Abstract
Background
Chemotherapy-induced peripheral neuropathy (CIPN) is a troublesome chronic symptom that has no proven pharmacologic treatment. The purpose of this double-blind randomized placebo-controlled trial was to evaluate a novel compounded topical gel for this problem.
Methods
Patients with CIPN were randomized to baclofen 10 mg, amitriptyline HCL 40 mg, and ketamine 20 mg in a pluronic lecithin organogel (BAK-PLO) versus placebo (PLO) to determine its effect on numbness, tingling, pain, and function. The primary endpoint was the baseline-adjusted sensory subscale of the EORTC QLQ-CIPN20, at 4 weeks.
Results
Data in 208 patients reveal a trend for improvement that is greater in the BAK-PLO arm over placebo in both the sensory (p=0.053) and motor subscales (p=0.021). The greatest improvements were related to the symptoms of tingling, cramping, and shooting/burning pain in the hands as well as difficulty in holding a pen. There were no undesirable toxicities associated with the BAK-PLO and no evidence of systemic toxicity.
Conclusion
Topical treatment with BAK-PLO appears to somewhat improve symptoms of CIPN. This topical gel was well tolerated, without evident systemic toxicity. Further research is needed with increased doses to better clarify the clinical role of this treatment in CIPN.
Keywords: CIPN, Topical gel, BAK-PLO
Background
Chemotherapy-induced peripheral neuropathy (CIPN) is a major dose-limiting side effect of many chemotherapeutic agents including vincristine, paclitaxel, cisplatin, and oxaliplatin [1–7]. The incidence of CIPN can be variable, ranging from 0% to 70% of patients receiving chemotherapy, but commonly occurs in 30–40% of patients. There are a number of factors that can influence the incidence of CIPN, in particular, patient age, dose intensity, cumulative dose, duration of therapy, administration of other neurotoxic agents, and pre-existing conditions such as diabetes and alcohol abuse [2, 3, 8–13]. Many symptoms of CIPN may resolve completely for some patients. However, CIPN is only partly reversible in other instances. In the worst instances, it does not appear to be reversible at all and can even increase over time [13].
CIPN can be extremely painful and/or disabling, causing significant loss of functional abilities and quality of life [14]. Neurotoxic chemotherapeutic agents may cause structural damage to peripheral nerves, resulting in aberrant somatosensory processing of the peripheral and/or central nervous system [7]. This resultant peripheral neuropathy can potentially affect both small fiber axons (temperature and pin prick) and large fiber sensory axons (vibration and proprioception) [14]. A common clinical course begins with tingling or paresthesias and dysesthesias, often located in the toes and fingers. These symptoms can then spread proximally to affect both lower and upper extremities in a characteristic “glove and stocking” distribution [15]. The pain associated with CIPN has not been adequately characterized nor clinically quantified. Sensory perceptions of patients are varied and can include reports of severe pain, shooting pain, burning sensations, numbness, tingling, increased response to painful stimuli, sensitivity to touch, and/or a combination of all of these sensations. It is not currently known whether all of these sensations (such as numbness, tingling, burning pain, allodynia, and hyperalgesia) have similar physiologic characteristics or trajectories. Until more is known, all possible symptom experiences should be evaluated both clinically and in research.
Current management strategies
There is no proven standard treatment to prevent or treat CIPN at this time [13], although preliminary data suggest that infusions of calcium and magnesium may reduce CIPN related to oxaliplatin, and studies are ongoing to evaluate this treatment for neuropathy related to taxanes [16]. Current prevention strategies mainly consist of chemotherapy dose reduction or lower dose intensities, particularly in those patients who are at higher risk to develop neurotoxic side effects. Patients who develop peripheral neuropathy while actively receiving neurotoxic chemotherapy often receive decreased doses or discontinuation of their chemotherapy treatment all together [6]. This strategy, decreasing chemotherapy dosing or cessation of treatment, can potentially impact tumor response, prognosis and, therefore, overall survival.
Several agents have been evaluated for the treatment of CIPN, mostly on the basis of their ability to impact diabetic or other forms of neuropathy [17–19]. Adjuvant analgesics, including tricyclic antidepressants and anticonvulsants, are among a few of these. To date, none of these agents, such as nortriptyline [20], gabapentin [21], or lamotrigine [22], have been found effective for treating CIPN in randomized, controlled trials. Other pharmaceutical agents such as amifostine [23] and glutamine [24] are theorized to be helpful, but randomized controlled trials have not yet been completed to evaluate their effectiveness.
Physiology of analgesia and neuropathic pain
One of the limitations in developing effective prevention or treatment strategies for CIPN is the lack of knowledge about the exact pathophysiology and trajectory of the development of CIPN. Strategies to understand the physiology of CIPN have utilized the science for neuropathic pain, which is admittedly only one element of CIPN symptoms. There are several potential physiologic mechanisms theorized [25, 26]. One of these involves the depletion of substance P, a neurotransmitter that affects small diameter sensory afferent nerves. Depleting substance P might be able to quiet the noxious activation of these nerves and decrease pain. There are numerous potential physiologic targets for neuropathic pain that may relate to CIPN. These include modulating voltage-gated sodium channels, inhibiting NMDA, turning on alpha 2-ADRENERGIC receptors and GABA (in particular GABA-B) receptors, and inhibiting histamine, to name only a few [25–27]. Agents exhibiting one or more of these mechanisms of action are available and can be used either orally or topically [27, 28].
Since neuropathic pain is complex and several physiologic pathways may be implicated, one approach to treating chemotherapy-induced neuropathy or neuropathic pain would be to employ agents with multiple mechanisms of action for treatment [28]. Combining several pharmacological agents with different mechanisms of action orally, however, could result in intolerable sedation and side effects, due to cumulative effects of analgesics agents. One strategy, to allow multiple agents with different mechanisms of action while minimizing intolerable side effects, is to use a topical approach for analgesia. The use of topical amitriptyline in combination with ketamine for diabetic neuropathy, postsurgical/posttraumatic neuropathic pain with allodynia, hyperalgesia, pinprick hyperesthesia [29–31], or postherpetic neuralgia [32] has been described.
Topical agents, that are locally absorbed, have been described in the literature related to pain [29, 33]. Pharmacokinetic data over 96 h in 36 healthy adults found topical 4% amitriptyline and topical 2% ketamine to have systemic concentrations well below that of systemically administered agents [33]. The permeability of a compound is due primarily to the concentration in the vehicle and its ability to cross the stratum corneum of the skin [34]. Therefore, it is theoretically possible that pain medications can be given in combinations to provide complementary mechanisms of action to have efficacy at the local level without the unwanted systemic toxicities. It is also quite possible that topical analgesics can have an impact on a wider range of CIPN symptoms than just neuropathic pain.
This current trial was developed to evaluate three topical analgesic agents for their effect on CIPN. These agents, baclofen, amitriptyline HCL, and ketamine, were chosen specifically because of their unique but complementary mechanisms of action [28–37]. Baclofen is a GABA receptor agonist [35, 36], amitriptyline HCL affects adenosine A receptors [29–31] and sodium channels and ketamine inhibits NMDA receptors [29–31, 37]. Therefore, there are three different pathways invoked that may provide additive or synergistic relief of neuropathy symptoms. The triple agent gel was developed by a compounding pharmacist in North Dakota and has been used in clinical practice to relieve symptoms of peripheral neuropathy from various etiologies. Based on published data showing the efficacy of topical amitriptyline and ketamine for neuropathic pain as well as positive outcomes in clinical practice for CIPN with this triple agent gel, a clinical trial was developed to formally evaluate the combination of baclofen, amitriptyline, and ketamine.
The purpose of this study was to conduct a randomized placebo-controlled clinical trial to evaluate a topical baclofen, amitriptyline HCL, and ketamine (BAK) gel to alleviate neuropathic pain, numbness, and/or tingling of CIPN. Secondary goals included the evaluation of function, general pain, and toxicity.
Materials and methods
Eligibility characteristics
Patients who had received, or were currently receiving, neurotoxic chemotherapy and had numbness, tingling, or pain associated with peripheral neuropathy for at least the previous month were eligible for this study. Participants had to rate their numbness/tingling/pain at a level of at least four out of ten on a 0–10 scale where zero was no neuropathy and ten was worst possible neuropathy. Also required was a life expectancy of at least 4 months and a serum creatinine less than 1.5 times the upper limits of normal. Neuropathy had to be limited to the hands and/or feet where the topical gel could be applied. Participants could not have a history of peripheral neuropathy from other causes nor pre-existing allergies to baclofen, amitriptyline, or ketamine. They could not be concurrently treated with any agent with suspected efficacy for neuropathy, such as anticonvulsants or tricyclic antidepressants. Participants must not have had a history of coronary artery disease. Written informed consent for participating in this trial was obtained for each patient, which was monitored by local institutional review boards as required by US federal regulations. Participants were stratified based on current versus only previous neurotoxic chemotherapy exposure, use of opioids or oral pain medication, baseline pain ratings, and whether they had previously tried pharmacologic treatment for their neuropathy. Randomization was done using dynamic allocation to balance marginal distributions of the stratification factors [38].
Study intervention
Participants were randomized to receive 1.31 g of a compounded gel containing 10 mg of baclofen, 40 mg of amitriptyline HCL, and 20 mg of ketamine versus an identical appearing placebo gel. Instructions were to apply one level spoonful of gel topically to each area of pain, numbness, and/or tingling, twice a day, in the morning and before bed, for 4 weeks duration. Participants were not allowed to treat more than four areas of pain, numbness, and/or tingling at a single time (i.e., a maximum of four spoonfuls of gel per application). A small subset of participants was asked to have blood drawn at the end of the 4 weeks to measure concentrations of drugs and their metabolites. Drug assignments to individual patients were accessible only by the North Central Treatment Group randomization office, study pharmacists, and the study statisticians. The BAK and placebo gel were compounded at Gateway HealthMart Pharmacy Laboratory in Bismarck, North Dakota. An Investigational New Drug Application was obtained for this trial, and the Food and Drug Administration specified the doses of the agents that were approved to be used in this study. These doses were lower than initially proposed, due to the lack of data on systemic absorption of this triple combination. Potency of the gel was evaluated every 3 months out to 1 year by Eagle Analytical. Full potency was sustained in the gel for each active agent throughout the 1-year period. The study was registered according to current US federal regulations. Funding for this study was provided through the National Cancer Institute's CCOP program.
The primary end point for the study was the changes in the sensory neuropathy subscale as measured by the European Organization for Research and Treatment of Cancer QLQ-CIPN20 (CIPN-20) [39] instrument from baseline to 4 weeks. The CIPN20 is a fairly new 20-item questionnaire evaluating various aspects of CIPN. It has three subscales assessing sensory (nine items), motor (eight items), and autonomic (three items) symptoms and functioning with each item measured on an ordinal 1–4 scale (1, not at all; 4, very much). The sensory subscale contains nine items, which cover the experience of numbness, tingling, and burning/shooting pain in the fingers/hands and the toes/feet (six questions). The remaining three questions ask about problems standing or walking due to difficulty feeling the ground, difficulty distinguishing between hot and cold, and difficulty hearing. The motor subscale asks about function such as being able to hold a pen, open jars, climb stairs, and cramping. The autonomic subscale evaluates erectile dysfunction, dizziness, and vision. Participants completed this questionnaire at baseline, before starting the study gel, and at 4 weeks. The EORTC QLQ-CIPN20 has been used in patients with cancer receiving a variety of chemotherapies and has been shown in pre-testing to have internal consistency reliability based on Cronbach's alpha coefficients of 0.82, 0.73, and 0.76 for the three subscales, respectively [39].
Other measurement instruments utilized in this trial include the Profile of Mood States (POMS) [3, 40], the Brief Pain Inventory [41], and the sensory neuropathy subsection of the NCI Common Terminology Criteria, version 3.0. Single numeric analogue questions [42–44] regarding the presence of numbness/tingling/pain (all included in one question) as well as various potential side effects, such as drowsiness, dry mouth, dizziness, constipation, nausea, headaches, and skin irritation, were also included. Participants rated the severity of these symptoms on a 0 to 10 scale, with 10 being the most severe. Adverse events were evaluated through the patient-reported questions mentioned above as well as being graded through the NCI Common Terminology Criteria, version 3.0. Toxicities specifically graded during the 4 weeks of the study included rash, constipation, dry mouth, confusion, and depressed level of consciousness.
Statistical analysis
The scoring algorithm for the parent instrument, the EORTC QLQ-C30, was applied for linearly converting items and subscales of CIPN-20 to 0–100 scales so that a high score corresponds to better condition or less symptom. The primary analysis was to compare changes from baseline at 4 weeks for the sensory neuropathy subscale of the CIPN-20. Effect size using Cohen's d was calculated using the mean difference between the arms for the subscale divided by the standard deviation. Supplementary analysis included changes from baseline at 4 weeks for the motor neuropathy and autonomic subscale of the CIPN-20, the POMS, single-item neuropathy question, and the Brief Pain Inventory. The change from baseline at 4 weeks for the numeric analogue scale and self-report toxicities was also compared between arms. A two-sided two-sample t test or Wilcoxon rank-sum test with an alpha of 0.05 was used for the primary statistical analysis. Wilcoxon rank-sum tests and chi-square tests were used for baseline patient characteristics. Descriptive statistics with 95% confidence intervals based on two-sample t test were used for secondary analyses due to their exploratory nature. Sixty-four patients per arm provided 80% power to detect a half standard deviation difference between arms, which is considered a moderate effect size.
Results
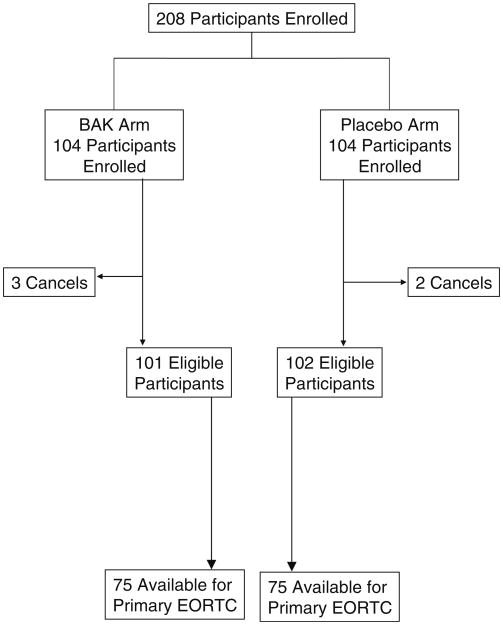
Two hundred and eight patients were enrolled into this study from February 22, 2008 to October 23, 2008 by 16 NCCTG institutions. There were five patients who withdrew from the study before starting study medication, leaving 101 patients in the BAK arm and 102 patients in the placebo arm. Baseline characteristics of the study population are listed in Tables 1 and 2, while the flow of participants is illustrated in the CONSORT diagram in Fig. 1. There were 26 participants in the BAK arm and 27 in the placebo arm who did not provide primary endpoint data. In the BAK arm, 11 refused due to experiencing an adverse event and 15 refused for non-specified reasons. In the placebo arm, eight refused due to an adverse event, one patient died, and 18 refused for non-specified reasons.
Table 1. Baseline characteristics.
| BAK (N=101) | Placebo (N=102) | P value | |
|---|---|---|---|
| Age | 0.21 | ||
| Mean (SD) | 59.9 (10.75) | 62.1 (10.27) | |
| Race | 0.48 | ||
| White | 94 (93%) | 90 (88%) | |
| Black or African American | 6 (5.9%) | 7 (6.9%) | |
| Asian | 0 (0%) | 2 (2%) | |
| Gender | 0.39 | ||
| Female | 66 (65%) | 60 (59%) | |
| Chemotherapy with neurotoxic agent | 0.64 | ||
| Non-active | 72 (71%) | 76 (75%) | |
| Current use of opioids or other pain meds | 0.77 | ||
| Yes | 39 (39%) | 42 (41%) | |
| Duration of pain or neuropathy symptoms | 0.05 | ||
| 1 to 3 months | 20 (20%) | 8 (8%) | |
| >3 to 6 months | 24 (24%) | 28 (28%) | |
| >6 months | 57 (56%) | 66 (65%) | |
| Baseline numbness/tingling/pain category | 0.75 | ||
| 4–7 | 76 (75%) | 74 (73%) | |
| 8–10 | 25 (25%) | 28 (28%) | |
| Previous peripheral neuropathy treatment | 1.0 | ||
| Yes | 17 (17%) | 18 (18%) | |
| Exposure to neurotoxic agents over lifetime | 0.88 | ||
| Single agent | 70 (69%) | 72 (71%) | |
| Multiple agents | 31 (31%) | 30 (29%) |
Table 2. Exposure to neurotoxic agents at baseline (participants may have been exposed to more than one agent).
| Agent | Previous treatment only percent of patients | Concurrent treatment percent of patients | ||
|---|---|---|---|---|
| BAK (N=101) | Placebo (N=102) | BAK (N=101) | Placebo (N=102) | |
| Vinca alkaloids | 3 | 5 | 3 | 5 |
| Oxaliplatin | 31 | 33 | 12 | 13 |
| Cisplatin | 13 | 21 | 4 | 2 |
| Taxanes | 40 | 41 | 12 | 5 |
| Thalidomide | 3 | 2 | 0 | 0 |
| Other | 7 | 3 | 2 | 5 |
Fig. 1. CONSORT diagram.
The primary analysis of the changes from baseline at 4 weeks for the sensory neuropathy subscale showed a trend in favor of the active arm, with the mean and SD of 8.1 (15.05) for the BAK arm and 3.8 (15.52) for the placebo arm, with an effect size of about 0.28 for the active arm over placebo (p= 0.053). For the motor neuropathy subscale, the mean change from baseline and SD were 7.1 (13.72) for BAK arm and 1.8 (14.05) for placebo arm, for an effect size of about 0.38 over placebo (p=0.021). The change in the autonomic subscale was not significantly different between the two study arms. These data are shown in Table 3.
Table 3. EORTC QLQ-CIPN20 mean change from baseline; range, 0–100, higher numbers are better.
| Mean change from baseline | BAK (N=75) | Placebo (N=75) | Difference (95% CI) | P value |
|---|---|---|---|---|
| Sensory subscale | 8.1 | 3.8 | 4.3 (−0.6, 9.3) | 0.053 |
| Motor subscale | 7.1 | 1.8 | 5.3 (0.9, 9.7) | 0.021 |
| Autonomic subscale | 3.3 | 1.7 | 1.6 (−4.0, 7.1) | 0.580 |
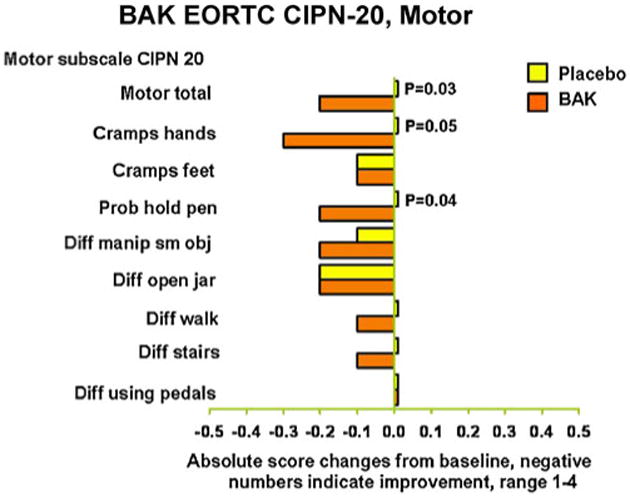
Further exploratory analyses were done looking at individual items of the sensory and motor neuropathy subscales, looking at the changes from baseline. In terms of the original 1–4 scale (Figs. 2 and 3), there were three areas that were improved with the BAK treatment without adjusting for multiple comparisons. These were tingling in fingers/hands, shooting or burning pain in fingers/hands, and the ability to hold a pen. Trends of improvement from BAK treatment were seen for several other items. In general, the benefits of therapy tended to be more impressive in the upper extremities, as opposed to the lower extremities.
Fig. 2. Sensory subscale of the EORTC QLQ-CIPN-20; change from baseline, lower numbers are better.
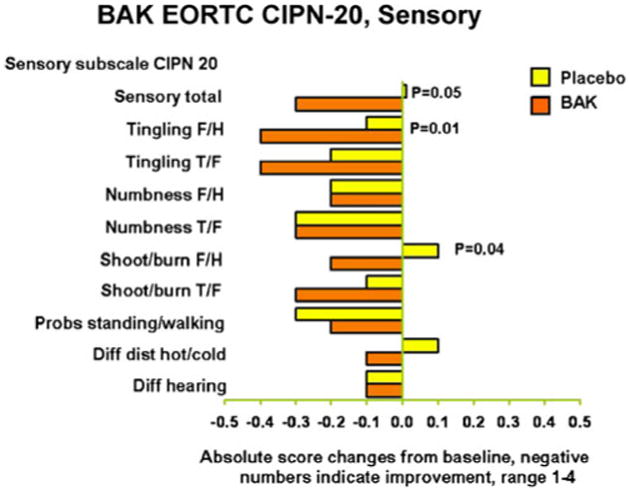
Fig. 3. Motor subscale of the EORTC QLQ-CIPN-20; change from baseline, lower numbers are better.
The sensory neuropathy change from baseline measured by the CTCAE was evaluated via two different ways due to the ordinal nature of toxicity data. Assuming equal intervals between grades, we have observed a marginally significant difference between the treatment and placebo arms (p=0.054). However, when we collapsed the change from baseline as positive change, no change, and negative change, analyzing as an ordinal scale (which is likely a more valid assumption), there was no significant differences between arms (p= 0.195). The single-item neuropathy question mean change from baseline at 4 weeks in the BAK arm was 11.2 (SD, 20.53) and 6.3 (SD, 23.60) in the placebo arm. Neither the Brief Pain Inventory nor the POMS were significantly different between the two study arms.
No significant differences in toxicities were observed between the BAK arm and the placebo throughout the 4 weeks of the study. These data are shown in Table 4 for the CTC graded toxicities and Table 5 for the self-report side effects.
Table 4. Maximum adverse events by arm related to central nervous system depression.
| BAK (N=101) | Placebo (N=102) | |
|---|---|---|
| Confusion | ||
| None | 95 (95%) | 97 (97%) |
| Mild (1) | 5 (5%) | 2 (2%) |
| Severe (3) | 0 | 1 (1%) |
| Low consciousness | ||
| None | 98 (98%) | 98 (98%) |
| Moderate | 1 (1%) | 2 (2%) |
| Severe | 1 (1%) | 0 (%) |
| Constipation | ||
| None | 76 (76%) | 74 (74%) |
| Mild | 18 (18%) | 22 (22%) |
| Moderate | 5 (5%) | 4 (4%) |
| Severe | 1 (1%) | 0 (0%) |
| Rash | ||
| None | 84 (84%) | 90 (90%) |
| Mild | 11 (11%) | 6 (6%) |
| Moderate | 5 (5%) | 4 (4%) |
| Dry mouth | ||
| None | 83 (83%) | 78 (78%) |
| Mild | 17 (17%) | 22 (22%) |
Table 5. Patient-reported side effects change from baseline at 4 weeks; range, 0–100, higher numbers are better.
| Side effect | BAK (N=101) | Placebo (N=102) | P value |
|---|---|---|---|
| Drowsiness | 10.7 | 8.4 | 0.50 |
| Concentration | 6.7 | 8.4 | 0.97 |
| Skin irritation | 1.6 | 4.7 | 0.47 |
| Dry mouth | 2.8 | 4.4 | 0.35 |
| Dizziness | 3.5 | 3.2 | 0.92 |
| Nausea | 3.2 | 1.4 | 0.53 |
| Headaches | 5.5 | 4.1 | 0.85 |
| Confusion | 1.4 | 2.8 | 0.12 |
| Constipation | 1.9 | 2.0 | 0.67 |
Blood was drawn during the double-blind phase on a small subset of participants (N=8) to evaluate systemic absorption. None of the four people taking placebo had detectable levels of any of the components of BAK. Of the four participants on active drug, two had undetectable levels of all three agents, one had barely detectable levels of amitriptyline that were not near therapeutic levels but no detectable ketamine or baclofen, and one had low therapeutic levels of baclofen but undetectable levels of amitriptyline and ketamine.
Discussion
This study demonstrated that BAK gel resulted in a trend toward more improvement in sensory neuropathy and a statistically significant improvement in motor neuropathy as measured by the EORTC QLQ-CIPN20. Though there were some statistically significant outcomes, the overall effect size was not large. While these results provide some support for the pre-study hypothesis that the BAK gel can decrease CIPN, the data from it fall short of convincingly proving this hypothesis.
There are a couple reasons that might explain why a more potent effect was not observed and why further study is needed. This study was developed based on the clinical experience of compounding pharmacists in North Dakota. Initially, based on clinical experience, the study proposed using 60 mg of amitriptyline HCl, 30 mg of ketamine, and 30 mg of baclofen. Due to insufficient data supporting the lack of systemic absorption, the Food and Drug Administration required the use of lower doses in this trial, which resulted in the dosing described in this manuscript. This lower dose may explain why more benefit was not seen.
A similar dose–effect issue may explain why the hands seemed to derive more benefit than the feet. Since one level spoonful was instructed to be applied to each area of neuropathy, it is possible that the feet were relatively underdosed based on surface area. In addition, it could be argued that the hands would likely have received a higher dose than the feet as the hands would have been used to rub the gel onto the feet, but not vice versa. Since separate questions for hands and feet are included in the sensory subscale, the under-dosing related to the feet could have impacted the effect size.
In addition, this group of agents was compounded in a pluronic lecithin organogel (PLO) gel, which was thought to be the best medium for topically absorbed agents. There were reports from the institutions participating in this trial that patients had difficulty working with the PLO gel and getting it to absorb into their skin. Therefore, future studies may wish to consider using a different liposomal transdermal base, such as Lipoderm, which may be easier for participants to rub into their skin.
It was not surprising that the autonomic subscale of the CIPN-20 measure was not positively impacted with the BAK treatment. The three items that comprise this subscale relate to systemic neuropathy troubles: erection (for males), dizziness, and vision. First, patients on this trial were only required to have neuropathy in their extremities. Therefore, it is likely that participants in this trial were not having difficulties in these areas. In addition, this supports that the study drugs were not systemically absorbed to any significant degree.
It is noteworthy that positive results on the Brief Pain Inventory similar to those for shooting/burning pain were not achieved. This is likely because the Brief Pain Inventory does not include language specific to neuropathic pain, while the CIPN20 uses the term “shooting/burning pain.”
About 25% of the participants in each arm did not provide data that enabled computation of the primary endpoint. As participants could be eligible for this study with advanced disease, going in and out of treatment, and experiencing any number of health problems, this percentage of non-evaluable patients is consistent with this situation.
As there are no other known effective treatments for the relief of established neuropathy symptoms from chemotherapy, the signal of potential benefit provided by these data warrant further study of this combination of topical baclofen, amitriptyline, and ketamine, specifically evaluating a higher dose of the agents in a more user-friendly compounding base.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-63848, CA-35195, CA-37417, CA-35448, CA-35267, CA-63849, CA-35113, CA-35103, CA-35415, CA-35431, and CA124477. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Footnotes
Additional participating institutions include the following: Cedar Rapids Oncology Program CCOP, Cedar Rapids, IA 52403, USA (Martin Wiesenfeld, MD); Geisinger Clinic and Medical Center CCOP, Danville, PA 17822, USA (Albert M. Bernath, Jr., M.D.); Rapid City Regional Hospital, Inc, Rapid City, SD 57701, USA (Richard Charles Tenglin, M. D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105, USA (Loren K. Tschetter, M.D.); Toledo Community Hospital Oncology Program (Paul L. Schaefer, M.D.); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN 55416, USA (Patrick J. Flynn, M.D.); Mayo Clinic, Scottsdale, AZ 85259-5404, USA (Tom R. Fitch, M.D.); and Hematology/Oncology Centers of the Northern Rockies, Billings, MT 59101, USA (Benjamin Marchello, M.D.).
Contributor Information
Debra L. Barton, Email: Barton.debra@mayo.edu, Mayo Clinic College of Medicine, 200 First Street, SW, Rochester, MN, 55905 USA.
Edward J. Wos, Medcenter One Health System, Bismarck, ND, USA
Rui Qin, Mayo Clinic College of Medicine, 200 First Street, SW, Rochester, MN, 55905 USA.
Bassam I. Mattar, Wichita CCOP, Wichita, KS, USA
Nathan Benjamin Green, Missouri Valley Cancer Consortium CCOP, Omaha, NE, USA.
Keith S. Lanier, Providence Oncology and Hematology Care Clinic, Portland, OR, USA
James Dewitt Bearden, III, Spartanburg Regional Medical Center, Spartanburg, SC, USA.
John W. Kugler, Illinois CancerCare, Peoria, IL, USA
Kay L. Hoff, Cancer Resource Center, Lincoln, NE, USA
Pavan S. Reddy, Wichita CCOP, Wichita, KS, USA
Kendrith M. Rowland, Jr, Carle Cancer Center, Urbana, IL, USA.
Mike Riepl, Gateway Health Mart Pharmacy, Bismarck, ND, USA.
Bradley Christensen, Mayo Clinic College of Medicine, 200 First Street, SW, Rochester, MN, 55905 USA.
Charles L. Loprinzi, Mayo Clinic College of Medicine, 200 First Street, SW, Rochester, MN, 55905 USA
References
- 1.Cavaletti G, Bogliun G, Marzorati L, et al. Peripheral neurotoxicity of Taxol in patients previously treated with cisplatin. Cancer. 1995;75:1141–1150. doi: 10.1002/1097-0142(19950301)75:5<1141::aid-cncr2820750514>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Cavaletti G, Fabbrica D, Minoia C, et al. Carboplatin toxic effects on the peripheral nervous system of the rat. Ann Oncol. 1998;9:443–447. doi: 10.1023/a:1008231925889. [DOI] [PubMed] [Google Scholar]
- 3.Ocean JA, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12:619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 4.Pace A, Bove L, Aloe A, et al. Paclitaxel neurotoxicity: clinical andneurophysiological study of 23 patients. Ital JNeurol Sci. 1997;18:73–79. doi: 10.1007/BF01999566. [DOI] [PubMed] [Google Scholar]
- 5.Rowinsky EK, Chaudry V, Cornblath DR, et al. Neurotoxicity of taxol. J Natl Cancer Inst Monogr. 1993;15:107–115. [PubMed] [Google Scholar]
- 6.Verstappen C, Heimans JJ, Hockman K, et al. Neurotoxic complications of chemotherapy in patients with cancer. Drugs. 2003;63(15):1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Windebank AJ. Chemotherapeutic neuropathy. Curr Opin Neurol. 1999;5:565–571. doi: 10.1097/00019052-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Campana WM, Eskeland N, Calcutt NA, et al. Prosaptide prevents paclitaxel neurotoxicity. Neurotoxicology. 1998;19:237–244. [PubMed] [Google Scholar]
- 9.Cliffer KD, Siuciak JA, Carson SR, et al. Physiological characterization of taxol-induced large-fiber sensory neuropathy in the rat. Ann Neurol. 1998;43:46–55. doi: 10.1002/ana.410430111. [DOI] [PubMed] [Google Scholar]
- 10.Guastalla JP, Pujade-Lauraine E, Weber B. Efficacy and safety of paclitaxel and carboplatin combination in patients with previously treated advanced ovarian carcinoma. Ann Oncol. 1998;9(9):37–43. doi: 10.1023/a:1008211909585. [DOI] [PubMed] [Google Scholar]
- 11.Polomano RC, Bennett GJ. Chemotherapy-evoked peripheral neuropathy. Pain Medicine. 2001;2:8–14. doi: 10.1046/j.1526-4637.2001.002001008.x. [DOI] [PubMed] [Google Scholar]
- 12.Pujude-Lauraine E, Guastalla JP, Weber B, et al. Efficacy and safety of the combination of paclitaxel/carboplatin in patients with previously treated advanced ovarian carcinoma: a multicenter French Groupe des investigateurs Nationauz pour l'etude des cancers ovariens phase II study. Semin Oncol. 1997;24:S15-30–S15-35. [PubMed] [Google Scholar]
- 13.Quasthoff S, Hartung HP. Chemotherapy induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 14.Envig AI, Wiernik PH, Wadler S, et al. Phase I study of paclitaxel (taxol) and granulocyte colony stimulating factor (g-csf) in patients with unresectable malignany. Invest New Drugs. 1998;16:29–36. doi: 10.1023/a:1006004809169. [DOI] [PubMed] [Google Scholar]
- 15.LoMonaco M, Milone M, Batocchi AP, et al. Cisplatin neuropathy: clinical course and neurophysiologic findings. J Neurol. 1992;239:199. doi: 10.1007/BF00839140. [DOI] [PubMed] [Google Scholar]
- 16.Nikcevich DA, Grothey A, Sloan JA, Kugler JW, Silberstein PT, Dentchev T, Wender DB, Novotny PJ, Windschitl HE, Loprinzi CL. Effect of intravenous calcium and magnesium (IV CaMg) on oxaliplatin-induced sensory neurotoxicity (sNT) in adjuvant colon cancer: results of the phase III placebo-controlled, double-blind NCCTG trial N04C7. J Clin Oncol. 2008;26 abstr 4009. [Google Scholar]
- 17.Ahmad M, Goucke CR. Management strategies for the treatment of neuropathic pain in the elderly. Drugs Aging. 2002;19(12):929–945. doi: 10.2165/00002512-200219120-00004. [DOI] [PubMed] [Google Scholar]
- 18.Argoff CE, Katz N, Backonja M. Treatment of postherpetic neuralgia:a review of therapeutic options. J Pain Symptom Manage. 2004;28(4):396–411. doi: 10.1016/j.jpainsymman.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Jensen PG, Larson JR. Management of painful diabetic neuropathy. Drugs Aging. 2001;18(10):737–749. doi: 10.2165/00002512-200118100-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hammack JE, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98(1–2):195–203. doi: 10.1016/s0304-3959(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 21.Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110(9):2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 22.Rao RD, Flynn PJ, Sloan J, et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double blind, placebo controlled NCCTG trial, N01C3. Cancer. 2008;112(21):2802–2808. doi: 10.1002/cncr.23482. [DOI] [PubMed] [Google Scholar]
- 23.Moore DH, Donnelly J, McGuire WP, et al. Limited access trial using amifostine for protection against cisplatin- and three-hour paclitaxel-induced neurotoxicity: a phase ii study of the Gynecologic Oncology Group. J Clin Oncol. 2003;21(22):4207–4213. doi: 10.1200/JCO.2003.02.086. [DOI] [PubMed] [Google Scholar]
- 24.Vahdat L, Papadopoulos K, Lange D, et al. Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clin Cancer Res. 2001;7:1192–1197. [PubMed] [Google Scholar]
- 25.Baron Mechanisms of disease: neuropathic pain, a clinical perspective. Nat Clin Pract Neurol. 2006;2(2):95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- 26.Jones M. Chronic neuropathic pain: pharmacological interventions in the new millennium. Int J Pharm Comp. 2000;4(1):6–15. [PubMed] [Google Scholar]
- 27.Sawynok J. Topical analgesics in neuropathic pain. Curr Pharm Des. 2005;11(23):2995–3004. doi: 10.2174/1381612054865019. [DOI] [PubMed] [Google Scholar]
- 28.Sawynok J. Topical and peripherally acting analgesics. Pharmacol Rev. 2003;55:1–20. doi: 10.1124/pr.55.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Lynch ME, Clark AJ, Sawynok J. A pilot study examining topical amitriptyline, ketamine and a combination of both in the treatment of neuropathic pain. Clin J Pain. 2003;19:323–328. doi: 10.1097/00002508-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Lynch ME, Clark AJ, Sawynok J, Sullivan MJL. Topical 2% amitriptyline and 1% ketamine in neuropathic pain syndromes. Anesthesiology. 2005;103(1):140–146. doi: 10.1097/00000542-200507000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical amitriptyline and ketamine in neurotopical amitriptyline and ketamine in neuropathic pain syndromes: an open label study. J Pain. 2005;6(10):644–649. doi: 10.1016/j.jpain.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Lockhart E. Topical combination of amitriptyline and ketamine for post herpetic neuralgia. J Pain. 2004;5:S182. [Google Scholar]
- 33.Everton D, Bhagwat D, Damask M. An open-label pharmacokinetic study in humans of a 4% amitriptyline, 2% ketamine topical cream. J Pain. 2007;8(4 suppl 1):S48. [Google Scholar]
- 34.Ceschel G, Bergamante V, Maffei P, et al. Solubility and transdermal permeation properties of dehydroepiandrosterone cyclodextrin complex from hydrophilic and lipophilic vehicles. Drug Delivery. 2005;12:275–280. doi: 10.1080/10717540500176563. [DOI] [PubMed] [Google Scholar]
- 35.Franek M, Vaculin S, Rokyta R. GABAB receptor agonist baclofen has non-specific antinociceptive effect in the model of peripheral neuropathy in the rat. Pysiol Res. 2004;53:351–355. [PubMed] [Google Scholar]
- 36.Smith GD, Harrison SM, Birch PJ, et al. Increased sensitivity to the antinociceptive activity of±baclofen in an animal model of chronic neuropathic, but not chronic inflammatory hyperalgesia. Neuropharmacology. 1994;33:1103–1108. doi: 10.1016/0028-3908(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 37.Gammaitoni A, Gallagher RM, Welz-Bosna M. Topical ketamine gel: possible role in treating neuropathic pain. Pain Medicine. 2000;1(1):97–100. doi: 10.1046/j.1526-4637.2000.00006.x. [DOI] [PubMed] [Google Scholar]
- 38.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 39.Postma TJ, Aaronson NK, Heimans JJ, EORTC Quality of Life Group et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 40.McNair DM, Loor M, Droppleman LF. Profile of mood states manual. Multi-Health Systems; New York: 2003. [Google Scholar]
- 41.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 42.Sloan JA, O'Fallon JR, Suman VJ, et al. Incorporating quality of life measurement into oncology clinical trials. Proc Am Stat Assoc. 1998;1998:282–287. [Google Scholar]
- 43.Grunberg SM, Groshen S, Steingass S. Comparison of conditional quality of life terminology and visual analogue scale measurements. Qual Life Res. 1996;5:65–72. doi: 10.1007/BF00435970. [DOI] [PubMed] [Google Scholar]
- 44.Gudex C, Dolan P, Kind P. Health state valuations from the general public using the visual analogue scale. Qual Life Res. 1996;5:521–531. doi: 10.1007/BF00439226. [DOI] [PubMed] [Google Scholar]