Abstract
Naturally occurring carbohydrate polymers are ubiquitous. They are assembled by polymerizing glycosyltransferases, which can generate polysaccharide products with repeating sequence patterns. The fidelity of enzymes of this class is unknown. We report a method for testing the fidelity of carbohydrate polymerase pattern deposition: we synthesized fluorosugar donors and used them as chain termination agents. The requisite nucleotide fluorosugars could be produced from a single intermediate using the Jacobsen catalyst in a kinetically controlled separation of diastereomers. The resulting fluorosugar donors were used by galactofuranosyltransferase GlfT2 from Myco-bacterium tuberculosis (M. tb), and the data indicate that this enzyme mediates the cell wall galactan production through a sequence-specific polymerization.
Polysaccharides exhibit great structural diversity. They vary in length, from the thousands of monosaccharide units found in cellulose1 and glycosaminoglycans2 to the hundreds found in bacterial lipopolysaccharide chains3 to smaller oligomers only tens of residues long.4 Frequently, these polysaccharides are constructed from more than one type of monosaccharide building block, alternating between two or more sugars in a repeating pattern. Examples of the alternating pattern are numerous. Hyaluronan, for instance, is a polymer found in synovial fluid containing alternating D-glucuronic acid and D-N-acetylglucosamine (GlcNAc) residues.2 The bacterial peptidoglycan alternates between GlcNAc and N-acetyl muramic acid residues,5 and repeating motifs are also a hallmark of bacterial O-antigen structure.3,6 Agarose, the primary component of agar, is a polymer that alternates between D- and L-galactopyranose derivatives.7 Additionally, some polymer sequences alternate not by the identity of the monosaccharides, but rather in the regiochemical linkages between identical monosaccharides. For example, the Escherichia coli K92 capsular polysaccharide consists of alternating NeuAc-α-(2,8)-NeuAc-α-(2,9) sialic acid residues.8 That such a diverse array of linkage patterns are manifest in Nature is remarkable when one considers that carbohydrate polymerization is a template-independent process. The increasing reports of bifunctional glycosyl-transferase activity, often in systems of medical importance,9 demand renewed attention to the regulation of pattern deposition.10 Moreover, while polysaccharides containing alternating repeats are pervasive, the fidelity of the carbohydrate polymerases that generate them is not known. Herein we highlight a method for assessing a carbohydrate polymerase’s sequence specificity. Using the polymerase GlfT2, we demonstrate the application of synthetic fluorosugar derivatives to probe polymerase fidelity.
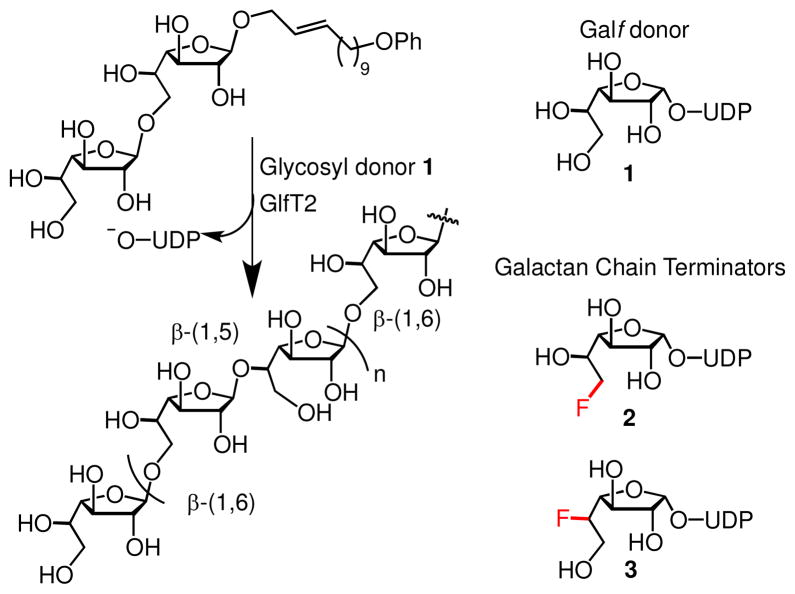
Carbohydrate polymerization is integral to M. tb cell wall biosynthesis. The cell wall contains a chain of 20–40 galactofuranose (Galf) residues termed the galactan, which is assembled largely through the polymerizing activity of the galactofuranosyltransferase GlfT2. GlfT2 acts on acceptor substrates bearing non-reducing Galf residues and elaborates them using the donor sugar UDP-Galf (1, Figure 1). A pattern is generated wherein adjacent galactose monomers are linked with alternating β-(1,5) and β-(1,6) regiochemistry.11–9,12 The Lowary group has provided evidence that GlfT2 generates regioisomeric linkages, but no direct scrutiny of linkage fidelity has been performed.12 We reasoned that this system could serve as a model for studying pattern generation in carbohydrate polymerases.
Figure 1.
Left: The polymerase GlfT2 transfers the donor nucleotide sugar UDP-Galf (1) to synthetic acceptor substrates to generate galactan. An alternating Galf-β-(1,5)-Galf-β-(1,6) pattern is generated. Right: Donors 2 and 3, bearing fluorine atoms in place of key hydroxyl groups, can be used as chain termination agents to determine the fidelity of pattern generation for carbohydrate polymerases such as GlfT2.
Our plan was to employ fluorinated UDP-galactofuranose analogs. Historically, fluorosugar analogs have served as versatile probes of glycosyltransferase mechanism. The substitution of fluorine in place of a hydroxyl group has several advantages. The electronegativity of fluorine preserves the conformational preference of carbohydrates, because it favors gauche interactions. Specifically, modeling and spectroscopy of 2-fluoroethanol and ethylene glycol demonstrate that the conformation and even the hydroxyl proton orientation are identical.13–14 This observation suggests that inductive effects on pKa and nucleophilicity can be approximated by fluorine substitution.15 Sterically, fluorine’s size would be easily accommodated. The location of the fluorine on the sugar, in turn, can have a dramatic impact on the glycosyltransferase activity. For instance, the oxocarbenium transition states of glycosylation reactions of fluorosugar donors with substitution at C2 are destabilized; therefore, fluorosugars can be used as competitive inhibitors.16 Metabolic precursors of fluorosugars can block glycan formation by disrupting nucleotide sugar biosynthesis.17–18
We envisioned fluorosugars could serve in another capacity. Specifically, we postulated that a glycosyl donor bearing fluorine at a site distal to the anomeric center should act as a substrate for carbohydrate polymerases and incorporate the fluorinated sugar into a growing chain. For an enzyme like GlfT2 that generates a carbohydrate sequence pattern, a fluorine atom positioned at the next glycosylation site would raise two possibilities: The remaining (unsubstituted) hydroxyl group could be glycosylated to generate a new glycosidic linkage with the “wrong” regiochemistry, or the complex could dissociate, terminating polymerization. Thus, the site of polymer termination can be used as a measure of the enzyme’s fidelity. The observation of long chains bearing consecutive β-(1,6) or β-(1,5) linkages would reveal GlfT2 to have low sequence specificity. In contrast, the observation of abruptly terminated galactan fragments would reveal GlfT2 to be highly sequence-specific.
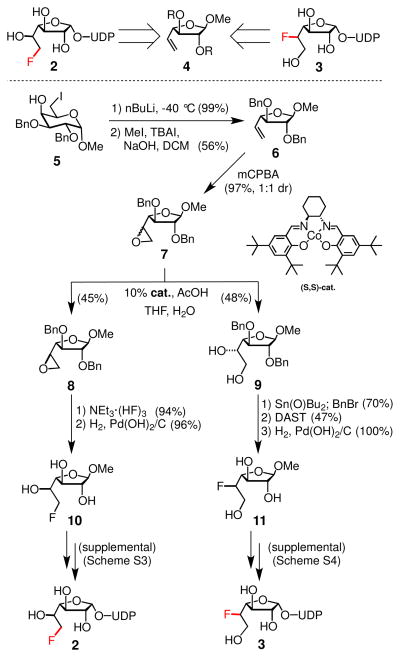
To determine the fidelity of GlfT2, we required access to 6-fluoro and 5-fluoro Galf donors 2 and 3 (Figure 1). The reported syntheses of Galf derivatives lack the flexibility we needed.19–20 Routes to compound 2 are not divergent, low-yielding, and exhibit little selectivity for forming the biologically active alpha anomer.19 A chemoenzymatic synthesis has been described that affords moderate yields.21–22 With regard to the synthesis of fluorosugar 3, we could find no reported routes. We sought to devise an efficient and stereoselective chemical synthesis of Galf derivatives in which fluorogroups at either C5 or C6 would be installed through a common intermediate (4, Figure 2).
Figure 2.
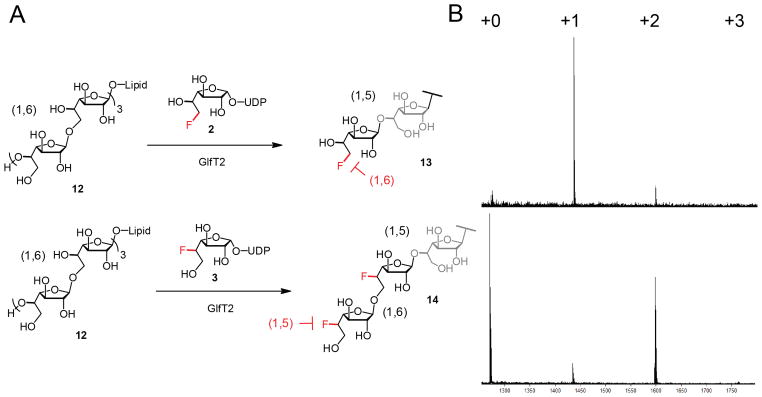
Fluorosugar donors disrupt the alternating linkage pattern and terminate polymerization. GlfT2 can elongate acceptor 12 by one residue when donor 2 is supplied (A, top), or by two residues when donor 3 is used (A, bottom). MALDI-TOF traces show chain termination is a result of pattern disruption (B).
Our desire to install fluoride at particular positions within the saccharide is the kind of challenge often encountered in carbohydrate synthesis: It is difficult to operate selectively on specific hydroxyl groups within a saccharide. One can differentiate the primary C6 hydroxyl group by its steric environment and the C1 position by its oxidation state, but the secondary C5 hydroxyl group has no obvious features that distinguish it from hydroxyl groups at the C2 or C3 positions. Approaches to dial in stereodefined functionality at these internal positions involve either de novo synthesis23 or strategically combining the reactivity of two positions.24–25 We envisioned a vinyl group precursor could lead to successful differentiation of C5: the C2 and C3 positions could be protected, while oxidation of the C5 and C6 positions would result in intermediates wherein we could exploit steric differences. The vinyl furanose precursor needed to test this strategy could be readily accessed. Specifically, commercially available α-D-methylgalactopyranoside could be converted to iodosugar 5 in four steps (supplemental S1) using a strategy developed for methyl-D-mannopyranoside.26 Treatment of iodosugar 5 with n-butyllithium generated a mixture of vinyl furanose anomers, and the predominant β-methylglycoside 6 can be isolated upon purification.
Direct, regioselective, disasteroselective hydroxyfluorination of alkenes remains at the synthetic frontier.27 Addition reactions of this type, which are neither regio- nor stereoselective, produce complex mixtures of isomers that are often difficult to separate. Diastereoselective reactions such as dihydroxylation or epoxidation of unactivated, monosubstituted olefins are challenging to optimize. Furthermore, because nucleophilic fluorination virtually always involves inversion of stereochemistry, both C5 epimers are required to access 2 and 3. To circumvent the necessity to develop two reactions with different diasteroselectivities, we surmised that an advantageous solution would be to use a kinetically controlled separation of diastereomers inspired by Jacobsen’s hydrolytic kinetic resolution.28 In this scenario, an epoxide and its ring opened counterpart would each be stereodefined intermediates for the synthesis of targets 2 and 3, respectively. This strategy was successful. Epoxidation of 6 gave a 1:1 diastereomeric mixture of epoxy sugars 7. Separation using Jacobsen’s cobalt (III) salen complex (10 mol%) allowed nearly quantitative isolation of the epoxy- D-galactose 8 and the L-altrofuranose diol 9. This strategy provided a point of divergence and addressed the regio-chemical and stereochemical challenges in a single transformation. Converting the products to fluorinated methylgalactofuranosides 10 and 11 was straightforward. The epoxide was regioselectively opened with hydrofluoric acid-triethylamine complex. Subsequent protecting group removal afforded a 6F-Galf derivative. Alternatively, the diol was subjected to a three-step sequence to provide 5F-Galf 11. When the 1H NMR spectrum of benzoylated 11 was compared to that of the analogous 2,3,6-tri-O-benzoyl-beta-D-methyl Galf, identical H4–H5 coupling constants were observed. (Figure S2) These results indicate that fluorination took place with inversion at the C5 stereocenter to generate 5-deoxy-5-fluoro-Galf (5F-Galf) methylglycoside. Installation of UDP was then performed to generate target fluorinated substrates 2 and 3 (Schemes S3 and S4).29–30,31
Fluorosugar donors in hand, we sought to test their activity as chain termination agents of GlfT2-catalyzed polymerization. Because GlfT2 is a bifunctional glycosyl-transferase, we hypothesize incorporation of a 5- or 6-fluorinated Galf residue into a polymerizing chain would block further propagation of the alternating pattern. If the enzyme generates only the alternating pattern (i.e., is sequence-specific), chain termination should occur when a fluorine atom is positioned to disrupt the required linkage. If the enzyme cannot err, incubation of GlfT2 with acceptor 12 and 6-fluoro donor 2 should result in the product of chain termination after one 6F-Galf residue is added (13, Figure 2A, top). Likewise, incubation with acceptor 12 and 5-fluoro donor 3 should result in chain termination after two 5F-Galf residues are added, generating truncated chain 14 (Figure 2A, bottom). Taken together, the pair of experimental outcomes indicates chain termination can serve as a measure of GlfT2 sequence fidelity.
GlfT2-catalyzed polymerization was evaluated using an in vitro assay.32 Recombinant His6-GlfT2 from M. tb was mixed at room temperature with synthetic hexasaccha-ride33 acceptor 12 and a sugar nucleotide donor. After 20 hours, the products were analyzed by matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry. When GlfT2 was exposed to 12 and the natural donor 1, the expected distribution of polymeric products was observed (Figure S5). When UDP-Galf was replaced with 2, however, a distinct termination of polymerization was observed in which the enzyme added a single fluorinated residue to the acceptor (Figure 2B, top). An abrupt termination of polymerization occurred just prior to construction of a “mistaken” linkage.
The failure of the fluoroadduct 13 to be elongated effectively could have been driven by GlfT2’s reticence to deviate from the alternating pattern or by its inability to act on a fluorinated acceptor. Fluorine is smaller than a hydroxyl group and it is less polar—either of these qualities might contribute to the enzyme’s proclivity to add a single residue to afford 13. We therefore carried out an analogous elongation experiment with 5-fluoro donor 3. Incubation of GlfT2 with donor 3 resulted in the appearance of a +2 product (Figure 2B, bottom). This result indicates the intermediate +1 fluorinated substrate can be elongated so long as the alternating pattern is not dirupted. The use of fluorinated donors provides GlfT2 with an optimal setting for elongation of a pattern-disrupted galactan. Still, residual product peaks corresponding to the first “mistake” can be observed only in trace amounts for each experiment. The results reveal that this enzyme, known to be notoriously promiscuous with respect to acceptor specificity, is remarkably chaste with regard to sequence specificity. Longer products were not observed in either case (Figure S6).34 In both experiments, chain termination occurred just prior to the generation of a mistaken linkage suggesting that the pattern of alternating β-(1,5) and β-(1,6) glycosidic bonds is essential to galactan biosynthesis. These results demonstrate that GlfT2 possesses a high degree of sequence specificity and, consequently, its bifunctional catalytic activities are interdependent for effective galactan polymerization. Precise regulation in the assembly of glycan sequence suggests a conserved and advantageous physiological role for galactan. Cell wall biosynthesis is indeed critical for M. tb viability. Two first-line antibiotics, isoniazid and ethambutol, are known to disrupt the mycolic acid and arabinan elements, respectively.35 Genetic knockout studies of GlfT2 and UGM, the latter of which generates UDP-Galf, are lethal to the organism,36 and small molecules that bind UGM inhibit growth.37 The dependence of GlfT2 on constructing alternating linkages suggests that chain termination agents could serve as antibiotics.
The strategy we describe evokes the work of Frederick Sanger,38 in which chain-terminating nucleosides are used to rapidly and accurately sequence nucleic acid polymers. Here, the concept is reimagined to gain insight into another class of biopolymer: the polysaccharide. In the case of polysaccharides, the influence of electronic effects on the conformation and the acidity of the glycosyl acceptor prompted us to employ fluorosugars rather than deoxy-sugars. The effectiveness of this approach is illustrated by the ability of GlfT2 to use either compound 2 or 3 to incorporate fluorosugars into the carbohydrate polymer. Chain termination occurred only when GlfT2 was challenged to deviate from the alternating pattern of regioisomers. The high fidelity of GlfT2 is especially intriguing given recent evidence indicating that the polymerase has but a single active site.9
The synthetic strategy used to generate fluorosugars 2 and 3, which converts both products of a kinetic disasteroselective hydrolysis to useful substrates, should be applicable to the assembly of other fluorinated carbohydrates. We anticipate that fluorosugars will serve as valuable probes of repeating motif specificity in other carbohydrate polymerases. In addition, fluorine is a useful probe in NMR, so the ability to enzymatically incorporate fluorinated sugars can facilitate the characterization of polysaccharide structure. Finally, we suggest that fluoro-sugar donors may be used to generate novel fluorinated carbohydrate-based materials.39
Supplementary Material
Scheme 1.
Fluorosugars 2 and 3 can be accessed from a common intermediate, vinyl furanose 4 (top). A kinetically controlled separation of epoxide diastereomers can be used as a synthetic divergence point and as a method to isolate stereodefined isomers 8 and 9 en route to the synthetic targets.
Acknowledgments
We thank Dr. R. A. Splain for the disaccharide precursors of the hexasaccharide used in the polymerization assays. The NIH is acknowledged for financial support (R01 AI063596). All compounds were characterized at the UW-Madison Chemistry Instrument Center, which is supported by the NSF (CHE-9208463 and CHE-9974839 and the NIH (NCRR 1S10RR024601).
Footnotes
SUPPORTING INFORMATION: Experimental details, 1H, 19F, 31P, 13C NMR spectra, MALDI traces. This material is available free of charge via the Internet.
References
- 1.Ross PMRaBM. Microbiol Rev. 1991;55:35. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weigel PH, DeAngelis PL. J Biol Chem. 2007;282:36777. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 3.Raetz CRH, Whitfield C. Annu Rev Biochem. 2002;71:635. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan PJ, Nikaido H. Annu Rev Biochem. 1995;64:29. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 5.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. FEMS Microbiol Rev. 2008;32:234. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 6.Woodward R, Yi W, Li L, Zhao G, Eguchi H, Sridhar PR, Guo H, Song JK, Motari E, Cai L, Kelleher P, Liu X, Han W, Zhang W, Ding Y, Li M, Wang PG. Nat Chem Biol. 2010;6:418. doi: 10.1038/nchembio.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCandless EL, Craigie JS. Annu Rev Plant Physiol. 1979;30:41. [Google Scholar]
- 8.Shen GJ, Datta AK, Izumi M, Koeller KM, Wong CH. J Biol Chem. 1999;274:35139. doi: 10.1074/jbc.274.49.35139. [DOI] [PubMed] [Google Scholar]
- 9.May JF, Levengood MR, Splain RA, Brown CD, Kiessling LL. Biochemistry. 2012;51:1148. doi: 10.1021/bi201820p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vionnet J, Vann WF. Glycobiology. 2007;17:735. doi: 10.1093/glycob/cwm032. [DOI] [PubMed] [Google Scholar]
- 11.Besra GS, Khoo KH, McNeil MR, Dell A, Morris HR, Brennan PJ. Biochemistry. 1995;34:4257. doi: 10.1021/bi00013a015. [DOI] [PubMed] [Google Scholar]
- 12.Rose NL, Completo GC, Lin SJ, McNeil M, Palcic MM, Lowary TL. J Am Chem Soc. 2006;128:6721. doi: 10.1021/ja058254d. [DOI] [PubMed] [Google Scholar]
- 13.Hagen K, Hedberg K. J Am Chem Soc. 1973;95:8263. [Google Scholar]
- 14.Guvench O, MacKerell AD. J Phys Chem A. 2006;110:9934. doi: 10.1021/jp0623241. [DOI] [PubMed] [Google Scholar]
- 15.O’Hagan D. Chem Soc Rev. 2008;37:308. [Google Scholar]
- 16.Withers SG, Street IP, Bird P, Dolphin DH. J Am Chem Soc. 1987;109:7530. [Google Scholar]
- 17.Pouilly S, Bourgeaux V, Piller F, Piller V. ACS Chem Biol. 2012 doi: 10.1021/cb200511t. [DOI] [PubMed] [Google Scholar]
- 18.Barthel SR, Antonopoulos A, Cedeno-Laurent F, Schaffer L, Hernandez G, Patil SA, North SJ, Dell A, Matta KL, Neelamegham S, Haslam SM, Dimitroff CJ. J Biol Chem. 2011;286:21717. doi: 10.1074/jbc.M110.194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peltier P, Daniellou R, Nugier-Chauvin C, Ferrières V. Org Lett. 2007;9:5227. doi: 10.1021/ol702392x. [DOI] [PubMed] [Google Scholar]
- 20.Peltier P, BeláHová M, Dianiaková P, Zhou R, Zheng RB, Pearcey JA, Joe M, Brennan PJ, Nugier-Chauvin C, Ferrières V, Lowary TL, Daniellou R, Mikuaová K. Chem Biol. 2010;17:1356. doi: 10.1016/j.chembiol.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Errey JC, Mukhopadhyay B, Kartha KPR, Field RA. Chem Commun. 2004:2706. doi: 10.1039/b410184g. [DOI] [PubMed] [Google Scholar]
- 22.Peltier P, Guégan JP, Daniellou R, Nugier-Chauvin C, Ferrières V. Eur J Org Chem. 2008;2008:5988. [Google Scholar]
- 23.Northrup AB, MacMillan DWC. Science. 2004;305:1752. doi: 10.1126/science.1101710. [DOI] [PubMed] [Google Scholar]
- 24.Halcomb RL, Danishefsky SJ. J Am Chem Soc. 1989;111:6661. [Google Scholar]
- 25.Sanders WJ, Kiessling LL. Tet Lett. 1994;35:7335. [Google Scholar]
- 26.Kumar V, Gauniyal HM, Shaw AK. Tetrahedron: Asymmetry. 2007;18:2069. [Google Scholar]
- 27.Furuya T, Kamlet AS, Ritter T. Nature. 2011;473:470. doi: 10.1038/nature10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaus SE, Brandes BD, Larrow JF, Tokunaga M, Hansen KB, Gould AE, Furrow ME, Jacobsen EN. J Am Chem Soc. 2002;124:1307. doi: 10.1021/ja016737l. [DOI] [PubMed] [Google Scholar]
- 29.de Lederkremer RM, Nahmad VB, Varela O. J Org Chem. 1994;59:690. [Google Scholar]
- 30.Marlow AL, Kiessling LL. Org Lett. 2001;3:2517. doi: 10.1021/ol016170d. [DOI] [PubMed] [Google Scholar]
- 31.Mohamady S, Desoky A, Taylor SD. Org Lett. 2011;14:402. doi: 10.1021/ol203178k. [DOI] [PubMed] [Google Scholar]
- 32.May JF, Splain RA, Brotschi C, Kiessling LL. Proc Natl Acad Sci U S A. 2009;106:11851. doi: 10.1073/pnas.0901407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levengood MR, Splain RA, Kiessling LL. J Am Chem Soc. 2011;133:12758. doi: 10.1021/ja204448t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higher mass peaks also would arise if GlfT2 generates a mistaken linkage on rare occasion when it first initiates polymerization from the non.
- 35.Young DB, Duncan K. Annu Rev Microbiol. 1995;49:641. doi: 10.1146/annurev.mi.49.100195.003233. [DOI] [PubMed] [Google Scholar]
- 36.Pan F, Jackson M, Ma Y, McNeil M. J Bacteriol. 2001;183:3991. doi: 10.1128/JB.183.13.3991-3998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dykhuizen EC, May JF, Tongpenyai A, Kiessling LL. J Am Chem Soc. 2008;130:6706. doi: 10.1021/ja8018687. [DOI] [PubMed] [Google Scholar]
- 38.Sanger F, Nicklen S, Coulson AR. Proc Natl Acad Sci U S A. 1977;74:5463. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiessling LL, Splain RA. Annu Rev Biochem. 2010;79:619. doi: 10.1146/annurev.biochem.77.070606.100917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.