Abstract
Adaptive behavior depends on the detection of potential errors so that ongoing behavior might be corrected. Here, we ask if basolateral amygdala (ABL) might serve this function by examining activity in rats performing a task in which errors were induced by pitting two behavioral responses against each other. This response competition or conflict was created by forcing rats to respond away from the direction in which they were freely choosing on the majority of trials. Rats were slower and less accurate on these incongruent trial types. We found that activity in ABL fired more strongly prior to errant responses, but did not signal the potential for errors on correctly performed incongruent trials. These data support a role for ABL in processing errors prior to their occurrence and suggest that ABL is not involved in monitoring conflict so that ongoing behavior might be corrected.
Keywords: amygdala, attention, error, conflict, single unit, rat
INTRODUCTION
Recent data demonstrate that basolateral amygdala (ABL) is involved in error-related functions, specifically when reward expectancies are violated (Belova et al., 2007; Roesch et al., 2010a; b; Tye et al., 2010). For example, we have previously shown that activity in ABL increases when rewards are unexpectedly delivered or omitted in a task in which expected reward varies in size and time to delivery (Roesch et al., 2010b). Others have reported increased activity in ABL when rats were expecting reward, but not delivered during extinction (Tye et al., 2010). In primates, unexpected delivery of appetitive and aversive outcomes during performance of a trace conditioning task with reversals caused amgydala neurons to fire more strongly than when the outcome was totally predictable (Belova et al., 2007). These reports suggest that ABL encodes reward expectancy errors in the service of enhanced attention or event processing on subsequent trials so that behavior can be adaptive when outcome contingencies change. Here, we further our investigation of error encoding in ABL.
Since ABL is strongly interconnected with anterior cingulate (ACC) we reasoned that ABL might contribute to error related functions commonly ascribed to ACC (Sripanidkulchai et al., 1984; Cassell & Wright, 1986; Dziewiatkowski et al., 1998). Several studies have shown that ACC is more active in situations in which an error is more probable (Pardo et al., 1990; Badgaiyan & Posner, 1998; Bush et al., 1998; Carter et al., 1998; Carter et al., 2000; Cohen et al., 2000; Botvinick et al., 2001; Braver et al., 2001; van Veen et al., 2001; Ito et al., 2003; Walton et al., 2004; Amiez et al., 2005; Brown & Braver, 2005; De Martino et al., 2006; Oliveira et al., 2007; Brown & Braver, 2008; Rudebeck et al., 2008; Rushworth & Behrens, 2008). The increased likelihood of errors in these studies is due to an induced competition or conflict between two behavioral responses. These tasks typically have participants override some type of automatic or habitual response in order to perform a task appropriate one. For example, in the well known Stroop task, subjects are shown a word, for example, ‘red’, and asked to identify what ink color the word was written in (e.g. ‘red’ written in green ink). In this situation, the prepotent or automatic response is to read the word (i.e. red) and the competing, task appropriate response, is to name the color of the ink (i.e. green). ACC has been proposed to ‘monitor’ such conflict during actual performance of the task.
Evidence for conflict between two competing or co-activated responses comes from behavioral measures of reaction time and accuracy. During high conflict situations (e.g. when the ink color is incongruent with the semantic meaning of the word), reactions times are slower and error rates are higher than when they are congruent (e.g. ‘red’ written in red ink). Importantly, there are several other ways to induce conflict, all with the common feature of slowing reaction times and reducing accuracy under high conflict conditions. In all of these studies, tasks capitalize on the difficulty of suppressing some automatic or habitual tendency instead of making the task imposed appropriate response. It is unclear what function ABL might play in this type of a situation, but considering its connectivity with ACC and its recently discovered role in reporting reward expectancy errors and attention (Belova et al., 2007; Roesch et al., 2010a; b; Tye et al., 2010), we hypothesized that it might also detect the likelihood of errors on high conflict trials.
To address this issue we devised a new task that created a habit like response by allowing rats, on the majority of trials, to choose between one of two response directions. On the remaining trials rats were forced to respond either toward (congruent) or away from (incongruent) the direction that they were choosing more often on free-choice trials. Rats were slower and less accurate on incongruent trials, suggesting that they had to override a potent inherent tendency to respond in their preferred direction in order to make the task imposed correct response. We found that activity in ABL fired more strongly prior to errant responses, suggesting that activity in ABL reflects that an error was about to occur. However, single neurons in ABL did not signal the potential for errors on correctly performed incongruent trials. These data support a role for ABL in processing errors prior to their occurrence, and suggests that it is not involved in monitoring conflict or allocating attention in the service of improved performance on behaviors currently being planned.
METHODS
Subjects
Four male Long-Evans rats were obtained at 175–200g from Charles River Labs. Rats were individually housed on a 12h light/dark cycle and tested during the light phase. All experiments were approved by the University of Maryland College Park under university and NIH guidelines.
Surgical procedures and histology
Surgical procedures followed guidelines for aseptic technique. Electrodes were manufactured and implanted as in prior recording experiments. Rats had a drivable bundle of 10–25µm diameter FeNiCr wires (Stablohm 675, California Fine Wire, Grover Beach, CA) chronically implanted in the left hemisphere dorsal to ABL (−3.0 mm posterior to bregma, −5.0 mm laterally, and −7.5 mm ventral to the brain surface). Immediately prior to implantation, these wires were freshly cut with surgical scissors to extend ~1 mm beyond the cannula and electroplated with platinum (H2PtCl6, Aldrich, Milwaukee, WI) to an impedance of ~300 kOhms. Cephalexin (15 mg/kg p.o.) was administered twice daily for two weeks post-operatively to prevent infection. The rats were then perfused and their brains removed and processed for histology (Roesch et al., 2007).
Behavioral task
Training and recording was conducted in aluminum chambers approximately 45.7 cm on each side with sloping walls narrowing to an area of 30.5 cm × 30.5 cm at the bottom. A central odor port was located above two adjacent fluid wells on a panel in the right wall of each chamber (Roesch et al., 2009). Two house lights were located above the panel. The odor port was connected to an air flow dilution olfactometer to allow the rapid delivery of olfactory cues. Task control was implemented via computer. Port entry, well entry, and licking were monitored by disruption of photobeams.
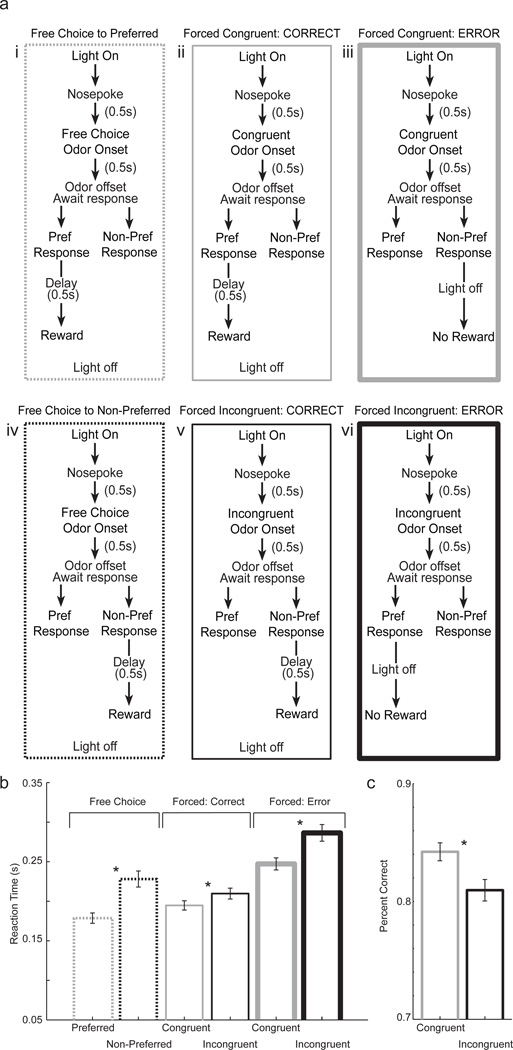
The basic design of a trial is illustrated in Figure 1A. Trials were signaled by illumination of the house lights inside the box. When these lights were on, nosepoke into the odor port resulted in delivery of the odor cue for 500 ms to a small hemicylinder located behind this opening starting 500 ms after entry into the odor port. One of three different odors was delivered to the port on each trial, in a pseudorandom order. At odor offset, the rat had 3 seconds to make a response at one of the two fluid wells located below the odor port. Reaction times were calculated as the time between the offset of the odor stimulus and the exit of the snout from the odor port. One odor instructed the rat to go to the left to receive reward (forced-choice trial), a second odor instructed the rat to go to the right to receive reward (forced-choice trial), and a third odor indicated that the rat could obtain reward at either well (free-choice trial). All correctly performed trials resulted in 1 bolus of 10% sucrose solution. On forced-choice trials, responding in the wrong direction resulted in no reward with an immediate correction trial. Odors were presented in a pseudorandom sequence such that the free-choice odor was presented on 12/20 trials and the left/right odors were presented in equal numbers in the remainder of trials (+/−1 over 250 trials). In addition, the same odor could be presented on no more than 3 consecutive trials. Odor identities did not change over the course of the experiment.
Fig. 1. Task and behavior.
(A) Trials were signaled by illumination of house lights inside the box. When these lights were on, nosepoke into the odor port resulted in delivery of the odor cue to a small hemicylinder located behind this opening. One of three different odors was delivered to the port on each trial, in a pseudorandom order. At odor offset, the rat had 3 s to make a response at one of the two fluid wells located below the port. Each well produced the same amount of reward (1 bolus). The free-choice odor signaled that reward would be available at either well (i, iv). Free choice trials were used as a measure of response preference. Other odors forced rats to go either to or away from this preference. On these trials, reward (1 bolus) was only available in the direction signaled by the odor. Correctly performed forced-choice trials toward the preferred direction are illustrated in thin gray and will be referred to as congruent (ii). Errors on these trials are illustrated in thick gray and are referred to as congruent errors (iii). Correctly performed forced-choice trials toward the non-preferred direction are illustrated by thin black lines and will be referred to as incongruent (v). Errors on these trials are illustrated by thick solid black lines and are referred to incongruent errors (vi). The free-choice odor was presented 12/20 trials and the two force-choice trials were equally presented in the remainder of trials. (B) The height of each bar indicates the mean reaction time for each trial type described in ‘A’ averaged across all recording sessions. (C) The height of each bar indicates mean percent correct when the rats were forced in the congruent and incongruent response direction. Asterisks indicate significant difference via ttest (p < 0.05). Error bars are standard error of the mean. Thick black = Incongruent errors; Thick gray = Congruent errors; Thin black = Correct incongruent; Thin gray = Correct congruent; Dashed black = Free choice to non-preferred; Dashed gray = Free choice to preferred.
During the first day of training rats were first taught to simply nose poke into the odor port and then go to the well for reward. On the second day, the free-choice odor was introduced and rats were free to respond to either well for reward. On each subsequent day, the number of forced-choice odors increased by 2 for each block of 20 trials. Once the rats were able to maintain accurate responding (> 60%) on forced-choice trials for at least 150–200 trials at the rate of 8 out of 20 total trials (~ 19 days), surgery was performed and recording sessions began 1–2 weeks later. Rats were water deprived (~30 min of free water per day) with free access on weekends. Rats were weighed weekly. No rat showed a significant decrease in weight over the course of the experiment.
Single-unit recording
Procedures were the same as described previously (Bryden et al., 2011). Wires were screened for activity daily; if no activity was detected, the rat was removed, and the electrode assembly was advanced 40 or 80µm. Otherwise active wires were selected to be recorded, a session was conducted, and the electrode was advanced at the end of the session. Neural activity was recorded using two identical Plexon Multichannel Acquisition Processor systems (Dallas, TX), interfaced with odor discrimination training chambers. Signals from the electrode wires were amplified 20X by an op-amp headstage (Plexon Inc, HST/8o50-G20-GR), located on the electrode array. Immediately outside the training chamber, the signals were passed through a differential pre-amplifier (Plexon Inc, PBX2/16sp-r-G50/16fp-G50), where the single unit signals were amplified 50X and filtered at 150–9000 Hz. The single unit signals were then sent to the Multichannel Acquisition Processor box, where they were further filtered at 250–8000 Hz, digitized at 40 kHz and amplified at 1–32X. Waveforms (>2.5:1 signal-to-noise) were extracted from active channels and recorded to disk by an associated workstation with event timestamps from the behavior computer. Waveforms were not inverted before data analysis.
Data analysis
Units were sorted using Offline Sorter software from Plexon Inc (Dallas, TX), using a template matching algorithm. Sorted files were then processed in Neuroexplorer to extract unit timestamps and relevant event markers. These data were subsequently analyzed in Matlab (Natick, MA). Reaction times were computed by taking the time from odor offset to odor port exit. Percent correct scores were computed by dividing the total number of trials in which a response was made to one of the fluid wells. The main analysis was performed on the epoch starting at odor onset and ending when the rat exited the odor port to examine activity prior to response to determine if activity was higher before incorrect compared to correct responses and before correctly performed incongruent versus congruent trial types. For analysis examining correlations between firing rate and reaction time we chose to examine 200 ms of activity immediately preceding odor port entry. Wilcoxon tests were used to measure significant shifts from zero in distribution plots (p < 0.05). T-tests or ANOVAs were used to measure within cell differences in firing rate (p < 0.05). Pearson Chi-square tests (p < 0.05) were used to compare the proportions of neurons.
RESULTS
Rats were trained on an odor discrimination task in which two odors signaled rats to respond to the left and right for reward (Fig 1). A third odor indicated a free-choice (reward in either well). Free choice trials were in the majority (12 out of 20 trials). Forced-left and forced-right trials occurred in equal proportions during the remaining trials (4 congruent and 4 incongruent). On average, rats performed 189 correct trials per session. Each day rats formed a natural bias toward one well. During the recording sessions, in which we recorded 295 neurons, rats formed a significant bias (chi-square; p < 0.05) toward left and right wells in 32 (41%) and 42 (54%) sessions, respectively. There were only 4 sessions in which side bias did not achieve significance. Biases were mostly stationary from day to day. Rats 11 and 16 significantly chose left more often than right in 29 out of 33 recording sessions (rat 11: 9 left versus 3 right; rat 16: 20 left versus 1 right). The other two rats significantly chose right over left in 38 out of 41 sessions (rat 12: 0 left versus 22 right; rat 15: 3 left versus 16 right).
It is unclear why rats preferred one side over the other because reward levels were equal between the two responses and rats alternated between two recording boxes. Side preference might reflect a number of different inherent factors including handedness, initial body position and exploration. Regardless of what the reason might be, what is important here is that responding in the preferred direction on free-choice trials was potent, possibly habitual, as indicated by faster reaction times as compared to responses made in the opposite direction. This is illustrated in Figure 1B, which plots the average of the mean reaction times across all recording sessions for free choices made to the side that was chosen more (preferred) and less (non-preferred) often (Fig 1B; ttest; t536 = 4.1008; p < 0.0001). For the remainder of the paper, the preferred (gray lines) and non-preferred (black lines) response will be defined as the direction that elicited the faster and slower reaction times, respectively, which was determined independently for each recording session.
Forced-choice trials signaled the rat to either make a response to (congruent; gray dashed) or away from (incongruent; black dashed) the rat’s preferred direction. On incongruent forced-choice trials rats had to override their prepotent tendency to respond in their preferred direction, thus creating a situation of response conflict (i.e. co-activation of competing responses). Consistent with this hypothesis, on correct incongruent forced-choice trials (i.e. rats were cued to respond opposite their preference; Fig. 1A:v), reaction times were significantly slower than on correct forced-choice trials made in the preferred direction (Fig. 1A:ii; congruent; Fig. 1B; gray vs black thin; ANOVA; main effect of congruence; F(1,1176) = 11.67; p < 0.001). Furthermore, rats were less accurate on incongruent trial types as compared to congruent forced-choice trials as illustrated in Figure 1C, which plots percent correct scores for incongruent and congruent trials (ttest; t294 = 3.74; p < 0.0001). Thus, with this procedure, we have created a conflicting situation that results in slower reaction times and worse performance on incongruent trials.
Finally, we examined reaction time on error trials in which rats made responses in the wrong direction on forced-choice trials. Interestingly, rats were significantly slower on error trials (Fig. 1B; thick lines; ANOVA; main effect of correctness; F(1,1176) = 66.43; p < 0.0001), with the slowest response being on incorrect incongruent trials (thick gray vs thick black; ttest; t294 = 4.16; p < 0.0001). Slower reaction times on error trials might reflect some errant processing of information or possibly that rats know that they are in the process of making a mistake and thus try to slow down. Previous work has shown that prior to correctly refraining from making a habitual response rats first slow that response for several trials before correctly inhibiting it (Schoenbaum et al., 2003; Setlow et al., 2003). Notably, slowing of reaction time on errors does not reflect lack of concentration, motivation or general lackadaisical performance because rats showed no significant difference in the speed at which they initiated correct and incorrect trials (light-on latency = time from house light onset to nose poke into odor port; ttest; error = 0.95 ms; correct = 0.94 ms; t294 = 0.15; p = 0.88).
Activity in ABL reflects the upcoming error
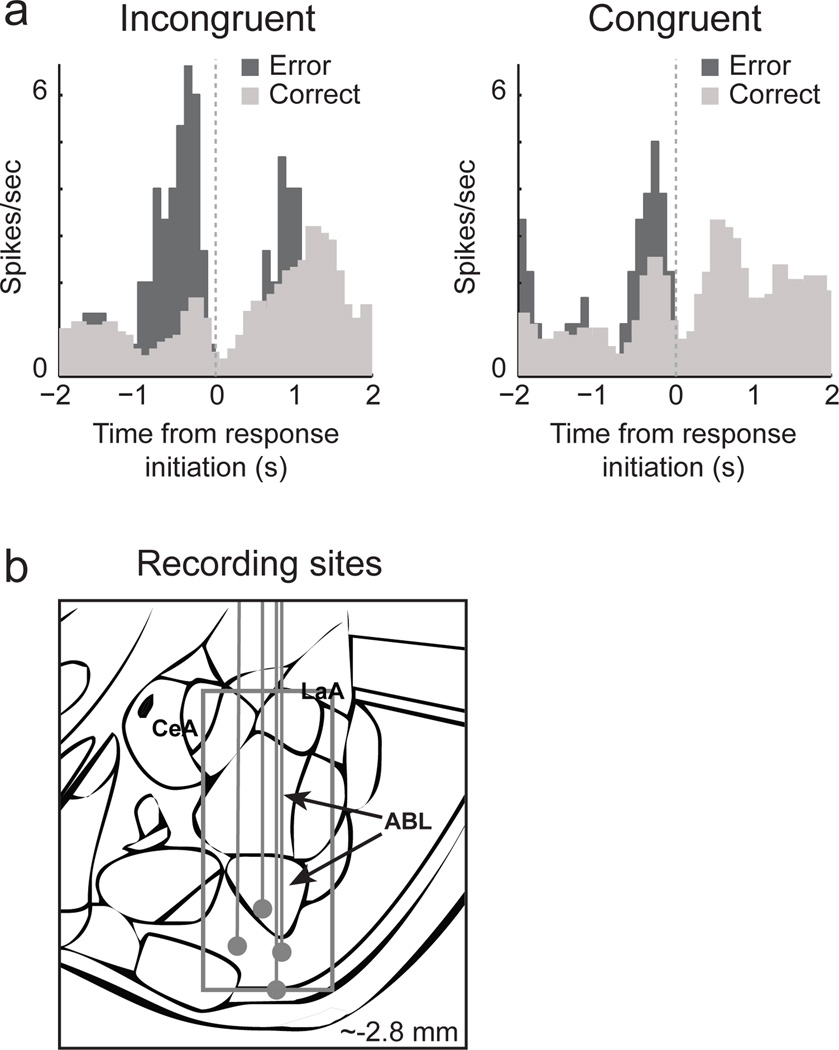
We recorded from 295 neurons in 4 rats during performance of this task. The locations of the recording sites are illustrated in Figure 2B. Many neurons appeared to fire more strongly prior to the response on forced-choice errors as illustrated by the single cell example in Figure 2A. Activity is aligned on odor port exit to demonstrate that changes in firing occurred before response initiation. This single cell fired more strongly prior to incongruent error responses compared to correctly performed incongruent trials. A similar but smaller effect was observed on congruent trials. Increases in activity preceded the initiation of the behavioral response and occurred long before any trial feedback was presented. Note, that activity during incongruent errors cannot simply reflect responses made in the opposite direction because activity was not high for correct congruent trials in which rats responding in the same direction (Fig. 2A; right panel; gray). Further, activity cannot reflect the identity of the odor that cued the incongruent response on errors because the same odor was presented on correctly performed incongruent trials (Fig. 2A; left panel; gray).
Fig. 2.
Activity of a single ABL neuron averaged over all trials aligned on odor port exit during forced-choice congruent and incongruent trials to demonstrate increased firing prior to response initiation on errors. Dark gray = error; Light gray = correct; Left and right columns represent incongruent and congruent trials, respectively. (B) Location of recording sites. Gray dots represent final electrode position. Gray box marks extent of recording sites. CeA, central nucleus of amygdala; LaA, lateral amygdala; ABL, basolateral amygdala.
To quantify this effect across the population of 295 cells we performed a 2-factor ANOVA with response direction (congruent versus incongruent) and correctness (correct versus incorrect) as factors during odor sampling (odor onset to odor port exit) for each of the 295 cells. Of the 295 neurons, 43 showed either a main or interaction effect with correctness, which is in excess of the frequency expected by chance alone (type 1 error; 10%; chi-square = 6.8; p < 0.01). Further breakdown revealed that 18 neurons showed a main effect of correctness alone, with the majority (n = 13) firing more strongly on error trials. Twenty-five neurons exhibited an interaction effect between correctness and congruence with no main effects. Of those, a post-hoc ttest analysis revealed that 11 neurons exhibited activity that was significantly higher during errors whereas 6 fired more strongly during correct trials (p < 0.05). For the remaining neurons whose activity showed a significant interaction but no significance in the post-hoc ttest analysis (n = 9), 7 and 2 neurons exhibited a larger difference between incorrect and correct trials on incongruent and congruent trials, respectively. Thus, overall, the number of neurons from this analysis that fired more strongly prior to incorrect versus correct trials were in the significant majority (24 fired significantly more strongly during error compared to correct trials; 11 fired significantly more strongly during correct compared to incorrect trials; chi-square = 4.75; p < 0.05).
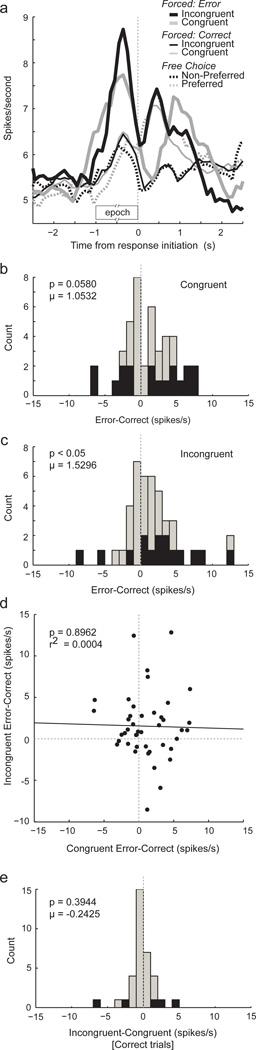
The average population histogram of these 43 cells is illustrated in Figure 3A. Activity was significantly stronger prior to the response for both incongruent (thick black) and congruent (thick gray) errors compared to their correct counterparts (thin solid; incongruent, t42 = 2.51, p < 0.05; congruent, t42 = 2.11, p < 0.05) during odor sampling (odor onset to port exit). Activity during incongruent and congruent conditions did not significantly differ from each other (t42’s < 0.66, p’s > 0.51).
Fig. 3.
Activity in ABL was modulated by error prior to response initiation. (A) Curves represent average population firing rate, aligned on odor port exit, for all cells that showed a significant interaction or main effect of correctness (n = 43) in a 2-factor ANOVA with congruency and correctness as factors (see text). Analysis epoch was from odor onset to response initiation (odor port exit). Line conventions are the same as in Figure 1. Preferred and non-preferred directions were determined as responses, averaged across the session, that elicited the fastest and slowest responses. Thick black = Incongruent errors; Thick gray = Congruent errors; Thin black = Correct incongruent; Thin gray = Correct congruent; Dashed black = Free choice to non-preferred; Dashed gray = Free choice to preferred. (B and C) Histograms depicting the distribution of firing rate indices (error minus correct) for congruent and incongruent trials, respectively. Activity was taken from odor onset to odor port exit. Black bars indicated significant difference at the single cell level (error versus correct; ttest; p < 0.05). (D) Scatter plot showing correlation between distributions illustrated in B and C. X-axis = congruent error minus congruent correct; Y-axis = incongruent error minus incongruent correct. (E) Histogram depicting the distribution of firing rate indices (incongruent minus congruent) for correct trials. Black bars indicated significant difference at single cell level (incongruent versus congruent; ttest; p < 0.05).
To further quantify these effects we plotted distributions representing the differences between incorrect and correct trials independently for congruent and incongruent forced-choice. Both distributions were shifted in the positive direction indicating increased firing on error trials (Fig. 3B–C); however the shift was only significant for incongruent trials (Fig. 3B–C; Wilcoxon; Incongruent: μ = 1.53 spikes/s; p < 0.05 ; Congruent: μ=1.05 spikes/s; p = 0.058). The number of single neurons that fired significantly more strongly before error trials were in the majority for both incongruent (12 vs 3; chi-square = 5.28; p < 0.05) and congruent (11 vs 7; chi-square = 0.85; p = 0.36) trial types, but again, this difference only achieved significance for incongruent trials.
Thus, from these analyses, we conclude that activity in ABL was significantly stronger prior to incongruent and congruent response errors, at both the population and single cell level, with a trend for stronger error encoding under incongruent trial types.
Interestingly, there was no correlation between firing related to errors on congruent and incongruent trials. This is illustrated in Figure 3D which plots the error distribution for congruent (Fig. 3B) and incongruent (Fig. 3C) trial types against each other. The relationship between the two was not correlated indicating that error modulation on incongruent and congruent trials did not tend to occur within single neurons (p = 0.8962; r2 = 0.0004). In other words, those neurons that fired more strongly on incongruent error trials do not necessarily show the same effect on congruent trials.
Activity in ABL does not reflect response conflict
As described above, activity in ABL reflects the upcoming error before it occurs. Here we asked if activity might also signal the potential for errors on higher conflict incongruent trials versus low conflict congruent trials when these trials were performed correctly. As described above, rats were slower on incongruent trials reflecting competition between two competing events (i.e. response conflict).
Surprisingly, comparison of correct incongruent (black thin) and correct congruent (gray thin) trial types in the population histogram (Fig. 3A) and in the single cell examples (Fig. 2A) suggested that activity was not significantly higher under incongruent trials types. To quantify the lack of effect we compared activity between correct incongruent and congruent trials types during odor sampling (odor onset to odor port exit). Again we computed a difference measure (incongruent – congruent) and performed ttests at the single cell level. The distribution for the 43 neurons is illustrated in Figure 3E. This distribution was not significantly shifted (Wilcoxin; μ = −0.24 spikes/s; p = 0.39) and the number of neurons that fired significantly more under correct incongruent trials was not in the significant majority (3 versus 2).
This effect was also not present in the population as a whole. Out of the 295 ABL cells, equal numbers of neurons fired significantly more strongly on incongruent and congruent trials during odor sampling than they did for their respective counterparts (10 versus 10) and the distribution representing differences between incongruent and congruent was not significantly shifted from zero (Wilcoxin; u = 0.05; p = 0.73). Thus, activity in ABL was not significantly stronger under incongruent as compared to congruent trial types in the subpopulation of 43 neurons that encoded errors or in the population as a whole.
Activity in ABL was positively correlated with reaction time on incongruent trials
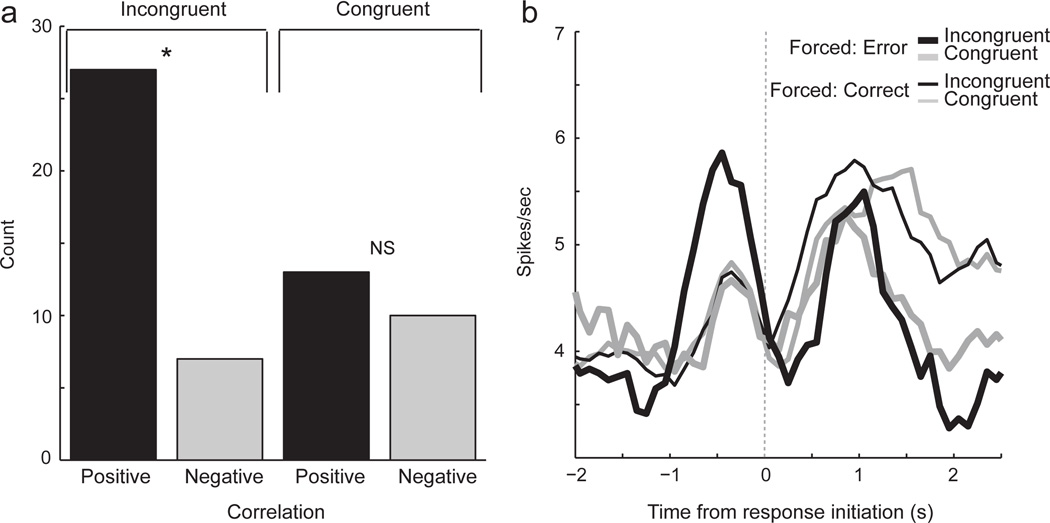
In the sections described above we demonstrate that rats were significantly slower on error trials. We also demonstrate that activity was high when those errors were about to be made. Here we ask if there might be a direct correlation between reaction time and firing in ABL at the single cell level. For this analysis we examined activity just prior to the initiation of the movement (200 ms) and performed a correlation analysis separately for incongruent and congruent trial types to avoid any confounds that might result from congruency effects on reaction time and firing rate. The number of neurons that showed a positive correlation (n = 40) between firing right and reaction time exceeded chance alone (chi-square = 46.7; p < 0.0001), whereas the number of those showing a negative correlation (n = 17) did not (chi-square = 0.65; p = 0.41). Under congruent trial types the number of neurons with activity that were positively and negatively correlated with reaction time did not significantly differ (Fig. 4A; 13 versus 10; chi-square = 0.37; p = 0.53), however, under incongruent trials the number of neurons that exhibited a positive correlation between firing rate and reaction time exceeded those with a significant negative correlation (Fig. 4A; 27 versus 7; chi-square = 11.6; p < 0.001). For these cells, when activity was high, reaction times were slow. As expected, when plotting these 27 cells, firing was stronger prior to incongruent errors (Fig. 4B; solid black). Note, as described in the sections above, these neurons exhibited no difference between incongruent (thin black) and congruent (thin gray) trials prior to the response when performed correctly. These results suggest that the firing of single neurons in ABL is closely tied to the slowing of reaction times during errors on incongruent forced-choice trials.
Fig. 4.
Activity was correlated with reaction time. (A) Correlation analysis between firing rate taken 200 ms prior to response initiation and reaction time (port exit minus odor offset). Analysis was performed independently for congruent and incongruent conditions for all ABL neurons. Height of each bar indicates the count of neurons that showed a significant positive or negative correlation between reaction time and firing rate. (B) Curves represent average population firing rate, aligned on odor port exit, for all cells that exhibited a significant positive correlation (n = 27). Activity was significantly higher for incongruent errors (thick black) than for congruent errors (thick gray; ttest; p < 0.05). There were no other differences in firing (p’s > 0.05). Thick black = Incongruent errors; Thick gray = Congruent errors; Thin black = Correct incongruent; Thin gray = Correct congruent.
DISCUSSION
Recent studies suggest that ABL is involved in detecting errors (Belova et al., 2007; Roesch et al., 2010b; Tye et al., 2010). These studies focus on prediction errors as defined as when an outcome, such as a reward, is delivered or not delivered unexpectedly on correctly performed trials. Here we hypothesized that ABL might detect errors related not just to violations in expectancies, but also to mistakes in performance as they were occurring. To induce errors we made animals respond on the minority of trials away from their response bias. Under these conflicting situations rats were significantly slower on correct trials and made more errors as compared to low conflict congruent trials.
Consistent with our hypothesis, activity in ABL increased firing prior to errant responses. Surprisingly, however, activity was not high during performance of correct incongruent trials. These two trial types were identical in that the rats were cued to respond away from the rats’ preferred direction. The only difference was whether or not the rat successfully was able to override the habitual response. This effect is different than what has been described for activity in ACC, which has been shown detect situations of high response conflict on correct trials in addition to responding when errors occur (Pardo et al., 1990; Badgaiyan & Posner, 1998; Bush et al., 1998; Carter et al., 1998; Carter et al., 2000; Cohen et al., 2000; Botvinick et al., 2001; Braver et al., 2001; van Veen et al., 2001; Ito et al., 2003; Walton et al., 2004; Amiez et al., 2005; Brown & Braver, 2005; De Martino et al., 2006; Oliveira et al., 2007; Brown & Braver, 2008; Rudebeck et al., 2008; Rushworth & Behrens, 2008). Higher activity in ACC is often correlated with better task performance suggesting that ACC is critical for signaling the need for increased attention so that ongoing behavior can be corrected. Our data suggests that ABL does not serve this function and only signals errors when it is too late to correct the current response. Such signals might be critical for processing events that follow errors so that future mistakes might not be made (Hall et al., 2005). Indeed, slower reaction times during error trials and its correlation with activity in ABL are consistent with this hypothesis.
In conclusion, we found that activity in ABL signals when an error is about to occur but does not signal the potential for errors (i.e. conflict) on correctly performed trials. Error signaling prior to the actual error has not been described previously in ABL. This likely reflects the fact that in previous work there was no way for the animal to realize that an error was about to occur because unexpected delivery and omission of reward occurred without warning (Belova et al., 2007; Calu et al., 2010; Roesch et al., 2010b; a; Tye et al., 2010). Here rats likely know, as indicated by slower reaction times, that an error was about to occur.
Together, these data suggest that ABL is involved in a trial-by-trial error assessment of performance so that behavior can be adaptive when habits have become detrimental to performance. It is unlikely that ABL is the brain area that actually marshals neural resources to resolve response conflict on the current or subsequent trials so that performance can be improved because changes in ABL have not been observed after prediction errors on subsequent trials (Belova et al., 2007; Roesch et al., 2010b; Tye et al., 2010) and activity was not high on correctly performed incongruent trials as described here. Instead, these data suggest that error signals in ABL must impact some downstream area, such as ACC, to regulate attention and resolve conflict (Ochsner et al., 2002; Dolcos & McCarthy, 2006; Etkin et al., 2006; Egner et al., 2008; Chiew & Braver, 2011).
ACKNOWLEDGEMENTS
This work was supported by grants from the NIDA (R01DA031695, MR).
REFERENCES
- Amiez C, Joseph JP, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan RD, Posner MI. Mapping the cingulate cortex in response selection and monitoring. Neuroimage. 1998;7:255–260. doi: 10.1006/nimg.1998.0326. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. A computational model of risk, conflict, and individual difference effects in the anterior cingulate cortex. Brain Res. 2008;1202:99–108. doi: 10.1016/j.brainres.2007.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden DW, Johnson EE, Diao X, Roesch MR. Impact of expected value on neural activity in rat substantia nigra pars reticulata. Eur J Neurosci. 2011;33:2308–2317. doi: 10.1111/j.1460-9568.2011.07705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Roesch MR, Haney RZ, Holland PC, Schoenbaum G. Neural correlates of variations in event processing during learning in central nucleus of amygdala. Neuron. 2010;68:991–1001. doi: 10.1016/j.neuron.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull. 1986;17:321–333. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. Neural circuitry of emotional and cognitive conflict revealed through facial expressions. PLoS One. 2011;6:e17635. doi: 10.1371/journal.pone.0017635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: who's in control? Nat Neurosci. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziewiatkowski J, Spodnik JH, Biranowska J, Kowianski P, Majak K, Morys J. The projection of the amygdaloid nuclei to various areas of the limbic cortex in the rat. Folia Morphol (Warsz) 1998;57:301–308. [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Hall G, Prados J, Sansa J. Modulation of the effective salience of a stimulus by direct and associative activation of its representation. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:267–276. doi: 10.1037/0097-7403.31.3.267. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Luthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Oliveira FT, McDonald JJ, Goodman D. Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action-outcome associations. J Cogn Neurosci. 2007;19:1994–2004. doi: 10.1162/jocn.2007.19.12.1994. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Esber GR, Schoenbaum G. All that glitters … dissociating attention and outcome expectancy from prediction errors signals. J Neurophysiol. 2010a;104:587–595. doi: 10.1152/jn.00173.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Esber GR, Schoenbaum G. Neural correlates of variations in event processing during learning in basolateral amygdala. J Neurosci. 2010b;30:2464–2471. doi: 10.1523/JNEUROSCI.5781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Singh T, Brown PL, Mullins SE, Schoenbaum G. Ventral striatal neurons encode the value of the chosen action in rats deciding between differently delayed or sized rewards. J Neurosci. 2009;29:13365–13376. doi: 10.1523/JNEUROSCI.2572-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in anterior piriform cortex versus orbitofrontal cortex during odor discrimination and reversal learning. Cereb Cortex. 2007;17:643–652. doi: 10.1093/cercor/bhk009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Bannerman DM, Rushworth MF. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci. 2008;8:485–497. doi: 10.3758/CABN.8.4.485. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Schoenbaum G, Gallagher M. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron. 2003;38:625–636. doi: 10.1016/s0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K, Sripanidkulchai B, Wyss JM. The cortical projection of the basolateral amygdaloid nucleus in the rat: a retrograde fluorescent dye study. J Comp Neurol. 1984;229:419–431. doi: 10.1002/cne.902290310. [DOI] [PubMed] [Google Scholar]
- Tye KM, Cone JJ, Schairer WW, Janak PH. Amygdala neural encoding of the absence of reward during extinction. J Neurosci. 2010;30:116–125. doi: 10.1523/JNEUROSCI.4240-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]