Introduction
Although not considered in the core symptomatology of autism, a variety of abnormal motor features are present in persons with autism and may interfere with adaptive fine motor tasks such as, buttoning, tying shoelaces, and writing; 1-3 gross motor tasks such as throwing, catching, jumping and running; 4 as well as social-communicative functions such as speech articulation, gesturing, and eye gaze coordination. 5 Results from two recent studies suggest impairments in movement preparation and coordination involving multiple joints in persons with Autism Spectrum Disorder (ASD). 6, 7 Informal estimates of prevalence of motor abnormalities in persons with autism are reported as high as 85% to 90%. 8, 9 In one study, reviews of medical charts of 200 children with ASD between 2 and 4 years of age revealed that 100% of the children exhibited problems with motor planning and sequencing. 10 Forty-eight percent of the children had severe difficulties in motor planning. Motor planning and other aspects of motor control, such as coordination between various components of a motor task, are essential for efficient movement. In another more recent study including 101 children with autism or ASD who were assessed with the Movement Assessment Battery for Children (M-ABC) 11, 79% were identified with definite movement problems (<5th centile) and another 10% had borderline problems (5-15th centile). As well, there was no dicernable differences between the proportion of children with autism and those in the broader ASD group that presented with definite movement problems. 12 Despite this evidence of abnormalities in motor control and the implications of these abnormalities for adaptive skills, fundamental mechanisms of motor control in children and young adults with ASD have received little research attention. These fundamental mechanisms include movement preparation and planning, coordination, execution, and adaptation. As far back as 1992, physical educators began to recognize that children with autism had deficiencies in motor skills. 13 Historically, most of the therapeutic interventions for children with autism have been provided by pediatric occupational therapists with evidence of intervention dating back 40 years. Around 20 years ago occupational therapists began using sensory integration therapy for children with ASD. 14-17 Not until recently, have pediatric physical therapists begun intervening to address some of the gross motor and motor control deficits in children with ASD. As a result there has been an increase in the referrals of children with ASD to pediatric physical therapists for improving overall motor performance and motor planning.
Precision grip is a simple motor task. It is fundamental to most adaptive motor tasks, such as, buttoning, tying shoelaces, and writing, which are reported to be problematic in children with ASD. 6 Additionally, precision grip has been extensively studied to provide insight to fundamental mechanisms of motor control in healthy children and adults, and clinical populations such as individuals with Down syndrome, 18, 19 cerebral palsy, 20-24 and patients with cerebellar dysfunction. 25, 26 The methodology for studying precision grip is proven and reliable. Thus, examining precision grip in ASD will hopefully improve our understanding of motor control and motor planning in this population and assist pediatric physical therapists in designing efficacious intervention strategies related to overall movement.
Precision grip requires coordination between a proximal component -the load force, and a distal component -the grip force. 27 Load force is produced by muscles around the shoulder and in the upper arm. 25 Load force acts parallel to the contact surface of the object, providing the lifting force for objects and countering the force of gravity. Grip force is a compressive force produced by muscles in the hand and forearm acting perpendicular to the contact surface of the object and provides the force for holding an object. 25 An indicator of motor coordination is the duration between grip and load force onset (i.e., onset latency). 27 This duration decreases, as motor control improves, indicating temporal coordination of onset of force production. In typically developing children younger than seven years, this onset latency is prolonged compared to older children; consequently, a large amount of grip force is produced before the onset of the load force. After age seven, onset latency decreases and coordination of grip and load forces improves. 27
Similar coordination mechanisms are employed during a reach-to-grasp task. The reaching component is controlled by the proximal musculature of the shoulder and elbow, while the grasping component is controlled by distal musculature of the forearm and hand. Jeannerod suggests that these two distinct and independent components are united by a higher order coordinative structure, 28 most likely the cerebellum. 29 Both a precision grip and a reach-to-grasp task require the coordination of proximal and distal components. Individuals with ASD are reported to have a significant delay between the onset of reaching for and grasping an object compared to individuals with typical development, 6 suggesting temporal dyscoordination. This finding raises the possibility that dyscoordination affects other movement components, such as those involved in precision grip. In fact, results from two recent studies suggest impairments in movement preparation and coordination of multiple joints during arm movement tasks in persons with ASD. 6, 7 Despite evidence suggesting motor disturbances, 2, 6, 7, 30 the control of precision grip in children and adolescents with ASD has not been specifically investigated.
Purpose
The purpose of this pilot study was to determine if there were abnormalities in the temporal coordination of grip and load forces during precision grip in individuals with high-functioning autism (ASD) as compared to typically-developing peers. We predicted that the profiles of participants with ASD would differ from the profiles of the comparison group in the following ways: 1) longer onset latencies between grip and load forces, 2) elevated grip force at load force onset, due to impaired timing of grip force, 3) elevated peak grip force amplitudes, and 4) longer time taken to reach peak grip force amplitude.
Methods
Participants
Twenty-six participants (13 with ASD and 13 age-and gender-matched peers developing typically) between 8-19 years of age were recruited. Subjects with autism were recruited through the Subject Registry Core of the University of North Carolina Neurodevelopmental Disorders Research Center, the Autism Society of North Carolina’s Chapel Hill and Durham chapters. Subjects with typical development were recruited through local community facilities (e.g., YMCA) (Table 1). Participants younger than 13 years of age were matched within six months of their chronological age, whereas older participants were matched within 12 months of their chronological age, with one exception (pair 11, Table 1). The mean age for the ASD group was 11 years 2 months (SD = 3.4 years; Range = 8y 2m-19y 1m), while the mean age for the comparison group was 10 years 8 months (SD = 3.1 years; Range: 8y 1m-18y 3m). A t-test confirmed that the groups were not significantly different with respect to chronological age [t (24) = 0.481, p = 0.635].
Table 1.
Participant Characteristics
| ID# | Age (years) | Sex | SCQ Score (ASD only) | Performance IQ (ASD only) | |
|---|---|---|---|---|---|
| Typical | ASD | ||||
| 1 | 16.3 | 16.3 | F | 30 | 94 |
| 2 | 18.3 | 19.1 | F | 31 | 70 |
| 4 | 8.1 | 8.2 | M | 23 | 102 |
| 5 | 9.3 | 9.4 | M | 22 | 121 |
| 6 | 9.8 | 9.8 | M | 18 | 83 |
| 7 | 8.6 | 8.8 | M | 25 | 70 |
| 8 | 9.1 | 9.1 | M | 17 | 70 |
| 9 | 9.3 | 9.3 | M | 23 | 114 |
| 10 | 10.3 | 10.6 | M | 16 | 70 |
| 11 | 10.8 | 14.6 | M | 33 | 92 |
| 12 | 13 | 13.2 | M | 28 | 82 |
| 13 | 8.3 | 8.3 | M | 32 | 102 |
| 15 | 9.6 | 9.6 | M | 21 | 122 |
(SCQ = Social Communication Questionnaire, ASD = Autism Spectrum Disorders)
Inclusion criteria for participants with ASD included those with: 1) a clinical diagnosis of Autistic Disorder, Asperger Disorder, or Pervasive Developmental Disorder, Not Otherwise Specified 31 from a licensed psychologist or physician; 2) a confirmed autism score of 15 or more on the Social Communication Questionnaire (SCQ) 32, a commonly used screening tool for ASD, (3) no known genetic/medical conditions (e.g., fragile X syndrome; seizure disorder); (4) a performance IQ ≥70 confirmed by records of testing within the past 3 years (records provided by parents), (5) no observable motor side effects due to antipsychotic medication; 33 (6) normal or corrected normal hearing and vision; (7) no musculoskeletal defects that might prevent completion of the grasping task; and (8) an ability to understand simple directions and perform the requested tasks. Inclusion criteria for the comparison group included those with (1) normal developmental history; (2) normal or corrected normal vision and hearing; and (3) no musculoskeletal injury that could prevent completion of the grasping task.
Experimental Apparatus
The grasping apparatus incorporated two load cells 34 placed orthogonal to each other (Figure 1). One load cell placed between two parallel vertical surfaces measured grip forces, and a second load cell placed horizontally below the grasping area measured load forces. An aluminum box (3.5 inches × 2.25 inches) was attached to the second load cell and was used to house the calibrated Newton weights without providing visual cues (shape or size) to the participants. The width of the grasping portion of the apparatus was 1.5 inches and its radius 0.5 inch. Its surface was lined with suede to have a coefficient of friction between the extremes of silk and sandpaper. 35 The erect height of the apparatus was 4.5 inches and its weight 3.6 Newton (N). Participants were instructed to lift the “box” from a standardized start position and place it on a target (blue rectangle) approximately 6 inches away. The trial was complete when the participant placed the apparatus on the blue target rectangle.
Figure 1.
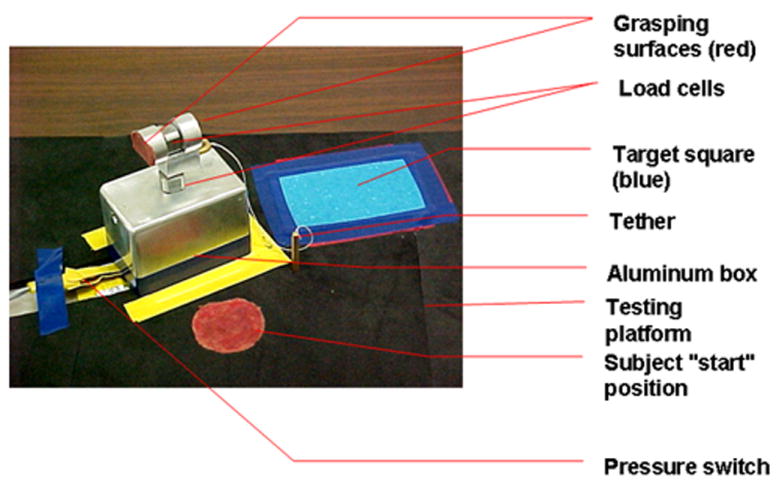
Experimental Apparatus
Data were collected using DATAPAC software 36 on a laptop computer equipped with an analog to digital board. The entire system was portable to facilitate data collection in an environment that was conducive to optimal performance and in a location convenient to the participants.
Procedures
The Committee for the Protection of Human Subjects at the University of North Carolina at Chapel Hill approved this study. After confirming eligibility, informed parental consent and minor participant’s assent were obtained. Data were collected onsite or at the participant’s residence or school. Parents of children in both groups completed the Edinburgh Handedness Inventory to determine the subject’s hand dominance. 37 In addition, the parents of the children in the ASD group completed the SCQ. 32. Participants were seated comfortably at a testing table with back and feet supported. The height of the table was adjusted to 3-5 inches above the elbow. 38 The experimental apparatus was placed on the table at a distance equivalent to 60% of the participant’s arm length, 39 in front of the participant at midline. The participant placed his/her dominant hand on a “start” position that was located 5 cm posterior to the experimental apparatus and marked using red adhesive tape to ensure procedural fidelity (Figure 1). The non-dominant hand was placed below the table on the participant’s ipsilateral thigh. Speed of the movement was self-selected.
The task was demonstrated using a precision grip (using the thumb and index finger to pick-up the object) followed by standardized oral instructions to “pick up the box, placing your thumb and pointer finger on the red circles, and put it down on the blue square”. Three practice trials and fifteen test trials (five blocked test trials, at each of three different apparatus loads 4.6N, 5.6N, and 7.6N in order) were completed by each participant. The standardized order of presentation was designed to discern the effect of anticipation with alternating loads (light-heavy-light) in future analyses. Grip and load forces of each participant were recorded as they grasped and lifted the experimental apparatus.
During the practice trials, if the primary investigator observed that the participant (1) failed to use a precision grip, (2) failed to grasp the apparatus on the grasping surfaces or, (3) failed to put down the apparatus on the designated area, “task-learning trials” with no attached weights were administered. The “task–learning trials” consisted of the investigator repeating the demonstration of the task, and providing additional verbal cueing and physical guidance as needed. Once the participant was consistent with the precision grip task (performed 2 trials with no errors), weights were added to the grasping apparatus and the participant continued independently with the practice trials. One subject from the ASD group required the “task learning trials”. The following were considered mistrials and the trial was repeated if the participant was: (1) not using a precision grip, (2) grasping the object in an area other than the grasping surface, or 3) throwing the object. All participants were rewarded with self-selected, age appropriate reinforcers (e.g., stickers, parent approved snacks) at the end of each block of trials.
Data Reduction
Analog data were collected at a sampling frequency of 125 Hz, amplified (1 volt represented 10N) and smoothed using a 20 Hz Butterworth low-pass filter with a roll-off of 35. 34 Dependent variables calculated to quantify grip and load force coordination included grip to load force onset latency, the grip force at onset of load force, the peak grip force, and the time to peak grip force (Figure 2).
Figure 2.
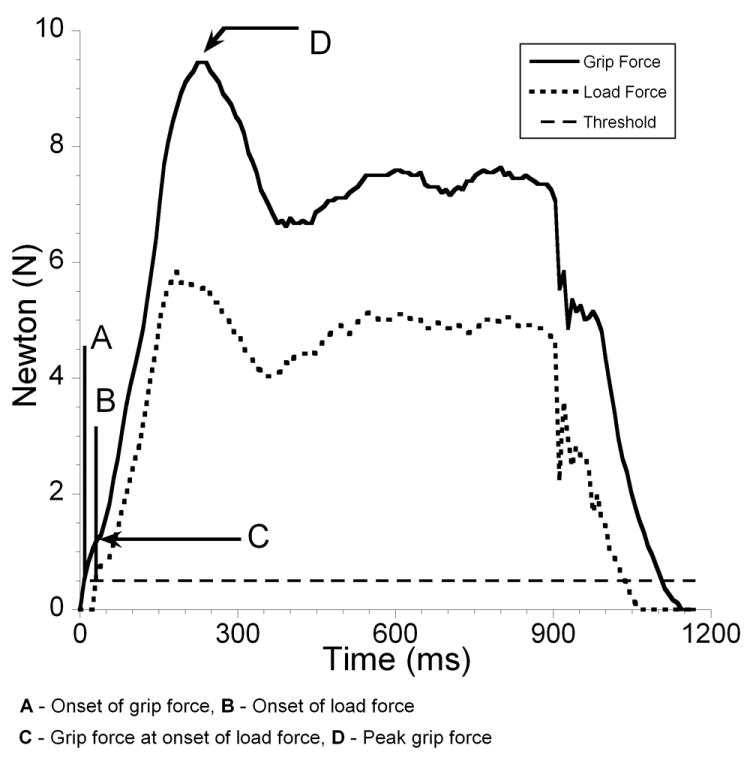
Typical Grip and Load Force Profiles
Grip to load force onset latency was defined as the duration between onset of grip force and onset of a load force against gravity. Grip and load force onset was defined as a positive increase of 0.05 volts above baseline. Peak grip force was defined as the maximum amplitude occurring closest in time after object lift-off (Figure 3a). Time to peak grip force was defined as the duration between the onset of grip force and peak grip forces. In some trials multiple peaks of grip force were observed, and in these trials the initial (primary) peak associated with object lift-off was considered as the peak grip force (Figure 3b). In some instances secondary peaks were greater than the primary peak associated with object lift-off. These secondary peaks occurred later in the task with respect to object lift-off and were not used in determining peak grip force.
Figure 3.
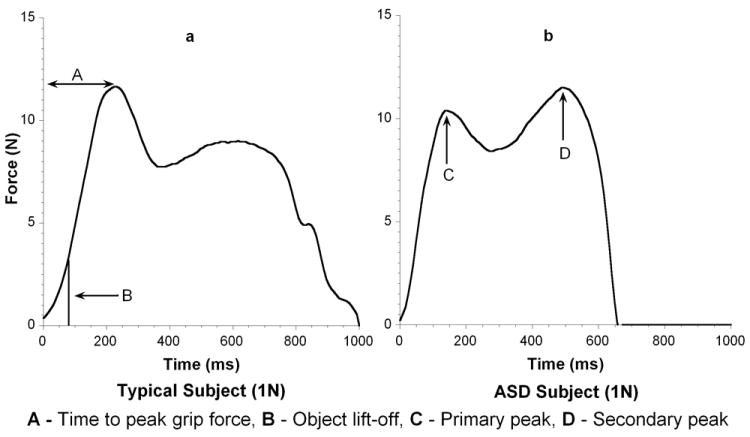
a and b Primary and Secondary Peak Grip Force
To determine the consistency of the output of the load cell, intraclass correlation coefficients [3,5] (ICCs) were computed for both load cells over 5 trials, across three known calibrated weights of 1N, 2N, and 5N. The ICC was used to compare load cell outputs across five 1-second intervals for each trial. The ICC values ranged from 0.92 to 0.99 for both load cells across the three test loads. The test-retest reliability was examined by collecting data from the same subject under identical experimental conditions on two occasions, 45 minutes apart. Data obtained from the two occasions were correlated. Pearson’s product-moment correlation coefficient (r) for peak grip force and grip force at onset of load force were r = 0.92 and r = 0.98, respectively. For the 1N, 2N, and 5N test loads, the absolute differences between the means on the two occasions were 0.6 N, 1.5 N, and 2.6 N, respectively, for peak grip force and 0.1 N, 0.1 N, and 0.4 N, respectively, for grip force at onset of load force.
Statistical Analysis
A 3-level Hierarchical Linear Model using SAS version 9.1 software was implemented, with trials nested within loads, and loads nested within participants. A separate model testing the difference between groups controlling for load was fit for each dependent measure (i.e., grip to load force onset latency, grip force at onset of load force, peak grip force, and time to peak grip force). Load by group interactions were evaluated and found to be non-significant in all cases and were not included in the final model.
Results
The dependent variables did not meet the assumptions for normality and homogeneity of variance; therefore, they were transformed using a logarithmic (log 10) transformation (Table 2). The standard deviations for all variables, except the peak grip force, are greater in the group with ASD. Grip to load force onset latencies were significantly longer in participants with ASD [F (1, 301) = 6.06, p = 0.0144] (Table 3). Grip forces at onset of load forces were significantly greater in participants with ASD [F (1, 301) = 4.64, p = 0.0321] (Table 3). Peak grip forces (PGF) and times to peak grip force (TPGF) were not significantly different between groups (Table 3). The main effect for load was statistically significant for both PGF and TPGF [F (2, 48) = 15.54, p < 0.001 and F (2, 48) = 10.65, p < 0.001 respectively]. Post hoc analysis revealed that PGF and TPGF for the 4.6N load were significantly less than for the 7.6N load (β = -0.11 p<0.001; β = -0.11, p<0.001 respectively). Also, the TPGF for the 5.6N load was significantly less than for the 7.6N load (β = -0.07, p=0.003).
Table 2.
Between Group Descriptive Statistics for Three Load Categories
| Load | Group | GLOT (ms) | GFATLF (N) | PGF (N) | TPGF (ms) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 1 | Autism | 147.5 | 212.1 | 2.9 | 2.1 | 10.2 | 2.8 | 368.4 | 237.3 |
| Typical | 70.4 | 61.8 | 2.0 | 1.3 | 9.8 | 2.8 | 307.8 | 89.0 | |
| 2 | Autism | 127.2 | 135.2 | 2.7 | 1.8 | 12.1 | 2.8 | 418.6 | 270.1 |
| Typical | 87.5 | 105.6 | 2.1 | 1.4 | 11.6 | 5.5 | 322.7 | 142.3 | |
| 4 | Autism | 171.9 | 146.8 | 3.3 | 2.1 | 13.5 | 3.2 | 488.1 | 234.4 |
| Typical | 82.8 | 66.6 | 2.4 | 1.9 | 12.0 | 3.4 | 379.1 | 132.3 | |
| Total | Autism | 148.8 | 169.0 | 2.9 | 2.0 | 11.9 | 3.2 | 424.4 | 251.3 |
| Typical | 80.2 | 80.2 | 2.2 | 1.6 | 11.1 | 4.1 | 336.3 | 126.1 | |
(GLOT = Grip to Load Force Onset Latency, GFATLF = Grip Force at Onset of Load Force, PGF = Peak Grip Force, TPGF = Time to Peak Grip Force)
Table 3.
Between Group ANOVA Table.
| F1,301 | Difference | SE | p | Converted Difference | |
|---|---|---|---|---|---|
| GLOT | 6.06 | 0.26 | 0.10 | 0.01* | 1.82 |
| GFATLF | 4.64 | 0.12 | 0.05 | 0.03* | 1.32 |
| PGF | 1.40 | 0.04 | 0.03 | 0.24 | 1.10 |
| TPGF | 1.59 | 0.07 | 0.05 | 0.20 | 1.17 |
Notes: adjusted for load
(GLOT = Onset Latency between Grip and Load forces, GFATLF = Grip Force at Onset of Load Force, PGF = Peak Grip Force, TPGF = Time to Peak Grip Force, SE = Standard Error)
significant at p<0.05
Discussion
The prolonged grip to load force onset latencies found in this sample of participants with ASD compared with typically developing peers suggests temporal dyscoordination in motor control. Similar findings of prolonged onset latencies between grip and load forces have been observed in persons with cerebellar abnormality, 25 cognitive deficits, 19 and impaired tactile sensitivity in the thumb and index finger. 35 The presence of cognitive delay and to some extent cerebellar abnormality are reported in persons with ASD. 12, 40, 41 Additionally, recent studies examining somatosensory thresholds have shown normal or enhanced perception 42-44 and are thus an unlikely explanation of motor dyscoordination. Abnormalities in the cytoarchitecture (i.e., increased density of cortical minicolumns) of brains of persons with autism have been discovered recently, 45, 46 which may result in problems with functional neural connectivity necessary for multisensory integration, social-communication, and motor coordination, as well as other adaptive functions. 43 Further research is needed to determine to what extent neural deficits may be related to the observed temporal dyscoordination in participants with ASD found in this study.
Grip force must be timed correctly with respect to anticipated object load. 25 At the start of a precision grip, object load is anticipated based on previous trials, not determined from feedback obtained during the trial. This is because insufficient time is available for feedback-based corrections to occur prior to load force onset. The elevated grip force at onset of load force found in persons with ASD relative to typically developing peers in this study may be due to either an inability to correctly time the grip force with respect to anticipated load, and/or inability to use prior experience to correctly anticipate the load. Such dyscoordination may result in hypermetria (i.e., increased amplitude of grip force, as a compensatory strategy to prevent slips and drops). Although timing was abnormal, participants with ASD were able to scale their grip force to changes in load. That is, larger grip forces were used for heavier loads; however, this scaling appeared inefficient, as forces used by these participants were greater than required for the task. These findings suggest that persons with ASD may have more problems in the planning or proactive stages of precision grip as compared to the feedback or reactive stages.
The group with ASD presented with larger standard deviations than the comparison group. Larger standard deviations are indicative of greater movement variability in the group with ASD. This is consistent with previously published findings. Mari et al., 6 reported increased variability in their group with autism across all their kinematic variables. This increased variability could be due to impairment in movement planning and/or error correction. Further research is required to investigate the source of movement variability in ASD.
This study had several limitations. The small sample size may have contributed to insufficient power for detection of differences in some of the components studied that had non-significant results, and limits generalizability of these findings to the population at large. Although the participants with ASD had performance IQs ≥70 (no evidence of intellectual disability per se), differences in cognitive profiles (relative strengths and weaknesses in skills) and disability status between the two groups may have influenced the results. Future research that includes a comparison group with a known intellectual disability matched on mental age to the ASD group could better determine the extent to which cognitive levels affect temporal dyscoordination. In addition, including a wider range of ages and levels of cognitive functioning (IQ) would yield more generalizable findings.
Moreover, future studies should investigate the role of somatosensory and multisensory processing differences in this population 42, 43, 47 on resultant motor performance, as well as the relationship between severity of autistic features and motor impairments 12. Our group has recently completed a study to determine if abnormalities in precision grip are unique to autism as compared to DD and to what extent age and severity are mediating variables. Although cerebellar dysfunction has been implicated in ASD and may be related to temporal dyscoordination in other populations, specific neural correlates underlying such motor control problems in persons with autism have not been determined. Functional magnetic resonance imaging studies should be ideal for addressing these types of research questions.
Conclusions
In conclusion, our sample of children and adolescents with high functioning ASD displayed temporal dyscoordination between grip and load forces while performing a precision grip task as compared to age-matched peers developing typically. These findings enhance our understanding of motor deficits in autism that may have diagnostic as well as clinical implications.
Acknowledgments
This project was funded in part by an internal seed grant through the Department of Allied Health Sciences and the Human Movement Science Student Research Fund at the University of North Carolina at Chapel Hill.
References
- 1.Gilotty L, Kenworthy L, Sirian L, Black DO, Wagner AE. Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychol. 2002;8:241–248. doi: 10.1076/chin.8.4.241.13504. [DOI] [PubMed] [Google Scholar]
- 2.Leary MR, Hill DA. Moving on: Autism and movement disturbance. Ment Retard. 1996;34:39–53. [PubMed] [Google Scholar]
- 3.Filipek PA, Accardo PJ, Baranek GT, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999;29:439–484. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- 4.Dyck MJ, Piek JP, Hay D, Smith L, Hallmayer J. Are abilities abnormally interdependent in children with autism? J Clin Child Adolesc Psychol. 2006;35:20–33. doi: 10.1207/s15374424jccp3501_3. [DOI] [PubMed] [Google Scholar]
- 5.Piek JP, Dyck MJ. Sensory-motor deficits in children with developmental coordination disorder, attention deficit hyperactivity disorder and autistic disorder. Hum Mov Sci. 2004;23:475–488. doi: 10.1016/j.humov.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Mari M, Castiello U, Marks D, Marraffa C, Prior M. The reach-to-grasp movement in children with autism spectrum disorder. Philos Trans R Soc Lond B Biol Sci. 2003;358:393–403. doi: 10.1098/rstb.2002.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ. Movement preparation in high-functioning autism and Asperger disorder: A serial choice reaction time task involving motor reprogramming. J Autism Dev Disord. 2001;31:79–88. doi: 10.1023/a:1005617831035. [DOI] [PubMed] [Google Scholar]
- 8.Wing L. Asperger’s syndrome: A clinical account. Psyshol Med. 1981;11:115–129. doi: 10.1017/s0033291700053332. [DOI] [PubMed] [Google Scholar]
- 9.Miyahara M, Tsujii M, Hori M, Nakanishi K, Kageyama H, Sugiyama T. Brief report: Motor in coordination in children with Asperger syndrome and learning disabilities. J Autism Dev Disord. 1997;27:595–603. doi: 10.1023/a:1025834211548. [DOI] [PubMed] [Google Scholar]
- 10.Greenspan S, Wieder S. Developmental patterns and outcomes in infants and children with disorders in relating and communicating: A chart review of 200 cases of children with autistic spectrum diagnoses. J Autism Dev Disord. 1997;1:87–141. [Google Scholar]
- 11.Henderson SE, Sugden DA. The Movement Assessment Battery for Children. Londaon, UK: The Pyschological Corporation; 1992. [Google Scholar]
- 12.Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, Baird G. Impairment in movement skills of children with autistic spectrum disorders. Dev Med Child Neurol. 2009;51(1):1–7. doi: 10.1111/j.1469-8749.2008.03242.x. [DOI] [PubMed] [Google Scholar]
- 13.Weber RC, Thorpe J. Teaching children with autism through task variation in physical education. Except Child. 1992;59:77–86. doi: 10.1177/001440299205900108. [DOI] [PubMed] [Google Scholar]
- 14.Watling RL, Dietz J. Immediate effect of Ayres’ssensory integration-based occupational therapy intervention on children with autism spectrum disorders. Am J Occup Ther. 2007;61:574–583. doi: 10.5014/ajot.61.5.574. [DOI] [PubMed] [Google Scholar]
- 15.Hodgetts S, Hodgetts W. Somatosensory stimulation interventions for children with autism: Literature review and clinical considerations. Can J Occup Ther. 2007;74:393–400. doi: 10.2182/cjot.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Watling R, Tomchek S, LaVesser P. The Commission on Practice. The scope of occupational therapy services for individuals with autism spectrum disorders across the lifespan. Am J Occup Ther. 2005;59:680–683. doi: 10.5014/ajot.59.6.680. [DOI] [PubMed] [Google Scholar]
- 17.Schaaf RC, Miller LJ. Occupational therapy using a sensory integrative approach for children with developmental disabilities. Ment Retard Dev Disabil Res Rev. 2005;11:143–148. doi: 10.1002/mrdd.20067. [DOI] [PubMed] [Google Scholar]
- 18.Latash ML. Learning motor synergies by persons with Down syndrome. J Intellect Disabil Res. 2007;51:962–971. doi: 10.1111/j.1365-2788.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 19.Cole KJ, Abbs JH, Turner GS. Deficits in the production of grip forces in Down syndrome. Dev Med Child Neurol. 1988;30:752–758. doi: 10.1111/j.1469-8749.1988.tb14637.x. [DOI] [PubMed] [Google Scholar]
- 20.Eliasson AC, Forssberg H, Hung YC, Gordon AM. Development of hand function and precision grip control in individuals with cerebral palsy: A 13-year follow-up study. Pediatrics. 2006;118:e1226–36. doi: 10.1542/peds.2005-2768. [DOI] [PubMed] [Google Scholar]
- 21.Gordon AM, Lewis SR, Eliasson AC, Duff SV. Object release under varying task constraints in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 2003;45:240–248. doi: 10.1017/s0012162203000471. [DOI] [PubMed] [Google Scholar]
- 22.Valvano J, Newell KM. Practice of a precision isometric grip-force task by children with spastic cerebral palsy. Dev Med Child Neurol. 1998;40:464–473. doi: 10.1111/j.1469-8749.1998.tb15397.x. [DOI] [PubMed] [Google Scholar]
- 23.Eliasson AC, Gordon AM, Forssberg H. Tactile control of isometric fingertip forces during grasping in children with cerebral palsy. Dev Med Child Neurol. 1995;37:72–84. doi: 10.1111/j.1469-8749.1995.tb11933.x. [DOI] [PubMed] [Google Scholar]
- 24.Gordon AM, Duff SV. Relation between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 1999;41:586–591. doi: 10.1017/s0012162299001231. [DOI] [PubMed] [Google Scholar]
- 25.Fellows SJ, Ernst J, Schwarz M, Topper R, Noth J. Precision grip deficits in cerebellar disorders in man. Clin Neurophysiol. 2001;112:1793–1802. doi: 10.1016/s1388-2457(01)00623-x. [DOI] [PubMed] [Google Scholar]
- 26.Muller F, Dichgans J. Dyscoordination of pinch and lift forces during grasp in patients with cerebellar lesions. Exp Brain Res. 1994;101:485–492. doi: 10.1007/BF00227341. [DOI] [PubMed] [Google Scholar]
- 27.Forssberg H, Eliasson AC, Kinoshita H, Johansson RS, Westling G. Development of human precision grip. I: Basic coordination of force. Exp Brain Res. 1991;85:451–457. doi: 10.1007/BF00229422. [DOI] [PubMed] [Google Scholar]
- 28.Jeannerod M. The timing of natural prehension movements. J Mot Behav. 1984;16:235–254. doi: 10.1080/00222895.1984.10735319. [DOI] [PubMed] [Google Scholar]
- 29.Zackowski KM, Thach WT, Jr, Bastian AJ. Cerebellar subjects show impaired coupling of reach and grasp movements. Exp Brain Res. 2002;146:511–522. doi: 10.1007/s00221-002-1191-9. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz C, Martineau J, Barthelemy C, Assaiante C. Motor control and children with autism: Deficit of anticipatory function? Neurosci Lett. 2003;348:17–20. doi: 10.1016/s0304-3940(03)00644-x. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 32.Rutter M, Bailey A, Berument SK, Lord C, Pickles A. Social Communication Questionnaire -Lifetime Version. Los Angeles, CA: Western Psychological Services; 2001. Prepublication edition. [Google Scholar]
- 33.Advokat CD, Mayville EA, Matson JL. Side effect profiles of atypical antipsychotics, typical antipsychotics, or no psychotropic medications in persons with mental retardation. Res Dev Disabil. 2000;21:75–84. doi: 10.1016/s0891-4222(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 34.Kistler Instrumentation Corporation. High impedanceload cell, part number: 9212; charge amplifier, part number: 5010B1. Amherst, NY: [Google Scholar]
- 35.Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56:550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- 36.Run Technologies. DATAPAC 2000. Laguna Hills, CA: 2000. [Google Scholar]
- 37.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 38.Bendix T. Adjustment of the seated workplace--with special reference to heights and inclinations of seat and table. Dan Med Bull. 1987;34:125–139. [PubMed] [Google Scholar]
- 39.Kuhtz-Buschbeck JP, Stolze H, Johnk K, Boczek-Funcke A, Illert M. Development of prehension movements in children: A kinematic study. Exp Brain Res. 1998;122:424–432. doi: 10.1007/s002210050530. [DOI] [PubMed] [Google Scholar]
- 40.Fatemi SH, Halt AR, Realmuto G, et al. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol. 2002;22:171–175. doi: 10.1023/A:1019861721160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Courchesne E, Townsend J, Saitoh O. The brain in infantile autism: Posterior fossa structures are abnormal. Neurology. 1994;44:214–223. doi: 10.1212/wnl.44.2.214. [DOI] [PubMed] [Google Scholar]
- 42.Blakemore SJ, Tavassoli T, Calo S, et al. Tactile sensitivity in Asperger syndrome. Brain Cogn. 2006;61:5–13. doi: 10.1016/j.bandc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Cascio C, McGlone F, Folger S, et al. Tactile perception in adults with autism: A multidimensional psychophysical study. J Autism Dev Disord. 2008;38:127–137. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL. Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res. 2007;1154:116–123. doi: 10.1016/j.brainres.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casanova MF, van Kooten IA, Switala AE, et al. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- 46.Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res Cogn Brain Res. 2003;17:651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- 47.Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory experiences questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. J Child Psychol Psychiatry. 2006;47:591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]


