Abstract
The adenosine A2A receptor (A2AR) is a potential drug target for the treatment of Parkinson’s disease and other neurological disorders. In rodents, the therapeutic efficacy of A2AR modulation is improved by concomitant modulation of the metabotropic glutamate receptor 5 (mGluR5). To elucidate the anatomical substrate(s) through which these therapeutic benefits could be mediated, pre-embedding electron microscopy immunohistochemistry was used to conduct a detailed, quantitative ultrastructural analysis of A2AR localization in the primate basal ganglia and to assess the degree of A2AR/mGluR5 colocalization in the striatum. A2AR immunoreactivity was found at the highest levels in the striatum and external globus pallidus (GPe). However, the monkey, but not the rat, substantia nigra pars reticulata (SNr) also harbored a significant level of neuropil A2AR immunoreactivity. At the electron microscopic level, striatal A2AR labeling was most commonly localized in postsynaptic elements (58% ± 3% of labeled elements), whereas, in the GPe and SNr, the labeling was mainly presynaptic (71% ± 5%) or glial (27% ± 6%). In both striatal and pallidal structures, putative inhibitory and excitatory terminals displayed A2AR immunoreactivity. Striatal A2AR/mGluR5 colocalization was commonly found; 60–70% of A2AR-immunoreactive dendrites or spines in the monkey striatum coexpress mGluR5. These findings provide the first detailed account of the ultrastructural localization of A2AR in the primate basal ganglia and demonstrate that A2AR and mGluR5 are located to interact functionally in dendrites and spines of striatal neurons. Together, these data foster a deeper understanding of the substrates through which A2AR could regulate primate basal ganglia function and potentially mediate its therapeutic effects in parkinsonism.
INDEXING TERMS: mGluR5, Parkinson’s disease, primate, immunogold, globus pallidus, substantia nigra, putamen
Adenosine is a ubiquitous neuromodulator that binds to at least four known G-protein-coupled receptors in the brain (A1, A2A, A2B, and A3). Because of its localization and functional interaction with dopamine D2 receptors (D2R), the A2A receptor has gained interest as a potential drug target for several disorders, including Parkinson’s disease (PD; Pinna, 2009), drug addiction (Brown and Short, 2008), sleep disorders, pain (Ferre et al., 2007b), and psychiatric disorders (Cunha et al., 2008).
In situ hybridization experiments (Schiffmann et al., 1991b; Dixon et al., 1996; Peterfreund et al., 1996; Svenningsson et al., 1997b, 1998; Kaelin-Lang et al., 2000), radioligand binding studies (Martinez-Mir et al., 1991; Calon et al., 2004), and light microscopic (LM) immunohistochemical data (Rosin et al., 1998) have revealed high levels of A2AR expression in the striatum, nucleus accumbens, external globus pallidus, and olfactory tubercle, with low expression levels elsewhere in the brain. Qualitative electron microscopic (EM) data have indicated that dendrites, spines, and terminals express A2AR immunoreactivity in the rat striatum (Hettinger et al., 2001). Furthermore, double in situ hybridization studies (Schiffmann et al., 1991a; Fink et al., 1992; Augood and Emson, 1994; Svenningsson et al., 1998) and double-labeling immunohistochemistry (Quiroz et al., 2009) in the rat striatum have shown that A2AR colocalizes with enkephalin and D2R, but not dopamine D1 receptor, substance P, or somatostatin, indicating a preferential expression of A2AR in indirect pathway (striatopallidal) medium spiny neurons (MSNs). These findings are also supported by tract-tracing studies showing a lack of A2AR expression in rat striatonigral neurons (Schiffmann and Vanderhaeghen, 1993).
In cell culture, A2ARs physically interact with D2Rs (Ferre et al., 1997, 1999, 2001; Franco et al., 2000; Hillion et al., 2002; Canals et al., 2003; Ciruela et al., 2004) and the group I metabotropic glutamate receptor 5 (mGluR5; Ferre et al., 1999, 2002; Cabello et al., 2009). In addition to their physical interactions, simultaneous activation of A2AR and mGluR5 results in synergistic functional effects that can be seen in several downstream biological processes, including decreased affinity of D2R for dopamine (Ferre et al., 1999), increased striatal c-Fos expression (Ferre et al., 2002), and increased cyclic adenosine monophosphate (cAMP) formation and striatal dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32) phosphorylation (Nishi et al., 2003). Most importantly, antagonists of A2AR decrease parkinsonian motor signs in animal models of PD (Grondin et al., 1999; Kanda et al., 2000; Bibbiani et al., 2003; Tanganelli et al., 2004; Pinna et al., 2007; Hodgson et al., 2010) and decrease alcohol self-administration in rodent models of alcoholism (Thorsell et al., 2007); two effects that are synergistically potentiated by the concomitant administration of mGluR5 antagonist (Coccurello et al., 2004; Kachroo et al., 2005; Adams et al., 2008).
Although the exact sites of functional interactions between these two receptors in the central nervous system remain to be determined, both A2AR and mGluR5 are heavily expressed in dendrites and spines of striatal MSNs, as revealed in single-labeling immuno-EM studies in rats and monkeys (Hettinger et al., 2001; Paquet and Smith, 2003). However, despite low levels of presynaptic mGluR5 reported in ultrastructural studies of the rat and monkey striatum (Shigemoto et al., 1993; Paquet and Smith, 2003), synaptosomal fractionation and immunofluorescence techniques have identified a large proportion of putative axon terminals in the rodent striatum (Rodrigues et al., 2005) and hippocampus (Tebano et al., 2005) that coexpress A2AR and mGluR5.
Thus, to gain a better understanding of the primate adenosinergic system and to characterize potential target sites where A2AR and mGluR5 antagonists could mediate their synergistic behavioral effects, we have conducted a detailed quantitative study of the ultrastructural localization of A2ARs in the basal ganglia and determined the degree of striatal A2AR/mGluR5 colocalization in rhesus monkeys using single and double immunohistochemistry at the EM level.
MATERIALS AND METHODS
Animals and tissue preparation
In total, four adult rhesus monkeys (two males, two females; 8–18 years old) and four adult female Sprague-Dawley rats were used in this study. All procedures were approved by the animal care and use committee of Emory University and conform to the U.S. National Institutes of Health guidelines. For immunohistochemistry, animals were deeply anesthetized with pentobarbital (100 mg/kg i.v.) and transcardially perfused with cold oxygenated Ringer’s solution, followed by a fixative containing 2% paraformaldehyde and 3.75% acrolein in a phosphate buffer (PB) solution. After perfusion, the brains were removed from the skull, coronally sliced into thick (~1 cm) blocks, and postfixed overnight in 2% paraformaldehyde. These thick blocks were then cut into 60-μm-thick coronal sections using a vibrating microtome and stored at −20°C in an antifreeze solution containing 30% ethylene glycol and 30% glycerol in PB. Prior to immunohistochemical (IHC) processing, sections were washed with phosphate-buffered saline (PBS; 0.01 M, pH 7.4), treated with a 1% sodium borohydride solution for 20 minutes, and then washed in PBS again.
For Western blot studies, one rat and one monkey were deeply anesthetized with pentobarbital (100 mg/kg i.v.) and killed by decapitation. Brains were rapidly removed from the skull and dissected. Samples were taken from the cerebral cortex, hippocampus, caudate nucleus, putamen (or striatum in rat), cerebellum, and ventral midbrain (containing the substantia nigra) and flash frozen in liquid nitrogen. Frozen samples were stored at −80°C until needed.
Antibody characterization
For the localization of the adenosine A2A receptor, a commercially available mouse monoclonal antibody (Millipore Corporation, Billerica, MA; catalog 05-717, lot 28777) was used (Table 1). This antibody was raised against the full-length human adenosine A2A receptor and was epitope-mapped to the sequence SQPLPGER in the third intracellular loop of the receptor. The specificity of this antibody has been previously characterized via Western blot in rat brain (band at 45 kDa), slot blot, IHC in transfected cells, and antibody preadsorption with IHC in rat brain slices (Rosin et al., 1998). This antibody does not label tissue from A2AR knockout mice (Day et al., 2003). In the present study, Western blots show high specificity of the antibody for A2AR in monkey tissue (Fig. 1B). Omission of the primary antibody from IHC processing of our monkey brain tissue resulted in the disappearance of immunostaining. When the primary antibody is preadsorbed with a synthetic peptide matching the epitope described above, there is a significant loss of neuropil staining, providing further evidence for the antibody’s specificity in monkeys.
TABLE 1.
Antibody Information
| Adenosine A2A receptor | SQPLPGER | Millipore, Billerica, MA; catalog 05-717, lot 28777; raised in mouse, monoclonal |
| Metabotropic glutamate receptor 5 | K-SSPKYDTLIIRDYTNSSSSL | Millipore; catalog 06-451; raised in rabbit, polyclonal |
Figure 1.
Antibody characterization and Western blot detection of A2AR in the monkey and rat. A: Ten micrograms of HEK293T cell lysates transfected with mock pcDNA3.1, A1, or A2A receptor cDNAs was subjected to SDS-PAGE and Western blotting with anti-A2AR antibody (1:2,000). Two specific bands, probably representing the unmodified and glycosylated versions of the A2A receptor, were detected at approximately 45 and 55 kDa, respectively, in the A2AR-transfected condition (top panel, lane 3) and not in mock- or A1R-transfected cell lysates (top panel, lanes 1 and 2). Probing samples for actin confirmed equal protein loading (bottom panel). B: Twenty micrograms of macaque brain region samples were subjected to SDS-PAGE and Western blotting with the anti-A2AR antibody (1:2,000). A2AR is enriched in the macaque striatum, with detection of A2AR at approximately 45 kDa and a minor band that may represent an A2AR degradation product at approximately 37 kDa (top panel). Longer exposures of the same blot revealed that A2AR is most enriched in CN > PU > SNr (middle panel). Probing samples for actin confirmed equal protein loading (bottom panel). C: Twenty micrograms of macaque or rat brain regions were subjected to SDS-PAGE. Western blotting with the anti-A2AR (1:2,000) revealed A2AR to be detectable in the macaque SNr but not the rodent SNr (top panel). Probing samples for actin confirmed equal protein loading (bottom panel). CN, caudate nucleus; CRBL, cerebellum; CTX, cortex; HPC, hippocampus; PU, putamen; SNr, substania nigra pars reticulata; ST, striatum; WB, Western blot.
For the localization of mGluR5, an affinity-purified polyclonal rabbit antibody (Millipore; catalog 06-451) raised against a 21-residue synthetic peptide (K-SSPKYDTLIIRDYTNSSSSL) corresponding to the C-terminal of mGluR5 with a lysine added to the N-terminal was used at a dilution of 1:5,000. The specificity of this antibody has been characterized by immunoblot on homogenate from cells transfected with the receptor and from rat brain areas known to express the receptor (band at 140 kDa; Marino et al., 2001). The antibody does not stain brain tissue from mGluR5 knockout mice (Kuwajima et al., 2004).
Western blots
HEK293T cells (ATCC, Manassas, VA) were maintained in DMEM GlutaMAX (Invitrogen, Carlsbad, CA), supplemented with 10% dialyzed fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin (Thermo Scientific, Waltham, MA) in a humidified incubator at 37°C and 5% CO2. Cells were transfected with plasmids encoding mock pcDNA3.1 (Invitrogen), human A1, or human A2A receptor cDNAs (Missouri S&T cDNA Resource Center, Rolla, MO) using the lipofectamine transfection method (Lipofectamine 2000; Invitrogen). After 24 hours of expression, cells were washed twice with ice-cold PBS supplemented with calcium and harvested in ice-cold harvest buffer containing 50 mM NaCl, 20 mM HEPES, 5 mM EDTA, and one protease inhibitor cocktail tablet (Roche Applied Science, Basel, Switzerland), diluted with dH2O to 50 ml, pH 7.4. Cell lysates were snap frozen at −80°C until needed.
To prepare brain tissue homogenates, monkey and rat brain tissue samples were thawed on ice, and each brain region was individually homogenized using a dounce homogenizer for 10 strokes in ice-cold harvest buffer. Prepared tissue homogenates were aliquotted, snap frozen in liquid nitrogen, and stored at −80°C until needed.
After thawing of HEK293T cell lysates and brain tissue homogenates on ice, bicinchoninic acid protein assays (Thermo Scientific) were performed to normalize protein across samples. Samples were then prepared for Western blotting by diluting with 6× Laemmli sample buffer to a final 1× concentration, followed by robust sonication on ice. Samples from the HEK293T cell lysates and brain tissue homogenates were loaded onto 4–20% Tris-glycine gels and subjected to SDS-PAGE. Proteins were then transferred to nitrocellulose membranes and visualized with Ponceau stain. Membranes were subjected to Western blotting to probe for the A2A receptor and to test the specificity of the anti-A2AR antibody. After blocking for 30 minutes in “blot buffer” containing 2% nonfat milk, 50 mM NaCl, 10 mM HEPES, and 0.1% Tween-20 in dH2O, membranes were incubated with the anti-A2AR antibody diluted 1:2,000 in blot buffer for 1 hour at room temperature. After washing in blot buffer, membranes were probed with anti-mouse HRP-conjugated secondary antibody (diluted in blot buffer, 1:4,000) for 30 minutes. Bands were visualized using an enhanced chemiluminescence kit (Thermo Scientific), and membranes were exposed to films for various times. Membranes were then stripped using Restore Buffer (Thermo Scientific) and probed with antiactin (Sigma, St. Louis, MO) to verify equal protein loading.
Single immunoperoxidase labeling for LM
To map the expression pattern of A2AR immunoreactivity in the monkey brain, 56 coronal brain sections from a single monkey, each separated by 300 μm, were simultaneously immunostained for A2AR, covering a total brain area that extends from approximately interaural +28 to +3 in the rostrocaudal plane according to the Paxinos stereotaxic atlas (Paxinos et al., 2000). After sodium borohydride treatment, sections were incubated for 1 hour in PBS containing 10% normal horse serum (NHS) for A2AR localization or normal goat serum (NGS) for mGluR5 localization, 1% bovine serum albumin (BSA), and 0.3% Triton X-100. Then, the sections were incubated in the primary antibody solution containing a 1:2,000 dilution of A2AR antibody, 1% NHS or NGS, 1% BSA, and 0.3% Triton X-100 in PBS for 24 hours at room temperature. Sections were then rinsed three times in PBS and incubated in the secondary antibody solution containing 1% normal horse or goat serum, 1% BSA, 0.3% Triton X-100, and biotinylated horse anti-mouse or goat anti-rabbit IgGs (Vector Laboratories, Burlingame, CA) at a dilution of 1:200 for 90 minutes at room temperature. After three rinses in PBS, sections were incubated for 90 minutes in the avidin-biotin peroxidase complex (ABC) solution at a dilution of 1:100 (Vectastain standard ABC kit, Vector Laboratories) including 1% BSA and 0.3% Triton X-100. For revelation, sections were first rinsed with PBS and Tris buffer (50 mM, pH 7.6), then incubated in a solution containing 0.025% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma), 10 mM imidazole, and 0.005% hydrogen peroxide in Tris buffer for 10 minutes. Finally, sections were rinsed in PBS, mounted onto gelatin-coated slides, dehydrated, and coverslipped with Permount.
Single immunoperoxidase labeling for EM
Sections containing the striatum, external globus pallidus (GPe), or substantia nigra from three monkeys were processed for the EM immunoperoxidase localization of A2AR. After sodium borohydride treatment, sections were transferred to a cryoprotectant solution containing 25% sucrose and 10% glycerol in PB (0.05 M, pH 7.4) for 20 minutes and then placed in a −80°C freezer for 20 min to permeabilize cell membranes. Brain sections were then thawed through washes in decreasing concentrations of cryoprotectant solution until being placed into PBS. The processing of sections was then identical to that used for LM up to the point of DAB revelation, with two important differences: 1) Triton X-100 was omitted from all solutions, and 2) sections were incubated in the primary antibody (1:1,000 dilution) solution for 48 hours at 4°C.
After DAB revelation, the tissue was rinsed in PB (0.1 M, pH 7.4) and treated with 1% osmium tetroxide for 20 minutes. It was then rinsed with PB and dehydrated with increasing concentrations of ethanol. Uranyl acetate (1%) was added to the 70% EtOH dehydration solution and incubated for 35 minutes to increase the contrast of membranes in the EM. After alcohol dehydration, sections were treated with propylene oxide and embedded in epoxy resin (Durcupan ACM; Fluka, Buchs, Switzerland) for at least 12 hours, mounted onto slides, and placed in a 60 °C oven for 48 hours to cure the resin.
Small blocks of tissue from the motor putamen, GPe, and substantia nigra pars reticulata (SNr) were cut out from the embedded sections and glued onto resin blocks for ultrathin sectioning with an ultramicrotome (Leica Ultracut T2). Sixty-nanometer-thick sections were collected from the surface of the tissue block to ensure that antibody penetration was optimal in the tissue analyzed in the EM. Sections were mounted on single-slot pioloform-coated copper grids, stained for 5 minutes with lead citrate, and examined on a Zeiss EM-10C transmission electron microscope. Electron micrographs were captured and saved with a CCD camera (DualView 300W; Gatan, Inc., Pleasanton, CA) controlled by DigitalMicrograph software (version 3.11.1; Gatan, Inc.).
Single pre-embedding immunogold labeling for EM
Two tissue sections at the level of the striatum from each of the three monkeys were used for the immunogold localization of A2AR. After having been pretreated with sodium borohydride and cryoprotectant solutions, as detailed above, brain sections were incubated for 30 minutes in PBS containing 5% dry milk and then rinsed in TBS-gelatin (Tris-buffered saline; 0.24% Tris, 0.1% fish gelatin, pH 7.6) buffer. Sections were then exposed to the primary antibody solution containing 1% dry milk and mouse anti-A2AR primary antibody (1:1,000) in TBS-gelatin buffer for 24 hours at room temperature. After rinsing with TBS-gelatin, sections were exposed to the secondary antibody solution containing 1% dry milk and horse anti-mouse IgGs conjugated to 1.4-nm-diameter gold particles (Nanoprobes, Yaphank, NY) at a concentration of 1:100 in TBS-gelatin for 2 hours at room temperature. After rinsing with TBS-gelatin and 2% sodium acetate buffer, gold particles were enlarged to 30–50 nm using the silver intensification method (HQ silver kit; Nanoprobes). Then, sections underwent osmification, dehydration, and embedding as detailed above for immunoperoxidase experiments, but with two important differences: 1) exposure to 0.5% osmium tetroxide for 10 minutes instead of 1% for 20 minutes and 2) exposure to 1% uranyl acetate for 10 minutes instead of 35 minutes. Ultrathin sections were then cut and collected as described above.
Dual pre-embedding immunogold/immunoperoxidase method for striatal colocalization of A2AR and mGluR5
To determine the degree and pattern of colocalization of A2AR and mGluR5 in the motor putamen, two sections from each of the three monkeys used for EM studies were processed for the dual localization of mGluR5 and A2AR immunoreactivity using the preembedding immunogold and immunoperoxidase methods. To assess the extent of colocalization and avoid interpretation problems resulting from the differential sensitivity of the peroxidase- or gold-labeled antibodies for their antigens, two different reactions were performed on separate sets of striatal sections. In the first series of sections, A2AR was labeled with peroxidase, and mGluR5 was revealed with gold; in the second series of incubations, A2AR was labeled with gold and mGluR5 with peroxidase.
The incubation procedures and preparation of brain sections for these double-labeling studies were similar to those used for the single pre-embedding immunogold and immunoperoxidase labeling, except that antibody solutions contained a cocktail of two different primary or secondary antibodies. A2AR primary antibody was used at a dilution of 1:1,000, and mGluR5 primary antibody was used at a dilution of 1:5,000. After secondary antibody incubations, the tissue was processed for silver intensification of gold particles, rinsed with PBS, and then exposed to ABC and DAB as described above for the single immunoperoxidase labeling procedure. After rinsing in PB, sections underwent osmification, dehydration, resin embedding, and ultrathin sectioning as described above.
Analysis of material
LM analysis
Brain sections mounted on glass slides were scanned at ×20 using a ScanScope CS scanning LM system (Aperio Technologies, Vista, CA). Digital representations of these slides were saved and analyzed in ImageScope software version 10.0.36.1805 (Aperio Technologies).
EM analysis
In most cases, the ultrathin sections analyzed in the EM were taken from at least two different tissue blocks per structure per animal to increase the sampling of tissue analyzed. From single immunoperoxidase-labeled sections, 100 micrographs per structure per animal of randomly encountered A2AR-labeled neuronal elements were captured at ×25,000, giving a total of 1,162 μm2 of tissue analyzed per structure per animal. Fifty micrographs per structure per animal were captured in the same manner for gold-labeled tissue, giving an area of 581 μm2 analyzed. In double-labeled tissue, 100 micrographs per structure per animal of randomly encountered A2AR-labeled neuronal elements were captured at ×25,000, irrespective of the presence or absence of mGluR5 labeling in the same element. As a prerequisite to ensure that the double-labeled tissue examined contained optimal immunostaining for both A2AR and mGluR5, micrographs were taken only from areas in which both the immunoperoxidase and immunogold deposits were visible. Dendrites and terminals were considered immunoreactive in gold-labeled tissue if they contained at least two gold particles, whereas, because of their relatively small surface area, spines and unmyelinated axons were categorized as immunoreactive if they were overlaid with a single gold particle or more. With these selection criteria, the overall proportions of A2AR-labeled spines and dendrites in the immunogold and immunoperoxidase material were closely related, supporting the reliability of the quantitative data collected across immunoperoxidase and immunogold material. Contrast and sharpness of the micrographs were adjusted to show better the morphological features of labeled elements.
RESULTS
Western blots
To demonstrate further the specificity of the A2AR antibody, we performed a series of immunoblot experiments probing transfected cell lysates and brain tissue homogenates. Lysates from HEK293T cells transfected with pcDNA3.1 (mock), human adenosine A1 receptor (A1R), or human A2AR were immunoblotted for detection of A2AR. Human receptors were used in order to provide further characterization of the antibody for use in immunohistochemistry of primate tissue as well as to confirm adenosine receptor subtype specificity in our hands, as previously reported (Rosin et al., 1998). Corresponding to the predicted molecular weight of the A2AR, a prominent band at about 45 kDa was detected only in the lysate expressing A2AR and not in the cell lysates expressing A1R or mock cDNA (Fig. 1A). The second band in the A2AR cell lysate, at about 55 kDa, presumably represents glycosylated A2AR, as suggested by Rosin et al. (1998). Probing for actin showed equal protein loading from all cell lines. This demonstrates the specificity of the antibody, in agreement with Rosin et al. (1998).
Western blotting for detection of A2AR in the monkey cortex, hippocampus, caudate nucleus, putamen, midbrain, and cerebellum provided further evidence for the specificity of this antibody in monkey tissue. Short exposures of these blots revealed specific bands, only in the lanes for the caudate nucleus and putamen, at the expected molecular weight for A2AR (45 kDa), as well as a lighter minor band at about 37 kDa, which may represent A2AR proteolytic degradation product, as suggested by Rosin et al. (1998) (Fig. 1B, top). However, longer exposures of the same blots revealed immunolabeling for A2AR in all structures sampled, the strongest of which (other than caudate and putamen) was from the ventral midbrain (SNr, Fig. 1B, bottom). Blots probed with an antiactin antibody demonstrated equal protein loading for all structures.
Immunoperoxidase A2AR labeling: light microscopy
At the LM level, immunoperoxidase labeling resulted in a dark brown deposit, which, in most labeled structures, was associated predominantly with fine neuropil processes. The most strongly labeled structures in the monkey brain were the striatum and GPe (Fig. 2). Dense, homogeneous labeling invaded the whole extent of the caudate nucleus, putamen, nucleus accumbens, and olfactory tubercle. Dense neuropil labeling in these structures precluded identification of labeled soma.
Figure 2.
Overall distribution of immunoperoxidase labeling for A2AR in the monkey basal ganglia. A–I: A series of low-power light micrographs of coronal brain sections (nine of 56 shown) showing A2AR immunoreactivity throughout the rostrocaudal extent of the monkey basal ganglia. The interaural stereotaxic coordinate for each section is indicated at the bottom right of each panel (Paxinos et al., 2000). AC, anterior commissure; CC, corpus collosum; CD, caudate nucleus; CTX, cortex; GPe, external globus pallidus; GPi, internal globus pallidus; Hip, hippocampus; IC, internal capsule; LV, lateral ventricle; NAc, nucleus accumbens; OT, optic tract; PUT, putamen; SN, substantia nigra; Th, thalamus. Scale bar = 5 mm.
In the GPe, intense labeling was found in neuropil processes that invaded the whole extent of the structure. The dense immunoreactivity associated with the GPe stood out next to the very low level of labeling in the neighboring GPi (Fig. 2D–F).
Another basal ganglia nucleus that displayed a significant level of A2AR immunoreactivity was the SNr (Figs. 2G–I, 3D). Although the SNr is not generally recognized as a major A2AR-containing structure in rodents, the monkey SNr displayed moderate neuropil immunoreactivity along its whole rostrocaudal extent, which stood out next to the lightly labeled substantia nigra pars compacta (Fig. 2G,H).
Figure 3.
A2AR immunolabeling in rat and monkey substantia nigra. A series of medium- to high-power micrographs depicting details of A2AR immunostaining in the rat (A–C) and monkey (D) basal ganglia. All sections in this figure were processed in the same immunohistochemical reaction. A: A2AR immunolabeling at the level of the rat striatum/GP. Note the strong immunoreactivity in the striatopallidal complex, comparable to the pattern of labeling in monkeys (see Fig. 2). B,C: Lack of A2AR immunolabeling in the rat SNr. C is a higher magnification image of the boxed area in B. D: A2AR immunolabeling in the monkey SNr. Note the higher degree of neuropil A2AR immunoreactivity in the monkey than in the rat SNr. AG, periaqueductal gray; CP, cerebral peduncle; CTX, cortex; GP, globus pallidus; SC, superior colliculus; SNr, substantia nigra pars reticulata; ST, striatum; Th, thalamus; VTA, ventral tegmental area. Scale bars = 1 mm.
Because previous immunohistochemical studies did not report the SNr as being enriched in A2AR immunoreactivity in the rat, we performed additional experiments in three rats to assess a possible species difference in the extent of A2AR immunoreactivity in the SNr between rodents and primates. When run together in the same antibody solutions and processed during the same immunohistochemical reaction, striatal and GPe neuropil in both species displayed strong A2AR labeling, and significant A2AR immunoreactivity was evident in the monkey, but not the rat, SNr (Fig. 3). To confirm this species difference in nigral expression of A2AR between monkey and rat, a Western blot was run for detection of A2AR in the monkey and rat striatum and SNr. Although high levels of receptor proteins were detected in both monkey and rat striatal tissue, A2AR was detected in the monkey, but not the rat, ventral midbrain samples containing the SNr (Fig. 1C). Also, the doublet of bands seen in monkey tissue representing whole A2AR and a proteolytic fragment was not evident in the rat striatum, in agreement with previous findings (Rosin et al., 1998). This may be due to a species difference in A2AR proteolytic processing or a number of other factors beyond the scope of the present study. Blotting for actin showed equal protein loading from both species.
In addition to basal ganglia nuclei, all layers of the cerebral cortex contained light perikaryal labeling, with relatively denser labeling in the underlying white matter, which appeared to be mostly glial in nature (Fig. 2). Other brain areas that contained lower levels of A2AR immunoreactivity in the monkey brain included the thalamus, hippocampus, subthalamic nucleus, and claustrum (Fig. 2). Brainstem regions posterior to the substantia nigra were largely devoid of A2AR immunoreactivity.
Ultrastructural localization of A2AR
Immunoperoxidase A2AR labeling
Although most of the A2AR labeling was found in the neuropil of basal ganglia nuclei, LM does not provide a level of resolution high enough to categorize the neuronal and glial elements with which these receptors are associated. To address this issue, we performed a quantitative EM analysis of the distribution of A2AR immunoreactivity in the monkey basal ganglia (Fig. 4). A2AR-immunostained sections from the putamen, GPe, and SNr were examined in the electron microscope. At the EM level, the immunoperoxidase labeling for A2AR could be identified as a dark, electron-dense, amorphous deposit associated with restricted compartments of neuronal and glial elements. The relative abundance of immunoreactive structures in each of these regions, identified based on specific ultra-structural features (Peters et al., 1991), was determined and plotted against each other in distribution histograms (Fig. 4E,F).
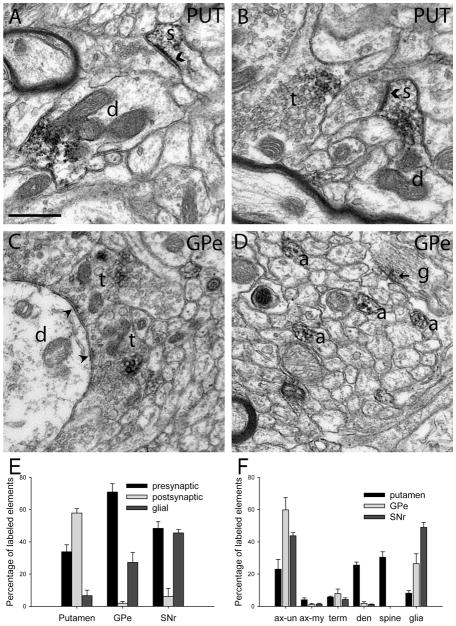
Figure 4.
Ultrastructural localization of A2AR in the monkey basal ganglia. Electron micrographs of neuronal elements immunolabeled for A2AR (A–D) and quantification of immunoperoxidase labeling in the postcommissural putamen, GPe, and SNr of rhesus monkeys (E,F). A: An A2AR-labeled dendrite (d) and spine (s) in the sensorimotor putamen. B: A labeled spine (s) protruding from an unlabeled dendrite (d) in the putamen. A labeled terminal (t) is also visible. C: Two immunoreactive putative GABAergic terminals (t) form symmetric synapses on an unlabeled dendrite (d) in the GPe. D: Labeled unmyelinated axons (a) in the GPe. A lightly labeled (arrow) fibrous astrocytic process (g) is also seen. E: Quantification of the percentages of labeled elements categorized as neuronal (presynaptic vs. postsynaptic) or glial in the monkey putamen and GPe. F: Breakdown of the percentages of each type of A2AR-labeled element in different basal ganglia nuclei. Percentage values refer to the number of specific labeled neuronal or glial elements over the total number of labeled elements examined in that structure (± SEM). Symmetric synapses are marked with arrowheads, and asymmetric synapses are marked with chevrons. Scale bar = 0.5 μm.
Putamen
The A2AR striatal labeling was localized mainly to post-synaptic elements, including dendritic spines and shafts, which accounted for 58% ± 3% of labeled elements in the putamen. On the other hand, 34% ± 4% of labeled striatal elements were presynaptic in nature, the majority of which were unmyelinated axons, although immunoreactive terminals were also encountered. Seventeen of the thirty-seven labeled terminals identified formed axodendritic symmetric synapses, 10 displayed asymmetric synapses, and the synaptic specialization of the remaining 10 boutons was not visible in the plane of section or not preserved well enough to be categorized as symmetric or asymmetric. Among the 10 A2AR-labeled terminals forming asymmetric synapses, eight were in contact with spines, whereas the other two formed synapses with dendritic shafts. Labeled glial processes were rare in the striatum (7% ± 3% of labeled elements) but represented a larger proportion of labeled elements in other structures examined (Fig. 4E,F).
GPe
In the GPe, the bulk of labeling was expressed presynaptically (71% ± 5% of labeled elements), largely in unmyelinated axons, with more modest labeling of axon terminals forming either symmetric (47 of 76 labeled terminals) or asymmetric (six of 76) synapses. The scarcity of labeled postsynaptic elements (2% ± 1%) indicates that A2AR immunoreactivity in the GPe is confined largely to afferent axons and terminals. On the other hand, immunoreactive glial processes were commonly found in the GPe (27% ± 6% of labeled elements). The glial immunoreactivity was associated either with large-diameter processes of fibrous astrocytes (Fig. 4D) or with thinner glial processes in close contact with neuronal elements.
SNr
As in the GPe, A2AR labeling in the SNr was composed primarily of presynaptic elements (48% ± 4% of labeled elements), with scarce postsynaptic immunoreactivity (6% ± 5% of labeled elements). Labeled terminals in the SNr formed predominantly axodendritic symmetric synapses (17 of 33 labeled terminals). The remaining boutons either formed asymmetric synapses (four of 33 labeled terminals) or did not establish clear synaptic junctions in the plane of section that was examined. The SNr contained a larger proportion of labeled glial elements (46% ± 2% of labeled elements) than other basal ganglia nuclei.
Immunogold A2AR labeling
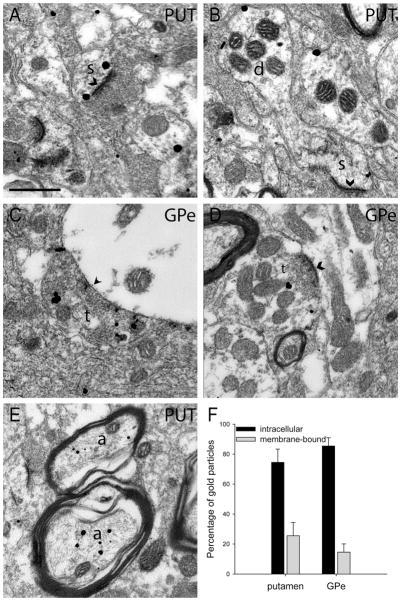
To characterize further the potential subsynaptic sites of action where adenosine could mediate its effects through A2AR activation in the striatopallidal complex, the immunogold method was used to provide a more accurate description of the subcellular and subsynaptic localization of A2AR in the postcommissural putamen and GPe (Fig. 5). The gold labeling in neuronal elements was divided into two major categories: intracellular or plasma membrane bound. The membrane-bound gold particles were further divided into three subcategories: synaptic (within or directly apposed to the synaptic active zone), perisynaptic (<20 nm outside of active zone), or extrasynaptic (>20 nm from the synapse). In both the putamen and GPe, 75–85% of A2AR-associated gold particles were found in the intracellular compartment (Fig. 5F). The difference between intracellular and membrane-bound gold particles was statistically significant in both structures (t-test, P < 0.05). There was no statistical difference in the pattern of labeling (intracellular vs. membrane bound) between the two structures (t-test, P = 0.355). Among membrane-bound gold particles, only 2.6% in the putamen and 1.5% in the GPe were found at synaptic and perisynaptic sites; the bulk of labeling was extrasynaptic in both nuclei. Glial gold labeling was not included in this analysis.
Figure 5.
EM immunogold labeling for A2AR. Electron micrographs of neuronal elements immunolabeled for A2AR (A–E) and quantification of immunogold labeling (F) in the putamen and GPe. A: Perisynaptic and intracellular labeling for A2AR in a striatal spine. B: A labeled spine protrudes from an immunoreactive dendrite (d) in the putamen. C: An A2AR-labeled putative inhibitory axon terminal (t) that forms a symmetric axodendritic synapse in the GPe. D: A labeled putative excitatory terminal (t) forming an asymmetric synapse with an unlabeled dendrite in the GPe. E: Two labeled myelinated axons (a) in the putamen. F: Quantification of intracellular vs. plasma membrane-bound A2AR immunogold labeling in the putamen and GPe. Symmetric synapses are marked with arrowheads, and asymmetric synapses are marked with chevrons. Scale bar = 0.5 μm.
A2AR/mGluR5 colocalization
Because A2AR and mGluR5 have been shown to interact physically in the rat striatum, we performed double-labeling EM colocalization experiments in the monkey putamen to identify the subcellular compartments where these interactions could potentially occur in primates. The degree of colocalization of A2AR and mGluR5 in the putamen was substantial, especially in postsynaptic elements (Fig. 6). When A2AR was labeled with peroxidase and mGluR5 with gold, 68% ± 5% of A2AR-containing spines and 60% ± 2% of dendrites also contained mGluR5 (N = 717 A2AR-immunoreactive elements). When A2AR was labeled with gold and mGluR5 with peroxidase, 73% ± 2% of A2AR-containing spines and 62% ± 1% of dendrites also contained mGluR5 (N = 904 A2AR-immunoreactive elements). Colocalization in presynaptic elements was much less frequent; only 5–15% of A2AR-containing axons and terminals also contained mGluR5, likely because mGluR5 is rarely found in presynaptic elements in the striatum (Paquet and Smith, 2003; Mitrano and Smith, 2007). Only 2–6% of all double-labeled elements observed were presynaptic in nature. There was no significant difference in the degree of colocalization in any type of element when A2AR was labeled with peroxidase vs. gold (t-test, P > 0.05), which validates the reliability of our double-labeling method.
Figure 6.
A2AR/mGluR5 double labeling in the motor putamen. Electron micrographs of A2AR/mGluR5 double-labeled neuronal elements in the monkey putamen (A–E) and quantification of colocalization (F). A–E: Examples of spines (s) and dendrites (d) colabeled for both A2AR and mGluR5 in the putamen. In each panel, arrows point to peroxidase deposits, arrowheads indicate immunogold labeling, and chevrons mark asymmetric synapses. In A and B, the immunoperoxidase deposit indicates A2AR immunoreactivity, whereas mGluR5 is labeled with gold particles. In C–E, the reaction was reversed, i.e., peroxidase is used to localize mGluR5, whereas A2AR is labeled with gold. A spine (s2) and dendrite (d2) single labeled for mGluR5 are also visible in D and E, respectively. F: Quantification of the percentage of A2AR-labeled neuronal elements that also express mGluR5 immunoreactivity in the motor putamen. Black bars represent the percentages of A2AR-immunoreactive elements that coexpress mGluR5 immunoreactivity in tissue dually stained for A2AR with peroxidase and mGluR5 with gold; gray bars represent the same quantitative assessment of colocalization in tissue double immunostained for A2AR with gold and mGluR5 with peroxidase. There was no statistical difference in the extent of colocalization between these two staining methods. Scale bar = 0.5 μm.
DISCUSSION
Our results provide the first detailed quantitative assessment of the ultrastructural localization of A2AR in the primate basal ganglia and its degree of subcellular colocalization with mGluR5 in the striatum. In addition to its well-recognized association with indirect striatofugal GABAergic neurons, our data reveal that A2ARs are also located to subserve presynaptic modulation of glutamatergic afferents to the striatum and GPe in nonhuman primates. In contrast to the case in rodents, a significant level of presynaptic A2AR immunoreactivity was found in putative GABAergic terminals in the monkey SNr, thereby raising the possibility that adenosine might influence basal ganglia outflow, not only through the indirect modulation of GPe or striatal activity but also via A2AR-mediated regulation of GABAergic transmission in the primate SNr. Furthermore, the extensive degree of A2AR and mGluR5 colocalization at the level of striatal spines and dendrites described here suggests that functional interactions between these two receptors most likely occur postsynaptically in the primate striatum, a situation different from the presynaptic interactions recently proposed for rodents on the basis of in vitro data (Rodrigues et al., 2005). In addition to a strong neuronal expression, A2ARs were also found to be significantly enriched in glial cells throughout the monkey basal ganglia, most particularly in the SNr, providing another substrate through which adenosine can mediate its effects on basal ganglia activity in the normal and diseased state.
A2AR: a marker of indirect striatopallidal neurons in primates and nonprimates
Previous studies have shown that A2AR mRNA and protein in the rodent and primate striatum colocalize with markers of indirect but not direct pathway MSNs (Schiffmann et al., 1991a; Fink et al., 1992; Augood and Emson, 1994; Svenningsson et al., 1997b, 1998; Quiroz et al., 2009). This selective expression makes A2AR a highly specific marker of indirect striatopallidal neurons in primates and nonprimates.
In line with these data, high levels of A2AR immunoreactivity were found in the monkey striatum and GPe, with very little labeling in the GPi. In agreement with previous rodent data (Hettinger et al., 2001), the majority of striatal A2AR immunoreactivity was located in postsynaptic structures in the monkey striatum, most likely belonging to indirect striatofugal neurons. When considered in conjunction with our findings that almost all neuronal A2AR immunoreactivity in the GPe was located in presynaptic elements and that 47 of 53 classifiable A2AR-labeled terminals in the GPe were putatively GABAergic and morphologically resembled striatal-like boutons, our data provide further evidence for strong A2AR expression in striatopallidal MSNs in nonhuman primates.
In the striatum, the preferential expression of A2AR in dendrites and spines suggests the possibility of postsynaptic A2AR-mediated effects on striatal projection neurons. However, many of the known effects of A2AR in striatal MSNs occur through modulation of other receptors with which A2AR interacts, such as dopamine D2 receptors, mGluR5, and CB1 receptors (Stromberg et al., 2000; Domenici et al., 2004; Ferre et al., 2007a; Tozzi et al., 2011). For example, although A2AR activation alone has no effect on the membrane potential of MSNs in rat striatal brain slices, it can block the ability of a D2 receptor agonist to suppress NMDA-induced down-state to upstate transitions (Azdad et al., 2009). Additionally, postsynaptic A2AR activation negatively modulates NMDA receptor postsynaptic currents in rat striatal slices (Norenberg et al., 1997, 1998; Wirkner et al., 2000; Gerevich et al., 2002; Wirkner et al., 2004). Although direct electrophysiological effects of postsynaptic A2ARs on MSNs have been rarely reported, the ability of these receptors to regulate biochemical indicators of MSN function, such as cAMP formation, phosphorylation of DARPP-32, and 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid receptor and L-type calcium channel function is well documented from in vitro and rodent studies (Schiffmann et al., 2007). However, little is known about A2AR function in the primate striatum.
The significant level of presynaptic A2ARs in striatal-like terminals in the monkey GPe is suggestive of modulatory sites of action that could regulate striatopallidal GABAergic transmission. Patch-clamp recording data from rat brain slices have, indeed, shown that A2AR agonist presynaptically enhances the amplitude of striatal-evoked IPSCs in GP neurons (Shindou et al., 2001). In line with these in vitro data, local infusions of A2AR agonist into the striatum or GP of unlesioned rats led to increased GABA concentrations in the GP, and systemic administration of A2AR antagonist reduced extracellular GABA levels in the GP of 6-hydroxydopamine (6-OHDA)-lesioned rats (Ochi et al., 2000). In vivo intrapallidal delivery of A2AR antagonist can also block the decrease in GP neuron firing rate induced by striatal stimulation in rats (Querejeta et al., 2010). Together, these findings demonstrate that A2AR subserves key regulatory functions of GABAergic transmission along the striatopallidal indirect pathway in rodents. Our EM data, combined with previous LM studies in monkeys (Svenningsson et al., 1998) and humans (Schiffmann et al., 1991b; Svenningsson et al., 1997a; Kaelin-Lang et al., 2000; Calon et al., 2004) demonstrate that these receptors are located to subserve similar functions at striatopallidal synapses in primates.
However, the expression of A2AR immunoreactivity in putative glutamatergic terminals in the monkey GPe suggests that the influence of adenosine-mediated effects through these receptors may go beyond the striatopallidal GABAergic system. Because there is no further evidence that A2AR activation modulates glutamatergic transmission in the GP, functional studies directly addressing this issue are warranted to understand better the complex and various mechanisms by which A2ARs modulate basal ganglia activity in normal and disease states.
A2AR expression in striatal terminals
In addition to a significant postsynaptic expression, A2AR immunoreactivity was also found frequently to be associated with both putative glutamatergic and GABAergic terminals as well as a large number of unmyelinated axons in the monkey putamen. Labeled myelinated axons were much less common but were present in the striatum (Fig. 5E). This pattern is reminiscent of what has been described in the rat striatum, except for the axonal labeling, which was not as prominent in rodents (Hettinger et al., 2001). A2A receptors have, indeed, been found to modulate presynaptically both excitatory and inhibitory transmission in the rodent striatum. For instance, local infusion of A2AR agonist into the rat striatum greatly increases extracellular glutamate levels (Popoli et al., 1995), whereas infusion of an A2AR antagonist attenuates the increase in striatal glutamate levels induced by cortical stimulation in mice (Quiroz et al., 2009). In line with these data, A2AR agonist increased, and A2AR antagonist decreased, the amplitude of EPSCs in mouse striatal slices through presynaptic regulation of cortical glutamatergic inputs (Quiroz et al., 2009). It is noteworthy that A2AR-mediated regulation of glutamatergic transmission might not only affect cortical afferents but also modulate thalamic inputs (Smith et al., 2004, 2009; Raju et al., 2006). The fact that some of the A2AR-labeled terminals formed asymmetric axodendritic synapses is, indeed, suggestive that a subset of thalamostriatal afferents from the caudal intralaminar thalamic nuclei expresses presynaptic A2ARs (Smith et al., 2009). Furthermore, we found that the monkey thalamus contains low to moderate levels of perikaryal A2AR labeling, in agreement with previous evidence for A2AR expression in the rodent and primate thalamus (Dixon et al., 1996; Svenningsson et al., 1997a; Rosin et al., 1998; Mishina et al., 2007).
In line with previous rodent studies (Hettinger et al., 2001), a subset of A2AR-positive terminals in the monkey striatum displayed the ultrastructural features of intrinsic GABAergic terminals, which most likely originate from axon collaterals of indirect pathway GABAergic striatal projection neurons. However, the A2AR-mediated regulation of GABAergic transmission in the striatum appears to be more complex than mere presynaptic effects and may involve coupling of the receptor to G-protiens other than Gs (Kirk and Richardson, 1995; Gubitz et al., 1996; Ravyn and Bostwick, 2006).
A2AR expression in the SNr
One of the interesting findings of our study was the evidence for significant expression of A2AR immunoreactivity in the monkey SNr, which in rodents is not a major site of A2AR mRNA or protein expression (Schiffmann et al., 1991a; Fink et al., 1992; Dixon et al., 1996; Rosin et al., 1998; Kaelin-Lang et al., 2000; Hettinger et al., 2001). On the other hand, previous studies have reported significant levels of A2AR ligand binding in the human SN using PET or autoradiography detection methods (Svenningsson et al., 1997a; Mishina et al., 2007).
At the ultrastructural level, the A2AR labeling in the monkey SNr was split almost equally between presynaptic (unmyelinated axons/terminals) and glial elements, with very little postsynaptic expression, indicating predominant presynaptic or glial regulatory functions of basal ganglia output neurons. It is known that some striatopallidal axons send collaterals to the SNr (Parent et al., 1995), and most of the identifiable synapses formed by the A2AR-positive SNr boutons in our analyses were of the symmetric type, so these data strongly suggest that much of the presynaptic labeling in the monkey SNr is accounted for by GABAergic axon collaterals of indirect pathway neurons. Given that the GPi is almost completely devoid of A2AR immunoreactivity in monkeys, these findings suggest that GABAergic axon collaterals of indirect striatopallidal neurons in the SNr might differ phenotypically from those in the GPi, thereby providing a substrate for specific A2AR-mediated regulation of striatal GABAergic inputs to the SNr. However, because a small number of labeled terminals in the SNr displayed features of glutamatergic boutons, A2AR-mediated regulation of excitatory transmission in the SNr cannot be ruled out.
A2AR in glia
Our findings provide further evidence that A2ARs mediate some of their physiological effects in the central nervous system through glial cells, a hypothesis that has been put forward in previous articles based largely on in vitro and in vivo data showing that adenosine and A2AR antagonists modulate astrocyte reactivity (Hindley et al., 1994; Brambilla et al., 2003; Dare et al., 2007) as well as glial glutamate release and uptake (Nishizaki et al., 2002). In monkeys, the basal ganglia nucleus most enriched in glial A2AR expression was the SNr, although the striatum and GP also contained a significant number of A2AR-positive glial processes. Glial A2ARs were also strongly expressed in the external capsule and subcortical white matter of rhesus monkeys. It is noteworthy that the relative prevalence of A2AR in glia appears to be higher in monkey than in rodent basal ganglia (Hettinger et al., 2001). Whether this represents a genuine species difference in A2AR expression indicating a more active role of glial A2AR in primates than nonprimates remains to be determined.
At the EM level, essentially all labeled large-diameter glial processes belonged to fibrous astrocytes, as can be determined by their content of densely packed intermediate filaments (Fig. 4D). However, many finer labeled glial processes were identified, which could belong to other glial cell types. A2ARs are considered important regulators of microglial reactivity and proliferation toward sites of brain injury or degeneration, thereby suggesting an additional role in neuroinflammation, brain repair, and cell death (Gebicke-Haerter et al., 1996). Because, we did not use specific markers of astrocytes or microglia in this study, our findings cannot confirm or reject evidence for microglial A2AR immunoreactivity in the basal ganglia of normal monkeys. However, the outcome may be different in animal models of basal ganglia disorders and in humans affected with chronic neurodegenerative diseases such as PD, insofar as microglia have been shown to upregulate A2AR expression when activated in an animal model of neuroinflammation (Orr et al., 2009).
Intracellular and extrasynaptic localization of A2AR in the striatum
Our immunogold data indicate that the majority (75–85%) of A2AR labeling in the striatum and GPe is located in the intracellular compartment of pre- or postsynaptic immunoreactive elements. This could be indicative of a high level of turnover of A2A receptors at the cell membrane. Alternatively, in vitro reports on neuronal and non-neuronal cultures suggest that intracellular A2ARs could be latent in storage awaiting certain conditions under which they could be mobilized to the plasma membrane, thereby increasing the neuron’s sensitivity to extracellular adenosine (Milojevic et al., 2006). It has also been found that A2ARs, like many G-protein-coupled receptors, internalize in response to agonist administration (Brand et al., 2008), suggesting that some of this intracellular labeling may be the result of an abnormal increase in the extracellular concentration of adenosine under hypoxic conditions at the time of perfusion (Hagberg et al., 1987), followed by activation and internalization of receptors. However, in vitro data argue against this hypothesis by showing that A2ARs mobilize to the plasma membrane under anoxic conditions (Arslan et al., 2002). It is unclear which of these processes would prevail in vivo.
Yet another possibility is that intracellular A2ARs have a function inside the cell. This is still a topic of great controversy, but a growing body of evidence suggests that intracellular G-protein-coupled receptors may have functional signaling roles at multiple intracellular sites, including the Golgi apparatus, endoplasmic reticulum, and cell nucleus (Boivin et al., 2008). Although intracellular functions of A2ARs have not been reported, it is worth noting that mGluR5, with which A2AR interacts (Ferre et al., 1999, 2002), is often localized on the nuclear membrane, through which it regulates nuclear calcium levels (O’Malley et al., 2003).
Among the plasma membrane-bound A2AR-associated gold particles, most (97–98%) were found at extrasynaptic sites, which is a common feature for many G-protein-coupled receptors (Galvan et al., 2006). These extrasynaptic A2A receptors most likely are activated only during high levels of neuronal metabolic activity when large-scale ATP hydrolysis leads to grossly increased extracellular adenosine levels across entire nuclei (Nordstrom et al., 1977), via bidirectional nonconcentrating nucleoside transporters (Geiger and Fyda, 1991). Astrocytes represent another likely source of adenosine for these extrasynaptic receptors, in that they can release both adenosine and ATP (which is rapidly converted to adenosine; Dunwiddie et al., 1997) via facilitated transport or a calcium-dependent vesicular mechanism, in response to hypoxia or chemical signals (Cunha, 2001; Martin et al., 2007; Parpura and Zorec, 2010). Extracellular adenosine levels in the striatum are in the low to middle nanomolar range under basal conditions (Ballarin et al., 1991), which is enough to produce a low tone of A2AR activation (the EC50 of adenosine at A2AR is ~700 nm; Dunwiddie and Diao, 1994; Fredholm et al., 2001). Under hypoxic conditions, striatal adenosine levels can increase into the micromolar range (Hagberg et al., 1987), probably activating most A2ARs.
Although they were rare (2–3%), we did find some pre-and postsynaptic A2A receptors at synaptic and perisynaptic sites in the monkey striatum, which is in agreement with previous rodent data (Ciruela et al., 2006). These A2A receptors most likely respond to synaptically released adenosine or ATP, which are coreleased with other neurotransmitters (Cunha and Ribeiro, 2000; Latini and Pedata, 2001), even during lower levels of synaptic activity.
Postsynaptic colocalization of A2AR and mGluR5 in striatal neurons: potential sites of functional interactions
As mentioned above, A2AR and mGluR5 physically interact and display a functional synergy whereby simultaneous activation or inhibition of both receptors produces greater effects than the sum of the modulation of each receptor alone (Ferre et al., 1999, 2002). This functional synergy is relevant to several biological phenomena and possibly therapeutic benefits of A2AR antagonists in movement disorders and other brain disorders (Coccurello et al., 2004; Kachroo et al., 2005; Adams et al., 2008). For instance, in the hippocampus, where A2AR and mGluR5 colocalize at individual glutamatergic synapses, the synergistic effects of both receptor agonists on their targets potentiate NMDA receptor activity more strongly than individual receptor activation, suggesting a possible role for A2AR/mGluR5 functional interactions in learning and memory (Tebano et al., 2005).
There is also evidence from in vitro data supporting functional A2AR/mGluR5 interactions in the rat striatum (Ferre et al., 1999, 2002; Nishi et al., 2003). However, in contrast to our findings that support a high level of A2AR/mGluR5 coexpression only in postsynaptic elements in the monkey striatum, other in vitro data have indicated that as much as 50% of striatal terminals coexpress mGluR5 and A2AR immunoreactivity in isolated striatal nerve terminal preparations (Rodrigues et al., 2005). These findings were corroborated in functional studies showing that agonists for A2AR and mGluR5 synergistically interact to enhance K+-induced glutamate release from rat synaptosomal preparations. However, these data are at odds with ultrastructural data indicating that striatal mGluR5 immunoreactivity is largely localized postsynaptically in rats (Shigemoto et al., 1993). Similarly, the ultrastructural localization of A2AR and mGluR5 described in the present study and our previous reports in the monkey striatum showing little evidence for presynaptic mGluR5 (Paquet and Smith, 2003; Mitrano and Smith, 2007) strongly suggest that the main target sites for functional interactions between these receptor subtypes are the spines and dendrites of striatal projection neurons. It is possible that the presynaptic synaptosomal membrane fractions prepared by Rodrigues et al. (2005) also included postsynaptic material containing mGluR5, leading to an overestimation of the prevalence of presynaptic mGluR5 immunoreactivity. Alternatively, the disparity could be due to a shift from a significant presynaptic mGluR5 expression in young animals (2–3 weeks old) to an almost exclusive postsynaptic localization in adults (Hubert and Smith, 2004). Whether the discrepancies rely on technical differences remains unknown, but we cannot rule out the possibility that the small amount of presynaptic mGluR5 in the adult striatum is functionally relevant, suggesting that striatal and pallidal axon terminals may be another potential site for A2AR/mGluR5 interactions. Although we did find presynaptic colocalization of the two receptors in the monkey putamen, it was rare, accounting for only 2–6% of striatal double-labeled elements.
It is worth noting that the pattern of striatal A2AR immunogold labeling (mainly postsynaptic, largely intracellular, with a smaller membrane-bound component composed mostly of extrasynaptic receptors) is very similar to the pattern of striatal mGluR5 immunogold labeling previously described for the monkey striatum (Paquet and Smith, 2003). The neuronal signaling via these large pools of extrasynaptic receptors could become very important during periods of high neuronal activity, such as in the parkinsonian state, when corticostriatal glutamatergic transmission is abnormally overactive (Calabresi et al., 1993). With large-scale breakdown of ATP and overflow of synaptic glutamate, these two groups of extrasynaptic receptors might be simultaneously activated and synergistically interact to increase greatly the excitability of indirect pathway MSNs. This scenario suggests a potential role for A2AR/mGluR5 interactions in PD pathophysiology.
Therapeutic relevance of A2AR/mGluR5 antagonists in PD
Antagonists at A2AR and mGluR5 both decrease motor deficits in experimental rodent models of parkinsonism (Breysse et al., 2002, 2003; Ossowska et al., 2002, 2005, 2007; Tanganelli et al., 2004; Pinna et al., 2007). When given together, A2AR and mGluR5 antagonists produce a synergistic improvement in locomotion of reserpinized mice (Kachroo et al., 2005) and in the performance of a reaction time task in 6-OHDA-treated rats (Coccurello et al., 2004). Despite some success in preclinical studies (Grondin et al., 1999; Kanda et al., 2000; Bibbiani et al., 2003), the administration of the A2AR antagonist istradefylline (also known as KW-6002), alone or in combination with levodopa, has had limited success in alleviating parkinsonian symptoms in PD patients (Bara-Jimenez et al., 2003; Hauser et al., 2003; Fernandez et al., 2010), although the antagonist appeared to be safe and well-tolerated in humans. Based on the rodent behavioral data and our findings suggesting potential sites for A2AR/mGluR5 interactions in the primate striatum, it appears reasonable to suggest that the combination of A2AR and mGluR5 antagonists might be a relevant nondopaminergic approach to PD pharmacotherapeutics with improved efficacy.
Another strong interest for mGluR5 therapeutics in Parkinson’s disease relates to the significant effect that these drugs have on the development and progression of L-DOPA-induced dyskinesias (Johnston et al., 2010; Morin et al., 2010). A2AR antagonists also have antidyskinetic properties, at least in animal models (Morelli et al., 2007), although the preclinical data have not led to conclusive evidence for antidyskinetic effects in PD patients (Bara-Jimenez et al., 2003; Hauser et al., 2008; LeWitt et al., 2008; Stacy et al., 2008). In addition to their potential to treat the motor symptoms of PD, A2AR and mGluR5 antagonists have neuroprotective effects against degeneration of midbrain dopaminergic neurons in toxin-based models of PD (Chen et al., 2001; Battaglia et al., 2004; Aguirre et al., 2005; Bove et al., 2005; Pierri et al., 2005; Masilamoni et al., 2009, 2011; Nobre et al., 2010).
Together these findings highlight the exciting possibility that A2AR/mGluR5 combination therapy might be used at various stages of the disease to treat multiple aspects of PD symptoms and pathology. The antiparkinsonian, antidyskinetic, and neuroprotective benefits of A2AR/mGluR5 combination therapy have never been tested in primates. With future research in these areas, it might be possible to take advantage of the synergy between these two receptors to improve PD pharmacotherapeutics greatly.
Acknowledgments
Grant sponsor: National Parkinson Foundation; Grant sponsor: National Institutes of Health; Grant number: RR00165 (to the Yerkes Primate Center); Grant sponsor: National Research Service Award; Grant number: F31 NS061520-01A2 (to J.W.B.); Grant sponsor: UDALL Parkinson’s Disease Center; Grant number: P50 NS071669.
We thank Jean-Francois Pare and Susan Jenkins for technical assistance.
LITERATURE CITED
- Adams CL, Cowen MS, Short JL, Lawrence AJ. Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol. 2008;11:229–241. doi: 10.1017/S1461145707007845. [DOI] [PubMed] [Google Scholar]
- Aguirre JA, Kehr J, Yoshitake T, Liu FL, Rivera A, Fernandez-Espinola S, Andbjer B, Leo G, Medhurst AD, Agnati LF, Fuxe K. Protection but maintained dysfunction of nigral dopaminergic nerve cell bodies and striatal dopaminergic terminals in MPTP-lesioned mice after acute treatment with the mGluR5 antagonist MPEP. Brain Res. 2005;1033:216–220. doi: 10.1016/j.brainres.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Arslan G, Kull B, Fredholm BB. Anoxia redistributes adenosine A2A receptors in PC12 cells and increases receptor-mediated formation of cAMP. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:150–157. doi: 10.1007/s002100100456. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Emson PC. Adenosine A2A receptor mRNA is expressed by enkephalin cells but not by somatostatin cells in rat striatum: a co-expression study. Brain Res. 1994;22:204–210. doi: 10.1016/0169-328x(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Azdad K, Gall D, Woods AS, Ledent C, Ferre S, Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology. 2009;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarin M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol Scand. 1991;142:97–103. doi: 10.1111/j.1748-1716.1991.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Sherzai A, Dimitrova T, Favit A, Bibbiani F, Gillespie M, Morris MJ, Mouradian MM, Chase TN. Adenosine A2A receptor antagonist treatment of Parkinson’s disease. Neurology. 2003;61:293–296. doi: 10.1212/01.wnl.0000073136.00548.d4. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Busceti CL, Molinaro G, Biagioni F, Storto M, Fornai F, Nicoletti F, Bruno V. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigrostriatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Neurosci. 2004;24:828–835. doi: 10.1523/JNEUROSCI.3831-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Petzer JP, Castagnoli N, Jr, Chen JF, Schwarzschild MA, Chase TN. A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson’s disease. Exp Neurol. 2003;184:285–294. doi: 10.1016/s0014-4886(03)00250-4. [DOI] [PubMed] [Google Scholar]
- Boivin B, Vaniotis G, Allen BG, Hebert TE. G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res. 2008;28:15–28. doi: 10.1080/10799890801941889. [DOI] [PubMed] [Google Scholar]
- Bove J, Serrats J, Mengod G, Cortes R, Tolosa E, Marin C. Neuroprotection induced by the adenosine A2A antagonist CSC in the 6-OHDA rat model of parkinsonism: effect on the activity of striatal output pathways. Exp Brain Res. 2005;165:362–374. doi: 10.1007/s00221-005-2302-1. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. Glia. 2003;43:190–194. doi: 10.1002/glia.10243. [DOI] [PubMed] [Google Scholar]
- Brand F, Klutz AM, Jacobson KA, Fredholm BB, Schulte G. Adenosine A2A receptor dynamics studied with the novel fluorescent agonist Alexa488-APEC. Eur J Pharmacol. 2008;590:36–42. doi: 10.1016/j.ejphar.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breysse N, Baunez C, Spooren W, Gasparini F, Amalric M. Chronic but not acute treatment with a metabotropic glutamate 5 receptor antagonist reverses the akinetic deficits in a rat model of parkinsonism. J Neurosci. 2002;22:5669–5678. doi: 10.1523/JNEUROSCI.22-13-05669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breysse N, Amalric M, Salin P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats. J Neurosci. 2003;23:8302–8309. doi: 10.1523/JNEUROSCI.23-23-08302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Short JL. Adenosine A2A receptors and their role in drug addiction. J Pharm Pharmacol. 2008;60:1409–1430. doi: 10.1211/jpp/60.11.0001. [DOI] [PubMed] [Google Scholar]
- Cabello N, Gandia J, Bertarelli DC, Watanabe M, Lluis C, Franco R, Ferre S, Lujan R, Ciruela F. Metabotropic glutamate type 5, dopamine D2 and adenosine A2A receptors form higher-order oligomers in living cells. J Neurochem. 2009;109:1497–1507. doi: 10.1111/j.1471-4159.2009.06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, Sancesario G, Bernardi G. Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson’s disease. Brain. 1993;116:433–452. [PubMed] [Google Scholar]
- Calon F, Dridi M, Hornykiewicz O, Bedard PJ, Rajput AH, Di Paolo T. Increased adenosine A2A receptors in the brain of Parkinson’s disease patients with dyskinesias. Brain. 2004;127:1075–1084. doi: 10.1093/brain/awh128. [DOI] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, Neve K, Fuxe K, Agnati LF, Woods AS, Ferre S, Lluis C, Bouvier M, Franco R. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr, Schwarzschild MA. Neuroprotection by caffeine and A2A adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci. 2001;21:RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Burgueno J, Casado V, Canals M, Marcellino D, Goldberg SR, Bader M, Fuxe K, Agnati LF, Lluis C, Franco R, Ferre S, Woods AS. Combining mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope-epitope electrostatic interactions between adenosine A2A and dopamine D2 receptors. Anal Chem. 2004;76:5354–5363. doi: 10.1021/ac049295f. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccurello R, Breysse N, Amalric M. Simultaneous blockade of adenosine A2A and metabotropic glutamate mGlu5 receptors increase their efficacy in reversing Parkinsonian deficits in rats. Neuropsychopharmacology. 2004;29:1451–1461. doi: 10.1038/sj.npp.1300444. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Ribeiro JA. ATP as a presynaptic modulator. Life Sci. 2000;68:119–137. doi: 10.1016/s0024-3205(00)00923-1. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Ferre S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des. 2008;14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiol Behav. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici MR, Pepponi R, Martire A, Tebano MT, Potenza RL, Popoli P. Permissive role of adenosine A2A receptors on metabotropic glutamate receptor 5 (mGluR5)-mediated effects in the striatum. J Neurochem. 2004;90:1276–1279. doi: 10.1111/j.1471-4159.2004.02607.x. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L. Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J Pharmacol Exp Ther. 1994;268:537–545. [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez HH, Greeley DR, Zweig RM, Wojcieszek J, Mori A, Sussman NM. Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial. Parkinsonism Rel Disorders. 2010;16:16–20. doi: 10.1016/j.parkreldis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Ferre S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferre S, Popoli P, Rimondini R, Reggio R, Kehr J, Fuxe K. Adenosine A2A and group I metabotropic glutamate receptors synergistically modulate the binding characteristics of dopamine D2 receptors in the rat striatum. Neuropharmacology. 1999;38:129–140. doi: 10.1016/s0028-3908(98)00154-3. [DOI] [PubMed] [Google Scholar]
- Ferre S, Popoli P, Gimenez-Llort L, Rimondini R, Muller CE, Stromberg I, Ogren SO, Fuxe K. Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinsonism Rel Disorders. 2001;7:235–241. doi: 10.1016/s1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- Ferre S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, Casado V, Fuxe K, Goldberg SR, Lluis C, Franco R, Ciruela F. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Ciruela F, Quiroz C, Lujan R, Popoli P, Cunha RA, Agnati LF, Fuxe K, Woods AS, Lluis C, Franco R. Adenosine receptor heteromers and their integrative role in striatal function. Sci World J. 2007a;7:74–85. doi: 10.1100/tsw.2007.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Diamond I, Goldberg SR, Yao L, Hourani SM, Huang ZL, Urade Y, Kitchen I. Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry implications for drug addiction, sleep and pain. Prog Neurobiol. 2007b;83:332–347. doi: 10.1016/j.pneurobio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Franco R, Ferre S, Agnati L, Torvinen M, Gines S, Hillion J, Casado V, Lledo P, Zoli M, Lluis C, Fuxe K. Evidence for adenosine/dopamine receptor interactions: indications for heteromerization. Neuropsychopharmacology. 2000;23(Suppl):S50–S59. doi: 10.1016/S0893-133X(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Galvan A, Kuwajima M, Smith Y. Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience. 2006;143:351–375. doi: 10.1016/j.neuroscience.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebicke-Haerter PJ, Christoffel F, Timmer J, Northoff H, Berger M, Van Calker D. Both adenosine A1- and A2-receptors are required to stimulate microglial proliferation. Neurochem Int. 1996;29:37–42. [PubMed] [Google Scholar]
- Geiger JD, Fyda DM. Adenosine transport in nervous tissues. In: Stone TW, editor. Adenosine in the central nervous system. London: Academic Press; 1991. pp. 1–23. [Google Scholar]
- Gerevich Z, Wirkner K, Illes P. Adenosine A2A receptors inhibit the N-methyl-D-aspartate component of excitatory synaptic currents in rat striatal neurons. Eur J Pharmacol. 2002;451:161–164. doi: 10.1016/s0014-2999(02)02301-4. [DOI] [PubMed] [Google Scholar]
- Grondin R, Bedard PJ, Hadj Tahar A, Gregoire L, Mori A, Kase H. Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology. 1999;52:1673–1677. doi: 10.1212/wnl.52.8.1673. [DOI] [PubMed] [Google Scholar]
- Gubitz AK, Widdowson L, Kurokawa M, Kirkpatrick KA, Richardson PJ. Dual signalling by the adenosine A2A receptor involves activation of both N- and P-type calcium channels by different G proteins and protein kinases in the same striatal nerve terminals. J Neurochem. 1996;67:374–381. doi: 10.1046/j.1471-4159.1996.67010374.x. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Andersson P, Lacarewicz J, Jacobson I, Butcher S, Sandberg M. Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. J Neurochem. 1987;49:227–231. doi: 10.1111/j.1471-4159.1987.tb03419.x. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Hubble JP, Truong DD. Randomized trial of the adenosine A2A receptor antagonist istradefylline in advanced PD. Neurology. 2003;61:297–303. doi: 10.1212/01.wnl.0000081227.84197.0b. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Shulman LM, Trugman JM, Roberts JW, Mori A, Ballerini R, Sussman NM. Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Mov Disord. 2008;23:2177–2185. doi: 10.1002/mds.22095. [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Hindley S, Herman MA, Rathbone MP. Stimulation of reactive astrogliosis in vivo by extracellular adenosine diphosphate or an adenosine A2 receptor agonist. J Neurosci Res. 1994;38:399–406. doi: 10.1002/jnr.490380405. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Bedard PJ, Varty GB, Kazdoba TM, Di Paolo T, Grzelak ME, Pond AJ, Hadjtahar A, Belanger N, Gregoire L, Dare A, Neustadt BR, Stamford AW, Hunter JC. Preladenant, a selective A2A receptor antagonist, is active in primate models of movement disorders. Exp Neurol. 2010;225:384–390. doi: 10.1016/j.expneurol.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Smith Y. Age-related changes in the expression of axonal and glial group I metabotropic glutamate receptor in the rat substantia nigra pars reticulata. J Comp Neurol. 2004;475:95–106. doi: 10.1002/cne.20163. [DOI] [PubMed] [Google Scholar]
- Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM. Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Pharmacol Exp Ther. 2010;333:865–873. doi: 10.1124/jpet.110.166629. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25:10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin-Lang A, Liniger P, Probst A, Lauterburg T, Burgunder JM. Adenosine A2A receptor gene expression in the normal striatum and after 6-OH-dopamine lesion. J Neural Transm. 2000;107:851–859. doi: 10.1007/s007020070037. [DOI] [PubMed] [Google Scholar]
- Kanda T, Jackson MJ, Smith LA, Pearce RK, Nakamura J, Kase H, Kuwana Y, Jenner P. Combined use of the adenosine A2A antagonist KW-6002 with L-DOPA or with selective D1 or D2 dopamine agonists increases antiparkinsonian activity but not dyskinesia in MPTP-treated monkeys. Exp Neurol. 2000;162:321–327. doi: 10.1006/exnr.2000.7350. [DOI] [PubMed] [Google Scholar]
- Kirk IP, Richardson PJ. Inhibition of striatal GABA release by the adenosine A2A receptor is not mediated by increases in cyclic AMP. J Neurochem. 1995;64:2801–2809. doi: 10.1046/j.1471-4159.1995.64062801.x. [DOI] [PubMed] [Google Scholar]
- Kuwajima M, Hall RA, Aiba A, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus. J Comp Neurol. 2004;474:589–602. doi: 10.1002/cne.20158. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005) Ann Neurol. 2008;63:295–302. doi: 10.1002/ana.21315. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci. 2001;21:7001–7012. doi: 10.1523/JNEUROSCI.21-18-07001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Martinez-Mir MI, Probst A, Palacios JM. Adenosine A2 receptors: selective localization in the human basal ganglia and alterations with disease. Neuroscience. 1991;42:697–706. doi: 10.1016/0306-4522(91)90038-p. [DOI] [PubMed] [Google Scholar]
- Masilamoni JG, Alagille D, Bogenpohl JW, Delevich K, Reddy V, Votaw JR, Tamagnan G, Wichmann T, Smith Y. Potential neuroprotective effects of metabotropic glutamate receptor type 5 (mGluR5) antagonist in MPTP-treated monkeys. Movement Disorders Society 13th International Congress; Paris, France. 2009. [Google Scholar]
- Masilamoni JG, Bogenpohl JW, Alagille D, Delevich K, Tamagnan G, Votaw JR, Wichmann T, Smith Y. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain. 2011;134(Pt 7):2057–2073. doi: 10.1093/brain/awr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojevic T, Reiterer V, Stefan E, Korkhov VM, Dorostkar MM, Ducza E, Ogris E, Boehm S, Freissmuth M, Nanoff C. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol. 2006;69:1083–1094. doi: 10.1124/mol.105.015818. [DOI] [PubMed] [Google Scholar]
- Mishina M, Ishiwata K, Kimura Y, Naganawa M, Oda K, Kobayashi S, Katayama Y, Ishii K. Evaluation of distribution of adenosine A2A receptors in normal human brain measured with [11C]TMSX PET. Synapse. 2007;61:778–784. doi: 10.1002/syn.20423. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Morelli M, Di Paolo T, Wardas J, Calon F, Xiao D, Schwarzschild MA. Role of adenosine A2A receptors in parkinsonian motor impairment and l-DOPA-induced motor complications. Prog Neurobiol. 2007;83:293–309. doi: 10.1016/j.pneurobio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Mori A, Shindou T, Ichimura M, Nonaka H, Kase H. The role of adenosine A2A receptors in regulating GABAergic synaptic transmission in striatal medium spiny neurons. J Neurosci. 1996;16:605–611. doi: 10.1523/JNEUROSCI.16-02-00605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin N, Gregoire L, Gomez-Mancilla B, Gasparini F, Di Paolo T. Effect of the metabotropic glutamate receptor type 5 antagonists MPEP and MTEP in parkinsonian monkeys. Neuropharmacology. 2010;58:981–986. doi: 10.1016/j.neuropharm.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Nishi A, Liu F, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci U S A. 2003;100:1322–1327. doi: 10.1073/pnas.0237126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizaki T, Nagai K, Nomura T, Tada H, Kanno T, Tozaki H, Li XX, Kondoh T, Kodama N, Takahashi E, Sakai N, Tanaka K, Saito N. A new neuromodulatory pathway with a glial contribution mediated via A2a adenosine receptors. Glia. 2002;39:133–147. doi: 10.1002/glia.10100. [DOI] [PubMed] [Google Scholar]
- Nobre HV, Jr, Cunha GM, de Vasconcelos LM, Magalhaes HI, Oliveira Neto RN, Maia FD, de Moraes MO, Leal LK, Viana GS. Caffeine and CSC, adenosine A2A antagonists, offer neuroprotection against 6-OHDA-induced neurotoxicity in rat mesencephalic cells. Neurochem Int. 2010;56:51–58. doi: 10.1016/j.neuint.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Nordstrom CH, Rehncrona S, Siesjo BK, Westerberg E. Adenosine in rat cerebral cortex: its determination, normal values, and correlation to AMP and cyclic AMP during shortlasting ischemia. Acta Physiol Scand. 1977;101:63–71. doi: 10.1111/j.1748-1716.1977.tb05984.x. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Wirkner K, Illes P. Effect of adenosine and some of its structural analogues on the conductance of NMDA receptor channels in a subset of rat neostriatal neurones. Br J Pharmacol. 1997;122:71–80. doi: 10.1038/sj.bjp.0701347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg W, Wirkner K, Assmann H, Richter M, Illes P. Adenosine A2A receptors inhibit the conductance of NMDA receptor channels in rat neostriatal neurons. Amino Acids. 1998;14:33–39. doi: 10.1007/BF01345239. [DOI] [PubMed] [Google Scholar]
- O’Malley KL, Jong YJ, Gonchar Y, Burkhalter A, Romano C. Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J Biol Chem. 2003;278:28210–28219. doi: 10.1074/jbc.M300792200. [DOI] [PubMed] [Google Scholar]
- Ochi M, Koga K, Kurokawa M, Kase H, Nakamura J, Kuwana Y. Systemic administration of adenosine A2A receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: a microdialysis study. Neuroscience. 2000;100:53–62. doi: 10.1016/s0306-4522(00)00250-5. [DOI] [PubMed] [Google Scholar]
- Orr AG, Orr AL, Li XJ, Gross RE, Traynelis SF. Adenosine A2A receptor mediates microglial process retraction. Nat Neurosci. 2009;12:872–878. doi: 10.1038/nn.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowska K, Konieczny J, Wardas J, Golembiowska K, Wolfarth S, Pilc A. The role of striatal metabotropic glutamate receptors in Parkinson’s disease. Amino Acids. 2002;23:193–198. doi: 10.1007/s00726-001-0128-0. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Konieczny J, Wolfarth S, Pilc A. MTEP, a new selective antagonist of the metabotropic glutamate receptor subtype 5 (mGluR5), produces antiparkinsonian-like effects in rats. Neuropharmacology. 2005;49:447–455. doi: 10.1016/j.neuropharm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Konieczny J, Wardas J, Pietraszek M, Kuter K, Wolfarth S, Pilc A. An influence of ligands of metabotropic glutamate receptor subtypes on parkinsonian-like symptoms and the striatopallidal pathway in rats. Amino Acids. 2007;32:179–188. doi: 10.1007/s00726-006-0317-y. [DOI] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–7669. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Charara A, Pinault D. Single striatofugal axons arborizing in both pallidal segments and in the substantia nigra in primates. Brain Res. 1995;698:280–284. doi: 10.1016/0006-8993(95)01017-p. [DOI] [PubMed] [Google Scholar]
- Parpura V, Zorec R. Gliotransmission: exocytotic release from astrocytes. Brain Res Rev. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. San Diego: Academic Press; 2000. p. 163. [Google Scholar]
- Peterfreund RA, MacCollin M, Gusella J, Fink JS. Characterization and expression of the human A2A adenosine receptor gene. J Neurochem. 1996;66:362–368. doi: 10.1046/j.1471-4159.1996.66010362.x. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. The fine structure of the nervous system: neurons and their supporting cells. xviii. New York: Oxford University Press; 1991. p. 494. [Google Scholar]
- Pierri M, Vaudano E, Sager T, Englund U. KW-6002 protects from MPTP induced dopaminergic toxicity in the mouse. Neuropharmacology. 2005;48:517–524. doi: 10.1016/j.neuropharm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Pinna A. Novel investigational adenosine A2A receptor antagonists for Parkinson’s disease. Expert Opin Invest Drugs. 2009;18:1619–1631. doi: 10.1517/13543780903241615. [DOI] [PubMed] [Google Scholar]
- Pinna A, Pontis S, Borsini F, Morelli M. Adenosine A2A receptor antagonists improve deficits in initiation of movement and sensory motor integration in the unilateral 6-hydroxydopamine rat model of Parkinson’s disease. Synapse. 2007;61:606–614. doi: 10.1002/syn.20410. [DOI] [PubMed] [Google Scholar]
- Popoli P, Betto P, Reggio R, Ricciarello G. Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur J Pharmacol. 1995;287:215–217. doi: 10.1016/0014-2999(95)00679-6. [DOI] [PubMed] [Google Scholar]
- Querejeta E, Martinez-Romero B, Miranda JE, Delgado A. Modulation of the striatopallidal pathway by adenosine A2A receptors depends on dopaminergic striatal input. Brain Res. 2010;1349:137–142. doi: 10.1016/j.brainres.2010.06.040. [DOI] [PubMed] [Google Scholar]
- Quiroz C, Lujan R, Uchigashima M, Simoes AP, Lerner TN, Borycz J, Kachroo A, Canas PM, Orru M, Schwarzschild MA, Rosin DL, Kreitzer AC, Cunha RA, Watanabe M, Ferre S. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. Sci World J. 2009;9:1321–1344. doi: 10.1100/tsw.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol. 2006;499:231–243. doi: 10.1002/cne.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravyn V, Bostwick JR. Functional coupling of the Galpha(olf) variant XLGalpha(olf) with the human adenosine A2A receptor. J Recept Signal Transduct Res. 2006;26:241–258. doi: 10.1080/10799890600710592. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA. Colocalization and functional interaction between adenosine A2A and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem. 2005;92:433–441. doi: 10.1111/j.1471-4159.2004.02887.x. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- Schiffmann SN, Vanderhaeghen JJ. Adenosine A2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. J Neurosci. 1993;13:1080–1087. doi: 10.1523/JNEUROSCI.13-03-01080.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991a;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Libert F, Vassart G, Vanderhaeghen JJ. Distribution of adenosine A2 receptor mRNA in the human brain. Neurosci Lett. 1991b;130:177–181. doi: 10.1016/0304-3940(91)90391-6. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Shindou T, Mori A, Kase H, Ichimura M. Adenosine A2A receptor enhances GABAA-mediated IPSCs in the rat globus pallidus. J Physiol. 2001;532:423–434. doi: 10.1111/j.1469-7793.2001.0423f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. T Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy M, Silver D, Mendis T, Sutton J, Mori A, Chaikin P, Sussman NM. A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology. 2008;70:2233–2240. doi: 10.1212/01.wnl.0000313834.22171.17. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Popoli P, Muller CE, Ferre S, Fuxe K. Electrophysiological and behavioural evidence for an antagonistic modulatory role of adenosine A2A receptors in dopamine D2 receptor regulation in the rat dopamine-denervated striatum. Eur J Neurosci. 2000;12:4033–4037. doi: 10.1046/j.1460-9568.2000.00288.x. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Hall H, Sedvall G, Fredholm BB. Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse. 1997a;27:322–335. doi: 10.1002/(SICI)1098-2396(199712)27:4<322::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, Fredholm BB. Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience. 1997b;80:1171–1185. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Aubert I, Burbaud P, Fredholm BB, Bloch B. Cellular distribution of adenosine A2A receptor mRNA in the primate striatum. J Comp Neurol. 1998;399:229–240. doi: 10.1002/(sici)1096-9861(19980921)399:2<229::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, Sandager Nielsen K, Ferraro L, Antonelli T, Kehr J, Franco R, Ferre S, Agnati LF, Fuxe K, Scheel-Kruger J. Striatal plasticity at the network level. Focus on adenosine A2A and D2 interactions in models of Parkinson’s disease. Parkinsonism Rel Disorders. 2004;10:273–280. doi: 10.1016/j.parkreldis.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Rebola N, Pepponi R, Domenici MR, Gro MC, Schwarzschild MA, Chen JF, Cunha RA, Popoli P. Adenosine A2A receptors and metabotropic glutamate 5 receptors are colocalized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-D-aspartate effects. J Neurochem. 2005;95:1188–1200. doi: 10.1111/j.1471-4159.2005.03455.x. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Johnson J, Heilig M. Effect of the adenosine A2A receptor antagonist 3,7-dimethyl-propargylxanthine on anxiety-like and depression-like behavior and alcohol consumption in Wistar rats. Alcohol Clin Exp Res. 2007;31:1302–1307. doi: 10.1111/j.1530-0277.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Di Filippo M, Tantucci M, Costa C, Borsini F, Ghiglieri V, Giampa C, Fusco FR, Picconi B, Calabresi P. The distinct role of medium spiny neurons and cholinergic interneurons in the D/AA receptor interaction in the striatum: implications for Parkinson’s disease. J Neurosci. 2011;31:1850–1862. doi: 10.1523/JNEUROSCI.4082-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner K, Assmann H, Koles L, Gerevich Z, Franke H, Norenberg W, Boehm R, Illes P. Inhibition by adenosine A2A receptors of NMDA but not AMPA currents in rat neostriatal neurons. Br J Pharmacol. 2000;130:259–269. doi: 10.1038/sj.bjp.0703234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner K, Gerevich Z, Krause T, Gunther A, Koles L, Schneider D, Norenberg W, Illes P. Adenosine A2A receptor-induced inhibition of NMDA and GABAA receptor-mediated synaptic currents in a subpopulation of rat striatal neurons. Neuropharmacology. 2004;46:994–1007. doi: 10.1016/j.neuropharm.2004.01.008. [DOI] [PubMed] [Google Scholar]