Abstract
The mechanism of HLA-DM (DM) activity is still unclear. We have shown that DM-mediated peptide release from HLA-DR (DR) is dependent on the presence of exchange peptide. However, DM also promotes a small amount of peptide release in the absence of exchange peptide. Here we show that SDS-PAGE separates purified peptide/DR1 complexes (pDR1) into two conformers whose ratio is peptide Kd-dependent. In the absence of exchange peptide, DM only releases peptide from the slower migrating conformer. Addition of exchange peptide converts the DM-resistant conformer to the slower migrating conformer, which is DM labile. Thus, exchange peptide generates a conformer of pDR1 which constitutes the intermediate for peptide exchange and the substrate for DM activity. The resolution of the intermediate favors the highest affinity peptide. However, once folded into the DM-resistant conformer, even low affinity peptides can be presented in the absence of free peptide, broadening the repertoire available for presentation.
The recognition of pathogen-derived peptides by CD4+ T cells is a crucial event in the initiation of an immune response. These peptides are presented in the context of class II MHC (MHCII) on antigen-presenting cells (APC). Antigen presentation is a multistep process involving the intracellular fragmentation of protein antigens, followed by binding of the derived peptide epitopes to MHCII molecules with the participation of the peptide-editing molecule HLA-DM (DM), and subsequent transport to the APC surface1. The MHCII–peptide–DM interaction is a key step in the selection of epitopes. However, the details of this process are still poorly understood and this impediment is reflected in the difficulty in approaching rational vaccine design or in epitope prediction2.
Using HLA-DR1 as our test system, we have shown that peptide binding is a distributive process in that all peptide residues can synergistically contribute binding energy. We have observed that within a certain range of Kd, peptide binding is highly cooperative. We interpret cooperativity as evidence of the folding process involving both peptide and MHCII that results in a stable complex3,4. More recently, we have observed that: 1) in the absence of an exchange peptide, the peptide-editing factor DM will release prebound peptide very poorly; 2) in the presence of an exchange peptide, DM-promoted release of the prebound peptide does not show cooperativity, indicating that the typical unfolding associated with peptide release does not happen; and 3) if the exchange peptide is of a lower affinity than the prebound peptide, exchange does not take place, even with excess exchange peptide5.
We have proposed that the outcome of epitope selection in the presence of DM may be considered the result of a “compare-exchange” mechanism involving the continuous formation of a metastable intermediate generated by the two peptides, DR and DM. DM would bind this intermediate, release the prebound peptide while putting MHCII molecule in an “exchangeable” conformer whose geometry allows for rebinding of the prebound peptide in the case that the exchange peptide does not succeed in folding into the binding groove5,6. The possibility of such an intermediate was also postulated for peptide exchange in the absence of DM7,8.
An important question in describing DM activity is whether the peptide/MHCII (pMHCII) complex needs to assume a specific conformation to be targeted by DM. Past reports have proposed that the conformation induced by an empty/flexible hydrophobic pocket 1 of DR1 is what DM recognizes9,10. Recent work has shown that DM would bind to DR conformers in which this critical part of the binding site is already vacant11.
Our compare exchange model presupposes that the presence of an exchange peptide is “sensed” by DM. Our observation of a small but measurable peptide release by DM in the absence of exchange peptide together with these previous reports suggests the hypothesis that two forms of the bound complex coexist, with one being the preferential target of DM action. We show here that this is indeed the case. Newly generated pMHCII complexes show two conformers on the basis of gel migration, with the slower migrating conformer being DM-sensitive. The level of the DM-labile conformer is a function of the peptide Kd. DM-labile conformer can be generated from the DM-resistant conformer by addition of an exchange peptide. We propose a more refined model of DM activity and the implications of this model are discussed in the context of epitope selection.
Results
pMHCII complexes are separated in two bands by SDS-PAGE under non-reducing conditions whose ratio is peptide affinity dependent
We have previously shown that in addition to strong DM activity in the presence of an exchange peptide, a small amount of DM activity can be observed in the absence of exchange peptide5. The interaction of peptides with MHCII molecules is cooperative, and we interpreted cooperativity as evidence for peptide binding as flexible process3,4. Flexibility during a ligand/receptor interaction has been indicated as the structural basis for the presence of multiple conformers for the same complex12 and multiple conformers of MHCII have indeed been reported9,13,14. In order to explain the exchange-peptide-independent activity of DM, we hypothesized that: the peptide/MHCII complex assumes different conformers; only one of these conformers constitutes a viable DM target; and if this conformer exists in the absence of an exchange peptide, DM will act on it. The first step in addressing this issue is to study MHCII conformers. We formed pMHCII complexes by incubating DR1 with three different HA306–319-derived substituted peptides of decreasing affinity. The peptides adopted for this analysis and relative Kd values are listed in Table I. The existence of multiple conformers was analyzed in SDS-PAGE under non-reducing conditions15. As shown in Figure 1A, two bands could be identified in each lane by visualizing the fluorescence from the bound peptide, with the slower migrating band (top arrow) showing different intensities. This finding is expected from the literature, and the slower migrating form has been called “floppy” and the faster migrating form “compact”15,16.
Table 1. Dissociation constant values of the peptides used in this study. Peptide affinity for DR1 was measured by equilibrium-based competitive binding assay as indicated in the Methods. Substitutions are marked in red; amino acid position within peptide sequence is indicated according to the P numbering system, assuming the Y308 as P1.
| Name | Peptide Sequence | Kd (nM) |
|---|---|---|
| HA306–319 | PKYVKQNTLKLAT | 73.4 ± 3.8 |
| P2 S | PKYSKQNTLKLAT | 97.2 ± 4 |
| P2,10 SG | PKYSKQNTLKLGT | 373.1 ± 15 |
| P2,7,10 SPG | PKYSDQNTPKLGT | 605.3 ± 40 |
| P2,3,7,10 SDPG | PKYSDQNTPKLGT | 3.13 ± 0.07 E+05 |
Figure 1. In the absence of exchange peptide, DM functions on a pMHCII conformer whose concentration is proportional to peptide Kd.
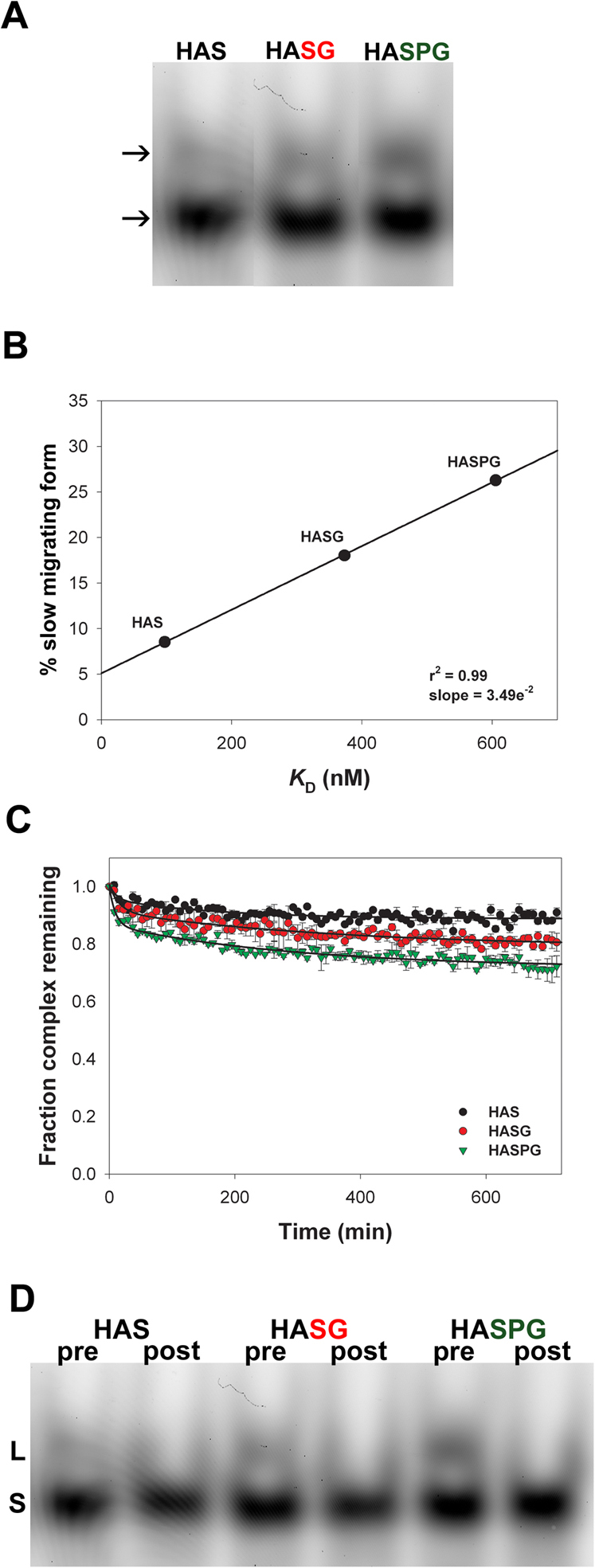
(A) SDS-PAGE analysis of purified peptide/MHCII complexes under non-reducing conditions. FAM-peptide/DR1 complexes were visualized by detecting the amount of fluorescence from the bound peptide. (B) Fraction of slower migrating form measured as the contribution of the upper band to total signal intensity for each lane of the gel in panel A is plotted against peptide Kd. The line indicates the fit to a linear regression. (C) FP measurement of peptide release in the presence of DM and in the absence of exchange peptide for the three complexes of panels A–B. Data are plotted as the fraction of complex remaining relative to t = 0. Reactions were performed in triplicate, and data points represent mean ± SD of three independent experiments. (D) SDS-PAGE visualization of L (labile) and S (stabile) conformers before and after peptide release by DM.
The relation between the fraction of the slower migrating form and the affinity of the peptide is shown in Figure 1B. Even though our analysis is based only on three peptides, the Pearson's coefficient indicates a strong linear correlation between peptide Kd and the amount of the slower migrating conformer, at least for the range of peptide affinity here considered.
DM promotes release of peptides from the slower migrating conformer in the absence of an exchange peptide
To investigate the relation between peptide/DR1 (pDR1) complex conformers and DM-mediated peptide release in the absence of an exchange peptide, we monitored peptide release from 100 nM of purified FAM-labeled pDR1 complexes in the presence of 300 nM of DM and in the absence of an exchange peptide (Figure 1C). The extent of release was associated with the Kd of the complexes. We analyzed pre- and post-DM incubation samples in a non-reducing SDS gel (Figure 1D). Clearly, only the faster migrating conformer was detectable when the DM-reaction reached plateau, regardless of the nature of the peptide. Thus, DM seemed to affect only the slower migrating conformer (Figure 1D). Therefore we identify the slower migrating conformer as DM-labile (L) and the faster as DM-stable (S).
An alternative explanation for the reduced release of peptides from MHCII in the presence of DM and in the absence of exchange peptide might be the formation of an equilibrium between peptide dissociation and re-association. In our experiments, the initial complex concentration is very low, reducing the probability of re-assocation by simple Brownian motion in solution. However, we directly tested this possibility by measuring the stability of the HASG/DR complex at 100 nM in the presence of DM without exchange peptide. Simultaneously, we measured the DM-mediated association of HASG to an equimolar amount of DR at the same concentration. We reasoned that if the reduced release of peptides from MHCII is due to rebinding of freshly dissociated peptides, we should reach the same equilibrium when allowing 100 nM of peptide to bind 100 nM of DR in the presence of DM in an association assay. As shown in Supplementary Fig. S1, and consistent with the off-rate shown in Figure 1C, DM-mediated peptide release in the absence of any unlabeled peptide reaches an equilibrium at 82% of bound peptide. On the contrary, during a loading assay, the fraction of bound peptide at the steady state is about 20%. The significant difference between peptide release and peptide binding at equilibrium indicates that the plateau established during a peptide off rate cannot be explained simply as a cycle of release and rebinding.
Exchange peptide is required for DM to promote release of prebound peptide from the faster migrating conformer
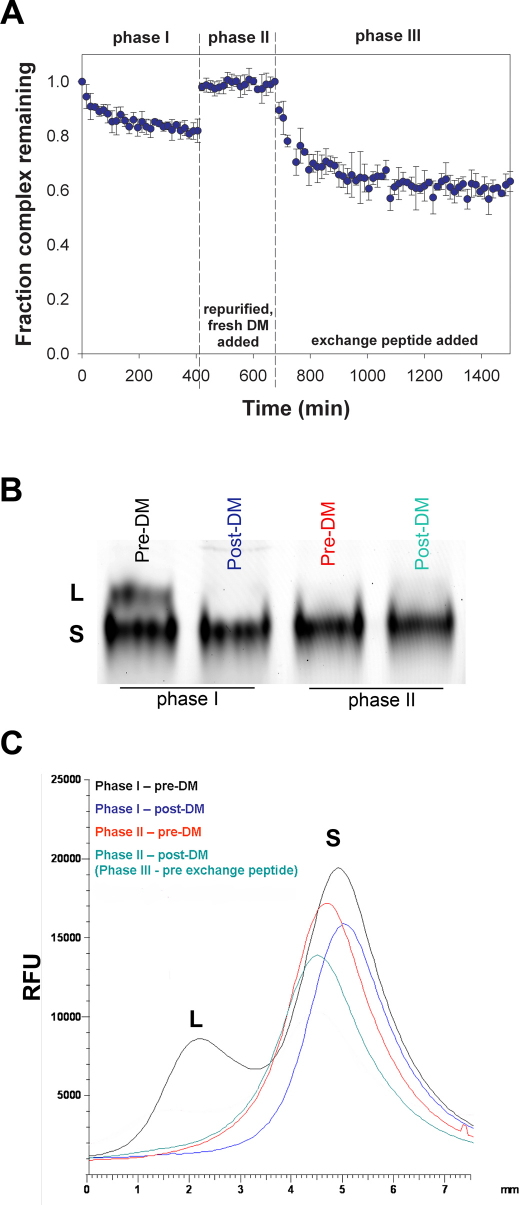
Having shown that DM can act on the L-conformer in the absence of exchange peptide, we wished to investigate the relation between the S conformer, the exchange peptide, and DM. To this aim, we enriched the S conformer of the HASG/DR1 complex by treatment with DM (Figure 2A, phase I). When the initial release had reached a plateau, DM was purified away from the pDR1 complex by anti-FLAG affinity chromatography and unbound peptide by filtration through a centrifugal filter device. A new release reaction was started by adding fresh DM in the absence of any exchange peptide (Figure 2A phase II). No further peptide release was detected, indicating that DM could not act on the S conformer. Non-reducing SDS-gel analysis showed only the S conformer (Figure 2B). This suggests that, at least for the time of our observation, the S-conformer did not spontaneously convert into the L. The scan of the gel shows the disappearance of the L and the stability of S conformers (Figure 2C). As expected, the addition of exchange peptide (unlabeled HASG) to the reaction resulted in the release of prebound peptide (Figure 2A phase III). This result indicates that only in the presence of an exchange peptide is the S conformer a target of DM activity.
Figure 2. DM promotes release of peptides from the S form of the complex only in the presence of an exchange peptide.
(A) FP analysis of peptide release was carried out in three phases. In phase I, purified FAM-HASG/DR1 complexes were co-incubated with 3 fold excess DM and in the absence of exchange peptide until reaction steady state was reached. Then DM was removed on an anti-FLAG column and free peptide by filtration though centrifugal filter device. Re-purified complexes were then co-incubated with DM (phase II) for 4 hours in the absence of exchange peptide, followed by addition of unlabeled HASG in equimolar amount as compared to complex concentration (phase III). Data are plotted as the fraction of complex remaining relative to t = 0. Reactions were performed in triplicate, and data points represent mean ± SD of three independent experiments. (B) – (C) SDS-PAGE gel analysis (B) and relative quantitation (C) of samples at the beginning and end of phase I (lane 1 and 2), and at the beginning and end of phase II (lane 3 and 4). L, DM labile conformer; S, DM-stabile conformer.
Exchange peptide generates a slowly migrating pMHCII conformer
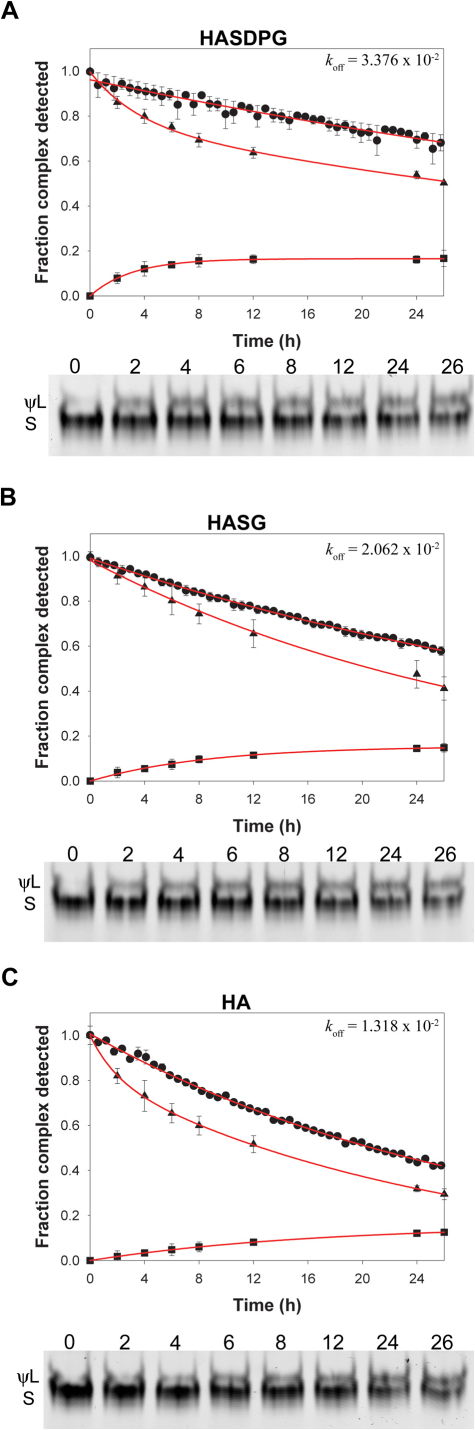
We have shown that the L-conformer is the target of DM in the absence of exchange peptide and that DM requires the presence of an exchange peptide to act on the S-conformer. Thus, we hypothesized that the exchange peptide converts complexes from S to L conformers as the simplest way to combine these two observations. We approached this issue by generating the S conformer of HASG/DR1 by DM treatment, isolating the complex, and incubating with an equimolar concentration of unlabeled exchange peptide in the absence of DM. Three peptides with decreasing affinity (HA, HASG, HASDPG) were used in separate reactions to probe for any correlation between the nature of the exchange peptide and the effect on the complex. The sequence and affinity values of the unlabeled peptides are listed in Table I. In Figure 3, peptide off-rates in the presence of equimolar amount of exchange peptide are shown (the fraction of HASG/DR1 complex remaining over time in the presence of HASDPG (A), HASG (B) and HA (C) is identified with round symbols). As evidenced by the different koff values, the rate of prebound peptide release is a function of the exchange peptide Kd. In the presence of the exchange peptides a weaker band with slower electrophoretic mobility is observed (gels below off-rate plots). This is similar to the L conformer shown in Figures 1 and 2 and will be temporarily referred to as the pseudo-labile (ψL) conformer. Three distinct observations can be made with regard to the release experiment shown in Figure 3: 1) the Kd of the exchange peptide is correlated to the rate of conversion from S conformer (identified with the triangles) to ψL (identified with the squares), as at any given time the fraction of complexes in S form available depends on the relative folding abilities of the two peptides5; 2) the overall loss of band intensity in each lane at t = 26 (ψL+S) normalized for the fluorescence intensity at t = 0, within experimental error and considering the different techniques adopted, is in keeping with the fraction of total complex remaining as the labeled peptide is exchanged (square + triangle = circle), and 3) the S/ψL ratio at t = 26 h is 6:1, 5:1 and 3.5:1 in the reactions containing SDPG, SG and HA respectively.
Figure 3. Presence of exchange peptide promotes conversion from S to ψL conformer.
Dissociation of FAM-HASG peptide from DR1 in the presence of unlabeled HASDPG (panel A), HASG (B) and HA (C). Peptide off-rate measured by FP is indicated with the circles  . Data is plotted as the fraction of complex remaining relative to t = 0. Below the peptide release plot, an SDS-PAGE is shown, monitoring the amount of S conformer of the HASG/DR1 complex over time and conversion to ψL conformer upon incubation with the respective exchange peptide. The quantitation of the S (
. Data is plotted as the fraction of complex remaining relative to t = 0. Below the peptide release plot, an SDS-PAGE is shown, monitoring the amount of S conformer of the HASG/DR1 complex over time and conversion to ψL conformer upon incubation with the respective exchange peptide. The quantitation of the S ( ) and ψL (
) and ψL ( ) conformers is reported in the same graph as the peptide off-rate, and data are plotted as the fraction of complex in that conformer relative to the S conformer measured at t = 0. Reactions were performed in triplicate, and data points represent mean ± SD of three independent experiments. Lines fit the data to a single or double exponential decay function.
) conformers is reported in the same graph as the peptide off-rate, and data are plotted as the fraction of complex in that conformer relative to the S conformer measured at t = 0. Reactions were performed in triplicate, and data points represent mean ± SD of three independent experiments. Lines fit the data to a single or double exponential decay function.
The conformer generated by the exchange peptide is DM-labile
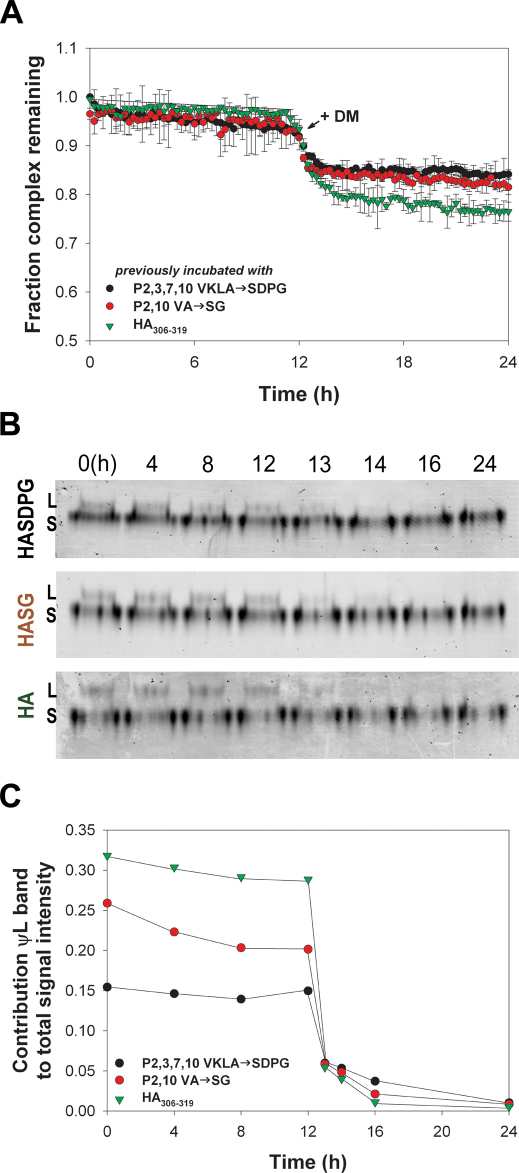
Next, we determined 1) the stability of the conformer generated by the addition of the exchange peptide, 2) whether the kinetic behavior of this specific conformer is a function of the nature of the exchange peptide by which it was generated, 3) whether the ψL band actually identifies DM-labile complexes, and 4) the relation between the amount of the ψL conformer and the amount of peptide release. To address these questions, we purified the HASG/DR1 complexes remaining at the end of the experiment shown in Figure 3 (t = 26 h) from free peptide by filtration, and we monitored peptide release in three new reactions containing 100 nM of purified complexes (Figure 4A). Conversion between conformers was analyzed by SDS-PAGE (Figure 4B). At t = 0, the ψL conformer contributes 15.4%, 25.8% and 31.8% of the total amount of complex generated in the presence of SDPG, SG and HA respectively (Figure 4B and C). The relative contribution of the ψL conformer to the total signal intensity (ψL/(S+ψL)) for each reaction in the first portion of this experiment showed little or no spontaneous release of peptide (Figure 4C). Therefore, the ψL form is relatively stable for at least 12 hours, regardless of the exchange peptide by which it was generated. At 12 hr we added DM to the reaction and monitored peptide release. The FP analysis showed a significant release of peptide (Figure 4A). The gel analysis indicates a loss of the ψL conformer (Figure 4B). The peptide release as the proportion of the starting concentration is equivalent to the proportion of ψL conformer present in each reaction at the time of DM addition. This means that all the peptide loss can account for the peptide in the ψL conformer (Figure 4C). These experiments indicate that: 1) ψL form is susceptible to DM activity, 2) the exchange peptide acts by promoting a conformational change of the complex from S to L conformer, and 3) that DM acts only on this conformer to promote peptide release.
Figure 4. The conformer generated in the presence of the exchange peptide is DM-labile.
(A) Release of peptide from HASG/DR1 complexes isolated at the end of the experiment shown in Figure 3 after purification from free peptide was monitored by FP. The nature of the exchange peptide present in reaction before purification is indicated in the legend. Data are plotted as the fraction of complex remaining relative to t = 0. Reactions were performed in triplicate, and data points represent mean ± SD of three independent experiments. The addition of DM to the three reactions is indicated. (B) L and S conformers of the complex before and after addition of DM were quantitated by SDS PAGE gel. The exchange peptide with which the complex was incubated prior purification is indicated on the left. (C) Modification of the ψL/S ratio analyzed by SDS-PAGE gel as shown in panel B. The nature of the exchange peptide present in reaction before purification is indicated in the legend. Data are plotted as contribution from the ψL band to total signal intensity in each lane.
Discussion
A number of kinetic measurements of peptide dissociation from pMHCII complexes provide compelling evidence for the existence of conformational isomers in solution9,13,14,17. There is evidence that T-cells can distinguish such conformers18,19. On electrophoresis gels, a slower and a faster migrating bands interpreted as evidence for a “floppy” and a “compact” form of pMHCII complexes have been found for I-Ad 15, I-Ek 16, I-Ak 20, I-Ab 21 and HLA-DR1. Thus, these two conformers appear to be a general feature of MHCII. These two conformers cannot be purely gel or detergent artifacts, since the transition from compact to floppy can be induced in lipid bilayers22 as well as in buffer in the absence of surfactant17. The slower migrating conformation has been indicated as an intermediate in the dissociation of faster migrating form into separate α and β chains17. The slower migrating conformer also constitutes a folding intermediate in the assembly of α and β chains with the peptide to form stabile conformer16. More importantly, a19F-NMR analysis of the PCC97–103/I-Ek complex revealed that the conformational isomerism is located in the N-terminal part of the complex13. Indeed, this is the area with the greatest flexibility, in particular for the DRα chain, as indicated by higher B-factors in crystal structure and by molecular dynamic simulations23.
Here we show that generating pMHCII complexes in a standard overnight binding reaction with excess peptide results in two conformers. The slower migrating band is DM-labile (L). We use the DM-lability of the slower migrating complex to enrich for the faster migrating, DM-stable (S) conformer. We show that the S conformer is stable in the presence of DM, until an exchange peptide is added. Incubation of a purified S conformer with different exchange peptides in the absence of DM results in the formation of a conformer with an electrophoretic mobility similar to L. Interestingly, the extent of generation of this L-like conformer is a function of the exchange peptide Kd. Finally, if the induced L+S mix is purified away from the exchange peptide and treated with DM, peptide release is equivalent to the amount of L conformer. Indeed, SDS-PAGE analysis shows that this release is correlated with the disappearance of the L conformer.
We propose that peptide exchange, either in the absence or in the presence of DM, is mediated through the slower migrating conformer, whose generation is a function of the availability of exchange peptide. The slower migrating conformer would be structurally different from the faster migrating conformer for a modified geometry of the binding groove, probably at the level of the α39–α54 region. Indeed, work based on the analysis of αF54-substituted DR1 molecules has recently shown that the interaction of the complex with DM is driven by a conformational change of the MHCII protein in the region of the α-subunit 310 helix and adjacent extended strand region24. The L-conformer observed in our experiments may functionally correspond to the conformation adopted by the mutated DR in those studies. The above conformational modification may promote a weakening of the H-bond network at the N-terminal of the complex and, depending on the distributed binding energy of the complex, trigger an initial DM-independent release of the peptide, leaving the P1 pocket emptied. The rearranged complex would feature a higher affinity for DM than the faster migrating conformer and would be susceptible to DM activity. As evidenced by a lack of cooperative effects in DM-mediated peptide release, DM would promote a dramatic structural change in the pMHCII complex that does not follow the usual energetic pathway of peptide/MHCII unfolding3,4. One possibility is that DM may promote a transient but catastrophic destabilization of the pre-bound peptide/MHCII complex, for instance through exposure of the N-terminal region to solvent. This destabilization may then be transmitted rapidly throughout the entire length of the peptide binding groove such that the typical peptide/MHCII unfolding is absent5. Under these conditions of widespread disruption of peptide/MHCII interactions, the probability of close proximity of the prebound and exchange peptides to a destabilized binding groove is enhanced. At this point the exchange peptide could be tested by the MHCII based on the ability to fold itself and the groove back into a stable conformation. The prebound peptide remains loosely tethered to the MHCII and would be able to rebind if the exchange peptide did not succeed in forming a closed, low-energy complex. We think that it is very unlikely that with DM still interacting with the MHCII, the exchange (or the prebound) peptide would (re)bind in an L-conformer. Rather, DM would be released from the complex once this assumes an S-conformer. The possibility of DM affecting both pre-bound peptide release as well as refolding of the complex around the best fitting peptide is intriguing and would agree with the evidence that DM “rescues” misfolded DR from aggregation25,26. However we do not currently have evidence supporting this double function for DM.
Recently, another model has been put forward based on DM binding of HLA-DR2 variants, indicating that DM binds specifically DR molecules in which the P1 interaction and the surrounding H-bond network have been destabilized due to system vibration11. We hypothesize that the αF54-substituted DR1 complexes, the partially emptied MHCII molecule, and our L-conformers are functionally equivalent. However, if system vibration were the unique reason for the formation of DM-labile conformers, we should expect spontaneous conversion of S into L conformer also in the absence of an exchange peptide. We could not detect such a conversion for the length of our observation. Formally, this could be explained postulating bidirectional interconversion (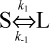 ) with k1 = k−1. However, the absence of peptide release from isolated S form of the complex in the presence of DM and absence of an exchange peptide supports the hypothesis that the half-life of the L conformer is ≥ 12 hours. Our observation is consistent with experiments showing a disproportionate stability of the H-bonds surrounding the P1 pocket24. The impact of the DR2 studies remains, in that they help identify, among all the possible scenarios, the direction of the peptide exchange reaction, with the prebound peptide releasing from the N-terminal and the exchange peptide attempting to bind starting from the P1 region.
) with k1 = k−1. However, the absence of peptide release from isolated S form of the complex in the presence of DM and absence of an exchange peptide supports the hypothesis that the half-life of the L conformer is ≥ 12 hours. Our observation is consistent with experiments showing a disproportionate stability of the H-bonds surrounding the P1 pocket24. The impact of the DR2 studies remains, in that they help identify, among all the possible scenarios, the direction of the peptide exchange reaction, with the prebound peptide releasing from the N-terminal and the exchange peptide attempting to bind starting from the P1 region.
We propose that the exchange peptide-induced conformer also constitutes the structural intermediate for peptide exchange in the absence of DM. The rate of accumulation of the ψL conformer is proportional to the affinity of the exchange peptide. Indeed, in the presence of a low-affinity peptide such as HASDPG, the conversion of HASG/DR1 complex from S to ψL reaches steady state at an earlier time point as compared to HA. We think that this phenomenon is related to the ability of an unlabeled exchange peptide with a higher affinity than the prebound FAM-peptide ( Kd,p < Kd,p* ) to replace the latter in the ψL conformer. On the contrary, an exchange peptide with a lower affinity than the prebound peptide (Kd,p > Kd,p*) would still be able to generate ψL conformers, but the ability to exchange is limited. As a consequence, ψL conformers containing prebound (fluorescent) peptide would accumulate at a faster rate (Scheme 1):
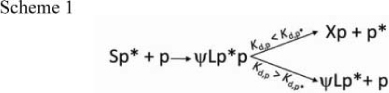 |
This process would be a DM-independent “compare-exchange” routine5, in which peptide exchange is a cooperative process based on the pre-bound peptide unfolding and the exchange peptide folding itself and the binding groove. A possible role for a two-peptide intermediate during peptide exchange in the absence of DM has been suggested by several groups in the past7,8.
The outcome of DM-mediated peptide exchange could be different from the intrinsic exchange depending on whether during intrinsic exchange the exchange peptide could bind both an L and S-conformer (“Xp” complex in Scheme 1). As shown here, DM would ignore the complex when it assumes an S conformer and would only exchange from the L conformer. In other words, DM would increase the fraction of S conformers at any point in time as compared to an intrinsic reaction. If intrinsic exchange also only happens on the L conformer, as we think likely, then the two modes of peptide exchange are equivalent; with DM acting as a catalyst of the intrinsic exchange by accelerating the release of the prebound peptide.
The observation of a subset of DM-labile conformers in the absence of any exchange peptide is the result of a standard peptide-binding assay in which the excess peptide acts as exchange peptide and thus induces the L-conformer in concentrations proportional to peptide Kd. While we do not have formal proof that that the L-conformer generated during a peptide binding step and that generated by adding peptide to the S-conformer are structurally identical, they appear to be functionally the same. However, we are currently in the process of isolating the different complexes generated in different conditions to further defining the nature of the L-conformer and provide a more refined mechanism of DM mediated peptide exchange.
The biological relevance of our data is that they indicate the mechanism by which peptides available for loading into MHCII can induce a conformational state of the pMHCII complex which, in turn, is amenable to DM-mediated peptide exchange. Because the exchange is a function of the peptide Kd and occurs as a simple pairwise comparison, the outcome is the loading of peptides based on Kd. The rapidity of the exchange allows for establishing an equilibrium representation of the peptides available in the endosome as a function of their affinity. This mechanism guarantees that even some low-affinity peptides can be maintained in the DM-stable conformer. This conformer would inform the epitope selection machinery that the complexes can be shifted to the membrane and represent the available peptide repertoire as possible TCR ligands.
Methods
Peptide Synthesis
Peptides derived from the sequence GPKYVKQNTLKLAT, representing residues 306–319 of the hemagglutinin protein from influenza A virus (H3 subtype), are described in Table 1. The N-terminal Gly facilitated labeling. Side chains in the HA peptide are numbered relative to the P1 Tyr residue27. N-terminal labeling with FAM (Molecular Probes) or LC-LC biotin (Pierce) was performed on the resin before deprotection, and then peptides were cleaved and purified by HPLC and confirmed by MALDI-TOF mass spectrometry (Protein Nucleic Acid Shared Facility – Medical College of Wisconsin).
Expression and Purification of Recombinant Soluble DR1 and DM Protein
Recombinant soluble empty (peptide free) DR1 was produced and immunoaffinity purified from a stably transfected Drosophila S2 insect cell line essentially as described27. It is known that DR1 produced in S2 cells might have insect-derived peptide loosely bound once purified. Whether the majority of purified dimers are “empty” DR has been questioned28. However, it has been shown that the binding properties of insect cell-derived MHCII molecules are comparable to the binding properties of α and β chains expressed in E. Coli and then refolded29. Soluble FLAG epitope tagged DM was isolated from a stably transfected Drosophila S2 cell line as described30. To avoid contamination with FLAG peptide, DM elution from the resin was performed with 0.1 M glycine HCl, pH 3.5. Both DR1 and DM proteins were purified and buffer exchanged into PBS (7 mM Na+/K+ phosphate, 135 mM NaCl, pH 7.4) using centrifugal ultra-filtration (Amicon). Purity (>95%) was confirmed by SDS-PAGE stained with GelCode Blue Stain Reagent (Pierce). DR1 and DM proteins were quantified by measuring the UV absorbance @ 280 nm using an E280 of 56340 M−1 cm−1 before use.
Competitive Peptide Binding Assay
To assess peptide affinity values, DR1 (20 nm) was incubated with 20 nm biotinylated HA peptide in PBS (0.1% BSA, 0.01% Tween 20, 0.1 mg/ml 4-(2-aminoethyl)-benzene sulfonyl fluoride, 0.1 mM iodoacetamide, 5 mM EDTA, 0.02% NaN3, pH 7.2) in the presence of varying amounts of inhibitor peptides at 37 C. Bound biotinylated peptide was detected using a solid-phase immunoassay and Eu2+ labeled streptavidin. Plates were read using a Wallac VICTOR counter (PerkinElmer Wallac). Data were fit to a logistic equation y = a/[1+(x/x0)b]. IC50 values were obtained from the curve fit of the binding data and converted to Kd values by using the Cheng-Prusoff equation Kd = (IC50)/(1 + [bHA]/Kd,bHA) in which Kd,bHA was set equal to 14 nM on the basis of the results of the direct binding of bio-HA peptide to DR1. Each point represents the mean and SD of three independent experiments performed in quadruplicate. Because pMHCII binding represents a multistep reaction, the IC50 for a competitive binding assay may not be directly proportional to the Kd. While this can be offset by long incubations relative to half-life, we study low-affinity peptides where half-lives are impossible to determine. Therefore, the values of affinity reported herein should be considered as apparent Kd values.
Formation of peptide/DR1 complexes
DR1/peptide complexes were formed by incubating 1 μM DR1 protein with a 10-fold molar excess of FAM-labeled peptide in 50 mM NaH2PO4 and 50 mM of sodium citrate (pH 5.3) and protease inhibitors for 16 h @ 37°C. DR1/peptide complexes were then purified from unbound peptide by buffer exchange into PBS with a Centricon-30 spin filter that had been pre-incubated with 25 mM MES (pH 6.5). Purified DR1/peptide complexes were then quantified by reading the UV absorbance @ 280 nm, factoring in an E280 of 1280 M−1 cm−1 for the Tyr residue and 10846 M−1 cm−1 for the fluorescein present in the bound peptide.
Fluorescence Polarization Dissociation Measurements
100 nM of purified DR1/peptide complexes were incubated with or without equimolar unlabeled exchange peptide and in the presence or in the absence of 3-fold excess DM, depending on the experimental conditions as indicated in Results. Reactions were performed at 37°C in 50 mM sodium citrate/sodium phosphate buffer at pH 5.0–5.3 and were covered with mineral oil to prevent evaporation. To avoid non-specific adherence of the protein, black polystyrene 96-well plates were used (Corning). Measurements were performed using a Wallac VICTOR counter (PerkinElmer Wallac) with the excitation wavelength = 485 nm and emission wavelength = 535 nm. Specific control groups included (a) protein only, (b) peptide only, and (c) buffer only, and were used for background correction. FP and anisotropy are mathematically related ways of expressing parallel:perpendicular emission ratios and are easily interconverted. Although FP is approximately linear with respect to the ratio of free:bound peptide, FP was converted to anisotropy (which is exactly linear) by the following equation A = 2*FP/(3-FP) where A is anisotropy and FP indicates fluorescence polarization in mP units. Anisotropy values were fitted either according to a single- or a bi- exponential decay model. Each experiment was performed in triplicate, and the reported dissociation rate reflects the mean ± SD of three independent experiments.
SDS-PAGE analysis of DM Mediated and Intrinsic Peptide Dissociation
At various time points, aliquots of the reaction analyzed by FP (15 μl) were removed and quenched with 0.5 M Tris-HCl (pH 8.0) in gel loading buffer (SDS 2%, glycerol 10%, β-mercaptoethanol 1%, EDTA 12.5 mM, bromophenol blue 0.02%) and immediately placed on ice. The aliquots were then loaded onto a non-reducing 5%-stacking/12%-running SDS-PAGE gel without prior sample boiling, and quickly separated by electrophoresis at 150 V for 30 min. FAM-peptide/DR1 complexes were then visualized using a Typhoon Trio (GE Healthcare Life Sciences) by measuring the amount of fluorescence from the bound peptide (excitation wavelength 488 nm, emission 535 nm) and analyzed with ImageQuant TL software. Each experiment was performed in triplicate, and the reported dissociation rate reflects the mean±SD of three independent experiments.
Author Contributions
A.F. performed the experiments, discussed the results and wrote the manuscript; J.G. discussed the results and wrote the manuscript.
Supplementary Material
Supplementary Figure 1
Acknowledgments
We thank Trudy Holyst for peptide synthesis, Dr. Lawrence Stern for DR1 expressing S2 cells, and Dr. Dennis Zaller for DM expressing S2 cells. This work was supported by NIH Grant R01AI63016.
References
- Watts C. & Powis S. Pathways of antigen processing and presentation. Rev Immunogenet 1, 60–74 (1999). [PubMed] [Google Scholar]
- Zhu S. et al. Improving MHC binding peptide prediction by incorporating binding data of auxiliary MHC molecules. Bioinformatics 22, 1648–55 (2006). [DOI] [PubMed] [Google Scholar]
- Anderson M. W. & Gorski J. Cooperativity during the formation of peptide/MHC class II complexes. Biochemistry 44, 5617–24 (2005). [DOI] [PubMed] [Google Scholar]
- Ferrante A. & Gorski J. Cooperativity of hydrophobic anchor interactions: evidence for epitope selection by MHC class II as a folding process. J Immunol 178, 7181–9 (2007). [DOI] [PubMed] [Google Scholar]
- Ferrante A., Anderson M. W., Klug C. S. & Gorski J. HLA-DM mediates epitope selection by a "compare-exchange" mechanism when a potential peptide pool is available. PLoS One 3, e3722 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A. & Gorski J. Cutting edge: HLA-DM-mediated peptide exchange functions normally on MHC class II-peptide complexes that have been weakened by elimination of a conserved hydrogen bond. J Immunol 184, 1153–8 (2010). [DOI] [PubMed] [Google Scholar]
- Tampe R., Clark B. R. & McConnell H. M. Energy transfer between two peptides bound to one MHC class II molecule. Science 254, 87–9 (1991). [DOI] [PubMed] [Google Scholar]
- de Kroon A. I. & McConnell H. M. Kinetics and specificity of peptide-MHC class II complex displacement reactions. J Immunol 152, 609–19 (1994). [PubMed] [Google Scholar]
- Sadegh-Nasseri S. et al. Conformational heterogeneity of MHC class II induced upon binding to different peptides is a key regulator in antigen presentation and epitope selection. Immunol Res 47, 56–64 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. L. & Sadegh-Nasseri S. HLA-DM recognizes the flexible conformation of major histocompatibility complex class II. J Exp Med 192, 1697–706 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders A. K. et al. HLA-DM captures partially empty HLA-DR molecules for catalyzed removal of peptide. Nat Immunol 12, 54–61 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. C. et al. Actin-binding cleft closure in myosin II probed by site-directed spin labeling and pulsed EPR. Proc Natl Acad Sci U S A 105, 12867–72 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt L., Boniface J. J., Davis M. M. & McConnell H. M. Conformational isomers of a class II MHC-peptide complex in solution. J Mol Biol 286, 207–18 (1999). [DOI] [PubMed] [Google Scholar]
- Schmitt L., Boniface J. J., Davis M. M. & McConnell H. M. Kinetic isomers of a class II MHC-peptide complex. Biochemistry 37, 17371–80 (1998). [DOI] [PubMed] [Google Scholar]
- Dornmair K. & McConnell H. M. Refolding and reassembly of separate alpha and beta chains of class II molecules of the major histocompatibility complex leads to increased peptide-binding capacity. Proc Natl Acad Sci U S A 87, 4134–8 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadegh-Nasseri S. & Germain R. N. A role for peptide in determining MHC class II structure. Nature 353, 167–70 (1991). [DOI] [PubMed] [Google Scholar]
- Dornmair K., Rothenhausler B. & McConnell H. M. Structural intermediates in the reactions of antigenic peptides with MHC molecules. Cold Spring Harb Symp Quant Biol 54 Pt 1, 409–16 (1989). [DOI] [PubMed] [Google Scholar]
- Lovitch S. B. & Unanue E. R. Conformational isomers of a peptide-class II major histocompatibility complex. Immunol Rev 207, 293–313 (2005). [DOI] [PubMed] [Google Scholar]
- Pu Z., Lovitch S. B., Bikoff E. K. & Unanue E. R. T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity 20, 467–76 (2004). [DOI] [PubMed] [Google Scholar]
- Srinivasan M., Marsh E. W. & Pierce S. K. Characterization of naturally processed antigen bound to major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A 88, 7928–32 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikoff E. K. et al. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med 177, 1699–712 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadegh-Nasseri S. & McConnell H. M. A kinetic intermediate in the reaction of an antigenic peptide and I-Ek. Nature 337, 274–6 (1989). [DOI] [PubMed] [Google Scholar]
- Yaneva R., Springer S. & Zacharias M. Flexibility of the MHC class II peptide binding cleft in the bound, partially filled, and empty states: a molecular dynamics simulation study. Biopolymers 91, 14–27 (2009). [DOI] [PubMed] [Google Scholar]
- Painter C. A. et al. Conformational lability in the class II MHC 310 helix and adjacent extended strand dictate HLA-DM susceptibility and peptide exchange. Proc Natl Acad Sci U S A 108, 19329–34 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt A. B. & Kropshofer H. HLA-DM - an endosomal and lysosomal chaperone for the immune system. Trends Biochem Sci 24, 150–4 (1999). [DOI] [PubMed] [Google Scholar]
- Vogt A. B., Moldenhauer G., Hammerling G. J. & Kropshofer H. HLA-DM stabilizes empty HLA-DR molecules in a chaperone-like fashion. Immunol Lett 57, 209–11 (1997). [DOI] [PubMed] [Google Scholar]
- Stern L. J. et al. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature 368, 215–21 (1994). [DOI] [PubMed] [Google Scholar]
- Aichinger G. et al. Major histocompatibility complex class II-dependent unfolding, transport, and degradation of endogenous proteins. J Biol Chem 272, 29127–36 (1997). [DOI] [PubMed] [Google Scholar]
- Joshi R. V., Zarutskie J. A., Stern L. J. A three-step kinetic mechanism for peptide binding to MHC class II proteins. Biochemistry 39, 3751–3762 (2000). [DOI] [PubMed] [Google Scholar]
- Sloan V. S. et al. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature 375, 802–6 (1995). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1