Abstract
In mammalian tissues, uptake of Ca2+ and Mn2+ by Golgi membranes is mediated by the secretory pathway Ca2+- ATPases, SPCA1 and SPCA2, encoded by the ATP2C1 and ATP2C2 genes. Loss of one copy of the ATP2C1 gene, which causes SPCA1 haploinsufficiency, leads to squamous cell tumors of keratinized epithelia in mice and to Hailey-Hailey Disease, an acantholytic skin disease, in humans. Although the disease phenotypes resulting from SPCA1 haploinsufficiency in mice and humans are quite different, each species-specific phenotype is remarkably similar to those arising as a result of null mutations in one copy of the ATP2A2 gene, encoding SERCA2, the endoplasmic reticulum (ER) Ca2+ pump. SERCA2 haploinsufficiency, like SPCA1 haploinsufficiency, causes squamous cell tumors in mice and Darier’s Disease, also an acantholytic skin disease, in humans. The phenotypic similarities between SPCA1 and SERCA2 haploinsufficiency in the two species, and the general functions of the two pumps in consecutive compartments of the secretory pathway, suggest that the underlying disease mechanisms are similar. In this review we discuss evidence supporting the view that chronic Golgi stress and/or ER stress resulting from Ca2+ pump haploinsufficiencies leads to activation of cellular stress responses in keratinocytes, with the predominance of pro-apoptotic pathways (though not necessarily apoptosis itself) leading to acantholytic skin disease in humans and the predominance of pro-survival pathways leading to tumors in mice.
Keywords: secretory pathway stress, Golgi stress, endoplasmic reticulum stress, acantholysis, Darier disease, cornification, unfolded protein response
1. Introduction
Maintenance of the appropriate concentrations of both cytosolic Ca2+ and luminal Ca2+ in secretory pathway organelles of mammalian cells requires the activities of three subfamilies of P-type Ca2+-transporting ATPases. These are the sarco(endo)plasmic Ca2+-ATPases (SERCA1-3; gene symbols ATP2A1-A3), the plasma membrane Ca2+-ATPases (PMCA1-4; ATP2B1-B4), and the Golgi or secretory pathway Ca2+-ATPases (SPCA1 and 2; ATP2C1 and ATP2C2). The structures, biochemical characteristics, and physiological functions of the SERCA and PMCA pumps have been reviewed extensively [1-4] and will not be discussed in this review. The focus here is on the SPCA family of Golgi Ca2+ pumps, with particular emphasis on disease phenotypes resulting from null mutations in the ATP2C1 (SPCA1) gene in mice and humans and their similarities to disease phenotypes resulting from null mutations in the ATP2A2 gene, which encodes SERCA2, the major endoplasmic reticulum (ER) Ca+ pump.
Null mutations in a single copy of the ATP2C1 or ATP2A2 genes have been shown to cause autosomal dominant skin diseases (Hailey-Hailey disease (HHD) and Darier’s disease (DD), respectively) in humans [5-7] and squamous cell tumors of keratinized epithelial cells in mice [8-11]. Although the phenotypes in humans and mice are very different, the primary genetic defect in each case leads to a reduction in the levels of functional Ca2+ pump in a compartment of the secretory pathway, and within each species the disease phenotypes are quite similar and involve keratinized epithelium. In this review we provide brief information about the identities and functions of the Golgi Ca2+ pumps, which have been reviewed by other investigators [1,12-15], and discuss evidence that secretory pathway stress, originating in either the Golgi or ER, and subsequent activation of Golgi and ER stress response pathways might serve as a common mechanism in the diseases resulting from Golgi or ER Ca2+ pump dysfunction.
2. The Golgi Ca2+ pump in yeast and mammals
The existence of a Golgi Ca2+ pump was first shown in biochemical studies of Golgi membranes from lactating bovine, rat, and mouse mammary glands. These included ATP-dependent Ca2+-uptake into purified Golgi vesicles [16-19], inhibition of Ca2+-ATPase activity and Ca2+-uptake by orthovanadate [19], and identification of a 100-kilodalton phosphorylated intermediate [17]. The Ca2+ affinity of the Golgi enzyme differed from that of the sarcoplasmic reticulum Ca2+ pump and the plasma membrane Ca2+ pump and, unlike the latter enzymes, it was not inhibited by quercetin [20]. These observations provided strong evidence for a Golgi P-type Ca2+-ATPase that was different from the SERCA and PMCA Ca2+ pumps.
The first molecular identification of the Golgi Ca2+ pump occurred in 1989 with the cloning of a putative yeast secretory pathway Ca2+ pump, termed PMR1 [21]. This was followed several years later by the cloning of a rat cDNA encoding a P-type ATPase, now termed SPCA1, which exhibited sufficient similarity to PMR1 to be considered its mammalian ortholog [22]. Later studies identified human [5,6] and C. elegans [23] SPCA1 orthologs and a second mammalian SPCA isoform, termed SPCA2 [24,25], and demonstrated that PMR1, SPCA1, and SPCA2 are expressed in Golgi membranes and function as Ca2+- and Mn2+-transporting ATPases [24-28]. Both SPCA1 and SPCA2 are expressed in lactating mammary glands [29,30] and SPCA2 is strongly induced during lactation [29], suggesting that the Golgi Ca2+ pump identified in the earlier studies of mammary glands [16-20] was a mixture of the two isoforms.
The presence of SPCA homologs in both higher and lower eukaryotes and the high degree of similarity to PacL, a bacterial Ca2+ pump [31], suggests that they have more ancient evolutionary origins than the SERCA and PMCA pumps. In plants, however, an SPCA homolog has not been identified, and the ECA3 Ca2+ pump, which is closely related to the SERCA pumps, is expressed in Golgi membranes and affects both Ca2+ and Mn2+ homeostasis [32]. The expression of a SERCA-like pump in plant Golgi membranes is consistent with evidence that, in addition to SPCA pumps, a thapsigargin-sensitive pump, presumably SERCA2, also contributes to Golgi Ca2+ stores in mammalian cells [28,33].
3. Cellular phenotypes resulting from knockdown or ablation of SPCA expression are consistent with secretory pathway stress and activation of stress responses
SPCAs from yeast, C. elegans, and mammals serve as Ca2+ and Mn2+ transporting ATPases in Golgi membranes [23,25,34-37] and thus function in maintaining the appropriate luminal Ca2+ and Mn2+ concentrations in this compartment of the secretory pathway. As discussed in this and the following section, ablation or reduction in SPCA activity causes Golgi dysfunction and stress, which in turn leads to activation of secretory pathway stress responses that appear to involve both survival and apoptotic mechanisms (summarized in Table 1).
Table 1.
Phenotypes resulting from SPCA-deficiency that are indicative of secretory pathway stress and activation of stress responses secondary to defective Ca2+ and Mn2+ sequestration
| I. Manifestations of secretory pathway (Golgi or ER) stress. | References |
|---|---|
| 1. Defective glycosylation, likely due to Mn2+ deficiency in Golgi | 21,26,38,41 |
| 2. Impaired proteolytic processing of proteins in Golgi, likely due to Ca2+ deficiency | 26, 38 |
| 3. Impaired trafficking of proteins in secretory pathway | 21,37,38,40-42 |
| 4. Impaired ERAD; selective for glycosylated substrate in mammalian cells | 38,41 |
| 5. Increased sensitivity to ER stress caused by tunicamycin, dithiothreitol, or thapsigargin | 26,41 |
| 6. Dilation of Golgi membranes and/or reduction in number of Golgi stacks | 11,58 |
| 7. Disaggregation or fragmentation of Golgi membranes | 11,37,40 |
| 8. Dilation of ER indicative of ER stress, secondary to Golgi stress | 11 |
| 9. Accumulation of cytosolic lipid droplets | 11,57 |
| II. Manifestations of Golgi stress responses | |
| 1. Increase in amount of Golgi membranes and Golgi-associated vesicles (survival response) | 11,58 |
| 2. Increased apoptosis | 11,42 |
In yeast, loss of PMR1 leads to glycosylation defects, impaired protein trafficking, and defective degradation of misfolded ER proteins [38]. In addition, absence of PMR1 causes growth inhibition and impairs proteolytic processing of α-factor, which is due at least in part to a reduction in the activity a Ca2+-dependent Golgi protease [26,38]. Both growth inhibition and the defect in proteolytic processing are partially suppressed by increasing the Ca2+ concentration of the medium [38]. These findings and the observation that expression of SERCA1, the rabbit skeletal muscle Ca2+ pump, can alleviate growth inhibition of pmr1 mutants [26] indicate that some of these phenotypes are due to a reduction of Ca2+ in the lumen of the Golgi. Mutations in PMR1 also lead to accumulation of cytosolic Mn2+ and cause hypersensitivity to Mn2+ toxicity [39], consistent with the suggestion that SPCAs function in Mn2+ detoxification [24], and increasing Mn2+ in the medium reverses defects in both N-linked and O-linked glycosylation [26]. These studies showed that the yeast SPCA homolog plays an important role in both Ca2+ and Mn2+ homeostasis in the Golgi apparatus, with primary effects on protein processing and trafficking.
Studies using keratinocytes from HHD patients have shown that loss of one copy of the SPCA1 gene (ATP2C1), which reduces SPCA1 expression to almost half of normal levels, causes a reduction in both the rate of Ca2+ sequestration and the levels of Ca2+ in Golgi vesicles [27], thus confirming a direct role for SPCA1 in maintaining Golgi Ca2+ stores in mammalian cells. Knockdown of SPCA1 expression in HeLa cells using RNA interference showed that SPCA1 mediates Ca2+ uptake in the Golgi, but also indicated that additional uptake is mediated by a SERCA pump [28]. Recent experiments confirmed these findings and showed that SPCA1 is expressed in the trans-Golgi compartment [37], consistent with earlier results [27]. More precise localization by immunogold labeling showed that SPCA1 is expressed in tubular parts of the trans-Golgi network and in tubular non-compact zones connecting the Golgi stacks, but little expression was observed in the cisternae [40]. RNA-interference experiments demonstrated that loss of SPCA1 affects both N-linked glycosylation [41] and trafficking of proteins through the secretory pathway [37,40,42].
In addition to maintenance of intra-Golgi Ca2+ and Mn2+, which has direct effects on Ca2+-dependent proteases [38] and Mn2+-dependent glycosyltransferases [43], there is also evidence that SPCA1 may affect cytosolic Ca2+ signaling [44-48]. A particularly interesting observation is that Ca2+ released from the Golgi leads to a localized increase in cytosolic Ca2+ that stimulates vesicle fusion, thus contributing to intra-Golgi transport of cargo [49], a finding that is consistent with the distribution of SPCA1 in the Golgi apparatus [40]. Thus, maintenance of luminal Ca2+ stores by SPCA1 may enable Ca2+ signaling events that directly affect vesicle trafficking and associated sorting of proteins through the secretory pathway. If so, then a deficiency in this signaling function likely contributes to the mislocalization of proteins that occurs in response to SPCA1 knockdown [37,41,42].
SPCA (PMR1) deficiency in yeast affects not only the Golgi, but also the ER, where it has a clear effect on ER stress responses. In yeast, PMR1 was shown to be identical to DER5, a gene involved in ER-associated protein degradation (ERAD) of misfolded proteins, and pmr1 mutants failed to efficiently degrade a mutant form of carboxypeptidase Y via the ubiqitin-proteosome pathway, apparently due to a failure in exporting the misfolded protein from the ER to the cytosol [26]. Null mutants were also more sensitive to treatment with dithiothreitol and tunicamycin [26], which cause ER stress due to accumulation of misfolded proteins in the ER, with subsequent activation of the unfolded protein response (UPR). Because both ERAD and the UPR are major components of the ER stress response, these findings suggest that loss of PMR1 activity in the Golgi can cause or exacerbate stress occurring in the ER, an earlier compartment of the secretory pathway, and impair the ability of the cell to adapt to ER stress.
Effects on the ER were also observed in mammalian cells. Knockdown of SPCA1 affected degradation of a mutant glycoprotein via ERAD, although it did not affect degradation of a non-glycoprotein substrate [41]. Signaling pathways that mediate ER stress responses were intact and functional; however, SPCA1-deficient cells were highly sensitive to treatment with tunicamycin or thapsigargin, both of which cause ER stress and activate ER stress responses [41]. The embryonic phenotype discussed below provides direct evidence of Golgi stress, secondary effects on the ER, and stress responses that can be categorized as survival responses or apoptotic responses.
4. Embryonic phenotypes resulting from ablation of SPCA1 and evidence of Golgi stress and activation of Golgi stress responses
Mouse embryos lacking SPCA1 appeared normal on embryonic day (ED) 8.5, but growth retardation and failure of neural tube closure were observed on ED 9.5 and embryolethality occurred between EDs 10 and 11 [11]. Loss of proteins involved in basic cellular functions often cause embryonic death around the time of implantation [50]; however, SPCA1-null embryos underwent blastocyst formation, implantation, development of the three germ layers, gastrulation, and major phases of organogenesis. This suggested that processing and trafficking of proteins through the secretory pathway, required for elaboration of cell surface proteins and secretion of extracellular matrix and signaling proteins, were relatively normal.
Some of the most common causes of embryolethality during the period in which SPCA1-null embryos died involve defects in hematopoiesis or the cardiovascular system [50]. However, red blood cells, the yolk sac, blood vessels, and heart appeared normal and supported circulation of blood [11]. The only structural defect was incomplete closure of the neural tube; however, bending occurred at dorsolateral and medial hinge points, suggesting that secretion of signaling factors required for neural plate bending [51] was occurring. Neural tube defects have a diversity of causes, including dysregulation of cell proliferation and/or apoptosis [52]. No reduction in mitotic index was observed; however, SPCA1-null embryos exhibited a significant increase in apoptosis in the neural tube and mesenchyme [11], suggesting that apoptosis was a major factor in both the neural tube defect and embryonic death. Increased apoptosis was also observed in neuronal cells subjected to RNA-mediated knockdown of SPCA1 in culture [42].
At the ultrastructural level, there were no apparent changes in desmosomes, junctional complexes, coated pits, mitochondria, or basement membranes [11]. This indicated that loss of SPCA1 either did not cause a massive perturbation of processing and trafficking of proteins in the secretory pathway or that compensation was occurring. SPCA1 mRNA was detected on ED 8.5, but not SPCA2 mRNA, suggesting that compensation for the loss of SPCA1-mediated Ca2+ uptake was due to other mechanisms. These may include the activity of a SERCA pump, which contributes to Golgi Ca2+ handling [28,33], to partial filling of Golgi Ca2+ stores by fusion with vesicles containing Ca2+ from the ER, and/or to additional adaptations in the Golgi, discussed below. Regarding the role of SPCA1 in uptake of Mn2+ required for glycosyltransferases [43], inositol 1,4,5-trisphosphate receptors are present in both ER and Golgi [53] and can mediate uptake of Mn2+ [54]. This alternative Mn2+ uptake route may be sufficient during embryogenesis.
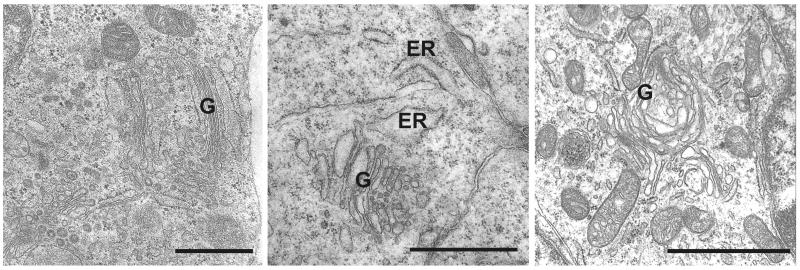
The most striking ultrastructural changes in SPCA1-null embryos involved the Golgi and provided clear evidence of Golgi stress. These included dilation of Golgi membranes, a reduction in the number of flat cisternae, an increase in the number of Golgi-associated vesicles, and other changes [11]. Although well-formed Golgi were occasionally observed (Fig. 1, left), they had fewer flat cisternae and a larger number of associated vesicles at the ends of the cisternae. The latter observation supports the suggestion [40,49] that Ca2+ release from Golgi stores served by SPCA1 contributes to membrane fusion. Dilated Golgi membranes were commonly observed (Fig. 1). Dilation of the rough ER, an indication of ER stress [55], and a reduction in the number of membrane-bound ribosomes, an indication of reduced ER-associated protein synthesis that is part of the ER stress response [56], were also observed (Fig. 1, middle). An additional indication of Golgi stress was the accumulation of excess lipid droplets in SPCA1-null cells [11]. This has also been observed in ATF6α knockout mice, where it was attributed to ER stress [57].
Fig. 1.
Golgi stress and Golgi stress responses in SPCA1-null embryos. Ultrastructural analysis revealed occasional examples of Golgi (G) with well-formed flattened stacks (left), but the number of cisternae were significantly reduced and the total amount of Golgi membranes and associated vesicles were significantly expanded (see morphometry in Ref. 11). Dilation of Golgi membranes (middle and right) was commonly observed and dilated rough ER (middle) was also observed. Dilation of Golgi and ER are indicators of Golgi and ER stress, and expansion of the Golgi is an apparent stress response favoring adaptation and survival (11). Bar = 1 micrometer
Given the critical role of the Golgi in the secretory pathway and both anterograde and retrograde trafficking between the ER and the Golgi, one would expect Golgi stress to affect the ER and to activate Golgi stress responses to deal with the perturbation. In fact, Golgi membranes were increased ~4-5 fold in SPCA1-null embryos [11], which should increase the capacity for processing and trafficking of proteins, and may be the major compensatory mechanism that allows embryonic development through EDs 10 and 11. Dilation and expansion of the amount of Golgi membranes were also observed in Tangier disease [58]. Like the ER, the Golgi appears to function in stress-sensing and to exhibit stress responses that allow a cell to either adapt to conditions causing insufficiency of Golgi function or undergo apoptosis [59-60]. Expansion of Golgi membranes is presumably part of a Golgi stress response, as it seems analogous to expansion of the ER, which occurs in response to ER stress (reviewed in 60-61). Expansion of the Golgi in SPCA1-null embryos, along with the induction of genes encoding Golgi proteins by XBP1 [62], an established regulator of ER biogenesis [63] that also causes expansion of the Golgi [64], suggest the existence of homeostatic mechanisms that adjust the amount of Golgi membranes to the needs of the cell, whether in response to Golgi stress or normal differentiation.
5. Haploinsufficiency of either SPCA1 or SERCA2 causes acantholytic skin disease in humans and squamous cell tumors in mice
Heterozygous loss-of-function mutations in SPCA1 cause HHD in humans [5,6] and squamous cell tumors in mice [11]. The phenotypes in both species are remarkably similar to those caused by loss-of-function mutations in a single copy of the SERCA2 gene, with DD in humans [7] and squamous cell tumors in mice [8,9]. In each case the genetic lesion is an autosomal dominant mutation of a P-type ATPase that sequesters Ca2+ in a compartment of the secretory pathway (Golgi or ER), and the only known target cell is the keratinocyte.
The most prominent characteristic of both HHD and DD, as discussed in numerous reviews [14,65-68], is the disruption of cell-cell contacts (acantholysis) in the suprabasal layer of the skin, which results from loss of desmosomal connections between cells. Ultrastructural studies of HHD [69] indicated that desmosomes and their connections with tonofilaments (keratin intermediate filaments) formed normally in non-lesional skin; however, in affected cells, tonofilaments separated from the desmosomes, followed by clumping of tonofilaments, loss of desmosomes, and acantholysis [69]. A similar study of DD patients indicated that the morphological changes in the suprabasal layer were similar to those of HHD and suggested that the loss of connection between tonofilaments and desmosomes preceded the loss of desmosomes [70,71]. This view has been supported by other investigators [72-74], although there are counterviews [75,76], and non-lesional skin of HHD patients is more fragile [76], indicating that the process of acantholysis is not identical in the two diseases.
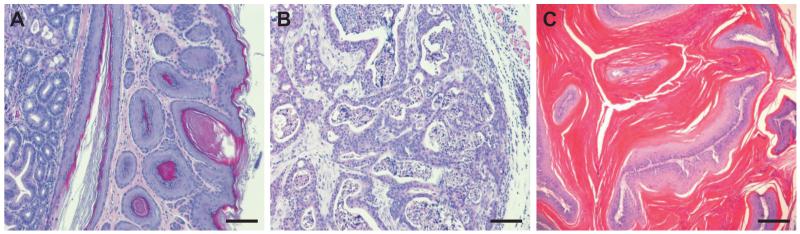
Squamous cell tumors, but not acantholytic skin diseases, were observed in SPCA1 and SERCA2 heterozygous mice. In the SERCA2 model, ~90% of the mice developed one or more squamous cell carcinomas or papillomas of the forestomach, oral mucosa, esophagus, or skin [8-10]. The wild-type allele was retained in the tumors, indicating that SERCA2 does not serve as a classical tumor suppressor and that haploinsufficiency is sufficient to cause cancer [9]. Sharp upregulation of H-ras and K-ras, with no apparent mutations, was part of the tumor mechanism. Upregulation of p53 was also observed and was proposed to slow tumor growth as loss of p53 resulted in growth of a massive tumor [9]. Similar tumors were observed in SPCA1 heterozygous mice (Fig. 2), but the incidence was lower (25%) [11]. The mechanisms have not been analyzed but are unlikely to involve loss of heterozygosity since the average age of onset was almost two years. The occurrence of tumors in SPCA1 heterozygous mice is consistent with the possibility that four cases of squamous cell tumors of the vulva in humans with HHD [77 and references therein] may be due to SPCA1 haploinsufficiency. Rare occurrances of squamous cell tumors have also been observed in DD [78 and references therein], and down-regulation of SERCA2 has been reported in human oral squamous cell tumors [79].
Fig. 2.
Squamous cell tumors in SPCA1 heterozygous mice. Carcinomas and papillomas were observed in keratinized epithelia of SPCA1 heterozygous mice (11), but the incidence was lower and the age of onset later than in SERCA2 heterozygous mice (8,9). A, Moderate hyperplasia of the forestomach in a 23-month-old heterozygous male; this phenotype was rarely observed in SPCA1 heterozygous mice, but was invariably observed in forestomach of SERCA2 heterozygous mice by 3 months of age. B, Poorly differentiated skin carcinoma in 22-month-old heterozygous male. C, Papilloma with severe hyperkeratosis from peri-anal region of 22-month-old heterozygous male. Bar = 100 micrometers in A and B, 200 micrometers in C.
There has been a great deal of investigation and speculation about the underlying mechanisms of HHD and DD [reviewed in 14, 65-68], but there is currently no consensus. Defects in Ca2+ signaling or defective folding, processing, and trafficking of cell adhesion proteins have been proposed, but there is little evidence that the Ca2+ signaling functions of SPCA1 and SERCA2 are similar, and production and assembly of desmosomes appears normal in HHD keratinocytes [80]. Several investigators have discussed the possibility that Golgi stress or ER stress might be involved in HHD [11] and DD [65,67,81], respectively.
6. Secretory pathway stress and stress responses as potential common factors in disease caused by SPCA1 and SERCA2 haploinsufficiency in humans and mice
Secretory pathway stress originating in the ER and activation of ER stress responses are major mechanisms in a wide range of diseases [82-84]. These include neurodegenerative diseases [85,86], cardiovascular disease [87,88], diabetes [89], obesity [90], inflammatory bowel disease [91], kidney disease [92], chronic obstructive pulmonary disease [93], skeletal disorders [94], and cancer [95,96]. Any of a number of perturbations of ER function elicits a complex set of ER stress responses involving activation of signaling pathways that can lead to correction of the deficit, thus favoring survival, and activation of pathways that can, under certain circumstances, lead to apoptosis [97]. In the initial stages of ER stress, pathways favoring both survival and apoptosis are activated, but if the cell is able to adapt, then pro-apoptotic pathways diminish and the execution phase of apoptosis is avoided [98Rutkowski06].
One of the most common experimental methods to induce ER stress is to inhibit SERCA2 with thapsigargin, an effect seen at even very low levels [98]. Thus, a reduction in SERCA2 activity in differentiating keratinocytes has the potential to cause ER stress and elicit ER stress responses [65,67,81]. Indeed, if ER function is affected by reduced sequestration of Ca2+ by SERCA2, then ER stress response pathways would be activated to at least some degree in keratinocytes of DD patients and SERCA2 heterozygous mice. Although less well understood, Golgi stress [59,60,99], such as that occurring in SPCA1 null embryos [11], also elicits stress responses (Table 1 and section 3). Furthermore, ~50% knockdown of SPCA1 in HeLa cells, comparable to the reduction in SPCA1 in HHD keratinocytes that reduces luminal Ca2+ [27], led to fragmentation of the Golgi and other indications of Golgi stress [37]. Thus, haploinsufficiency of both SERCA2 and SPCA1 causes secretory pathway stress, which in turn would be expected to activate stress response pathways that lead to survival or apoptosis.
The role of ER stress and related adaptive stress responses, such as the UPR, in cancer are well established and result from activation of survival pathways and suppression of apoptotic pathways [95,96]. The absence of ras and p53 mutations in tumors of SERCA2 heterozygous mice suggested a novel mechanism involving a global change in tumorigenic potential of the keratinized epithelium [9], which would be consistent with chronic ER stress and activation of survival responses favoring tumorigenesis. The Golgi is also involved in stress sensing and can mount stress responses that affect tumorigenesis [100]. For example, polo-like kinase 3 is involved in Golgi fragmentation during mitosis and apoptosis and functions as a tumor suppressor [100]. If survival responses to secretory pathway stress in mouse keratinocytes, originating in either the ER or Golgi, are favored over apoptotic responses, then these stress responses could be part of the mechanism of tumorigenesis in SERCA2 and SPCA1 mutant mice and in the few squamous cell tumors in HHD [77] and DD [78] patients.
As in many other diseases [82-96], chronic secretory pathway stress and activation of stress responses could be also part of the underlying mechanisms of HHD and DD. Acantholytic skin disease in humans is quite different from the phenotype of squamous cell tumors in mice; however, because ER stress leads to activation of pathways favoring either adaptation or apoptosis, it is possible that differences in the balance between these stress responses in mouse and human keratinocytes account for the differences in phenotype.
Manifestations of HHD and DD first occur in suprabasal keratinocytes as they begin differentiation. Keratinocyte differentiation involves i) the synthesis and trafficking of structural proteins, enzymes, and lipids needed to form the mature corneocyte and ii) a novel form of programmed cell death [101-103]. The UPR, a component of the ER stress response, regulates transcriptional programs that expand the secretory pathway [104] and plays a role in the differentiation of a number of cell types [105,106], including human epidermal keratinocytes [107]. ER or Golgi Ca2+ pump dysfunction causes secretory pathway stress and could therefore interfere with normal utilization of the stress response pathways during differentiation of keratinocytes. If there were insufficient reserve for adaptive stress responses, then apoptotic responses might be favored. Even if apoptotic stimuli did not reach a threshold sufficient for the execution phase, they could interfere with the specialized process of programmed cell death in keratinocytes [101-103], which must occur properly if the cell is to become a functional, but non-living corneocyte. Caspases involved in apoptosis contribute to cornification [108-109] and desmosomal proteins are particularly susceptible to cleavage during apoptosis [110-111].
Interestingly, desmoplakin, the desmosomal component to which keratin intermediate filaments attach, is cleaved efficiently by caspase 2 [111], an initiator caspase localized to the Golgi. Finally, there is evidence that apoptosis occurs in lesional tissue of both DD and HHD patients [112] and the reduced expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL in DD lesions [113] is consistent with activation of apoptotic responses in this disease.
7. Concluding remarks
The studies discussed in this review have shown that haploinsufficiency of either SPCA1 or SERCA2 causes acantholytic skin disease in humans and squamous cell tumors in mice. Both Ca2+ pumps serve in a compartment of the secretory pathway; keratinocytes are the affected cell type in both species; and dysfunction involving either pump leads to secretory pathway stress and elicits secretory pathway stress responses. These commonalities suggest that the underlying disease mechanisms are similar and, like so many other diseases [82-96], involve secretory pathway stress and activation of powerful stress response signaling pathways. These pathways contribute to either adaptation and cell survival or to apoptosis and cell death. Differential activities of prosurvival or proapoptotic arms of the stress response pathways in human and mouse keratinocytes may be the basis of the dramatic species differences in disease phenotypes.
Acknowledgements
The authors were supported by NIH grants HL061974, DK050594, and ES006096.
References
- [1].Brini M, Carafoli E. Calcium pumps in health and disease. Physiol. Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- [2].Strehler EE, Filoteo AG, Penniston JT, Caride AJ. Plasma-membrane Ca2+ pumps: structural diversity as the basis for functional versatility. Biochem. Soc. Trans. 2007;35:919–922. doi: 10.1042/BST0350919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Møller JV, Olesen C, Winther AM, Nissen P. The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q. Rev. Biophys. 2010;1:1–66. doi: 10.1017/S003358351000017X. [DOI] [PubMed] [Google Scholar]
- [4].Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc. Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- [5].Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, Ogawa H, Ikeda S, Mauro T, Epstein EH., Jr. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat. Genet. 2000;24:61–65. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- [6].Sudbrak R, Brown J, Dobson-Stone C, Carter S, Ramser J, White J, Healy E, Dissanayake M, Larrègue M, Perrussel M, Lehrach H, Munro CS, Strachan T, Burge S, Hovnanian A, Monaco AP. Hailey-Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca2+ pump. Hum. Mol. Genet. 2000;9:1131–1140. doi: 10.1093/hmg/9.7.1131. [DOI] [PubMed] [Google Scholar]
- [7].Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, Smith M, Munro CS, O’Donovan M, Craddock N, Kucherlapati R, Rees JL, Owen M, Lathrop GM, Monaco AP, Strachan T, Hovnanian A. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat. Genet. 1999;21:271–277. doi: 10.1038/6784. [DOI] [PubMed] [Google Scholar]
- [8].Liu LH, Boivin GP, Prasad V, Periasamy M, Shull GE. Squamous cell tumors in mice heterozygous for a null allele of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump. J. Biol. Chem. 2001;276:26737–26740. doi: 10.1074/jbc.C100275200. [DOI] [PubMed] [Google Scholar]
- [9].Prasad V, Boivin GP, Miller ML, Liu LH, Erwin CR, Warner BW, Shull GE. Haploinsufficiency of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump, predisposes mice to squamous cell tumors via a novel mode of cancer susceptibility. Cancer Res. 2005;65:8655–8661. doi: 10.1158/0008-5472.CAN-05-0026. [DOI] [PubMed] [Google Scholar]
- [10].Hong JH, Yang YM, Kim HS, Lee SI, Muallem S, Shin DM. Markers of squamous cell carcinoma in sarco/endoplasmic reticulum Ca2+ ATPase 2 heterozygote mice keratinocytes. Prog. Biophys. Mol. Biol. 2010;103:81–87. doi: 10.1016/j.pbiomolbio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- [11].Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, Doetschman T, Prasad V, Shull GE. Loss of the Atp2c1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J. Biol. Chem. 2007;282:26517–26527. doi: 10.1074/jbc.M703029200. [DOI] [PubMed] [Google Scholar]
- [12].Wuytack F, Raeymaekers L, Missiaen L. Molecular physiology of the SERCA and SPCA pumps. Cell Calcium. 2002;32:279–305. doi: 10.1016/s0143416002001847. [DOI] [PubMed] [Google Scholar]
- [13].Van Baelen K, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, Parys JB, Raeymaekers L, Wuytack F. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim. Biophys. Acta. 2004;1742:103–112. doi: 10.1016/j.bbamcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- [14].Missiaen L, Raeymaekers L, Dode L, Vanoevelen J, Van Baelen K, Parys JB, Callewaert G, De Smedt H, Segaert S, Wuytack F. SPCA1 pumps and Hailey-Hailey disease. Biochem Biophys Res Commun. 2004;322:1204–1213. doi: 10.1016/j.bbrc.2004.07.128. [DOI] [PubMed] [Google Scholar]
- [15].Missiaen L, Dode L, Vanoevelen J, Raeymaekers L, Wuytack F. Calcium in the Golgi apparatus. Cell Calcium. 2007;41:405–416. doi: 10.1016/j.ceca.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [16].Baumrucker CR, Keenan TW. Membranes of mammary gland. X. Adenosine triphosphate dependent calcium accumulation by Golgi apparatus rich fractions from bovine mammary gland. Exp. Cell Res. 1975;90:253–260. doi: 10.1016/0014-4827(75)90314-6. [DOI] [PubMed] [Google Scholar]
- [17].Neville MC, Selker F, Semple K, Watters C. ATP-dependent calcium transport by a Golgi-enriched membrane fraction from mouse mammary gland. J. Membr. Biol. 1981;61:97–105. doi: 10.1007/BF02007636. [DOI] [PubMed] [Google Scholar]
- [18].West DW. Energy-dependent calcium sequestration activity in a Golgi apparatus fraction derived from lactating rat mammary glands. Biochim. Biophys. Acta. 1981;673:374–386. doi: 10.1016/0304-4165(81)90469-4. [DOI] [PubMed] [Google Scholar]
- [19].Virk SS, Kirk CJ, Shears SB. Ca2+ transport and Ca2+-dependent ATP hydrolysis by Golgi vesicles from lactating rat mammary glands. Biochem. J. 1985;226:741–748. doi: 10.1042/bj2260741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Watters CD. A Ca2+-stimulated adenosine triphosphatase in Golgi-enriched membranes of lactating murine mammary tissue. Biochem. J. 1984;224:39–45. doi: 10.1042/bj2240039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, Davidow LS, Mao JI, Moir DT. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989;58:133–45. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- [22].Gunteski-Hamblin AM, Clarke DM, Shull GE. Molecular cloning and tissue distribution of alternatively spliced mRNAs encoding possible mammalian homologues of the yeast secretory pathway calcium pump. Biochemistry. 1992;31:7600–7608. doi: 10.1021/bi00148a023. [DOI] [PubMed] [Google Scholar]
- [23].Van Baelen K, Vanoevelen J, Missiaen L, Raeymaekers L, Wuytack F. The Golgi PMR1 P-type ATPase of Caenorhabditis elegans. Identification of the gene and demonstration of calcium and manganese transport. J. Biol. Chem. 2001;276:10683–10691. doi: 10.1074/jbc.M010553200. [DOI] [PubMed] [Google Scholar]
- [24].Xiang M, Mohamalawari D, Rao R. A novel isoform of the secretory pathway Ca2+,Mn2+-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J. Biol. Chem. 2005;280:11608–11614. doi: 10.1074/jbc.M413116200. [DOI] [PubMed] [Google Scholar]
- [25].Vanoevelen J, Dode L, Van Baelen K, Fairclough RJ, Missiaen L, Raeymaekers L, Wuytack F. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J. Biol. Chem. 2005;280:22800–22808. doi: 10.1074/jbc.M501026200. [DOI] [PubMed] [Google Scholar]
- [26].Dürr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Behne MJ, Tu CL, Aronchik I, Epstein E, Bench G, Bikle DD, Pozzan T, Mauro TM. Human keratinocyte ATP2C1 localizes to the Golgi and controls Golgi Ca2+ stores. J. Invest. Dermatol. 2003;121:688–694. doi: 10.1046/j.1523-1747.2003.12528.x. [DOI] [PubMed] [Google Scholar]
- [28].Van Baelen K, Vanoevelen J, Callewaert G, Parys JB, De Smedt H, Raeymaekers L, Rizzuto R, Missiaen L, Wuytack F. The contribution of the SPCA1 Ca2+ pump to the Ca2+ accumulation in the Golgi apparatus of HeLa cells assessed via RNA-mediated interference. Biochem. Biophys. Res. Commun. 2003;306:430–436. doi: 10.1016/s0006-291x(03)00977-x. [DOI] [PubMed] [Google Scholar]
- [29].Faddy HM, Smart CE, Xu R, Lee GY, Kenny PA, Feng M, Rao R, Brown MA, Bissell MJ, Roberts-Thomson SJ, Monteith GR. Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem. Biophys. Res. Commun. 2008;369:977–981. doi: 10.1016/j.bbrc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reinhardt TA, Lippolis JD. Mammary gland involution is associated with rapid down regulation of major mammary Ca2+-ATPases. Biochem. Biophys. Res. Commun. 2009;378:99–102. doi: 10.1016/j.bbrc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- [31].Berkelman T, Garret-Engele P, Hoffman NE. The pacL gene of Synechococcus sp. strain PCC 7942 encodes a Ca2+-transporting ATPase. J. Bacteriol. 1994;176:4430–4436. doi: 10.1128/jb.176.14.4430-4436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mills RF, Doherty ML, López-Marqués RL, Weimar T, Dupree P, Palmgren MG, Pittman JK, Williams LE. ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol. 2008;146:116–128. doi: 10.1104/pp.107.110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vanoevelen J, Raeymaekers L, Parys JB, De Smedt H, Van Baelen K, Callewaert G, Wuytack F, Missiaen L. Inositol trisphosphate producing agonists do not mobilize the thapsigargin-insensitive part of the endoplasmic-reticulum and Golgi Ca2+ store. Cell Calcium. 2004;35:115–121. doi: 10.1016/j.ceca.2003.08.003. [DOI] [PubMed] [Google Scholar]
- [34].Mandal D, Woolf TB, Rao R. Manganese selectivity of pmr1, the yeast secretory pathway ion pump, is defined by residue gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport. J. Biol. Chem. 2000;275:23933–23938. doi: 10.1074/jbc.M002619200. [DOI] [PubMed] [Google Scholar]
- [35].Dode L, Andersen JP, Raeymaekers L, Missiaen L, Vilsen B, Wuytack F. Functional comparison between secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and sarcoplasmic reticulum Ca2+-ATPase (SERCA) 1 isoforms by steady-state and transient kinetic analyses. J. Biol. Chem. 2005;280:39124–39134. doi: 10.1074/jbc.M506181200. [DOI] [PubMed] [Google Scholar]
- [36].Dode L, Andersen JP, Vanoevelen J, Raeymaekers L, Missiaen L, Vilsen B, Wuytack F. Dissection of the functional differences between human secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and 2 isoenzymes by steady-state and transient kinetic analyses. J. Biol. Chem. 2006;281:3182–3189. doi: 10.1074/jbc.M511547200. [DOI] [PubMed] [Google Scholar]
- [37].Lissandron V, Podini P, Pizzo P, Pozzan T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc. Natl. Acad. Sci. U S A. 2010;107:9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Antebi A, Fink GR. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 1995;15:1382–1388. doi: 10.1128/mcb.15.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Micaroni M, Perinetti G, Berrie CP, Mironov AA. The SPCA1 Ca2+ pump and intracellular membrane trafficking. Traffic. 2010;11:1315–1333. doi: 10.1111/j.1600-0854.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- [41].Ramos-Castañeda J, Park YN, Liu M, Hauser K, Rudolph H, Shull GE, Jonkman MF, Mori K, Ikeda S, Ogawa H, Arvan P. Deficiency of ATP2C1, a Golgi ion pump, induces secretory pathway defects in endoplasmic reticulum (ER)-associated degradation and sensitivity to ER stress. J. Biol. Chem. 2005;280:9467–9473. doi: 10.1074/jbc.M413243200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sepúlveda MR, Vanoevelen J, Raeymaekers L, Mata AM, Wuytack F. Silencing the SPCA1 (secretory pathway Ca2+-ATPase isoform 1) impairs Ca2+ homeostasis in the Golgi and disturbs neural polarity. J. Neurosci. 2009;29:12174–82. doi: 10.1523/JNEUROSCI.2014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Varki A. Factors controlling the glycosylation potential of the Golgi apparatus. Trends Cell Biol. 1998;8:34–40. doi: 10.1016/s0962-8924(97)01198-7. [DOI] [PubMed] [Google Scholar]
- [44].Callewaert G, Parys JB, De Smedt H, Raeymaekers L, Wuytack F, Vanoevelen J, Van Baelen K, Simoni A, Rizzuto R, Missiaen L. Similar Ca2+-signaling properties in keratinocytes and in COS-1 cells overexpressing the secretory-pathway Ca2+-ATPase SPCA1. Cell Calcium. 2003;34:157–162. doi: 10.1016/s0143-4160(03)00070-8. [DOI] [PubMed] [Google Scholar]
- [45].Harper C, Wootton L, Michelangeli F, Lefièvre L, Barratt C, Publicover S. Secretory pathway Ca2+-ATPase (SPCA1) Ca2+ pumps, not SERCAs, regulate complex [Ca2+]i signals in human spermatozoa. J. Cell Sci. 2005;118:1673–1685. doi: 10.1242/jcs.02297. [DOI] [PubMed] [Google Scholar]
- [46].Foggia L, Aronchik I, Aberg K, Brown B, Hovnanian A, Mauro TM. Activity of the hSPCA1 Golgi Ca2+ pump is essential for Ca2+-mediated Ca2+ response and cell viability in Darier disease. J. Cell Sci. 2006;119:671–679. doi: 10.1242/jcs.02781. [DOI] [PubMed] [Google Scholar]
- [47].Lai P, Michelangeli F. Changes in expression and activity of the secretory pathway Ca2+ ATPase 1 (SPCA1) in A7r5 vascular smooth muscle cells cultured at different glucose concentrations. Biosci. Rep. 2009;29:397–404. doi: 10.1042/BSR20090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Baron S, Struyf S, Wuytack F, Van Damme J, Missiaen L, Raeymaekers L, Vanoevelen J. Contribution of intracellular Ca2+ stores to Ca2+ signaling during chemokinesis of human neutrophil granulocytes. Biochim. Biophys. Acta. 2009;1793:1041–1049. doi: 10.1016/j.bbamcr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- [49].Micaroni M, Perinetti G, Di Giandomenico D, Bianchi K, Spaar A, Mironov AA. Synchronous intra-Golgi transport induces the release of Ca2+ from the Golgi apparatus. Exp. Cell Res. 2010;316:2071–2086. doi: 10.1016/j.yexcr.2010.04.024. [DOI] [PubMed] [Google Scholar]
- [50].Copp AJ. Death before birth: clues from gene knockouts and mutations. Trends Genet. 1995;11:87–93. doi: 10.1016/S0168-9525(00)89008-3. [DOI] [PubMed] [Google Scholar]
- [51].Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Sonic hedgehog and the molecular regulation of mouse neural tube closure. Development. 2002;129:2507–2517. doi: 10.1242/dev.129.10.2507. [DOI] [PubMed] [Google Scholar]
- [52].Copp AJ. Neurulation in the cranial region--normal and abnormal. J. Anat. 2005;207:623–635. doi: 10.1111/j.1469-7580.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dolman NJ, Tepikin AV. Calcium gradients and the Golgi. Cell Calcium. 2006;40:505–512. doi: 10.1016/j.ceca.2006.08.012. [DOI] [PubMed] [Google Scholar]
- [54].Renard-Rooney DC, Hajnóczky G, Seitz MB, Schneider TG, Thomas AP. Imaging of inositol 1,4,5-trisphosphate-induced Ca2+ fluxes in single permeabilized hepatocytes. Demonstration of both quantal and nonquantal patterns of Ca2+ release. J. Biol. Chem. 1993;268:23601–23610. [PubMed] [Google Scholar]
- [55].Marutani T, Yamamoto A, Nagai N, Kubota H, Nagata K. Accumulation of type IV collagen in dilated ER leads to apoptosis in Hsp47-knockout mouse embryos via induction of CHOP. J. Cell Sci. 2004;117:5913–5922. doi: 10.1242/jcs.01514. [DOI] [PubMed] [Google Scholar]
- [56].Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- [57].Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, Harada A, Mori K. Induction of liver steatosis and lipid droplet formation in ATF6α-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol. Biol. Cell. 2010;21:2975–2986. doi: 10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Orsó E, Broccardo C, Kaminski WE, Böttcher A, Liebisch G, Drobnik W, Götz A, Chambenoit O, Diederich W, Langmann T, Spruss T, Luciani MF, Rothe G, Lackner KJ, Chimini G, Schmitz G. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat. Genet. 2000;24:192–196. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- [59].Hicks SW, Machamer CE. Golgi structure in stress sensing and apoptosis. Biochim. Biophys. Acta. 2005;1744:406–414. doi: 10.1016/j.bbamcr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- [60].Yoshida H. ER stress response, peroxisome proliferation, mitochondrial unfolded protein response and Golgi stress response. IUBMB Life. 2009;61:871–879. doi: 10.1002/iub.229. [DOI] [PubMed] [Google Scholar]
- [61].Federovitch CM, Ron D, Hampton RY. The dynamic ER: experimental approaches and current questions. Curr. Opin. Cell Biol. 2005;17:409–414. doi: 10.1016/j.ceb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- [62].Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, Brewer JW. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J. Biol. Chem. 2007;282:7024–7034. doi: 10.1074/jbc.M609490200. [DOI] [PubMed] [Google Scholar]
- [63].Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tigges M, Fussenegger M. Xbp1-based engineering of secretory capacity enhances the productivity of Chinese hamster ovary cells. Metab. Eng. 2006;8:264–272. doi: 10.1016/j.ymben.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [65].Foggia L, Hovnanian A. Calcium pump disorders of the skin. Am. J. Med. Genet. C Semin. Med. Genet. 2004;131C:20–31. doi: 10.1002/ajmg.c.30031. [DOI] [PubMed] [Google Scholar]
- [66].Dhitavat J, Fairclough RJ, Hovnanian A, Burge SM. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey-Hailey disease. Br. J. Dermatol. 2004;150:821–828. doi: 10.1111/j.1365-2133.2004.05904.x. [DOI] [PubMed] [Google Scholar]
- [67].Hovnanian A. Darier’s disease: from dyskeratosis to endoplasmic reticulum calcium ATPase deficiency. Biochem. Biophys. Res. Commun. 2004;322:1237–1244. doi: 10.1016/j.bbrc.2004.08.067. [DOI] [PubMed] [Google Scholar]
- [68].Szigeti R, Kellermayer R. Autosomal-dominant calcium ATPase disorders. J. Invest. Dermatol. 2006;126:2370–2376. doi: 10.1038/sj.jid.5700447. [DOI] [PubMed] [Google Scholar]
- [69].Wilgram GF, Caulfield JB, Lever WF. An electron-microscopic study of acantholysis and dyskeratosis in Hailey and Hailey’s disease. J. Invest. Dermatol. 1962;39:373–381. [PubMed] [Google Scholar]
- [70].Caulfield JB, Wilgram GF. An electron-microscope study of dyskeratosis and acantholysis in Darier’s disease. J. Invest. Dermatol. 1963;41:57–65. [PubMed] [Google Scholar]
- [71].Wilgram GF, Weinstock A. Advances in genetic dermatology. Dyskeratosis, acantholysis, and hyperkeratosis, with a note on the specific role of desmosomes and keratinosomes in the formation of the horny layer. Arch. Dermatol. 1966;94:456–479. doi: 10.1001/archderm.94.4.456. [DOI] [PubMed] [Google Scholar]
- [72].Gottlieb SK, Lutzner MA. Hailey-hailey disease--an electron microcopic study. J. Invest. Dermatol. 1970;54:368–376. doi: 10.1111/1523-1747.ep12259067. [DOI] [PubMed] [Google Scholar]
- [73].Gottlieb SK, Lutzner MA. Darier’s disease. An electron microscopic study. Arch. Dermatol. 1973;107:225–230. doi: 10.1001/archderm.107.2.225. [DOI] [PubMed] [Google Scholar]
- [74].Hashimoto K, Fujiwara K, Tada J, Harada M, Setoyama M, Eto H. Desmosomal dissolution in Grover’s disease, Hailey-Hailey’s disease and Darier’s disease. J. Cutan. Pathol. 1995;22:488–501. doi: 10.1111/j.1600-0560.1995.tb01145.x. [DOI] [PubMed] [Google Scholar]
- [75].Burge SM, Schomberg KH. Adhesion molecules and related proteins in Darier’s disease and Hailey-Hailey disease. Br. J. Dermatol. 1992;127:335–343. doi: 10.1111/j.1365-2133.1992.tb00451.x. [DOI] [PubMed] [Google Scholar]
- [76].Metze D, Hamm H, Schorat A, Luger T. Involvement of the adherens junction-actin filament system in acantholytic dyskeratosis of Hailey-Hailey disease. A histological, ultrastructural, and histochemical study of lesional and non-lesional skin. J. Cutan. Pathol. 1996;23:211–222. doi: 10.1111/j.1600-0560.1996.tb01469.x. [DOI] [PubMed] [Google Scholar]
- [77].von Felbert V, Hampl M, Talhari C, Engers R, Megahed M. Squamous cell carcinoma arising from a localized vulval lesion of Hailey-Hailey disease after tacrolimus therapy. Am. J. Obstet. Gynecol. 2010;203(3):e5–7. doi: 10.1016/j.ajog.2010.06.041. [DOI] [PubMed] [Google Scholar]
- [78].Matsui K, Makino T, Nakano H, Furuichi M, Sawamura D, Shimizu T. Squamous cell carcinoma arising from Darier’s disease. Clin. Exp. Dermatol. 2009;34(8):e1015–6. doi: 10.1111/j.1365-2230.2009.03682.x. [DOI] [PubMed] [Google Scholar]
- [79].Endo Y, Uzawa K, Mochida Y, Shiiba M, Bukawa H, Yokoe H, Tanzawa H. Sarcoendoplasmic reticulum Ca2+ ATPase type 2 downregulated in human oral squamous cell carcinoma. Int. J. Cancer. 2004;110:225–231. doi: 10.1002/ijc.20118. [DOI] [PubMed] [Google Scholar]
- [80].Bernards M, Korge BP. Desmosome assembly and keratin network formation after Ca2+/serum induction and UVB radiation in Hailey-Hailey keratinocytes. J. Invest. Dermatol. 2000;114:1058–1061. doi: 10.1046/j.1523-1747.2000.00960-2.x. [DOI] [PubMed] [Google Scholar]
- [81].Onozuka T, Sawamura D, Goto M, Yokota K, Shimizu H. Possible role of endoplasmic reticulum stress in the pathogenesis of Darier’s disease. J. Dermatol. Sci. 2006;41:217–220. doi: 10.1016/j.jdermsci.2005.12.002. [DOI] [PubMed] [Google Scholar]
- [82].Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- [83].Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- [84].Hosoi T, Ozawa K. Endoplasmic reticulum stress in disease: mechanisms and therapeutic opportunities. Clin. Sci. (Lond) 2010;118:19–29. doi: 10.1042/CS20080680. [DOI] [PubMed] [Google Scholar]
- [85].Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. doi:10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nassif M, Matus S, Castillo K, Hetz C. Amyotrophic lateral sclerosis pathogenesis: A journey through the secretory pathway. Antioxid. Redox Signal. 2010;13:1955–1989. doi: 10.1089/ars.2009.2991. [DOI] [PubMed] [Google Scholar]
- [87].Minamino T, Kitakaze M. ER stress in cardiovascular disease. J. Mol. Cell. Cardiol. 2010;48:1105–1110. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- [88].Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ. Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Thomas SE, Dalton LE, Daly ML, Malzer E, Marciniak SJ. Diabetes as a disease of endoplasmic reticulum stress. Diabetes Metab. Res. Rev. 2010;26:611–621. doi: 10.1002/dmrr.1132. [DOI] [PubMed] [Google Scholar]
- [90].Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- [91].McGuckin MA, Eri RD, Das I, Lourie R, Florin TH. ER stress and the unfolded protein response in intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G820–G832. doi: 10.1152/ajpgi.00063.2010. [DOI] [PubMed] [Google Scholar]
- [92].Dickhout JG, Krepinsky JC. Endoplasmic reticulum stress and renal disease. Antioxid. Redox Signal. 2009;11:2341–2352. doi: 10.1089/ars.2009.2705. [DOI] [PubMed] [Google Scholar]
- [93].Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A, Wise R, Tuder R, Biswal S. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am. J. Respir. Crit. Care Med. 2009;180:1196–1207. doi: 10.1164/rccm.200903-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [94].Henriques-Pons A, Nagaraju K. Nonimmune mechanisms of muscle damage in myositis: role of the endoplasmic reticulum stress response and autophagy in the disease pathogenesis. Curr. Opin. Rheumatol. 2009;21:581–587. doi: 10.1097/BOR.0b013e3283319265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat. Rev. Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- [96].Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur. J. Pharmacol. 2009;625:234–46. doi: 10.1016/j.ejphar.2009.06.064. [DOI] [PubMed] [Google Scholar]
- [97].Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem. Sci. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- [98].Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4(11):e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Maag RS, Hicks SW, Machamer CE. Death from within: apoptosis and the secretory pathway. Curr. Opin. Cell Biol. 2003;15:456–461. doi: 10.1016/s0955-0674(03)00075-9. [DOI] [PubMed] [Google Scholar]
- [100].Wlodkowic D, Skommer J, McGuinness D, Hillier C, Darzynkiewicz Z. ER-Golgi network-a future target for anti-cancer therapy. Leuk. Res. 2009;33:1440–1447. doi: 10.1016/j.leukres.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- [102].Raj D, Brash DE, Grossman D. Keratinocyte apoptosis in epidermal development and disease. J. Invest. Dermatol. 2006;126:243–257. doi: 10.1038/sj.jid.5700008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lippens S, Hoste E, Vandenabeele P, Agostinis P, Declercq W. Cell death in the skin. Apoptosis. 2009;14:549–569. doi: 10.1007/s10495-009-0324-z. [DOI] [PubMed] [Google Scholar]
- [104].Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ma Y, Shimizu Y, Mann MJ, Jin Y, Hendershot LM. Plasma cell differentiation initiates a limited ER stress response by specifically suppressing the PERK-dependent branch of the unfolded protein response. Cell Stress Chaperones. 2010;15:281–293. doi: 10.1007/s12192-009-0142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Firtina Z, Duncan MK. Unfolded Protein Response (UPR) is activated during normal lens development. Gene Expr. Patterns. 2010 doi: 10.1016/j.gep.2010.10.005. (2010) [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Sugiura K, Muro Y, Futamura K, Matsumoto K, Hashimoto N, Nishizawa Y, Nagasaka T, Saito H, Tomita Y, Usukura J. The unfolded protein response is activated in differentiating epidermal keratinocytes. J. Invest. Dermatol. 2009;129:2126–2135. doi: 10.1038/jid.2009.51. [DOI] [PubMed] [Google Scholar]
- [108].Weil M, Raff MC, Braga VM. Caspase activation in the terminal differentiation of human epidermal keratinocytes. Curr. Biol. 1999;9:361–364. doi: 10.1016/s0960-9822(99)80162-6. [DOI] [PubMed] [Google Scholar]
- [109].Chaturvedi V, Sitailo LA, Bodner B, Denning MF, Nickoloff BJ. Defining the caspase-containing apoptotic machinery contributing to cornification in human epidermal equivalents. Exp. Dermatol. 2006;15:14–22. doi: 10.1111/j.0906-6705.2005.00383.x. [DOI] [PubMed] [Google Scholar]
- [110].Weiske J, Schöneberg T, Schröder W, Hatzfeld M, Tauber R, Huber O. The fate of desmosomal proteins in apoptotic cells. J. Biol. Chem. 2001;276:41175–41181. doi: 10.1074/jbc.M105769200. [DOI] [PubMed] [Google Scholar]
- [111].Aho S. Plakin proteins are coordinately cleaved during apoptosis but preferentially through the action of different caspases. Exp. Dermatol. 2004;13:700–707. doi: 10.1111/j.0906-6705.2004.00217.x. [DOI] [PubMed] [Google Scholar]
- [112].Gniadecki R, Jemec GB, Thomsen BM, Hansen M. Relationship between keratinocyte adhesion and death: anoikis in acantholytic diseases. Arch. Dermatol Res. 1998;290:528–532. doi: 10.1007/s004030050347. [DOI] [PubMed] [Google Scholar]
- [113].Pasmatzi E, Badavanis G, Monastirli A, Tsambaos D. Reduced expression of the antiapoptotic proteins of Bcl-2 gene family in the lesional epidermis of patients with Darier’s disease. J. Cutan. Pathol. 2007;34:234–238. doi: 10.1111/j.1600-0560.2006.00600.x. [DOI] [PubMed] [Google Scholar]