Isolated cells were resuspended in growth medium (Dulbecco's modified Eagle's medium, 10% [vol/vol] fetal bovine serum, 50 unit/mL penicillin G, 50 μg/mL streptomycin, 5 μg/mL gentamicin), preplated for 2 h at 37°C to reduce the fraction of faster-adhering fibroblasts, and then mixed with hydrogel solution to form engineered muscle networks. All experiments involving animals conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, Revised 1996) and animal protocols approved by Duke Animal Care and Use Committee.
Mesoscopic hydrogel molding
Tissue mold fabrication
Polydimethylsiloxane (PDMS) tissue molds containing an array of staggered hexagonal posts with specified dimensions were fabricated using the high aspect-ratio photolithography technique as previously described.20 Briefly, a 1.5 mm thick layer of SU-8 100 photoresist (Microchem) was coated on a 3″ silicon wafer and exposed to UV light through a photomask containing a pattern of hexagonal holes corresponding to posts with different lengths (0.6, 1.2, or 1.8 mm) and otherwise identical dimensions (Fig. 1A1–A3). Exposed wafers were etched with PGMEA solution (Sigma) to remove uncross-linked photoresist and silanized overnight. PDMS (Dow Corning) solution was then double-cast off the patterned silicon wafers to yield final tissue molds.
FIG. 1.
Fabrication of engineered muscle networks with controllable pore elongation (A1–A3) Photomasks used to create arrays of staggered hexagonal posts of different length. Post length, (A1) PL=0.6 mm; (A2) PL=1.2 mm; (A3) PL=1.8 mm. Post width, PW=0.2 mm, horizontal post spacing hPS=0.5 mm, vertical post spacing vPS=0.3 mm are the same in A1-3. Scale bars, 1 mm (200 μm in magnified regions). (B1–B3) Master silicon templates containing posts of different lengths (B1, PL=0.6 mm; B2, PL=1.2 mm; B3=1.8 mm). Scale bars, 2 mm (1 mm in magnified regions). (C1–C3) Two-week-old skeletal muscle networks attached to nylon (Cerex®) frames and pinned inside the polydimethylsiloxane molds obtained by double-casting off the master silicon templates in B1–B3. Networks are composed of single tissue bundles shown in light micrographs. Scale bars, 2 mm in left panels, 200 μm in right panels. Color images available online at www.liebertonline.com/tea
Cell seeding and tissue cultivation
Before cell seeding, PDMS molds were plasma-etched, sterilized, and coated with 0.2% (w/v) pluronic (Invitrogen) to prevent hydrogel adhesion. A nylon (Cerex®) frame was pinned within the mold as a tissue anchor to provide mechanical support and facilitate the handling and transfer of muscle networks. A mixture of freshly isolated neonatal rat skeletal myoblasts (NRSKMs; 10 million/mL), 2×growth medium, bovine fibrinogen (4 mg/mL) (Sigma), Matrigel (10% (v/v); BD) and thrombin (0.2 unit/mg fibrinogen) (Sigma) was injected into the PDMS mold, polymerized at 37°C for 45–60 min, and cultured in static conditions for 7–21 days (i.e., 1–3 weeks), first 4 days in growth medium and subsequent 3–17 days in differentiation medium containing 3% horse serum to promote the fusion and differentiation of myoblasts into myofibers. Culture medium was supplemented with 1 mg/mL aminocaproic acid (Sigma) to prevent degradation of fibrin.
Morphometric assessment of engineered muscle networks
Measurement of tissue thickness
3D video-rate optical coherence tomography (OCT)22 was used to noninvasively acquire volume images of muscle networks on culture day 14. Tissue thickness was measured by averaging multiple OCT cross-sections of the sample using ImageJ, as previously described.20
Measurement of pore dimensions, porosity, and tissue bundle width
Pore dimensions (pore length pl and pore width pw), porosity (%P), and tissue bundle width (bw) were measured from composite confocal microscopic F-actin images (2.5×magnification) of the entire muscle network using ImageJ software. Pore elongation was defined as pl/pw. Porosity was determined by calculating the fraction of acellular area in the network. In addition, the initial pore dimensions (pl0 and pw0), pore elongation (pl0/pw0), bundle width (bw0), and porosity before gel compaction (%P0) were analogously derived from the corresponding photomask patterns.
Estimation of tissue volume
The tissue volume of a muscle network (Vn) was estimated as (tissue area×average thickness), that is, (total area×porosity×average thickness).
Quantitative immunofluorescence assessment
Immunostaining procedure
Muscle networks were fixed with 4% formaldehyde for 2 h at 4°C, permeabilized with 0.1% Triton-X, blocked with 20% chicken serum in 1% bovine serum albumin, and incubated with primary antibodies (rabbit anti-myogenin; Santa Cruz) overnight at 4°C. Secondary antibodies (Alexa488 and Alexa594, Invitrogen) were then applied together with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining and Alexa488/594-conjugated phalloidin (Invitrogen) for F-actin staining for 2–3 h at room temperature.20 Images were acquired using a Zeiss confocal microscope (LSM510).
Quantification of Cell Alignment
The degree of cell alignment in muscle networks was quantified using an image intensity gradient algorithm23 from confocal F-actin images acquired at 5× or 10×magnification, as previously described.20 Briefly, local cell orientation vectors were calculated in image subregions and the deviation of the vector angles from the mean angle of all the vectors (MeanAngDev) was calculated using circular statistics to obtain a measure of the cell alignment (with 0° and 45° deviations respectively representing perfect and random alignments). The degree of cell alignment was defined as 1–MeanAngDev/45° (0: random orientation; 1: perfect alignment). Global cell alignment of the entire network was estimated by quantifying and averaging the degree of alignment in three repeating rectangular subunits, each subunit containing one central elliptical pore.20 Local cell alignment was determined in both the central portion of “tissue bundle” regions between the elliptical pores and the “node” regions that connected tissue bundles.
Quantification of myogenin index
The myogenin index was measured as previously described20 by dividing the number of myogenin-positive nuclei with the total number of DAPI-labeled nuclei in 20×confocal microscope images acquired at three different tissue depths (50, 100, and 150 μm) in each of three bundle and three node regions per network and four networks per group using MetaMorph (Molecular Devices).
DNA content quantification
Muscle networks were rapidly frozen in liquid nitrogen. DNA was isolated from each network using a DNeasy blood and tissue kit (Qiagen) and eluted in 200 μL AE buffer (10 mM Tris-Cl, 0.5 mM ethylenediaminetetraacetic acid, pH 9.0). The concentration of the isolated DNA was measured using a spectrometer (Nanodrop 1000) to obtain the total DNA content of a muscle network.
Isometric contractile force measurement
On culture day 14, muscle networks were removed from PDMS molds, transferred into a temperature-controlled chamber, and immersed in warm, non-oxygenated culture medium (36°C±1°C) that contained 1.8 mM Ca2+. The networks were separated from the surrounding nylon frame on two opposite sides and the frame was cut to allow tissue contraction. Of the other two perpendicular sides, one side was fixed to the chamber and the other was attached to a floating PDMS holder connected to a sensitive force transducer (provided by Dr. Robert Dennis at UNC-Chapel Hill). After 10 min equilibration in warm medium, the length of muscle networks was set to the cultivation length (L0=6 mm). A single electrical pulse (amplitude: 3.6 V/cm; duration: 5 ms) was applied by a pair of platinum electrodes to elicit isometric muscle contraction (twitch). Parameters of a single twitch including amplitude (At), the time-to-peak twitch (TPT, from the onset of electrical stimulus to the time of peak twitch) and half relaxation time (RT1/2, from the time of peak twitch to 50% recovery) were derived from the force traces, as previously described.24 The network was then stimulated every 5 min by a 1 s long pulse train with increasing frequency (5, 10, 20, 40, and 60 Hz) until tetanus was reached. Parameters of tetanus including peak amplitude (AT) and the tetanus-to-twitch ratio (TtR=AT/At) were calculated from the force traces. At the end of each experiment, the twitch amplitude was reassessed and compared to the initially measured value to ensure that experimental procedure had no adverse effect on the tissue function.
Statistical analysis
Data are expressed as mean±standard deviation. Statistical significance was determined by student t test between two groups and one-way analysis of variance (ANOVA) (with Tukey post hoc test) among three and more groups. Paired (student t-test) or repeated measure (ANOVA) comparison among different groups was conducted for data acquired from independent cell isolations. Differences were considered to be significant when p<0.05.
Results
Engineered muscle networks with controllable pore elongation
Skeletal muscle networks with different pore elongation were formed by casting a mixture of NRSKMs, fibrin gel, and Matrigel in PDMS molds containing posts of three distinct lengths. The exact lengths of microfabricated posts were 0.62±0.02 mm (Fig. 1B1), 1.19±0.03 mm, (Fig. 1B2) and 1.81±0.02 mm (Fig. 1B3). After 2 weeks of culture, the formed muscle tissue networks made using longer posts appeared to have more elongated elliptical pores and thinner tissue bundles surrounding the pores (Fig. 1C1–C3). Quantitatively, the PLs of 0.6, 1.2, and 1.8 mm yielded pore lengths (pls) of 0.59±0.01, 1.21±0.01, and 1.84±0.02 mm, respectively (n=8 per group), demonstrating that the pore length could be precisely controlled by altering the length of posts that created them. Longer posts also yielded wider pores and narrower (more compacted) tissue bundles (Supplementary Fig. S1B; Supplementary Data are available online at www.liebertonline.com/tea). The elongation (length-to-width ratio) of elliptical pores significantly increased from 2.1±0.2, to 2.9±0.2 and 3.3±0.2, for PLs of 0.6, 1.2, and 1.8 mm, respectively (Supplementary Fig. S1B inset). Increase in PL also resulted in a significant increase in tissue porosity from 13.9%±0.9%, to 28.4%±3.5%, and 42.0%±1.8%, for PL=0.6, 1.2, and 1.8 mm, respectively (n=5 per group, Supplementary Fig. S1C). Compared with the initial porosities estimated by the ratio of post area (white area in the photomask, Fig. 1A1–A3) to the total mold area (i.e., 10.7%, 14.7%, and 17%, for PL=0.6, 1.2, and 1.8 mm, respectively), longer posts led to a higher fold increase of porosity due to the cell-mediated gel compaction (1.3±0.1, 1.9±0.2, and 2.5±0.1-fold increase, for PL=0.6, 1.2, and 1.8 mm, respectively).
Simultaneously, tissue thickness measured by OCT was comparable in skeletal muscle networks made with different PLs, amounting in average to 232±5 μm (n=3 per group). Total tissue volumes estimated as tissue area×thickness decreased from 9.51±0.62 to 8.46±0.61 and 6.72±0.42 mm3 for PL=0.6, 1.2, and 1.8 mm, respectively (n=5 per group, Supplementary Fig. S1D). Compared with the initial cell/gel volumes of 55.85, 53.56, and 51.88 mm3 (for PL=0.6, 1.2, and 1.8 mm, respectively) injected to fill the tissue molds, the final tissue volumes significantly decreased with the increase in PL by 83.0%±1.1%, 84.2%±1.1%, and 87.1%±0.8%, respectively, showing that the longer posts also yielded a higher degree of cell-mediated gel compaction.
Effect of pore elongation on global and local myofiber alignment
Myofiber alignment, as one of the critical determinants of the muscle force generation, was expected to differ in networks with different PLs. As the PL increased from 0.6 to 1.2, and 1.8 mm, the degree of global myofiber alignment estimated from F-actin confocal images (Fig. 2A, B1) significantly increased from 0.31±0.03 to 0.51±0.03 and 0.58±0.03, respectively (Fig. 2C1). Simultaneously, the local myofiber alignment in tissue bundles (Fig. 2B2) increased from 0.47±0.08 to 0.74±0.05 and 0.80±0.03 and was significantly higher compared with the global alignment (Fig. 2C2). In contrast, the degree of myofiber alignment in node regions (Fig. 2B3) was unaffected by changes in PL and was smaller compared to that of the tissue bundles and the global alignment (Fig. 2C2).
FIG. 2.
Quantification of cell alignment in engineered muscle networks with different pore elongations (A) Composite confocal image of an F-actin stained tissue network showing repetitive subunits (yellow dashed lines) used for the alignment analysis. Scale bar, 1 mm. (B1) Map of myofiber orientation vectors in a representative subunit used to derive global myofiber alignment. Myofiber orientation vectors were determined for each 36×36 μm2 square pixel (blue square). (B2, B3) Orientation vector maps in bundle (B2) and node (cyan square in B3) regions used to derive the local myofiber alignments. Rectangular inset (yellow frame) shows 15 square pixels with corresponding myofiber orientation vectors. Scale bars, 100 μm. (C1,C2) The degrees of global (C1) and local (C2) alignment quantified in muscle networks made using different PLs. *Significantly different from the other two groups. Color images available online at www.liebertonline.com/tea
Effect of pore elongation on total myonuclear number
Beside the degree of myofiber alignment, the total number of myofibers (i.e., muscle contractile units) was another factor that could independently affect force generation capacity of the muscle networks. Since no methods exist to accurately count all myofibers in 3D muscle constructs, we assessed the total myofiber number indirectly by calculating the total number of myofiber nuclei (myonuclei) per network. First, the total number of all nuclei in the muscle network (Ntotal) was obtained by dividing the total DNA content of the network (DNAtotal) by the DNA content per nucleus (DNAnucleus). The DNAnucleus was derived using a standard curve of the DNA contents from known numbers of freshly isolated, single-nucleated NRSKMs, and amounted to 2.2±0.5 pg/nucleus, similar to the other reports for primary skeletal muscle cells.25 From these measurements, the obtained values for average DNAtotal of 2.49±0.59, 2.86±0.59, and 2.44±0.60 μg and corresponding Ntotal of 1.13±0.27, 1.30±0.27, and 1.11±0.27 million (for PL of 0.6, 1.2, and 1.8 mm, respectively) were found to be similar among the three groups.
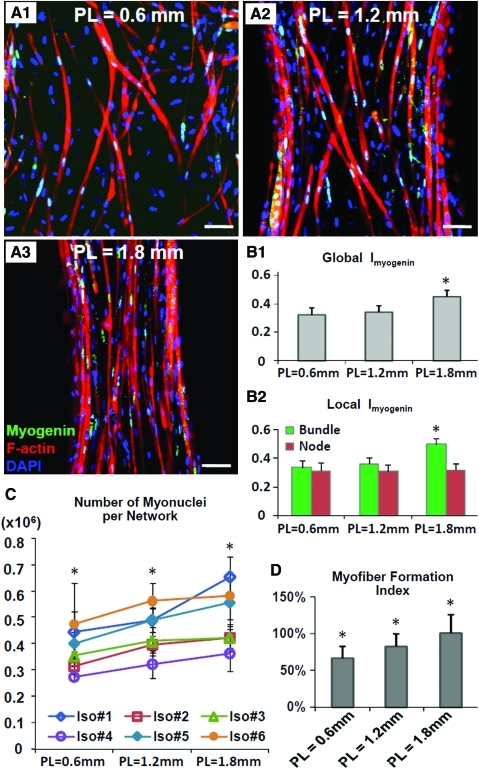
Myonuclei were identified as myogenin-positive nuclei, which both in our previous20 and in current study were found to be primarily localized in multinucleated myofibers (Fig. 3A1–A3). The ratio of myogenin-positive versus total (DAPI labeled) nuclei, termed myogenin index (Imyogenin) was used to estimate the fraction of myonuclei in the muscle networks and was found to be significantly higher in PL=1.8 mm group compared to the other two groups (32.7%±5.0%, 34.2%±4.6%, and 44.9%±4.4% for PL of 0.6, 1.2, and 1.8 mm, respectively, Fig. 3B1). This increase in global Imyogenin in the networks with longer pores resulted from a selective Imyogenin increase in the bundle regions rather than the node regions (Fig. 3B2) combined with an increase in the percent of network area occupied by the bundles (55%±2%, 61%±1%, and 72%±1% for PL of 0.6, 1.2, and 1.8 mm, respectively). The total myonuclei number per network, Nmyo-n, was obtained as the product of global Imyogenin and Ntotal in individual networks (six independent cell isolations with 18 networks per group) and was found to significantly increase for longer PLs (Nmyo-n= 0.37±0.09, 0.44±0.09, 0.50±0.12 million for PL of 0.6, 1.2, and 1.8 mm, respectively, Fig. 3C). Finally, to assess overall efficiency of myogenic differentiation in engineered muscle networks as a function of pore length, we calculated “myofiber formation index” by dividing total myonuclei number in 2-week-old muscle networks with the total initial number of seeded cells (calculated by multiplying cell seeding density by the initial network volume, Supplementary Fig. S1D). We found that the myofiber formation index significantly increased with the increase in PL (66.3%±15.8%, 82.4%±17.4%, and 100%±25% for PL of 0.6, 1.2, and 1.8 mm, respectively, Fig. 3D).
FIG. 3.
Estimation of the total myonuclear number and efficiency of myofiber formation in engineered muscle networks with different pore elongations (A1–A3) Co-staining of myogenin and 4′,6-diamidino-2-phenylindole (DAPI) shows that virtually all of myogenin-positive nuclei reside in F-actin labeled myotubes. The density and alignment of myotubes are higher in networks made using longer posts. Scale bar, 50 μm. (B1,B2) Comparison of Imyogenin (the ratio of myogenin-positive/total nuclei) in networks with different pore elongations shown averaged for the entire network (B1) and specifically in bundle and node regions (B2). (C) Numbers of myogenin-positive myonuclei per network (Nmyo-n) assessed in six independent cell isolations. Each data point represents average from three networks. (D) Comparison of myofiber formation efficiency in networks with different elongations with each bar representing the average from 18 networks from six isolations. *Significantly different from the two other groups. Color images available online at www.liebertonline.com/tea
Effect of pore elongation on contractile force generation of muscle networks
Our morphometric measurements revealed that both the myofiber alignment and myonuclear number were increased in muscle networks with more elongated pores. On the other hand, changes in pore elongation did not affect the formation of muscle sarcomeres, with all networks exhibiting a high percentage (>95%) of cross-striated myofibers (Supplementary Fig. S2). To examine how the contractile function of engineered muscle networks was affected by their structural differences, the twitch amplitudes of isometric contractile force in 1-, 2-, and 3-week-old networks, termed “network force” (Fn), were measured along the long axis of elliptical pores (Fig. 4A). It was found that Fn was increased by 3.0±0.4-fold in 2-week compared with 1-week-old muscle networks, but was not further changed in 3-week-old networks (Supplementary Fig. S3). Two-week-culture period was thus selected for all further experiments. The Fn values in 2-week-old networks were found to increase with increase in PL, amounting to 0.79±0.13, 1.02±0.27, and 1.22±0.22 mN for PL of 0.6, 1.2, and 1.8 mm, respectively (repeated measure ANOVA with Tukey's post hoc test was used to analyze three networks per group in each of six independent isolations, Fig. 4B). On the other hand, changes in pore elongation had no effect on twitch kinetic parameters (TPT and RT1/2) and exerted only a small effect on tetanus-to-twitch ratio (TtR=1.91±0.14, 1.83±0.16, and 1.75±0.15 for PL of 0.6, 1.2, and 1.8 mm, respectively, Fig. 4C).
FIG. 4.
Contractile force generation in engineered muscle networks with different pore elongations (A) Force measurement setup with a muscle network mounted in the chamber and connected to the force transducer. The two sides of the nylon frame perpendicular to the force measurement direction are cut in half. Generated contractile force was measured along the long axis of elliptical pores. Scale bar, 2 mm. (B) Twitch amplitude of network force (Fn) assessed in six independent cell isolations. Each data point represents average from three networks. *Significantly different from the two other groups. (C) Comparison of twitch-to-tetanus ratio (TtR), time to peak twitch (TPT), and half-relaxation time (RT1/2) in networks with different pore elongations with each bar representing the average from 18 networks from six isolations. #Significantly different from PL=1.8 mm group. Color images available online at www.liebertonline.com/tea
Effect of pore elongation on force per myonucleus
We further reasoned that in addition to the increased myofiber alignment and number, higher contractile force in networks with longer pores could be also caused by the structural and functional changes at the single myofiber level, including the increased force production of individual myofibers (e.g., through upregulation of contractile proteins, and/or maturation of excitation-contraction coupling) and/or enhanced force transmission from a myofiber to extracellular matrix (e.g., through upregulation of cell adhesion proteins). To test for this possibility, we determined “force per myonucleus” (fmyo), defined as the average force generated by each myofiber nucleus independent of the number and alignment of myofibers. Specifically, the muscle network was divided into equal volume elements with dimensions of 36 μm×36 μm×average tissue thickness (Fig. 5A1, A2). “Element force” (fe) was defined as the amplitude of force along average myofiber direction within a volume element (Fig. 5A3). “Force per myonucleus” (fmyo) was then calculated by dividing fe with the estimated number of myonuclei in a volume element (Nmyo-e), as follows.
FIG. 5.
Force per myonucleus (fmyo) in engineered muscle networks with different pore elongations (A1–A3) Derivation of “element force” (fe). (A1) The “network force” (Fn) is measured along the long axis of elliptical pores. The red dashed square denotes a repeating subunit in the network shown in A2. Scale bar, 2 mm. (A2) Force generated by repeating subunit is Fs. Yellow squared region is magnified in A3. (A3) In individual volume elements, the orientation vectors (blue lines) indicate the average myofiber directions. The “element force” (fe) is defined along the corresponding orientation vector and its projection angle on Fn is α. Scale bar, 50 μm. (B) Element force assessed in six independent cell isolations. Each data point represents average from three networks. *Significantly different from the two other groups. (C) Force per myonucleus assessed in six independent cell isolations. Each data point represents average from three networks. No significant difference was found among three groups. Color images available online at www.liebertonline.com/tea
To estimate the element force, fe, the networks were divided into repeating rectangular subunits that were assumed to have approximately identical myofiber orientations and contribute the same force, Fs, to the total measured force Fn (Fig. 5A1, A2). The fe was then derived from the following equation:
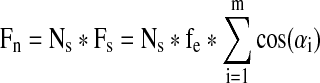 |
(1) |
where αi is the projection angle of fe on the direction of Fn (Fig. 5A3), Ns is the total number of subunits in the network, and m is the total number of volume elements in a subunit. The projection angles αi were obtained from the orientation maps of F-actin labeled myofibers used to determine local and global myofiber alignment (Fig. 2). The average fe obtained in six independent cell isolations (from three networks per group in each isolation) were found to significantly increase with the increase in PL from 34.2±5.5 to 43.7±11.7, and 63.1±11.5 nN for PL=0.6, 1.2, and 1.8 mm, respectively (Fig. 5B). The number of nuclei in the volume element, Nmyo-e, was then calculated by dividing the total number of myonuclei (Nmyo-n) by the number of volume elements in the muscle network (Ne, obtained by dividing the network volume with that of the volume element). Finally, the average force per myonucleus calculated as fmyo=fe/Nmyo-e was found to be comparable in the three groups, amounting to 3.15±0.95, 2.91±1.27, and 3.00±1.06 nN per myonucleus for PL=0.6, 1.2, and 1.8 mm, respectively (Fig. 5C). This result showed that the larger contractile force measured in muscle networks with longer pores was a result of the increase in myofiber alignment and the number of myonuclei per network, with no significant roles played by potential changes in the cellular force generation and/or transmission.
To further estimate the respective contributions of myofiber alignment and myonuclei number to the contractile force generated from the muscle networks (Fn), the Equation 1 was reformulated as follows:
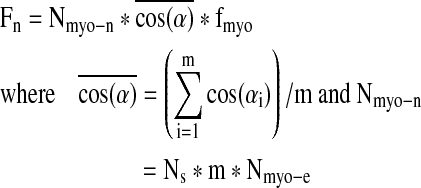 |
(2) |
The (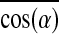 ) represented the contribution of global myofiber alignment to the measured force Fn and amounted to 0.78±0.03, 0.89±0.01, and 0.91±0.01 for PL=0.6 mm, 1.2 mm, and 1.8 mm, respectively. Since fmyo did not significantly change with PL, fractional changes in Fn (i.e., Δ Fn/Fn) between different PLs could be attributed to corresponding fractional changes in the myofiber number Nmyo-n (i.e., Δ Nmyo-n/Nmyo-n) and alignment
) represented the contribution of global myofiber alignment to the measured force Fn and amounted to 0.78±0.03, 0.89±0.01, and 0.91±0.01 for PL=0.6 mm, 1.2 mm, and 1.8 mm, respectively. Since fmyo did not significantly change with PL, fractional changes in Fn (i.e., Δ Fn/Fn) between different PLs could be attributed to corresponding fractional changes in the myofiber number Nmyo-n (i.e., Δ Nmyo-n/Nmyo-n) and alignment 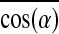 (i.e.,
(i.e.,  ). Specifically, from PL=0.6 mm to 1.2 mm, the 29.3% increase in Fn was synergistically caused by 19.9% increase in Nmyo-n and 14.1% increase in
). Specifically, from PL=0.6 mm to 1.2 mm, the 29.3% increase in Fn was synergistically caused by 19.9% increase in Nmyo-n and 14.1% increase in 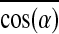 , while from PL=1.2 to 1.8 mm, the 18.5% increase in Fn was synergistically caused by 12.3% increase in Nmyo-n and 2.4% increase in
, while from PL=1.2 to 1.8 mm, the 18.5% increase in Fn was synergistically caused by 12.3% increase in Nmyo-n and 2.4% increase in 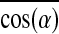 , suggesting a higher relative contribution of the increased myofiber number versus alignment to the measured force increase in networks with longer pores.
, suggesting a higher relative contribution of the increased myofiber number versus alignment to the measured force increase in networks with longer pores.
Discussion
We have previously described a mesoscopic hydrogel molding approach for engineering of porous skeletal muscle tissues with thickness, size, pore dimensions, and local and global myofiber alignment that could be precisely and reproducibly controlled by use of a computer-assisted microfabrication process.20 In the current study, we presented a detailed structure-function analysis of the roles that changes in tissue pore geometry play in the engineered muscle morphology and generation of contractile force. It was found that the use of longer PDMS posts for muscle network fabrication yielded: (1) larger and more elongated tissue pores and lower total tissue volume, (2) increased local and global myofiber alignment due to increased static strain between more distant post ends, (3) increased myonuclear volume density due to increased hydrogel compaction, (4) increased total myonuclear number (Nmyo-n) due to increased overall efficiency of myofiber formation, and, importantly, (5) increased amplitude of total generated isometric contractile force (Fn) without any change in average force production at the cellular level (fmyo).
Although the engineered muscle networks with more elongated pores had larger tissue porosity (Supplementary Fig. S1C) and a reduced volume (Supplementary Fig. S1D), they generated larger contractile forces (Fn) due to a synergistic increase in total myofiber alignment and myonuclei number. In fact, the major relative contributor to the increased Fn in the networks with longer pores was the increase in the myonuclei number. Previous studies in 2D cultures have shown that unidirectional alignment of skeletal myoblasts by different topographic cues (e.g., microgrooves or electrospun fibers)14–16 or patterned lines of extracellular matrix proteins (e.g., laminin)17 can promote myoblast fusion and differentiation into multinucleated myotubes. Further, studies utilizing 3D cell culture such as collagen or fibrin-based bioartificial muscle bundles have shown that static uniaxial tension imposed on myoblasts can also enhance their fusion into 3D aligned myofibers.4,8,19 In our study, increased pore elongation within the fibrin-based muscle networks yielded enhanced cell-mediated gel compaction and formation of thinner and longer tissue bundles in which both the increased cell alignment and higher uniaxial tension imposed on cells promoted myoblast fusion and formation of aligned and differentiated myofibers which contained myogenin-positive nuclei. Interestingly, since the myofiber alignment in tissue bundles was significantly increased between PL=0.6 and 1.2 mm, and only slightly increased between PL=1.2 and 1.8 mm (Fig. 2C2), the resulting increase in myofiber formation efficiency between PL=0.6 and 1.2 mm networks was likely caused by both the enhanced cell alignment and tissue tension, while the further increase in myofiber formation between PL=1.2 and 1.8 mm networks mainly resulted from the increased uniaxial tension rather than cell alignment. In general, our finding that aligned tissue structure in engineered skeletal muscle yields more efficient fusion and differentiation of myoblasts is conceptually similar to previous studies in engineered cardiac muscle that showed beneficial effects of aligned tissue structure on the formation of functional junctions between cardiomyocytes.26
Although the use of PDMS molds with PL >1.8 mm may further enhance the efficiency of myofiber formation (through higher strains imposed on cells) and to a less extent myofiber alignment, these beneficial effects on the total force generation would be countered by the resulting increase in tissue porosity and decrease in tissue volume, and the decrease in bundle width which may also compromise the mechanical integrity of the network. On the other hand, our recent study24 has shown that variations of hydrogel matrix composition in engineered muscle bundles, and in particular, the increase in Matrigel concentration, significantly reduced the degree of cell-mediated gel compaction, while simultaneously inducing myofiber hypertrophy, prolongation of intracellular Ca2+ transients, and significant increase in generated contractile force. Thus, one potential strategy to further enhance force generating capacity of engineered muscle networks would be to combine an increase in PL with optimization of hydrogel composition, which together could yield: (1) enhanced efficiency of myofiber formation through increased strain in bundle regions, (2) reduced degree of cell-mediated gel compaction with preserved tissue volume and bundle width, and (3) increased force production at the cellular level (fmyo) due to myofiber hypertrophy and enhanced intracellular Ca2+ handling. In addition, optimization of hydrogel stiffness27 through use of different cross-linking agents28 or techniques, such as ruthenium-catalyzed photo cross-linking,29 may additionally enhance efficiency of myoblast fusion and differentiation, and further enhance the contractile function of engineered muscle networks.
While this study explored the roles of engineered muscle geometry in contractile force production, a number of additional factors will need to be considered to eventually enable the translation of this or any other skeletal muscle tissue engineering technique into clinical practice. For example, while our engineered muscle networks show evidence for the spontaneous formation of postsynaptic and vascular structures (Supplementary Fig. S4), further optimization of these processes in vitro will be required to allow the efficient survival and functional integration of tissue constructs in the host neuromuscular system. The use of angiogenic and/or neurotrophic factors,7,30 electrical and mechanical stimulation,9,16,31 and coculture with vascular and/or neuronal cells6,32,33 are just some of the potential strategies to achieve this goal. Development of improved immunosuppression regimes34 and cell sources with robust regenerative capacity (e.g., from human pluripotent stem cells35) are some of the other important milestones toward the therapeutic use of tissue engineering to restore lost muscle function.
Conclusions
In this study, we showed that local geometrical conditions during 3D assembly of engineered skeletal muscle can influence the formation and alignment of differentiated muscle fibers, thus significantly affecting muscle contractile function. In particular, longer pores in engineered muscle networks acted to reduce the total tissue volume, but enhanced the generation of contractile force by increasing both the global myofiber alignment along the direction of force measurement and the local myoblast alignment and imposed strains which together enhanced the efficiency of myofiber formation. Future optimization of pore geometry and hydrogel matrix composition and stiffness is expected to further augment the force generating capacity of engineered muscle networks, thereby establishing their use as a model system for basic studies of muscle function, and potentially, as an in vivo graft for muscle tissue repair.
Supplementary Material
Acknowledgments
We thank Dr. Robert Dennis for providing the force transducer and Hanford Hendargo for conducting OCT measurement. This work was supported by Lew's fellowship from the Center for Biomolecular and Tissue Engineering at Duke University to W.B. and NIH grant AR055226 from National Institute of Arthritis and Musculoskeletal and Skin Disease to N.B.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bach A.D. Beier J.P. Stern-Staeter J. Horch R.E. Skeletal muscle tissue engineering. J Cell Mol Med. 2004;8:413. doi: 10.1111/j.1582-4934.2004.tb00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bian W. Bursac N. Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE Eng Med Biol Mag. 2008;27:109. doi: 10.1109/MEMB.2008.928460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das M. Rumsey J.W. Bhargava N. Stancescu M. Hickman J.J. Skeletal muscle tissue engineering: a maturation model promoting long-term survival of myotubes, structural development of the excitation-contraction coupling apparatus and neonatal myosin heavy chain expression. Biomaterials. 2009;30:5392. doi: 10.1016/j.biomaterials.2009.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheema U. Yang S.Y. Mudera V. Goldspink G.G. Brown R.A. 3-D in vitro model of early skeletal muscle development. Cell Motil Cytoskeleton. 2003;54:226. doi: 10.1002/cm.10095. [DOI] [PubMed] [Google Scholar]
- 5.Rossi C.A. Flaibani M. Blaauw B. Pozzobon M. Figallo E. Reggiani C., et al. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011;25:2296. doi: 10.1096/fj.10-174755. [DOI] [PubMed] [Google Scholar]
- 6.Koffler J. Kaufman-Francis K. Yulia S. Dana E. Daria A.P. Landesberg A., et al. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci U S A. 2011;108:14789. doi: 10.1073/pnas.1017825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borselli C. Storrie H. Benesch-Lee F. Shvartsman D. Cezar C. Lichtman J.W., et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107:3287. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y.C. Dennis R.G. Larkin L. Baar K. Rapid formation of functional muscle in vitro using fibrin gels. J Appl Physiol. 2005;98:706. doi: 10.1152/japplphysiol.00273.2004. [DOI] [PubMed] [Google Scholar]
- 9.Powell C.A. Smiley B.L. Mills J. Vandenburgh H.H. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol. 2002;283:C1557. doi: 10.1152/ajpcell.00595.2001. [DOI] [PubMed] [Google Scholar]
- 10.Saladin K.S. Muscular tissue. In: Watnick M., editor. Anatomy and Physiology: The Unity of Form and Function. 6th. New York: McGraw-Hill; 2010. pp. 402–422. [Google Scholar]
- 11.Lieber R.L. Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Azizi E. Brainerd E.L. Roberts T.J. Variable gearing in pennate muscles. Proc Natl Acad Sci U S A. 2008;105:1745. doi: 10.1073/pnas.0709212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNally E.M. Pytel P. Muscle diseases: the muscular dystrophies. Annu Rev Pathol. 2007;2:87. doi: 10.1146/annurev.pathol.2.010506.091936. [DOI] [PubMed] [Google Scholar]
- 14.Wang P.Y. Yu H.T. Tsai W.B. Modulation of alignment and differentiation of skeletal myoblasts by submicron ridges/grooves surface structure. Biotechnol Bioeng. 2010;106:285. doi: 10.1002/bit.22697. [DOI] [PubMed] [Google Scholar]
- 15.Huang N.F. Patel S. Thakar R.G. Wu J. Hsiao B.S. Chu B., et al. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006;6:537. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- 16.Liao I.C. Liu J.B. Bursac N. Leong K.W. Effect of Electromechanical Stimulation on the Maturation of Myotubes on Aligned Electrospun Fibers. Cell Mol Bioeng. 2008;1:133. doi: 10.1007/s12195-008-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark P. Coles D. Peckham M. Preferential adhesion to and survival on patterned laminin organizes myogenesis in vitro. Exp Cell Res. 1997;230:275. doi: 10.1006/excr.1996.3429. [DOI] [PubMed] [Google Scholar]
- 18.Collinsworth A.M. Torgan C.E. Nagda S.N. Rajalingam R.J. Kraus W.E. Truskey G.A. Orientation and length of mammalian skeletal myocytes in response to a unidirectional stretch. Cell Tissue Res. 2000;302:243. doi: 10.1007/s004410000224. [DOI] [PubMed] [Google Scholar]
- 19.Rhim C. Lowell D.A. Reedy M.C. Slentz D.H. Zhang S.J. Kraus W.E., et al. Morphology and ultrastructure of differentiating three-dimensional mammalian skeletal muscle in a collagen gel. Muscle Nerve. 2007;36:71. doi: 10.1002/mus.20788. [DOI] [PubMed] [Google Scholar]
- 20.Bian W. Bursac N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials. 2009;30:1401. doi: 10.1016/j.biomaterials.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bian W. Liau B. Badie N. Bursac N. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat Protoc. 2009;4:1522. doi: 10.1038/nprot.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yelbuz T.M. Choma M.A. Thrane L. Kirby M.L. Izatt J.A. Optical coherence tomography: a new high-resolution imaging technology to study cardiac development in chick embryos. Circulation. 2002;106:2771. doi: 10.1161/01.cir.0000042672.51054.7b. [DOI] [PubMed] [Google Scholar]
- 23.Karlon W.J. Covell J.W. McCulloch A.D. Hunter J.J. Omens J.H. Automated measurement of myofiber disarray in transgenic mice with ventricular expression of ras. Anat Rec. 1998;252:612. doi: 10.1002/(SICI)1097-0185(199812)252:4<612::AID-AR12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Hinds S. Bian W. Dennis R.G. Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials. 2011;32:3575. doi: 10.1016/j.biomaterials.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayward L.J. Zhu Y.Y. Schwartz R.J. Cellular localization of muscle and nonmuscle actin mRNAs in chicken primary myogenic cultures: the induction of alpha-skeletal actin mRNA is regulated independently of alpha-cardiac actin gene expression. J Cell Biol. 1988;106:2077. doi: 10.1083/jcb.106.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black L.D., 3rd Meyers J.D. Weinbaum J.S. Shvelidze Y.A. Tranquillo R.T. Cell-induced alignment augments twitch force in fibrin gel-based engineered myocardium via gap junction modification. Tissue Eng Part A. 2009;15:3099. doi: 10.1089/ten.tea.2008.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engler A.J. Griffin M.A. Sen S. Bonnemann C.G. Sweeney H.L. Discher D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akpalo E. Larreta-Garde V. Increase of fibrin gel elasticity by enzymes: a kinetic approach. Acta Biomater. 2010;6:396. doi: 10.1016/j.actbio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Syedain Z.H. Bjork J. Sando L. Tranquillo R.T. Controlled compaction with ruthenium-catalyzed photochemical cross-linking of fibrin-based engineered connective tissue. Biomaterials. 2009;30:6695. doi: 10.1016/j.biomaterials.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bezakova G. Helm J.P. Francolini M. Lomo T. Effects of purified recombinant neural and muscle agrin on skeletal muscle fibers in vivo. J Cell Biol. 2001;153:1441. doi: 10.1083/jcb.153.7.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita H. Nedachi T. Kanzaki M. Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp Cell Res. 2007;313:1853. doi: 10.1016/j.yexcr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Bach A.D. Beier J.P. Stark G.B. Expression of Trisk 51, agrin and nicotinic-acetycholine receptor epsilon-subunit during muscle development in a novel three-dimensional muscle-neuronal co-culture system. Cell Tissue Res. 2003;314:263. doi: 10.1007/s00441-003-0757-6. [DOI] [PubMed] [Google Scholar]
- 33.Larkin L.M. Van der Meulen J.H. Dennis R.G. Kennedy J.B. Functional evaluation of nerve-skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim. 2006;42:75. doi: 10.1290/0509064.1. [DOI] [PubMed] [Google Scholar]
- 34.Webber A. Hirose R. Vincenti F. Novel strategies in immunosuppression: issues in perspective. Transplantation. 2011;91:1057. doi: 10.1097/TP.0b013e3182145306. [DOI] [PubMed] [Google Scholar]
- 35.Barberi T. Bradbury M. Dincer Z. Panagiotakos G. Socci N.D. Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.