Abstract
Although the cochlea is an amplifier and a remarkably sensitive and finely tuned detector of sounds, it also produces conspicuous mechanical and electrical waveform distortions1. These distortions reflect non-linear mechanical interactions within the cochlea. By allowing one tone to suppress another (masking effect), they contribute to speech intelligibility2. Tones can also combine to produce sounds with frequencies not present in the acoustic stimulus3. These sounds compose the otoacoustic emissions that are extensively used to screen hearing in newborns. As both cochlear amplification and distortion originate from the outer hair cells, one of the two types of sensory receptor cells, it has been speculated that they stem from a common mechanism. Here, the non-linearity underlying cochlear waveform distortions is shown to rely on the presence of stereocilin, a protein defective in a recessive form of human deafness4. Stereocilin was detected in association with horizontal top connectors5-7, lateral links that join adjacent stereocilia within the outer hair cell’s hair bundle, and these links were absent in stereocilin-null mutant mice. These mice become progressively deaf. At the onset of hearing, however, their cochlear sensitivity and frequency tuning were almost normal, although masking was much reduced and both acoustic and electrical waveform distortions were completely lacking. From this unique functional situation, we conclude that the main source of cochlear waveform distortions is a deflection-dependent hair bundle stiffness resulting from constraints imposed by the horizontal top connectors, and not from the intrinsic non-linear behaviour of the mechanoelectrical transducer channel.
The cochlea is a highly-sensitive and sharply-tuned sound detector. It contains two types of sensory cells: outer hair cells (OHCs) that locally amplify and sharpen sound-induced mechanical stimulation of the cochlear partition, and inner hair cells (IHCs) that transmit sensory information to the brain. OHCs have been proposed to supply forces for amplification by changing the length and stiffness of their lateral wall in response to changes in membrane potential (a process known as electromotility)8-11, or by active movement of their apically-located mechanosensory hair bundle12,13. The OHC hair bundle is composed of actin-filled stereocilia arrayed in three rows of increasing height. The tallest stereocilia are embedded in the tectorial membrane, an acellular gel overlying the sensory epithelium. The stereocilia are coupled together by the tip link, that extends from the tip of a stereocilium to the side of the adjacent taller one and may gate the mechanoelectrical transducer (MET) channels12, and by zipper-like horizontal top connectors that join the upper parts of adjacent stereocilia within and between rows5-7 (supplementary Fig. 1a).
Mutations in the gene encoding stereocilin (supplementary Fig. 1b), a protein of the hair bundle, cause prelingual hearing impairment in man4. We engineered mutant mice with a frame-shifting deletion in the stereocilin gene (supplementary Fig. 2). In postnatal day 60 (P60) stereocilin-null (Strc−/−) mice, auditory brainstem responses to tones in the 5-40 kHz frequency range showed increased thresholds, up to 60 dB (data not shown). As these threshold shifts suggested a possible failure of the cochlear amplifier14,15, we studied cochlear sensitivity, frequency selectivity, as well as waveform distortions and masking interactions among spectral components of a complex sound15, all ascribed to the functioning of the OHCs.
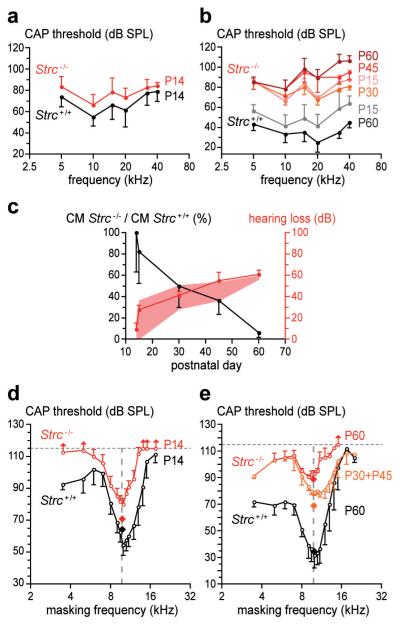
The signal recorded by a round-window electrode contains the compound action potential (CAP) from the cochlear nerve, representing synchronous neural activity in response to short tone bursts. CAP thresholds, by assessing cochlear sensitivity, allow estimation of amplification due to OHCs, normally ~60 dB at frequencies > 10 kHz16. P14-15 mice still have immature CAP thresholds (Fig. 1a vs 1b), but their mechanical and electrical cochlear responses are adult-like (see Figs 1d, 1e, 2a), indicating the immaturity of their CAP thresholds is likely to be neural in origin. At P14, the difference between CAP thresholds in Strc−/− mice and those of Strc+/+ mice was not statistically significant, less than 10 dB on average in the 5-40 kHz range (Fig. 1a, supplementary Fig. 3). From P15 on, progressive hearing loss appeared in Strc−/− mice, increasing from a flat 25 dB to a ~60-dB ceiling above 10 kHz at P60 (Fig. 1b, supplementary Fig. 3).
Figure 1. Hearing sensitivity and frequency tuning in stereocilin-null versus wild-type mice.
(a) Mean CAP thresholds (±sd; n=7) in P14 mice. The difference between Strc+/+ and Strc−/− mice is non-significant (p>0.05). (b) Mean CAP thresholds (±sd; n=5) in P15 to P60 mice. (c) Ratio of CM amplitudes in Strc−/− vs Strc+/+ mice at low frequencies (black curve), with predicted (pink shading) and actual (red curve) hearing loss. Differences, predicted vs actual, are non-significant (p>0.05). (d) CAP tuning curves in P14 mice (±sd; n=7). Q10 dB (ratio of test frequency to CAP tuning curve width at 10 dB above its tip) is similar in Strc−/− (Q10 dB = 3.1) and Strc+/+ (Q10 dB = 3.2) mice. (e) CAP tuning curves (±sd) in P60 Strc+/+ mice (Q10 dB = 3.4, n=5) and Strc−/− mice (P30 + P45, Q10 dB = 2.2, n=4; P60, Q10 dB = 3.1, n=4). Diamonds indicate test-tone levels. Vertical arrows indicate lack of masking in some animals for a masking-tone level of 115 dB SPL.
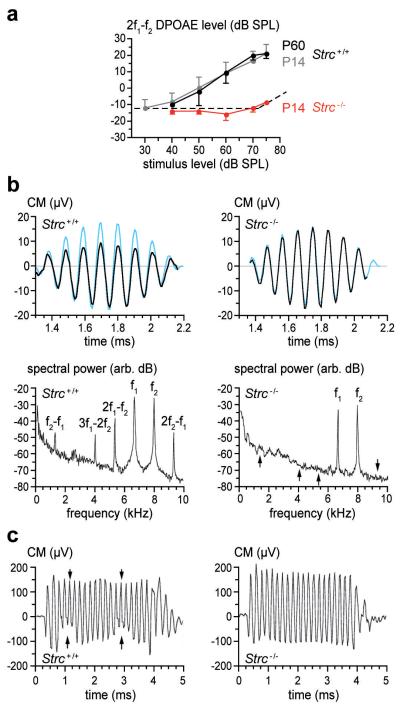
Figure 2. Waveform distortions in wild-type and stereocilin-null mice.
(a) DPOAE amplitude (mean ±sd) at frequency 2f1-f2 as a function of sound pressure level common to stimuli f1 and f2 (f2/f1 = 1.20) in Strc+/+ (P14, P60) and Strc−/− (P14) mice (n=8). Dotted line: noise floor. (b) Temporal waveforms and frequency spectra (black lines) of the CM in P14 mice, in response to one cycle of the beat of a pair of pure-tone stimuli (6.7 and 8 kHz, 65 dB SPL each, blue traces in upper panels, scaled to the CM response of the Strc+/+ ear). Arrows in Strc−/− CM frequency spectrum mark positions of intermodulation frequencies. (c) Amplitude modulation (arrows) of the CM response to a 5 kHz, 70 dB tone-burst by two periods of a 95 dB SPL infrasound (550 Hz), in P14 mice.
The round-window electrode also collects the cochlear microphonic potential (CM), a phasic response reflecting MET currents from basal-coil OHCs. As low-frequency tones are not amplified by basal-coil OHCs, the amplitude of the CM response to these tones is proportional to the number of functional MET channels17. This number gradually decreased from P15 to P60 in Strc−/− mice (Fig. 1c). Models of active OHC operation (supplementary Fig. 4) predict how much gain the residual active MET channels would generate17. A statistically significant difference was not found between the predicted and observed auditory thresholds (Fig. 1c), which suggests that decreased number of functional MET channels in OHCs accounts for the increased CAP thresholds in Strc−/− mice.
How well OHCs determine frequency selectivity, a task dependent on amplification, can be addressed by measuring how a continuous masker tone at varying frequency and level affects the CAP response to a test tone-burst presented simultaneously and just above threshold18. Masking occurs when the masker enters the frequency band of the cochlear filter centred on the test tone. We studied masking at 10 kHz (Fig. 1d,e) and 20 kHz (not shown). In P14 Strc−/− mice, CAP tuning curves revealed the persistence of fine frequency selectivity, as the quality factor of tuning, Q10 dB, remained similar to that of controls. CAP tuning curves also provide information on the strength of masking: the more upshifted their tip relative to the test tone level, the weaker the masking. Masking was weakened in P14 Strc−/− mice (Fig. 1d). Despite similar probe-tone levels, high-frequency maskers had to be >20 dB louder than in controls to provide the same CAP decrease. Simultaneous masking is due to two mechanisms2,19. One is neuronal and due to the masker producing action potentials that place neurons in the refractory period, and therefore unable to respond to the test tone (line-busy masking). Since stereocilin was not detected in the cochlear ganglion neurons (data not shown), this process should be preserved in Strc−/− mice. The other mechanism, suppressive masking, is due to non-linear interactions between masker and test tones decreasing the mechanical response to the test tone. This suppressive masking shows up in the basilar membrane mechanical response18. Of note, the extent of the decrease of the masking strength observed in Strc−/− mice is reminiscent of the suppression of the chinchilla basilar membrane motion induced by a high- or low-frequency tone at levels similar to those used here20. This suggests Strc−/− mice totally lack suppressive interactions, the persisting masking thus being due to the line-busy mechanism only.
The mechanical non-linearity that contributes to masking may also distort acoustic waveforms, so that in response to two-tone stimuli (frequencies f1, f2), distortion-product otoacoustic emissions (DPOAEs) at intermodulation frequencies (2f1-f2, f2-f1, etc) are propagated backward and detected as combination tones in the ear canal21,22. From P14 on, control mice presented DPOAE components well above the noise floor. In contrast, Strc−/− mice had no detectable DPOAEs up to 80 dB SPL (Fig. 2a).
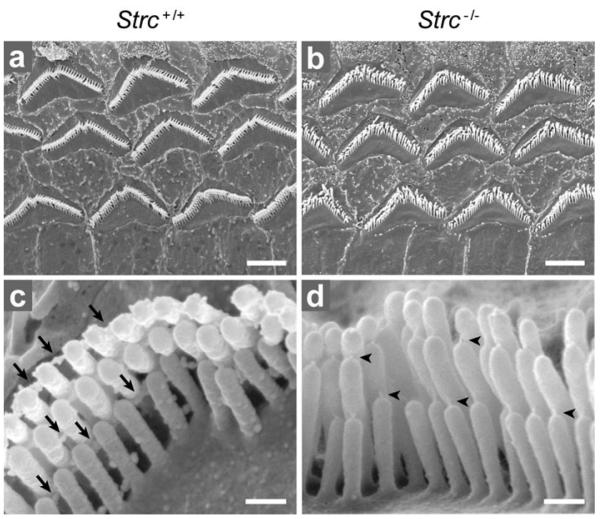
The complete absence of suppressive masking and DPOAEs in Strc−/− mice as early as P14, despite normal hearing thresholds and tuning curves, prompted a search for electrical distortion products. These show up as a levelling out of the CM waveforms that originate from MET currents through OHCs (Fig. 2b). In response to tonal stimuli at frequency f in P14 control mice, CM exhibited harmonics 2f and 3f at about −30 dB relative to the levels of the fundamental frequency (not shown). For two-tone stimuli with neighbouring frequencies f1 and f2, P14 control mice exhibited CM waveforms with multiple inter-modulation components reaching −12 to −15 dB relative to stimulus levels (Fig. 2b). Finally, when an infrasound bias (<0.6 kHz) was mixed with an audible frequency (5-10 kHz), the CM in P14 control mice showed periodic suppressive amplitude modulation, even for biasing levels as low as 85 dB SPL (Fig. 2c). In contrast, P14 Strc−/− mice showed none of these CM waveform distortions whatever the intensity of the acoustic stimulation up to 100 dB SPL (Fig. 2b,c). Histological examination of the cochlea in P14 Strc−/− mice did not reveal any gross structural anomalies. Scanning electron microscopy showed the tectorial membrane extended across the sensory epithelium, as in wild-type mice. Hair-bundle imprints corresponding to the anchoring points of the tallest OHC stereocilia23 were, however, not observed (not shown). Nonetheless, CM waveforms had the same phase relative to sound in Strc−/− and control mice (supplementary Fig. 5) indicating that, in both cases, OHCs were driven by the displacement of the cochlear partition, and not its velocity as they are in TectaΔENT/ΔENT mice, in which the tectorial membrane is detached from the sensory epithelium24. Strikingly, in all OHCs, but not in IHCs, the tops of the stereocilia rows were less clearly aligned in Strc−/− compared to Strc+/+ mice (Fig. 3b). In keeping with the apparently loose connection between adjacent stereocilia, the horizontal top connectors that normally develop from P9 on and are fully mature at P147 could not be detected in P14 Strc−/− OHCs by scanning (Fig. 3d) and transmission (not shown) electron microscopy. By contrast, the tip links were still present (Fig. 3d).
Figure 3. OHC hair bundle morphology in P14 wild-type and stereocilin-null mice.
(a, b) Low-magnification scanning electron micrographs showing the three rows of OHCs. The tops of stereocilia are less clearly aligned in the Strc−/− mouse (b) than in the Strc+/+ mouse (a). (c, d) Detailed views of single OHC hair bundles. Top connectors (arrows in c) are not detected in the Strc−/− mouse, while tip links (arrowheads in d) are present. Scale bars: 2.5 μm (a, b), 0.25 μm (c, d).
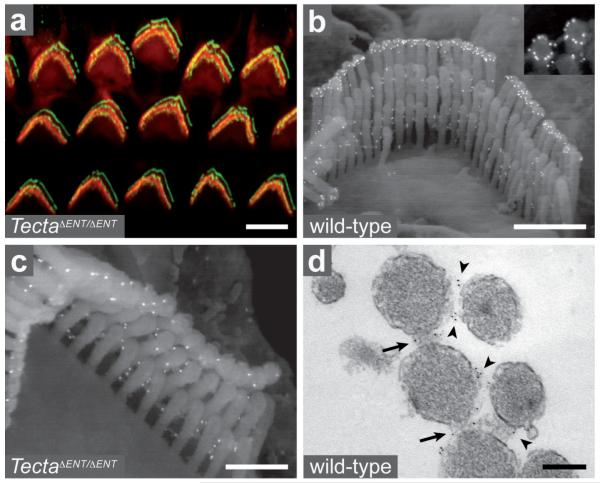
The distribution of stereocilin was analysed in P14 wild-type mice, and compared to that in TectaΔENT/ΔENT mice24. Stereocilin was detected in the hair bundles of OHCs only. It was present in the distal regions of all stereocilia rows, in both wild-type and TectaΔENT/ΔENT mice (Fig. 4a and data not shown). Scanning immunoelectron microscopy revealed stereocilin was distributed in a ring around the tip of each stereocilium from the tallest row in the OHCs of wild-type mice, but not of TectaΔENT/ΔENT mice (Fig. 4b,c), suggesting stereocilin is involved in the contact these stereocilia establish with the tectorial membrane. In addition, stereocilin was detected between all OHC stereocilia, both in wild-type and TectaΔENT/ΔENT mice (Fig. 4b,c). The protein was found to be associated with the horizontal top connectors by transmission immunoelectron microscopy (Fig. 4d). In OHCs, these links are characterised by a central density (refs. 5-7 and supplementary Fig. 1a) that may be contributed by stereocilin, predicted to be an extracellular protein according to its amino-acid sequence.
Figure 4. Immunodetection of stereocilin in wild-type and TectaΔENT/ΔENT OHCs.
(a) Confocal image of TectaΔENT/ΔENT OHC hair bundles (P14) stained for stereocilin (green) and actin (red). (b, c) Immunogold scanning electron micrographs of wild-type and TectaΔENT/ΔENT OHC hair bundles (P14) labelled for stereocilin. A ring-shaped labelling is seen around the tip of the tallest stereocilia in wild-type (b, inset) but not TectaΔENT/ΔENT mice (c). In contrast, the labelling between stereocilia is seen both in the wild-type and TectaΔENT/ΔENT mice. (d) Transmission electron micrograph of a stereocilin-labelled OHC hair bundle (P22, transverse section). Top connectors within (arrows) and between (arrowheads) rows are labelled. Scale bars: 5 μm (a), 1 μm (b), 0.5 μm (c), 0.2 μm (d).
The presence of DPOAEs in TectaΔENT/ΔENT mice25 indicates the tectorial membrane is not essential for DPOAE production. The absence of all intermodulation and harmonic components in the responses of P14 Strc−/− mice, despite preserved amplification and tuning, thus suggests that their OHC hair bundles lack the very element at the origin of cochlear waveform distortions. This element is commonly thought to be the MET channel16,26 for the following two reasons. Firstly, the MET current relates to hair bundle deflection via the Boltzmann’s distribution of the MET channels’ open probability27. This sigmoidal transfer function converts sinusoidal hair bundle deflection into distorted MET currents. Secondly, the thermodynamics of shuffling of the MET channels from open to closed states introduces a non-linear, deflection-dependent stiffness16,28. In Strc−/− mice, one might assume that the MET transfer function has become linear over the waveform-distortion-free 30-100 dB SPL range. The corollary of this, however, is a reduction of the energy difference between the open and closed states relative to the thermal energy kT26 that would then prevent MET channels from operating normally, which they do in P14 Strc−/− mice. The non-linear source of all cochlear waveform distortions that also permits suppressive masking should thus come not from the intrinsic working properties of the MET channels, but from a non-conductive hair-bundle stiffness impinging the response of the MET channels. Being the missing structure in waveform-distortion-free Strc−/− mice, horizontal top connectors qualify as an important source of non-linear stiffness, either directly or indirectly by providing the necessary condition for another mechanical element to operate non-linearly. The bending of neighbouring stereocilia toward each other that is observed when stereocilin-associated lateral links extend further towards the stereocilia base in mouse models of Usher syndrome type I (data not shown and supplementary Fig. 6) supports the importance of these links in hair bundle non-conductive stiffness. The lateral links and pivots of OHC stereocilia are well-acknowledged contributors to the component of the hair bundle stiffness derived from the restoring force. Being reportedly large13,27,29,30, this stiffness component would indeed provide a more suitable basis for the large waveform distortions and the strong suppressive masking observed in normal cochleae, than the conductive stiffness related to the MET channels.
METHODS SUMMARY
Production of Strc knockout mice (Supplementary Fig. 2)
Stereocilin knockout mice (Strctm1Ugds/tm1Ugds, referred to as Strc−/−) were produced using the Cre-lox system. We engineered mice in which exons 2 and 3 of Strc were floxed (loxP-Strc). Strc−/− mice were obtained by crossing these mice with a transgenic mouse expressing the Cre recombinase early and ubiquitously.
Recordings of cochlear microphonics, compound action potentials, and otoacoustic emissions
An electrode was placed in the round-window niche of anaesthetised mice. The receptor currents of basal sensory cells and the synchronous activity of the cochlear nerve were recorded in response to tone-bursts and mixtures of tone-bursts and interfering tones. The sensitivity, frequency selectivity, susceptibility to masking and ability of waveforms to exhibit distortion were compared in Strc−/− vs Strc+/+ mice. A miniature microphone probe in the external ear canal allowed combination tones in response to two-tone stimuli to be recorded as otoacoustic emissions.
Histological analysis and immunolocalisation
The morphology of the cochlear sensory epithelia was studied in Strc+/+ and Strc−/− mice using scanning electron microscopy. For immunolocalisation of stereocilin, we used two affinity-purified polyclonal rabbit antibodies, anti-B and anti-D, raised against the synthetic peptides CFLSPEELQSLVPLSD (amino-acids 970-985) and EQLAYLSPEQRRAVA (amino-acids 1753-1767) derived from the mouse stereocilin sequence (AF375593), respectively (Supplementary Fig. 1b). The distribution of stereocilin was analysed using confocal immunofluorescence microscopy, scanning and transmission immunoelectron microscopy.
METHODS
Generation of Strctm1Ugds/tm1ugds knockout mice (Supplementary Fig. 2)
We designed a targeting vector in which exons 2 and 3 of Strc and the hygromycin selection cassette were flanked with loxP sites. Electroporation of the linearised vector into CK 35 embryonic stem (ES) cells31 (derived from a 129/Sv mouse embryo) plated on hygromycin selective medium resulted in approximately 300 recombinant ES cell clones, of which five were correctly targeted. Two independent recombinant ES cell lines carrying the floxed allele were selected and injected into C57BL/6J host blastocysts. Mating of male chimeras with C57BL/6J females produced heterozygous animals. Integration of the recombinant DNA construct was confirmed by Southern blot analysis and PCR amplification of genomic DNA extracted from mice’s tails. For Southern blot analysis, genomic DNA was digested with Bgl II and transferred on Immobilon-NY+ (Millipore) membrane. The membrane was probed with a PCR-amplified fragment obtained by using primers 5′-GAGCTTCTGTCCAGTGATAGTTCAG-3′ and 5′-TGCTTAGGAAGCTTTCTGCAGCATGGG-3′. The PCR primers used to genotype the floxed Strc allele were HR (5′-TGGACGTAAACTCCTCTTCAGACC-3′), located in the hygromycin resistance gene, and Strc-R1 (5′-AGGCTGAGCCCACAGCACAAAG-3′), located in Strc intron 4 outside the targeting vector. Mice heterozygous for the floxed Strc allele were mated with the PGK-Crem transgenic mouse strain carrying the Cre recombinase gene driven by the early acting phosphoglycerate kinase-1 gene promoter32. Targeted deletion of exons 2 and 3 was confirmed by PCR analysis. Routine genotyping of animals for the Strctm1Ugds allele was carried out by two PCR amplifications, using forward primer Strc-F1 (5′-GGGCTCTGAGGAGGCTCTTTGGG-3′), located in exon 2, or Strc-F2 (5′-TGGGATTTGAACTCAGGTTGCTAGG-3′), located in intron 1, and reverse primer Strc-R2 (5′-CAGAGGCACACCTCTGCTCAGG-3′), located in exon 4. All studies were performed on mixed C57BL/6J-129/Sv genetic backgrounds.
For RT-PCR analysis, total RNA was isolated from single inner ears of P15 Strc+/+, Strc+/− and Strc−/− mice, and forward primer 5′-53-TCTAGGCCCAGTGTGCACCT, located in exon 1, and reverse primer 5′-1192-GGCAGAGCAAGTAGATGGAGAAGTTGG, located in exon 4, were used for amplification. The amplicons were gel-purified and sequenced.
Recordings of cochlear microphonics (CM), compound action potentials (CAP) and distortion-product otoacoustic emissions (DPOAE)
For CAP, CM and DPOAE measurements, mice were anaesthetised with ketamine and levomepromazin. CAP and CM were collected between a silver electrode inserted in the round window niche and a vertex needle electrode. CAP and CM were evoked by tone-bursts (2-period rise–fall, 20-period plateau, repetition rate 20/s, sound level varying from 10 to 115 dB SPL in 5 dB steps). Masking and biasing were produced by adding to the test tone-burst a continuous pure tone with variable frequency and level (2.5 dB steps). The response from the electrodes was amplified (gain 5,000), filtered (0.02–30 kHz), digitally converted and averaged using a computerised data-acquisition system. Visual inspection was used to determine the CAP thresholds, or that CAP amplitude was halved in the presence of an increasingly loud masker. The DPOAE at frequency 2f1-f2 was recorded in response to two equal level primary tones, f1 and f2, with f2/f1 = 1.20. Ear-canal sound pressure was amplified (x100) and averaged (0.5 s). The amplitudes of the DPOAE at 2f1-f2 and of the background noise were extracted by Fast-Fourier transform. For CM waveform distortion measurements, the response from the electrode to one or two primary tones (levels ranging from 50 to100 dB SPL) was Fourier-transformed and analysed at harmonic and intermodulation frequencies. Statistical differences were evaluated using Student’s t-test.
Antibodies and immunolabelling studies
Two rabbit immune sera, anti-B and anti-D, were produced against synthetic peptides B (NH2-970-CFLSPEELQSLVPLSD-COOH) and D (NH2-1753-EQLAYLSPEQRRAVA-COOH) derived from the mouse stereocilin amino-acid sequence, respectively (see supplementary Fig. 1b). The antibodies were affinity-purified on the corresponding peptides, and their specificity was verified by immunoblotting and immunostaining of transfected cells (Hela, Cos7, MDCK) producing full-length stereocilin, and by the loss of the inner ear immunolabelling in Strc−/− mice. Both antibodies gave similar staining patterns, but immunolabelling with anti-D required a pretreatment with methanol (Triton X-100 was ineffective), which precluded subsequent phalloidin staining. The results presented are those obtained with the anti-B antibody. For immunofluorescence detection, we used Alexa Fluor 488-conjugated goat anti-rabbit F(ab’)2 IgG fragment (1:800, Molecular Probes). Actin was labelled with TRITC-conjugated phalloidin (1 μg/ml, Sigma/Aldrich). Samples were processed as described33, with the following modifications. Inner ears were fixed immediately upon removal by immersion in 4% paraformaldehyde in PBS pH 7.4 (PFA/PBS) for 30 min at room temperature. After three rinses in PBS, cochlear sensory areas were microdissected and re-fixed in PFA/PBS for 30 min. For subsequent immunolabelling with anti-D, tissues were immersed in methanol (−20°C) for 3 to 5 min. The tissues were preincubated in PBS containing 20% goat serum for 1 hour at room temperature, prior to overnight incubation with the anti-stereocilin antibodies (~1 μg/ml) in PBS containing 1% bovine serumalbumine (PBS/BSA). Whole mount preparations were analysed with a laser scanning confocal microscope (LSM-510, Zeiss). Immunolabelling for SEM was done as for immunofluorescence detection. Primary antibodies were detected with protein A-conjugated 15 nm colloidal gold particles (EM Lab, Utrecht, The Netherlands; diluted 1/60 in PBS/BSA). Finally, samples were post-fixed for 1 hour in 2.5% glutaraldehyde in PBS before proceeding to SEM.
- 31.Kress C, Vandormael-Pournin S, Baldacci P, Cohen-Tannoudji M, Babinet C. Nonpermissiveness for mouse embryonic stem (ES) cell derivation circumvented by a single backcross to 129/Sv strain: establishment of ES cell lines bearing the Omd conditional lethal mutation. Mamm. Genome. 1998;9:998–1001. doi: 10.1007/s003359900914. [DOI] [PubMed] [Google Scholar]
- 32.Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- 33.Michel V, et al. Cadherin 23 is a component of the transient lateral links in the developing hair bundles of cochlear sensory cells. Dev. Biol. 2005;280:281–294. doi: 10.1016/j.ydbio.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Delmaghani S, et al. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat. Genet. 2006;38:770–778. doi: 10.1038/ng1829. [DOI] [PubMed] [Google Scholar]
Scanning electron microscopy
Samples were processed as described34. When immunolabelling was coupled to SEM, dried specimens were mounted on colloidal silver adhesive (Quick drying silver paint, Agar Scientific) to enhance their conductivity. Specimens were then coated with 30 nm of carbon and imaged using a Yttrium Aluminium Garnet (YAG) detector.
Transmission electron microscopy
For immunogold electron microscopy, pre-embedding labelling using the anti-B antibody was done as described33. Procedures for transmission electron microscopy were as described7.
Supplementary Material
Acknowledgements
We thank M. Lenoir, S. Guadagnini and M.-C. Prévost for advice on scanning electron microscopy, E. Perret and P. Roux for advice on confocal microscopy, S. Chardenoux, S. Nouaille and A. Mallet for technical help, Y. Lallemand for providing us with PGK-Crem mice, N. Michalski for help with figure drawing and J. Boutet de Monvel for critical reading of the manuscript. This work was supported by the European Commission FP6 Integrated Project EuroHear, Fondation Raymonde et Guy Strittmatter, Région Ile-de-France (Programme Sésame), and the Wellcome Trust.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
REFERENCES
- 1.Goldstein JL. Auditory nonlinearity. J. Acoust. Soc. Am. 1967;41:676–689. doi: 10.1121/1.1910396. [DOI] [PubMed] [Google Scholar]
- 2.Delgutte B. Physiological models for basic auditory percepts. In: Hawkins HH, McMullen TA, Popper AN, Fay RR, editors. Auditory Computation. Springer; New York: 1996. pp. 157–220. [Google Scholar]
- 3.Kemp DT. Stimulated acoustic emissions from within the human auditory system. J. Acoust. Soc. Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- 4.Verpy E, et al. Mutations in a new gene encoding a protein of the hair bundle cause non-syndromic deafness at the DFNB16 locus. Nat. Genet. 2001;29:345–349. doi: 10.1038/ng726. [DOI] [PubMed] [Google Scholar]
- 5.Furness DN, Hackney CM. Cross-links between stereocilia in the guinea pig cochlea. Hear. Res. 1985;18:177–188. doi: 10.1016/0378-5955(85)90010-3. [DOI] [PubMed] [Google Scholar]
- 6.Tsuprun V, Santi P. Structure of outer hair cell stereocilia side and attachment links in the chinchilla cochlea. J. Histochem. Cytochem. 2002;50:493–502. doi: 10.1177/002215540205000406. [DOI] [PubMed] [Google Scholar]
- 7.Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J. Comp. Neurol. 2005;485:75–85. doi: 10.1002/cne.20513. [DOI] [PubMed] [Google Scholar]
- 8.Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- 9.Ashmore JF. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J. Physiol. 1987;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He DZ, Dallos P. Somatic stiffness of cochlear outer hair cells is voltage-dependent. Proc. Natl. Acad. Sci. USA. 1999;96:8223–8228. doi: 10.1073/pnas.96.14.8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberman MC, et al. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- 12.Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature. 2005;433:880–883. doi: 10.1038/nature03367. [DOI] [PubMed] [Google Scholar]
- 14.Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J. Neurophysiol. 1978;41:365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- 15.Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear. Res. 1984;16:55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- 16.Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol. Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patuzzi RB, Yates GK, Johnstone BM. Outer hair cell receptor current and sensorineural hearing loss. Hear. Res. 1989;42:47–72. doi: 10.1016/0378-5955(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 18.Dallos P, Cheatham MA. Compound action potential (AP) tuning curves. J. Acoust. Soc. Am. 1976;59:591–597. doi: 10.1121/1.380903. [DOI] [PubMed] [Google Scholar]
- 19.Moore BCJ. Cochlear hearing loss. Whurr Publishers Ltd; London: 1998. [Google Scholar]
- 20.Ruggero MA, Robles L, Rich NC. Two-tone suppression in the basilar membrane of the cochlea: mechanical basis of auditory-nerve rate suppression. J. Neurophysiol. 1992;68:1087–1099. doi: 10.1152/jn.1992.68.4.1087. [DOI] [PubMed] [Google Scholar]
- 21.Kim DO, Molnar CE, Matthews JW. Cochlear mechanics: nonlinear behavior in two-tone responses as reflected in cochlear-nerve-fiber responses and in ear-canal sound pressure. J. Acoust. Soc. Am. 1980;67:1704–1721. doi: 10.1121/1.384297. [DOI] [PubMed] [Google Scholar]
- 22.Robles L, Ruggero MA, Rich NC. Two-tone distortion on the basilar membrane of the chinchilla cochlea. J. Neurophysiol. 1997;77:2385–2399. doi: 10.1152/jn.1997.77.5.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura RS. Hairs of the cochlear sensory cells and their attachment to the tectorial membrane. Acta Otolaryngol. 1966;61:55–72. doi: 10.3109/00016486609127043. [DOI] [PubMed] [Google Scholar]
- 24.Legan PK, et al. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 2000;28:273–285. doi: 10.1016/s0896-6273(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 25.Lukashkin AN, Lukashkina VA, Legan PK, Richardson GP, Russell IJ. Role of the tectorial membrane revealed by otoacoustic emissions recorded from wild-type and transgenic Tecta(deltaENT/deltaENT) mice. J. Neurophysiol. 2004;91:163–171. doi: 10.1152/jn.00680.2003. [DOI] [PubMed] [Google Scholar]
- 26.Patuzzi R. Cochlear micromechanics and macromechanics. In: Dallos P, Popper AN, Fay RR, editors. The cochlea. Springer; New York: 1996. pp. 186–257. [Google Scholar]
- 27.van Netten SM, Kros CJ. Gating energies and forces of the mammalian hair cell transducer channel and related hair bundle mechanics. Proc. Biol. Sci. 2000;267:1915–1923. doi: 10.1098/rspb.2000.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard J, Hudspeth AJ. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron. 1988;1:189–199. doi: 10.1016/0896-6273(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 29.Flock A, Strelioff D. Graded and nonlinear mechanical properties of sensory hairs in the mammalian hearing organ. Nature. 1984;310:597–599. doi: 10.1038/310597a0. [DOI] [PubMed] [Google Scholar]
- 30.Fettiplace R. Active hair bundle movements in auditory hair cells. J. Physiol. 2006;576:29–36. doi: 10.1113/jphysiol.2006.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.