To the Editor: Avian malaria is an insect-borne disease induced by a so far unknown number of protozoan blood parasites of the genera Plasmodium and Haemoproteus (hematozoa) (1,2). The unintentional introduction of P. relictum to the Hawaiian Islands, USA, has had fatal effects for the native bird fauna (3). In Europe, asymptomatic blood infections by hematozoa have been regularly observed, with an especially high prevalence in songbirds (4). However, numerous outbreaks of fatal protozoan infections have been reported over the past 40 years, mainly among psittacines of Australia that have been kept in aviaries (5,6). Diagnosis in all these cases was based on histopathologic detection of protozoan cyst-like structures of unexplained origin in the heart and skeletal muscles and, to a lesser extent, in other organs. In most cases, the protozoans were identified as members of the genus Leucocytozoon because of their morphologic features. Recent studies suggest that these cases may, in fact, have been infections of Besnoitia spp. (Sarcocystidae) or other unknown hematozoa (5); however, genetic evidence is lacking.
In August 2010, sudden deaths of parrots were noticed in 2 separate aviaries in northern Germany and Switzerland (Technical AppendixTable). Nine yellow-crowned parakeets (Cyanoramphus auriceps), 3 barred parakeets (Bolborhynchus lineola), and 2 budgerigars (Melopsittacus undulatus) died within 2–5 days after a history of reduced general condition and reduced activity and food intake before death. In addition, 2 budgerigars and 1 barred parakeet in the aviary in Germany showed lethargy and reduced food intake for 2 weeks but fully recovered. About half of the birds were juvenile. No new birds had been introduced into the aviaries during the previous 24 months.
Necropsy and histologic examination of 7 animals with fatal disease showed numerous large cyst-like protozoan structures (size up to 800 µm in diameter; Technical Appendix Figure) in myocardial and skeletal muscles and, to a lesser extent, in the lung and the smooth muscles of the intestinal tract without obvious signs of inflammation. The cyst-like structures had a thick eosinophilic outer wall, were partly compartmented by internal septae, and were filled with many merozoites. Surrounding muscle fibers were degenerated or necrotic and, in some cases, associated with hemorrhage. Blood smears of clinically affected animals screened for ≈5 × 105 cells each did not show parasites. To further characterize the parasites, we carried out a nested PCR and subsequent DNA sequencing as described (7). Notably, phylogenetic comparison of 479 bp of the mitochondrial cytochrome b gene derived from protozoan cyst-like structures with known sequences of avian hematozoa found 99%–100% homology of parasites from both outbreaks with the avian malaria parasites (Haemoproteus spp.) of European songbirds (Figure). Identical cytochrome b sequences were detected in a yellow-crowned parakeet from Switzerland (CYAUR1), a budgerigar from Germany (MEUND1), and a Haemoproteus sp. (TUPHI1) previously found in the blood of a song thrush (Turdus philomelos) in Bulgaria. The sequence derived from the barred parakeet (BOLIN1) of the German outbreak was identical with H. minutus of the common blackbird (T. merula). In fact, different psittacine species of the German outbreak were infected with different Haemoproteus spp. Because all affected parrots had been bred in Europe and had no contact to imported birds, these results suggest that infection was the result of previously unknown cross-species transmission of Haemoproteus spp. between birds of only distantly related orders (8,9).
Figure.
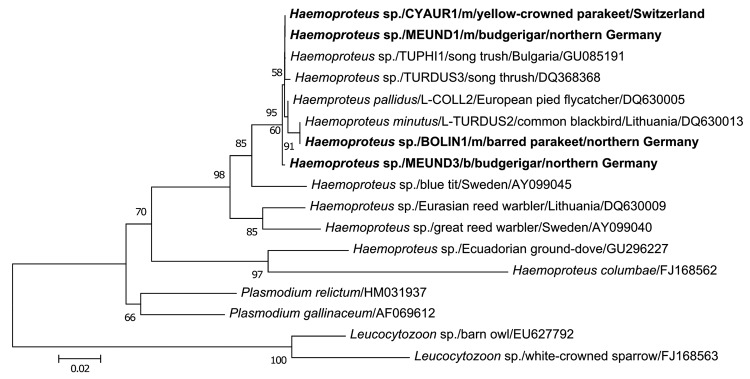
Phylogenetic relationships based on alignment of 479 bp of the cytochrome b gene of Haemoproteus spp. isolated from megalomeronts (m) of infected muscles and blood (b) of parrots with related hematozoan parasites in GenBank and the database MalAvi (http://mbio-serv4.mbioekol.lu.se/avianmalaria; 10). Nucleotide distance values of the maximum likelihood phylogenetic tree were calculated under the HKY substitution model. New sequences of Haemoproteus spp. from parrots of this study are shown in boldface. Two distinct species of the genus Leucocytozoon served as outgroup of the phylogenetic tree. The branch lengths are proportional to the degree of inferred evolutionary change as shown by the scale bar, and the numbers indicate bootstrap values (1,000 replicates). While the cytochrome b sequences CYAUR1, MEUND1, and BOLIN1, respectively, found matching sequences, MEUND3 showed closest sequence similarities with Haemoproteus spp. of the lineage COLL2, which depict a wider host breadth among songbirds (http://mbio-serv4.mbioekol.lu.se/avianmalaria). The isolates of Haemoproteus spp. from psittacine birds were deposited into GenBank under accession nos. HQ398207–HQ398212.
Blood samples from surviving, asymptomatic animals from the German outbreak were tested cytologically and by nested PCR for the presence of Haemoproteus spp. PCR identified Haemoproteus sequences in the blood of 3 of 26 psittacines, although parasitic structures were not identifiable in blood smears. Retrieved sequences were identical with that of MEUND1, except for a single-nucleotide polymorphism in 1 sequence (MEUND3; Figure). A latent infection of these animals therefore seems possible and may constitute a potential risk for further horizontal transmission in aviaries by blood-sucking insects such as biting midges (Culicoides), the vectors for Haemoproteus spp. of passerine birds in Europe (2).
In conclusion, we identified the cause of a previously unexplained lethal disease of captive parrots in Europe, induced by numerous large cyst-like megalomeronts in several organs, including the heart. Morphologically, the parasitic structures were strikingly similar to yet undetermined parasites of numerous previous outbreaks (5,6). Genetically, the parasites had 99%–100% homology to known Haemoproteus spp. from wild European songbirds. The avian malaria parasites identified are highly prevalent in the native songbird population but generally do not cause overt disease or death in their natural hosts. In contrast, the cases reported here suggest that these parasites that have adapted to European songbirds may cause fatal outbreaks in native psittacines of Australia, New Zealand, and South America that are raised in captivity. These findings also show that preexisting pathogens may be a potential hazard for invading species. Avian malaria should therefore be considered a threat for exotic parrots in Europe until results of further epidemiologic and experimental studies are available. Because many European bird species have been introduced to the native range of the psittacines studied here, a concern has been expressed that these parasites already have become established in these areas and are affecting the natural populations.
Supplementary Material
Table depicting complete list of animals in the affected aviaries and an image of the Myocardium of yellow-crowned parakeet that is severely infected with numerous large megalomeronts of Haemoproteus spp. .
Footnotes
Suggested citation for this article: Olias P, Wegelin M, Zenker W, Freter S, Gruber AD, Klopfleisch R. Avian malaria deaths in parrots, Europe [letter]. Emerg Infect Dis [serial on the Internet]. 2011 May [date cited]. http://dx.doi.org/10.3201/eid1705.101618
References
- 1.Atkinson CT, van Riper C III. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In Loye JE, Zuk M, editors. Bird–parasite interactions. Ecology, evolution, and behavior. New York: Oxford University Press; 1991. p. 19–48. [Google Scholar]
- 2.Martinsen ES, Perkins SL, Schall JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol. 2008;47:261–73. 10.1016/j.ympev.2007.11.012 [DOI] [PubMed] [Google Scholar]
- 3.van Riper C III, van Riper SG, Goff ML, Laird M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr. 1986;56:327–44. 10.2307/1942550 [DOI] [Google Scholar]
- 4.Scheuerlein A, Ricklefs RE. Prevalence of blood parasites in European passeriform birds. Proc Biol Sci. 2004;271:1363–70. 10.1098/rspb.2004.2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett GF, Peirce MA, Ashford RW. Avian haematozoa: mortality and pathogenicity. J Nat Hist. 1993;27:993–1001. 10.1080/00222939300770621 [DOI] [Google Scholar]
- 6.Stidworthy MF, Greenwood AG. Deaths in aviary birds associated with protozoal megaloschizonts. Vet Rec. 2006;159:606. 10.1136/vr.159.18.606 [DOI] [PubMed] [Google Scholar]
- 7.Hellgren O, Waldenström J, Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol. 2004;90:797–802. 10.1645/GE-184R1 [DOI] [PubMed] [Google Scholar]
- 8.Križanauskienė A, Hellgren O, Kosarev V, Sokolov L, Bensch S, Valkiūnas G. Variation in host specificity between species of avian hemosporidian parasites: evidence from parasite morphology and cytochrome b gene sequences. J Parasitol. 2006;92:1319–24. 10.1645/GE-873R.1 [DOI] [PubMed] [Google Scholar]
- 9.Ricklefs RE, Fallon SM. Diversification and host switching in avian malaria parasites. Proc Biol Sci. 2002;269:885–92. 10.1098/rspb.2001.1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bensch S, Hellgren O, Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resources. 2009;9:1353–8. 10.1111/j.1755-0998.2009.02692.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table depicting complete list of animals in the affected aviaries and an image of the Myocardium of yellow-crowned parakeet that is severely infected with numerous large megalomeronts of Haemoproteus spp. .


