Abstract
Background
Combined transperineal prostate brachytherapy (TPPB) and external beam radiation (EBRT) is widely used for treatment of prostate cancer. Long-term efficacy and toxicity results of a multicenter Phase II trial assessing combination of EBRT and TPPB boost with androgen deprivation therapy (ADT) for intermediate-risk prostate cancer are presented.
Methods
Intermediate-risk patients per MSKCC/NCCN criteria received six months of ADT, 45 Gy EBRT to the prostate and seminal vesicles, followed by TPPB with I125(100 Gy) or Pd103(90 Gy). Toxicity was graded using NCI CTC version 2 and RTOG late radiation morbidity scoring systems. Disease free survival (DFS) was defined as time from enrollment to progression (biochemical, local, distant or prostate cancer death). In addition to the protocol definition of biochemical failure (3 consecutive PSA rises >1.0ng/ml after 18 months from treatment start), the 1997 ASTRO consensus and Phoenix definitions were also assessed in defining DFS. The Kaplan-Meier method was used to estimate DFS and overall survival.
Results
61/63 enrolled patients were eligible. Median follow-up was 73 months. Late grade 2 and 3 toxicity, excluding sexual dysfunction, occurred in 20% and 3% of patients. Six year DFS applying the protocol definition, 1997 ASTRO consensus, and Phoenix definitions was 87.1%, 75.1%, and 84.9%. 6 deaths occurred, only one was attributed to prostate cancer. 6 year overall survival was 96.1%.
Conclusions
In a cooperative setting, combination of EBRT and TPPB boost plus ADT resulted in excellent DFS with acceptable late toxicity for patients with intermediate-risk prostate cancer.
Keywords: prostate, brachytherapy, radiation, cooperative group trial, hormonal therapy
INTRODUCTION
Combined external beam with brachytherapy boost is commonly used for treatment of prostate cancer. Particularly for patients with intermediate risk disease, this approach allows for coverage of the prostate and seminal vesicles with margin achieved with external beam radiation combined with a high dose boost to the prostate with brachytherapy.
While routinely used as an approach to prostate cancer therapy since the late 1980’s, reports of results of this approach in the 1990’s were limited to single institution series. These series indicated that combined modality therapy could be safely administered with promising efficacy results.1,2,3 In recognition of the growing use and success of this approach to treatment of prostate cancer at individual centers of excellence, in the late 1990’s both the Cancer and Leukemia Group B and the Radiation Therapy Oncology Group initiated multi-center phase II trials to assess the use of combined modality therapy as applied across a broad range of institutions through the cooperative research group mechanism.
CALGB 99809 was specifically designed to assess the feasibility, toxicity, and efficacy of combined modality therapy for treatment of patients with intermediate-risk prostate cancer. Patients were uniformly treated with external beam radiation to the prostate and seminal vesicles followed by low dose rate (LDR) prostate brachytherapy boost with 6 months of androgen deprivation therapy (ADT). Long-term results of this study are now presented.
METHODS
Patient eligibility
All patients had histologically confirmed adenocarcinoma of the prostate. A combination of clinical stage, pretreatment prostate-specific antigen (PSA), and biopsy Gleason score was used to define patients with clinically localized intermediate-risk prostate cancer criteria as per the Memorial Sloan–Kettering Cancer Center risk stratification criteria as follows: clinical T1 or T2 classification with PSA ≥10 and <20 ng/mL and Gleason score ≤6 or PSA <10 ng/mL and Gleason score ≥7, or T3a with PSA <10 and Gleason score ≤6 were eligible for the study. Patients with clinical evidence of nodal disease, N1, or evidence of metastases, M1, were excluded.
An Eastern Cooperative Oncology Group performance status of 0–2 was required. Patients had no prior treatment for prostate cancer except <4 weeks of androgen deprivation therapy (ADT), no prior transurethral resection of the prostate, and a prostate size determined by transrectal ultrasound at the time of biopsy or subsequent imaging of <60 cc. Six months of luteinizing hormone-releasing hormone (LHRH) agonist therapy with either leuprolide acetate or goserelin acetate was administered before initiation of external beam radiation. External beam radiation was required to start within a month of initiation of LHRH agonist therapy. Four weeks of oral anti-androgen therapy with either flutamide or bicalutamide was recommended but not required at the start of luteinizing hormone-releasing hormone agonist therapy to prevent a testosterone flare.
Enrolling institutions had experience with prostate ultrasound brachytherapy with at least 50 documented cases performed before registering patients on study. All participants signed an institutional review board–approved, protocol-specific informed consent form in accordance with federal and institutional guidelines. Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. Statistical analyses were performed by CALGB statisticians.
Treatment parameters
EBRT inclusive of the prostate and seminal vesicles was administered with three-dimensional conformal technique using ≥6 MV photons to 45 Gy in 25 fractions. International Commission on Radiation Units and Measurements 50 guidelines and nomenclature were used for the study. The prescribed dose was defined on the central axis at the intersection of the beams. A total dose variation of −5% to +7% from the prescription point dose was allowed. The clinical target volume (CTV) was defined as the prostate and seminal vesicles. A planning target volume (PTV) was applied to the CTV such that a block margin of 1.5–2 cm was applied around the CTV. Dose to 25% of the bladder and 50% of the rectum, defined 2 cm above to 2 cm below the CTV in the anteroposterior projection, was limited to 45 Gy. A daily fraction of 1.8 Gy was administered 5 days per week for 5 weeks.
Brachytherapy was performed 2–4 weeks after completion of EBRT by interstitial implantation using either I125 or Pd103. Preplanning was required via transrectal ultrasound performed within 2 weeks prior to implant; however, intraoperative adjustment in planning was allowed. The brachytherapy CTV was defined as the prostate identified via transrectal ultrasound with no margin. Prescribed dose to the CTV defined per International Commission on Radiation Units and Measurements’ 58 guidelines as the transrectal ultrasound–defined prostate was 100 Gy for I125 (AAPM TG43) or 90 Gy for Pd103 (as per dosimetric information provided by the vendor) in addition to 45 Gy EBRT. Source strength for I125 was required to be 0.30–0.50 U (0.24–0.40 mCi) and for Pd103 1.04–1.30U (0.8–1.0 mCi). A post-implant computed tomography scan was obtained 3–5 weeks after implant with axial images ≤5 mm thickness obtained from at least 2 cm cephalad to the base of the prostate to 2 cm caudad from the apex of the prostate.
Toxicity analysis
Toxicity was graded according to the NCI Common Toxicity Criteria Version 2 and the Radiation Therapy Oncology Group late radiation morbidity scoring systems. Late toxicity was defined as any toxicity persisting or occurring after 9 months following prostate implant. Patients were seen in follow-up per protocol guidelines at 3–5 weeks post implant, then at 3-month intervals until 2 years out from implant, every 6 months for years 2–4, and annually thereafter. At each follow-up through year 4, PSA, testosterone, digital rectal exam, hemoccult, and toxicity analysis including American Urologic Association symptom assessment were required. A central review of toxicity reporting was performed by the study chair (MH) to ensure accuracy and uniformity of toxicity scoring with any changes made in scoring documented with supporting information provided by the submitting institution to ensure transparency of the audit process.
Statistical design and data analysis
The primary end points were acute and late toxicity of EBRT and brachytherapy in patients with clinically localized intermediate-risk prostate cancer who underwent androgen deprivation. Acute toxicities were defined according to the CALGB expanded CTC version 2 and late toxicities were defined according to the RTOG Late Radiation Morbidity Scoring Criteria. Secondary endpoints were biochemical failures, disease free survival (DFS) and overall survival (OS). DFS was defined as time from enrollment to first observed progression (biochemical, local, distant) or prostate cancer death. In addition to the protocol definition of biochemical failure (3 consecutive PSA rises over 1.0ng/ml after 18 months from start of treatment), the 1997 ASTRO consensus definition, and Phoenix (nadir + 2) definition were also used in defining DFS to facilitate comparison with other studies.
The target sample size was 50 patients. Allowing for a 15% ineligibility rate, the total sample size was 60 patients. Sample size determination was based on the late toxicity end point. A single-stage design was used to test the null hypothesis that the late unacceptable radiation toxicity probability at 2 years (p) is p ≤ 0.05 vs. the alternative hypothesis that toxicity probability at 2 years is p ≥ 0.15. Unacceptable toxicity was defined as Grade 3 or higher toxicity, excluding sexual function. If at least 5 patients experienced late radiation toxicities at 2 years, the null hypothesis would be rejected. This design had a power of 89% and a type I error rate of 0.10. Acute and late toxicities at 2 years were estimated by proportions and 95% confidence intervals (CI) based on the binomial distribution. The Kaplan-Meier product-limit method was used to estimate the DFS and OS distributions.
As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating institutions at least once every three years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 29 patients (46%) of the 63 patients under this study.
RESULTS
Sixty-three patients were enrolled; 61 eligible patients with confirmed valid on-study forms were included in the analysis. The median follow-up time among living patients was 73 months (range: 0–96 months). A total of 83% of patients received brachytherapy with I125 and 17% with Pd 103. Patient and tumor characteristics are shown in Table 1.
Table 1.
Baseline characteristics for eligible patients enrolled in Cancer and Leukemia Group B study 99809.
| All patients | |
|---|---|
| N=61 | |
| Age (Years)α | 67 (49–82) |
| Gleason Score of Tumor | |
| 4–6 | 7 (11.5%) |
| 7 | 42 (68.9%) |
| 8–10 | 12 (19.7%) |
| T stage (Clinical) (N=59) | |
| I | 32 (54.0%) |
| II | 27 (46.0%) |
| PSAα | 6.1 (1.6–18.7) |
Median (Range)
Both acute and late toxicity were assessed. Acute grade 2 and 3 toxicity occurred in 25% (95% CI=14%-35%) and 7% (95% CI=0%-13%) of patients respectively. Urinary frequency/urgency (18%), urinary retention (7%), and proctitis (diarrhea, bleeding, and/or pain) (7%) were the most commonly reported grade 2 toxicities. Acute grade 3 toxicity was limited to dysuria (3%), urinary frequency/urgency (2%), and thrombosis (2%). A complete list of grade ≥2 acute toxicities is shown in Table 2. Late grade 2 and 3 toxicity occurred in 20% (95% CI=10%-30%) and 3% (95% CI=0%-8%) of patients respectively. The most common late grade 2 toxicities were urinary frequency/urgency (7%), urinary retention (5%), urinary incontinence (5%), and proctitis (diarrhea, bleeding, and/or pain) (5%). Late grade 3 toxicity was limited to the 2 patients who experienced grade 3 acute dysuria which persisted despite medications. In both cases the dysuria resolved with longer term follow-up. In one case resolution occurred spontaneously and in the second, dysuria resolved after a course of antibiotic therapy. This patient was treated empirically with antibiotics for dysuria in the first few months after brachytherapy and due to persistant dysuria was re-treated with antibiotic therapy a year and a half after treatment. Notably, urinalysis was culture negative on both occasions. A complete list of grade ≥2 late toxicities is shown in Table 3.
Table 2.
Acute grade 2 and higher treatment-related toxicities.
| Grade of Adverse Event | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2- Mod | 3-Severe | 4-LifeThr | 5-Lethal | Total | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Non-Hematologic Adverse Events | |||||||||
| RTOG/EORTC Acute Radiation Morbidity Scoring Scheme | |||||||||
| Constitutional Symptoms | |||||||||
| Fatigue (asthenia, lethargy, malaise) | 2 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Endocrine | |||||||||
| Adrenal insufficiency | 1 | (2%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Hot flashes/flushes | 3 | (5%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Gastrointestinal | |||||||||
| Flatulence | 1 | (2%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Proctitis | 4 | (7%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Pain | |||||||||
| Pain | 2 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Renal/Genitourinary | |||||||||
| Bladder spasms | 1 | (2%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Dysuria (painful urination) | 3 | (5%) | 2 | (3%) | 0 | (0%) | 0 | (0%) | 61 |
| Incontinence | 3 | (5%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Urinary frequency/urgency | 11 | (18%) | 1 | (2%) | 0 | (0%) | 0 | (0%) | 61 |
| Urinary retention (including neurogenic bladder) | 4 | (7%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Vascular | |||||||||
| Thrombosis/thrombus/embolism | 0 | (0%) | 1 | (2%) | 0 | (0%) | 0 | (0%) | 61 |
| SUMMARY | |||||||||
| Maximum Overall AE | 15 | (25%) | 4 | (7%) | 0 | (0%) | 0 | (0%) | 61 |
Table 3.
Late grade 2 and higher treatment-related toxicities.
| Grade of Adverse Event | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2- Mod | 3-Severe | 4-LifeThr | 5-Lethal | Total | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Hematologic Adverse Events | |||||||||
| Hemoglobin | 1 | (2%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| SUMMARY | |||||||||
| Maximum Hematologic AE | 1 | (2%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Non-Hematologic Adverse Events | |||||||||
| RTOG/EORTC Late Radiation Morbidity Scoring Scheme | |||||||||
| Bladder- Late RT Morbidity Scoring | 1 | (2%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Small/Large intestine-Late RT Morbidity Scori | 1 | (2%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Constitutional Symptoms | |||||||||
| Fatigue (asthenia, lethargy, malaise) | 2 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Endocrine | |||||||||
| Hot flashes/flushes | 1 | (2%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Gastrointestinal | |||||||||
| Proctitis | 2 | (3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Pain | |||||||||
| Pain | 1 | (2%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Renal/Genitourinary | |||||||||
| Dysuria (painful urination) | 0 | (0%) | 2 | (3%) | 0 | (0%) | 0 | (0%) | 61 |
| Incontinence | 3 | (5%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Urinary frequency/urgency | 4 | (7%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| Urinary retention (including neurogenic bladd | 3 | (5%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 61 |
| SUMMARY | |||||||||
| Maximum Overall AE | 12 | (20%) | 2 | (3%) | 0 | (0%) | 0 | (0%) | 61 |
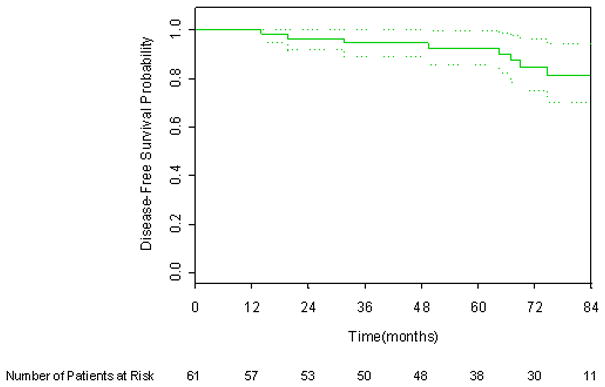
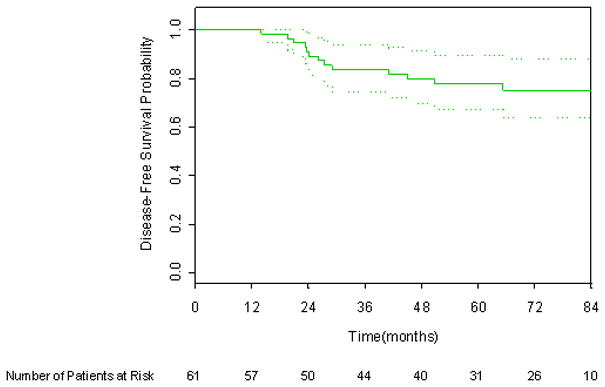
Six year DFS applying the protocol definition, 1997 ASTRO consensus definition, and Phoenix definition of biochemical failure was 87.1%, 75.1%, and 84.9% respectively, as shown in Figures 1 through 3. There were six deaths, only one of which was attributed to prostate cancer. The 6-year overall survival rate was 96.1%, as shown in Figure 4.
Figure 1.
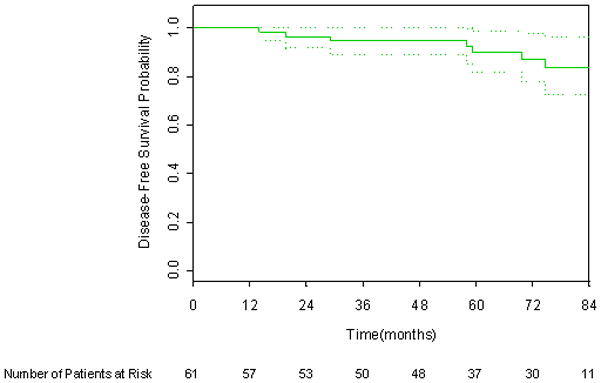
Kaplan-Meier disease-free survival curve incorporating the CALGB protocol definition. 95% CI bars are shown.
Figure 3.
Kaplan-Meier disease-free survival curve incorporating the Phoenix (nadir +2) definition. 95% CI bars are shown.
Figure 4.
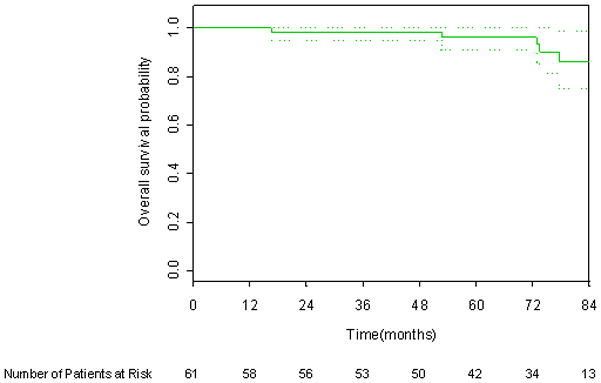
Kaplan-Meier overall survival curve. 95% CI bars are shown.
DISCUSSION
While EBRT combined with LDR brachytherapy is a common approach to treatment of prostate cancer, only two phase II studies conducted by cooperative groups have been completed, including CALGB 99809. The other study, RTOG 0019, similar to CALGB 99809, was designed to assess toxicity and participants were followed up to provide an initial assessment of treatment efficacy. RTOG 0019 included patients with clinical stage cT1c or T2a disease and either a Gleason score of 6 or lower and PSA levels of 10–20 ng/mL, or a Gleason score of 7 and PSA levels up to 20 ng/mL. All 138 patients were treated with EBRT to the prostate and seminal vesicles, which comprised 45 Gy three-dimensional conformal EBRT, followed 2–6 weeks later by LDR brachytherapy boost with 108 Gy I125. Use of up to 6 months of androgen deprivation therapy (ADT) was left to the discretion of the treating physicians. Late genitourinary toxic effects were graded according to the Common Toxicity Criteria Version 2.0, and all other toxic effects were evaluated according to the RTOG and European Organization for Research and Treatment of Cancer late radiation morbidity scoring system. In an initial report, median follow-up was 19 months, and acute grade 3 toxic events were documented in 7.6% of patients. No grade 4 or 5 acute toxic events or late grade 4 or 5 toxic events were observed.4 Increased toxicity was noted in a subsequent report with a median follow-up of 49 months, but was deemed acceptable. Grade 3 genitourinary and gastrointestinal side effects occurred in 10.8% and 3.1% of patients respectively, and grade 4 genitourinary side effects occurred in 2% of patients. The rate of grade 3 or higher gastrointestinal and/or genitourinary toxic effects at 4 years was estimated to be 15%, which was higher than the estimate in RTOG series of EBRT or LDR brachytherapy alone. Biochemical recurrence was defined according to either the 1997 ASTRO consensus definition or the Phoenix definition. The estimated 4-year rate of freedom from biochemical recurrence according to the ASTRO and Phoenix criteria were 81% and 86% respectively.5
To date, expectations for treatment outcomes have been primarily based on retrospective, single-institution analyses. Sylvester and co-workers reported long-term results on 223 patients who underwent EBRT to 45 Gy, the majority of whom received treatment to a limited pelvic field followed by brachytherapy boost with either I125 (108 Gy) or Pd103 (90 Gy). Biochemical recurrence was defined according to a modified ASTRO consensus criterion of two consecutive rises in PSA levels. With median follow-up of 9 years, applying the D’Amico and Memorial Sloan–Kettering Cancer Center risk stratification criteria the 15-year rates of biochemical freedom from recurrence for patients at low risk in the Sylvester et al trial were 86% and 88%, for those at intermediate risk 80% and 80%, and for those at high risk 68% and 53%.6 Dattoli et al reported on patients with median follow-up of 9.5 years. Patients received a median dose of 41 Gy EBRT to a limited pelvic field up to the common iliac lymph nodes, followed by a brachytherapy boost with Pd103 (80–90 Gy).7 Biochemical failure was assessed per the 1997 ASTRO consensus definition, nadir +2 definition, and absolute PSA >0.2 ng/ml at last follow-up. Actuarial rates of freedom from biochemical failure at 14 years were 87% for patients at intermediate risk and 72% for those at high risk without significant variance identified when applying the three definitions of biochemical failure. National Comprehensive Cancer Network guidelines were used for risk stratification (Intermediate risk: T2b to T2c, or Gleason 7, or PSA 10–20 ng/mL; High-risk: T3a or Gleason 8–10, or PSA > 20 mg/mL). Notably, the absolute risk of biochemical failure decreased progressively and fell to less than 1% after 6 years. Other researchers have reported similarly favorable findings in studies with shorter follow-up.8,9,10,11
There are three phase III trials inclusive of men with intermediate disease contempary with 99809 assessing external beam with or without ADT. A trial from the Dana-Farber Cancer Institute included 206 patients with cT1b–cT2b (AJCC 4th edition criteria) with PSA ≥10 and ≤40 ng/ml or PSA ≥ 7 treated with 70 Gy +/− 6 months of total ADT. With median follow-up of 4.5 years a survival benefit was noted for patients receiving ADT with 5 year overall survival of 88% with no deaths due to prostate cancer. Survival without salvage ADT was 82% at 5 years for patients receiving ADT.12 An update with median follow-up of 7.6 years reported estimated 8 year overall survival of 74% with prostate cancer specific mortality of 3% for patients on the ADT arm of the trial.13 The Trans-Tasman Group reported results on 818 men cT2b-cT4 (AJCC 4th edicition criteria) randomized to 66 Gy alone or with either 3 or 6 months of ADT. Only 18% of patients, however, were defined as intermediate risk. For patients receiving 6 months ADT, 5 year disease specific survival, freedom from salvage treatment, and prostate cancer specific survival were 52%, 78%, and 94% respectively.14 Preliminary results of RTOG 9408 were reported in 2009. 1,979 men were enrolled including 1,068 patients with intermediate-risk disease, defined as Gleason score 7 or Gleason of 6 or less and either a PSA of 10–20 or T2b disease. Patients received 66.6 Gy +/− 4 months of ADT. Eight year overall survival with vs. without ADT was 72% vs.66% which was statistically significant.15
A challenge in evaluating efficacy of combined modality therapy inclusive of ADT is the potential for misinterpretation of PSA rise due to testosterone rebound after cessation of ADT with biochemical failure. Distinguishing benign rise in PSA from biochemical recurrence is further complicated by the well documented phenomenon of PSA bounce after brachytherapy.16,17,18 The 1997 ASTRO consensus definition of biochemical failure has been shown to over call biochemical failure for patients who are treated with either ADT or brachytherapy when longer term analyses allowing for subsequent decline in PSA are performed.19,20 To address this concern, the protocol definition of PSA failure, 3 consecutive PSA rises over 1.0ng/ml after 18 months from start of treatment, was developed and applied in analysis. Subsequently, the Phoenix definition, nadir +2, was found to have improved accuracy in defining biochemical failure.20 To facilitate comparisons with past and future studies both the 1997 ASTRO consensus definition and the Phoenix definition were assessed along with the protocol definition. The finding of a lower rate of biochemical control in the current study using the 1997 ASTRO consensus definition in comparison with the other two definitions is therefore not unexpected. Ultimately, impact on survival is the most important assessment of efficacy and only a phase III trial will satisfactorily assess survival.
Treatment efficacy is important, however the impact of any treatment on disease control or eradication has to be considered in the larger context of toxicity and quality of life. Given that prostate cancer presents without significant symptoms for most men with clinically localized disease and that survival is typically protracted, treatment related toxicity is an important factor to be considered in choosing an approach to disease management. The long-term rates of grade 2 and 3 toxicity in the current study compare well to the other completed co-operative group study.5 We previously reported on the primary study endpoint, rate of grade ≥3 toxicity with 39 months median follow-up.21 Now with nearly double the median follow-up time only a modest increase in toxicity was noted, and only in the grade 2 category, with an increase from 13% in our initial report to 21% in this report. It is noted that median time to late GU and GI toxicity is approximately 18 months.22,23,24,25 Therefore, with 73 months median follow-up we believe it is very unlikely that significantly more toxicity will manifest with further follow-up. It is also important to recognize that IMRT was not allowed on CALGB 99809 as guidelines for use of IMRT in cooperative group trials had yet to be developed at the time of study inception. The use of IMRT and improved brachytherapy techniques has potential to further reduce toxicity.
The excellent results of CALGB 99809 in terms of both DFS and toxicity are notable, however there are several limitations of the study that should be recognized. Enrolling institutions all had experience with use of TPPB as there was a requirement that each participating site have a minimum of 50 prior cases in order to accrue patients on this study. Implant quality was excellent as assessed on central review with a median V90 of 98% and only one implant did not meet the minimum study criteria for V90 of 80%. Therefore, the results may not be applicable to centers with less prostate brachytherapy experience. The study also required the use of ADT. While ADT has been shown to improve overall survival in three randomized studies of intermediate risk patients treated with external beam radiation,12,14,15 no randomized studies investigating the use of hormonal therapy have been performed for patients undergoing brachytherapy. Given the modest doses of external beam radiation used on these trials, one hypothesis is that with dose escalation currently in common use, ADT will not be necessary. A new RTOG study, RTOG 0815, has been designed to address this question. Given the potential side effects of ADT, combined external beam and brachytherapy may be a more attractive therapeutic approach if ADT could be omitted. Impotentcy is a common concern for patients contemplating treatment for prostate cancer. Use of ADT coupled with lack of utilization of a validated instrument to assess sexual function made assessment of the impact of combined EBRT with brachytherapy boost difficult on the present study. The total number of patients is also modest albeit sufficient to address the primary study objective. Also, the favorable efficacy findings would be strengthened by confirmatory findings of other multi-institutional studies such as RTOG 0815 and RTOG 0232 which was designed to compare brachytherapy alone vs. external beam radiation with brachytherapy boost for patients with intermediate risk disease. Results from both of these studies will not be available for many years, however the current study provides valuable insight into results achievable with combined modality therapy across a broad range of institutions.
CONCLUSIONS
In a cooperative setting, combination of EBRT and TPPB boost with 6 months of ADT resulted in excellent DFS with acceptable acute and late toxicity for patients with intermediate-risk prostate cancer. Enrollment of intermediate risk patients onto phase III trials assessing combined EBRT with TPPB boost should be encouraged.
Figure 2.
Kaplan-Meier disease-free survival curve incorporating the 1997 ASTRO Consensus definition. 95% CI bars are shown.
Acknowledgments
This work was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Monica M. Bertagnolli, MD, Chair) and to the CALGB Statistical Center (Stephen George, PhD, CA33601).
The research for CALGB 99809 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Monica M. Bertagnolli, MD, Chair) and to the CALGB Statistical Center (Stephen George, PhD, CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Christiana Care Health Services, Inc. CCOP, Wilmington, DE–Stephen Grubbs, M.D., supported by CA45418; Dana-Farber Cancer Institute, Boston, MA–Harold J Burstein, M.D., Ph.D., supported by CA32291; Duke University Medical Center, Durham, NC–Jeffrey Crawford, M.D., supported by CA47577; Missouri Baptist Medical Center, St. Louis, MO–Alan P. Lyss, M.D., supported by CA114558-02; Nevada Cancer Research Foundation CCOP, Las Vegas, NV–John A. Ellerton, M.D., supported by CA35421; Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, M.D., supported by CA59518; Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC–James N. Atkins, M.D., supported by CA45808; State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, M.D., supported by CA21060; University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, M.D., supported by CA31983; University of Massachusetts Medical School, Worcester, MA–William V. Walsh, M.D., supported by CA37135; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO–Michael C Perry, M.D., supported by CA12046; University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, M.D., supported by CA47559; University of Vermont, Burlington, VT–Steven M Grunberg, M.D., supported by CA77406; Wake Forest University School of Medicine, Winston-Salem, NC–David D Hurd, M.D., supported by CA03927
Footnotes
The authors have no financial conflicts to disclose.
References
- 1.Dattoli M, Wallner K, Sorace R, et al. 103Pd brachytherapy and external beam irradiation for clinically localized, high-risk prostate carcinoma. Int J Radiat Oncol Biol Phys. 1997;39(3):776–7. doi: 10.1016/0360-3016(96)00214-3. [DOI] [PubMed] [Google Scholar]
- 2.Ragde H, Blasko JC, Grimm PD, et al. Brachytherapy for clinically localized prostate cancer: results at 7- and 8-year follow-up. Semin Surg Oncol. 1997;13(6):438–43. doi: 10.1002/(sici)1098-2388(199711/12)13:6<438::aid-ssu8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Stone NN, Stock RG. Prostate brachytherapy: treatment strategies. J Urol. 1999;162(2):421–6. doi: 10.1016/s0022-5347(05)68574-6. [DOI] [PubMed] [Google Scholar]
- 4.Lee WR, DeSilvio M, Lawton C, et al. A phase II study of external beam radiotherapy combined with permanent source brachytherapy for intermediate-risk, clinically localized adenocarcinoma of the prostate: preliminary results of RTOG P-0019. Int J Radiat Oncol Biol Phys. 2006;64(3):804–9. doi: 10.1016/j.ijrobp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Lee WR, Bae K, Lawton C, et al. Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy: analysis of Radiation Therapy Oncology Group study 0019. Cancer. 2007;109(8):1506–12. doi: 10.1002/cncr.22560. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester JE, Grimm PD, Blasko JC, et al. 15-Year biochemical relapse free survival in clinical Stage T1-T3 prostate cancer following combined external beam radiotherapy and brachytherapy; Seattle experience. Int J Radiat Oncol Biol Phys. 2007;67(1):57–64. doi: 10.1016/j.ijrobp.2006.07.1382. [DOI] [PubMed] [Google Scholar]
- 7.Dattoli M, Wallner K, True L, et al. Long-term outcomes after treatment with brachytherapy and supplemental conformal radiation for prostate cancer patients having intermediate and high-risk features. Cancer. 2007;110(3):551–5. doi: 10.1002/cncr.22810. [DOI] [PubMed] [Google Scholar]
- 8.Critz FA, Williams WH, Levinson AK, et al. Simultaneous irradiation for prostate cancer: intermediate results with modern techniques. J Urol. 2000;164(3 Pt 1):738–41. doi: 10.1097/00005392-200009010-00028. [DOI] [PubMed] [Google Scholar]
- 9.Merrick GS, Butler WM, Wallner KE, et al. Impact of supplemental external beam radiotherapy and/or androgen deprivation therapy on biochemical outcome after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2005;61(1):32–43. doi: 10.1016/j.ijrobp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Singh AM, Gagnon G, Collins B, et al. Combined external beam radiotherapy and Pd-103 brachytherapy boost improves biochemical failure free survival in patients with clinically localized prostate cancer: results of a matched pair analysis. Prostate. 2005 Jan 1;62(1):54–60. doi: 10.1002/pros.20118. [DOI] [PubMed] [Google Scholar]
- 11.Stock RG, Cahlon O, Cesaretti JA, et al. Combined modality treatment in the management of high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59(5):1352–9. doi: 10.1016/j.ijrobp.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 12.D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004 Aug 18;292(7):821–7. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 13.D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008 Jan 23;299(3):289–95. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 14.Denham JW, Steigler A, Lamb DS, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005 Nov;6(11):841–50. doi: 10.1016/S1470-2045(05)70348-X. [DOI] [PubMed] [Google Scholar]
- 15.Jones C, et al. Short term androgen deprivation prior to and during radiation therapy improves overall survival in patients with t1b-t2b adenocarcinoma of the prostate and PSA ≤ 20: initial results of RTOG 94-08 [abstract] ASTRO. 2009 [Google Scholar]
- 16.Stock RG, Stone NN, Cesaretti JA. Prostate-specific antigen bounce after prostate seed implantation for localized prostate cancer: descriptions and implications. Int J Radiat Oncol Biol Phys. 2003;56(2):448–53. doi: 10.1016/s0360-3016(02)04470-x. [DOI] [PubMed] [Google Scholar]
- 17.Cavanagh W, Blasko JC, Grimm PD, et al. Transient elevation of serum prostate-specific antigen following (125)I/(103)Pd brachytherapy for localized prostate cancer. Semin Urol Oncol. 2000;18(2):160–5. [PubMed] [Google Scholar]
- 18.Critz FA, Williams WH, Benton JB, et al. Prostate specific antigen bounce after radioactive seed implantation followed by external beam radiation for prostate cancer. J Urol. 2000;163(4):1085–9. [PubMed] [Google Scholar]
- 19.Thames H, Kuban D, Levy L, et al. Comparison of alternative biochemical failure definitions based on clinical outcome in 4839 prostate cancer patients treated by external beam radiotherapy between 1986 and 1995. Int J Radiat Oncol Biol Phys. 2003;57(4):929–43. doi: 10.1016/s0360-3016(03)00631-x. [DOI] [PubMed] [Google Scholar]
- 20.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Hurwitz MD, Halabi S, Ou SS, et al. Combination external beam radiation and brachytherapy boost with androgen suppression for treatment of intermediate-risk prostate cancer: an initial report of CALGB 99809. Int J Radiat Oncol Biol Phys. 2008;72(3):814–9. doi: 10.1016/j.ijrobp.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Benoit RM, Naslund MJ, Cohen JK. Complications after prostate brachytherapy in the Medicare population. Urology. 2000;55(1):91–6. doi: 10.1016/s0090-4295(99)00122-3. [DOI] [PubMed] [Google Scholar]
- 23.Zelefsky MJ, Fuks Z, Wolfe T, et al. Locally advanced prostatic cancer: long-term toxicity outcome after three-dimensional conformal radiation therapy--a dose-escalation study. Radiology. 1998;209(1):169–74. doi: 10.1148/radiology.209.1.9769828. [DOI] [PubMed] [Google Scholar]
- 24.Zelefsky MJ, Hollister T, Raben A, et al. Five-year biochemical outcome and toxicity with transperineal CT-planned permanent I-125 prostate implantation for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2000;47(5):1261–6. doi: 10.1016/s0360-3016(00)00550-2. [DOI] [PubMed] [Google Scholar]
- 25.Giordano SH, Lee A, Kuo YF, et al. Late gastrointestinal toxicity after radiation for prostate cancer. Cancer. 2006;107(2):423–32. doi: 10.1002/cncr.21999. [DOI] [PubMed] [Google Scholar]


