Abstract
Sarcomas are malignant neoplasms originating from mesodermal tissues and constitute less than 1% of body’s tumors, including those of the head and neck region. 5–15% of adult sarcomas are in the head and neck region (20% from bones and cartilages and 80% in soft tissues). Commonly encountered sarcomas in the head and neck region are - osteosarcoma, rhabdomyosarcoma, malignant fibrous histiocytoma, fibrosarcoma and angiosarcoma. This article reviews the available literature on head and neck sarcomas.
Keywords: Soft tissue tumor, Neck mass, Sarcoma
Introduction
Sarcomas are malignant neoplasms originating from mesodermal tissues that constitute connective tissues of the body [1]. They are rare group of malignancies that constitute less than 1% of body’s tumors, including those of the head and neck region [2–5]. 5–15% of adult sarcomas are in the head and neck region, while 20% of them arise from bones and cartilages and 80% arise in soft tissues [3, 6–9]. Of soft tissues sarcomas, 80–90% affect adults and 10–20% are seen in children [6]. 7% of all pediatric malignancies are soft tissue sarcomas [7]. Bone sarcomas are very rare (2600 cases are diagnosed with bone sarcoma in United States of America), constituting only less than 0.2% of malignant tumors [10]. Soft tissue sarcomas (STS), have tri-modal age distribution with peaks in less than 10 years of age, between 11 and 40 years and the last is over 40 years [6]. In head and neck region, based on histological subtyping 50% of sarcomas are: osteosarcoma, rhabdomyosarcoma, malignant fibrous histiocytoma, fibrosarcoma and angiosarcoma [6] (Fig. 1).
Fig. 1.
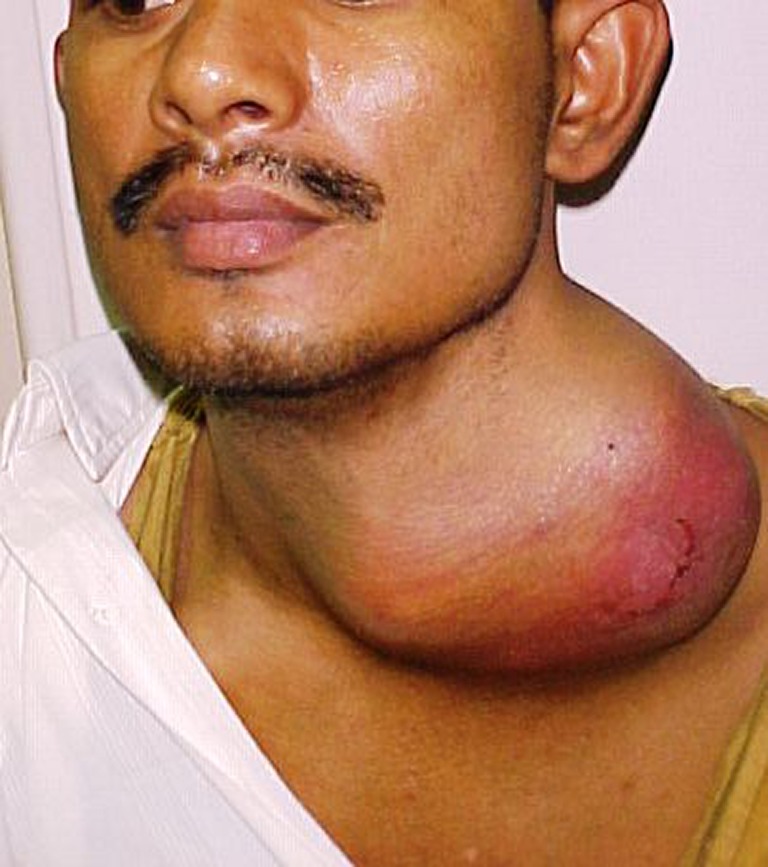
27 year old boy with soft tissue sarcoma of left neck
Etiology
The exact etiology of head and neck soft tissue sarcoma (HN-STS) is not known. However the etiology of sarcomas can be summarized as - idiopathic, genetically predisposing, radiation exposure, viral and chemical carcinogenic induced [11]. Several diseases, chemicals and viruses are considered possible causes. Genetic predisposition [12] is seen in individuals, such as those suffering from neurofibromatosis transforming to malignant peripheral nerve sheath tumor. Patients diagnosed with the Li-Fraumeni syndrome, and children with retinoblastoma who have higher chances of getting osteosarcoma, rhabdomyo-sarcoma, and fibrosarcoma. Also there is link between patients diagnosed with soft tissue sarcomas with Gardner’s syndrome and nevoid basal cell carcinoma syndrome.Radiation exposure is also a contributing factor resulting in late sarcomas. Peyton Rous showed a filterable cell-free (viral) transmission of a solid tumor sarcoma to chickens Certain human viruses are documented to be linked to sarcomas, such as HIV and Kaposi sarcoma-associated herpes virus (KSHV or HHV8)link to Kaposi sarcoma and that of leiomyosarcoma of the liver in pediatric patients to Epstein-Barr virus (EBV) infection [13, 14]. Foreign body associated sarcoma such as angiosarcoma have been documented from previously placed aortic vascular prostheses [15]. Chemicals like urethane, ethylene derivatives, and polycyclic hydrocarbons have also been reported to increase the risk of STS at sites other than head and neck region [11].
Classification
Histopathology plays a major role in classification of sarcoma [16]. Based on their origin, sarcomas are classified into: soft tissue sarcomas (STS) and bone sarcomas (BS).Due to their mesenchymal origin, sarcomas involving cartilage and nerve tissues are included in the soft tissue subcategory. Radiation induced sarcoma (RIS) is a name given to a separate category, as it can arise in soft tissues or bone (Table 1).
Table 1.
Classification of soft tissue sarcoma
| Soft tissue sarcomas |
|---|
| Angiosarcoma |
| Hemangiopericytoma |
| Synovial sarcoma |
| Chondrosarcoma |
| Rhabdomyosarcoma |
| Malignant schwannoma |
| Liposarcoma |
| Leiomyosarcoma |
| Fibrosarcoma |
| Malignant Fibrous Histiocytoma |
| Alveolar soft part sarcoma |
| Kaposi sarcoma |
| Radiation induced sarcoma |
Staging
American Joint Committee on Cancer (AJCC) and International Union Against Cancer (UICC) TNM staging system for soft tissue sarcomas is based on the tumor size, lymph nodes involvement and distant metastasis (Table 2) [17] whereas, Memorial Sloan-Kettering staging system for soft tissue sarcomas is based on size, depth of invasion and grade of the sarcoma (Table 3) [18]:
Table 2.
TNM classification
| Primary tumor (T) | Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor | |
| T1 | Tumor <5 cm in greatest dimension | |
| T1a: superficial T 1b: deep | ||
| T2 | Tumor >5 cm in greatest dimension | |
| T1a: superficial T 1b: deep | ||
| Regional lymph nodes (N) | Nx | Lymph nodes cannot be assessed |
| N0 | No lymph node metastasis | |
| N1 | Lymph node metastasis present | |
| Distant metastasis (M) | Mx | Distant metastasis cannot be assessed |
| M0 | No distant metastasis | |
| M1 | Distant metastasis present |
The grading of STS is based on histological differentiation as follow:
Gx: grade cannot be assessed
G 1: well-differentiated
G 2: moderately differentiated
G 3: poorly differentiated
G 4: undifferentiated
Combining staging and grading results in the following stage grouping:
IA (G1-2, T1a-b, N0, M0) - Low-grade, small, and superficial or deep tumor
IB (G1-2, T2a-b, N0, M0) - Low-grade, large, and superficial or deep tumor
IIA (G3-4, T1a-b, N0, M0) - High-grade, small, and superficial or deep tumor
IIB (G3-4, T2a, N0, M0) - High-grade, large, and superficial tumor
III (G3-4, T2b, N0, M0) - High-grade, large, and deep tumor
(any G, any T, N1, M0) – Lymph node metastasis
IV (any G, any T, any N, M1) - Distant metastasis
Table 3.
Memorial Sloan-Kettering staging system for soft tissue sarcomas
| Prognostic Feature | Favorable | Unfavorable |
|---|---|---|
| Size (cm) | Less than 5 cm | Greater than 5 cm |
| Depth of invasion | Superficial | Deep |
| Grade | Favorable | High |
| Stage groupings based on factors listed above | ||
| Stage 0 | 3 Favorable signs | |
| Stage I | 2 Favorable signs | |
| Stage II | 1 Unfavorable sign or 1 favorable sign and 2 unfavorable signs | |
| Stage III | 3 Unfavorable signs | |
| Stage IV | Distant metastases | |
Molecular Biology
The molecular biology of the soft tissue sarcomas has been studied but it is not completely understood. The classical techniques of molecular biology analysis, such as polymerase chain reaction (PCR), reverse transcriptase polymerase chain reaction (RT-PCR), fluorescent in situhybridization (FISH), Comparative GenomicHybridization (CGH), have been used to detect the alteration at molecular level in STS cases.
Mutation screening and gene sequencing using these techniques usually requires fresh or frozen tissue, but are increasingly applicable to formalin-fixed, paraffin-embedded material. A worse prognosis in adult soft tissue sarcomas is associated with alteration of INK4A and INK4B genes on chromosome 9p21 resulting in loss of p16 and p19 ARF function [19]. Interestingly, higher grade and poorer prognosis in extremity soft tissue sarcomas is associated with over expression of cyclin D1 [20].Fibromatosis and fibrosarcoma can be genetically differentiated from each other by the use of fluorescent in situ hybridization (FISH) technique [21]. The loss of the short arm of chromosome 17, point mutation of TP53, and homozygous loss of both alleles has been reported in soft tissue sarcomas [22].
Clinical Presentation
Soft tissue sarcomas, as a group, show a biphasic age distribution—80% to 90% affect adults, whereas 10% to 20% are seen in the pediatric age group. Age is an important determinant of histological type of soft tissue sarcoma (Table 4) [11].
Table 4.
Histology of soft tissue sarcoma based on age
| Under 10 Years | Rhabdomyosarcoma, Neuroblastoma, Fibrosarcoma |
|---|---|
| Between 11 and 40 Years | Synovial sarcoma, Epithelioid sarcoma, Clear-cell sarcoma, Nerve sheath sarcoma, Extra skeletal Ewing’s sarcoma, Alveolar soft-part sarcoma |
| Over 40 Years | Malignant fibrous histiocytoma, All other types. |
The most common symptom of head neck soft tissue sarcoma is a painless mass (in 80% of cases). Pain could be present occasionally and it is the most common presenting symptom in bone sarcomas [23]. Visual disturbance, epistaxis, chronic sinusitis, otolagia, sensory and/or motor disturbances are the other presenting features.
Workup and Investigations
The detailed history and physical examination play a major role in the diagnosis of the head and neck sarcomas.It is recommended to stage these lesions prior to any biopsy because the process of staging may show findings that change the differential diagnosis and more importantly, tissue changes after open biopsy may hamper the ability of imaging studies to define the local extent of disease [11]. Imaging plays a major role in defining the extent of the tumor to nearby vital structures for treatment planning and deciding surgical approach decision. The imaging modalities best indicated for head and neck sarcoma are similar to those elsewhere in the body. Magnetic resonance imaging (MRI) is generally superior to computed tomography scans (CT scan) in soft tissue sarcomas. CT scan is preferred to assess bone involvement. CT scan reconstruction is to be considered in the treatment planning. Due to the complex anatomy in the head and neck region, combined MRI and CT scan are recommended [11]. In working up a sarcoma case, CT scan of the chest is important to assessmetastatic lesions to the lungs. Positron-emission tomography (PET) using fluorodeoxyglucose (FDG) is a functional technique that evaluates glucose utilization by the tumor, and a significant finding seen in STS is that the higher the uptake by the tumor, the higher the grade it is and vice versa [24].
Histological examination of a biopsy specimen is the only reliable technique currently available that can lead to a definitive diagnosis [11]. The recommended biopsy techniques for the head and neck sarcomas include fine needle aspiration biopsy (FNAB) that is easy, fast and cheap technique that can be performed in clinical setting. It is good for identifying the presence of malignant cells but it is poor for grading of sarcoma. Core-needle biopsy (Tru-cut biopsy), is a good alternative as they can provide enough tissues for accurate histopathological studies and grading of head and neck sarcoma [25]. Excisional biopsies should be reserved for selected masses smaller than 3 cm in diameter [11]. If the tumor is accessible in head and neck region, an incisional biopsy can be contemplated. It allows for adequate sampling of viable tumor tissue under direct vision and ensures optimal hemostasis [11]. For deep seated tumor or those near vital neurovascular structures, in those situations, ultrasound or CT Scan guided biopsies are the recommended techniques.
Treatment of Head and Neck Sarcomas
The classical treatment modalities employed in head and neck sarcoma are: surgery, radiotherapy and/or chemotherapy. Treatment of sarcomas is dictated by tumor type, stage, location, size and patient age [26]. Due to the anatomical complexity and surrounding vital structures in the head and neck region, wide excision with adequate margin is not possible in all cases. Resection of gross tumor with post-operative adjuvant therapy is the treatment in most cases [27].
Adjuvant post-operative radiation therapy is often utilized if margins are close or the histology of the lesion is high-grade. Adjuvant brachytherapy has been shown to improve local control after complete resection of high-grade sarcomas [27, 28]. Chemotherapy alone has no role in managing HN-STS [29]
Surgery
Adequate surgical excision is not applicable in most of head and neck sarcomas due to the complex anatomy and close proximity of major vital structures to primary tumor. When the surgical margins are not adequately free, post-operative radiotherapy and / or chemotherapy should be considered [11]. Taking into consideration that neck lymph node metastasis in head and neck sarcomas is about 3%, neck dissection is indicated only in cases where palpable lymph node is identified in the neck or electively in sarcoma at high risk of nodal metastasis [11]. Lymph node metastasis from primary sarcoma in head and neck is more with embryonic rhabdomyosarcoma, epithelioid sarcoma, clear cell sarcoma, synovial cell sarcoma and vascular sarcoma [27]. Regional metastasis of head and neck sarcomas is almost exclusively in high-grade sarcomas [29]. The presence of nodal metastasis is a clear signal of distant micro-metastasis [11].
Radiation Therapy
Radiotherapy raises certain reservations in head and neck sarcoma treatment due to the well-documented radiation induced sarcoma (RIS). There is no role for elective neck irradiation prophylactically in head and neck sarcomas, due to the low incidence of positive lymph nodes in the neck, Radiotherapy is indicated as follows in head and neck sarcoma [27] for high grade sarcomas, positive surgical margins, lesions larger than 5 cm and recurrent lesions. The total recommended radiation dose for low-grade sarcomas is 6000 cGY and 6500 cGY for high-grade sarcomas. Positive margins indicate an additional 500–1000 cGY but total dose not exceeding 7500 cGY. Recurrent sarcomas are the only indication for neoadjuvant radiation therapy in head and neck sarcoma [27].
Chemotherapy
Soft tissue sarcoma in head and neck region responds very well to chemotherapy as their counterparts in other parts in the body [30]. It provides improved local control especially if combined with radiation therapy where wide excision is not possible. It is also indicated for high-grade soft tissue sarcomas along with radiation therapy and surgery [27]. Pre-operative chemo-radiotherapy has been tried to shrink large soft tissue sarcomas specially those near vital structures prior to surgery [30]. The indications to chemotherapy in the head and neck sarcoma are unresectable head and neck sarcoma, sarcomas with extension to the unusual locations(skull base) and aggressive sarcomas [27].
Prognosis
The 5 years survival rate for head and neck sarcoma is between 49 and 55% [27, 29, 30]. Two factors that play a major role in survival in head and neck sarcomas are local control and distant metastasis. The size of the primary tumor does not correlate with the local outcome. The risk of distant metastasis especially with high-grade sarcomas of the head and neck necessitates the need for complete screening protocols. The lung is the most common site of distant metastasisin head and neck sarcomas.
Conclusions
Head and neck sarcomas are group of malignant neoplasms that affect critical structural units of head and / or neck that can result in grave consequences if they are not diagnosed and managed properly in timely fashion.Proper biopsy and diagnostic imaging following a complete history and physical examination leads to correct diagnosis and successful treatment of the sarcoma. The emerging molecular biology studies are promising area of future research with potential influence on early detection and prognosis of head and neck sarcomas. Multidisciplinary management of HN-STS cases requires establishment and execution of detailed treatment plan in a coordinated timely manner for successful local-regional control with minor functional and/or cosmetic deficits as result of treatment.
References
- 1.Gorsky M, Epstein JB. Craniofacial osseous and chondromatous sarcomas in British Columbia, a review of 34 cases. Oral Oncol. 2000;36:27–31. doi: 10.1016/S1368-8375(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 2.Sturgis EM, Potter BO. Sarcomas of the head and neck region. Curr Opin Oncol. 2003;15:239–252. doi: 10.1097/00001622-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Wanebo HJ, Koness RJ, MacFarlane JK, Elber FR, Byers RM, Elias G, Spiro RH. Head and neck sarcoma: report of the head and neck sarcoma registry. Head Neck. 1992;14:1–7. doi: 10.1002/hed.2880140102. [DOI] [PubMed] [Google Scholar]
- 4.Pellitteri PK, Ferlito A, Bradley PJ, Shaha AR, Rinaldo A. Management of sarcomas of the head and neck in adults. Oral Oncol. 2003;39:2–12. doi: 10.1016/S1368-8375(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 5.Gorsky M, Epstein JB. Head and neck and intra-oral soft tissue sarcomas. Oral Oncol. 1998;34:292–296. [PubMed] [Google Scholar]
- 6.Kraus DH, Dubner S, Harrison LB, et al. Prognostic factors for recurrence and survival in head and neck soft tissue sarcomas. Cancer. 1994;74:697–702. doi: 10.1002/1097-0142(19940715)74:2<697::AID-CNCR2820740224>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Andrassy RJ. Advances in the surgical management of sarcomas in children. Am J Surg. 2002;184:484–491. doi: 10.1016/S0002-9610(02)01100-5. [DOI] [PubMed] [Google Scholar]
- 8.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS, Evans HL. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservationsurgery and radiation therapy. Cancer. 2003;97:2530–2543. doi: 10.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]
- 9.Tran LM, Mark R, Meier R, Calcaterra TC, Parker RG. Sarcomas of the head and neck. Prognostic factors and treatment strategies. Cancer. 1992;70:169–177. doi: 10.1002/1097-0142(19920701)70:1<169::AID-CNCR2820700127>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ, et al. Cancer statistics. CA Can J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 11.Patel SG, Shaha AR, Shah JP. Soft tissue sarcoma of the head and neck: an update. Am J Otolaryngol. 2001;22(1):2–18. doi: 10.1053/ajot.2001.20699. [DOI] [PubMed] [Google Scholar]
- 12.Zahm SH, Fraumeni JF., Jr The epidemiology of soft tissue sarcoma. Sem Oncol. 1997;24:504–514. [PubMed] [Google Scholar]
- 13.Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342(14):1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- 14.Timmons C, Dawson D, Richards C, Andrews W, Katz J. Epstein-Barr virus-associated leiomyosarcomas in liver transplantation recipients. Origin from either donor or recipient tissue. Cancer. 1995;76:1481–1489. doi: 10.1002/1097-0142(19951015)76:8<1481::AID-CNCR2820760828>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 15.Weiss W, Riles T, Gouge T, Mizrachi H. Angiosarcoma at the site of a Dacron vascular prosthesis: a case report and literature review. J Vasc Surg. 1991;14:87–91. doi: 10.1016/0741-5214(91)90158-Q. [DOI] [PubMed] [Google Scholar]
- 16.Shellenberger TD, Sturgis EM. Sarcomas of the head and neck region. Curr Oncol Rep. 2009;2:135–142. doi: 10.1007/s11912-009-0020-8. [DOI] [PubMed] [Google Scholar]
- 17.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 7. Hoboken: Wiley-Blackwell; 2009. pp. 157–161. [Google Scholar]
- 18.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg. 1988;12:326–331. doi: 10.1007/BF01655665. [DOI] [PubMed] [Google Scholar]
- 19.Orlow I, Drobnjak M, Zhang ZF, Lewis J, Woodruff JM, Brennan MF, Cordon-Card C. Alternation of INK4A and INK4B genes in adult soft tissue sarcoma, effect on survival. J Natl Cancer Inst. 1999;91:73–79. doi: 10.1093/jnci/91.1.73. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Lewis JJ, Brennan MF, Woodruff JM, Dudas M, Cordon-Cardo C. Overexpression of cyclin D1 in association with poor prognosis in extremity soft tissue sarcoma. Clin Cancer Res. 1998;4:2377–2382. [PubMed] [Google Scholar]
- 21.Schofield D, Fletcher JA, Grier HE, Yunis EJ. Fibrosarcoma in infants and children. Am J Surg Pathol. 1994;18:14–24. doi: 10.1097/00000478-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hogendroon PCW, Collin F, Daugaard S, Dei Tos AP, Fisher C, Schneider U, Sciot R. Changing concepts in pathological basis of soft tissue and bone sarcoma treatment. Eur J Cancer. 2004;40:1644–1654. doi: 10.1016/j.ejca.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Wanebo HJ. Head and neck sarcoma. In: Johnson JT, Didolker MS, editors. Head and neck cancer. Amsterdam: Elsevier Science; 1993. pp. 39–47. [Google Scholar]
- 24.Nieweg O, Hoekstra H, Pruim J (1994) In vivo grading of soft tissue sarcomas with positron emission tomography. Proc Ann Meet Soc Surg Oncol 225
- 25.Heslin MJ, Lewis JJ, Woodruff JM, Brennan MF. Core needle biopsy for diagnosis of extremity soft tissue sarcoma. Ann Oncol. 1997;4:425–431. doi: 10.1007/BF02305557. [DOI] [PubMed] [Google Scholar]
- 26.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 27.Tran LM, Mark R, Meier R, Calcaterra TC, Parker RG. Sarcoma of the head and neck, prognostic factors and treatment strategies. Cancer. 1992;70:169–177. doi: 10.1002/1097-0142(19920701)70:1<169::AID-CNCR2820700127>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Farhood AI, Hajdu SI, Shiu MH, Strong EW. Soft tissue sarcomas of the head and neck in adults. Am J Surg. 1990;160:365–369. doi: 10.1016/S0002-9610(05)80544-6. [DOI] [PubMed] [Google Scholar]
- 29.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of extremity. N Engl J Med. 1986;314:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 30.Jeannon JP, Irish J, Osullivan B, Goh C, Brown DH, Neligan P, Gullane P. Adult soft tissue sarcomas of the head and neck. Asian J Surg. 2002;25:5–12. [PubMed] [Google Scholar]


